Abstract
Study Objectives:
To explore the association between the non-apnea sleep disorder (NSD) and autoimmune diseases.
Design:
Cohort study.
Setting:
Nationwide database research.
Participants:
84,996 adult patients with NSD diagnoses recorded in the Taiwan National Health Insurance Research Database between 2000 and 2003, after excluding those with antecedent autoimmune diseases. A comparison cohort of 84,996 participants was formed by age-, gender-, income-, and urbanization-matched controls.
Interventions:
None.
Measurements and Results:
The two cohorts were followed up for occurrence of autoimmune diseases, including rheumatoid arthritis (RA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), Sjögren's syndrome (SS), and systemic sclerosis (SSc). A Cox proportional hazards regression model was used for muti-variate adjustment. In patients with NSD, the overall risk for incident autoimmune diseases was significantly higher than in controls (adjusted hazard ratio [HR] = 1.47, 95% confidence interval [CI] = 1.41–1.53). With regard to individual diseases, the risks for SLE, RA, AS and SS among NSD patients were also significantly higher than in controls (HR [95% CI] for SLE, RA, AS, and SS were 1.81 [1.50–2.18], 1.45 [1.36–1.54], 1.53 [1.38–1.70], and 1.51 [1.43–1.60], respectively), whereas the increased risk for SSc did not reach statistical significance (HR: 1.36 [0.82–2.26]).
Conclusion:
Patients with non-apnea sleep disorder were associated with a higher risk for developing autoimmune diseases.
Citation:
Hsiao YH, Chen YT, Tseng CM, Wu LA, Lin WC, Su VY, Perng DW, Chang SC, Chen YM, Chen TJ, Lee YC, Chou KT. Sleep disorders and increased risk of autoimmune diseases in individuals without sleep apnea. SLEEP 2015;38(4):581–586.
Keywords: sleep disorder, non-apnea sleep disorder, autoimmune disease, autoimmunity
INTRODUCTION
Sleep has a vital and widespread impact on human immunity.1 Several animal and human studies have shown insufficient sleep may enhance the susceptibility to infection.2,3 However, the deranged immunity caused by sleep deprivation may not only be restricted to the dampened response against pathogens, but may also involve breakdown of immunologic self-tolerance, thereby driving development of autoimmune diseases.4
In a murine model of systemic lupus erythematosus (SLE), sleep deprivation contributed to an earlier onset of the disease.4 Epidemiologic evidence evaluating the influence of sleep disorders on the susceptibility of autoimmune diseases is limited to sleep apnea,5 whereas other sleep disorders were not thoroughly studied, for example, insomnia, the most common sleep disorder. We hypothesized sleep disorders other than sleep apnea—non-apnea sleep disorders (NSD)—would predispose occurrence of autoimmune diseases. We thus conducted this cohort study to explore their relationship by utilizing a nationwide database.
MATERIALS AND METHODS
Data Source
The National Health Insurance (NHI) program in Taiwan, which was launched by the government under the principle of mandatory and universal enrollment for more than 23 million people beginning March 1, 1995, provided adequate health care and covered approximately 98.4% of Taiwanese in 2007.6 The National Health Research Institute (NHRI) organized the entire NHI Research Database (NHIRD) computerized claims data, including demographic data, treatment, and diagnosis by International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM),7 and the database is one of the largest nationwide population-based databases in the world. A consistent encryption procedure was applied to all the cohort data, including each patient's original identification, to protect privacy. In addition, the linkage of claims belonging to the same patient is feasible within the NHIRD datasets. In the current study, we used the Longitudinal Health Insurance Database (LHID) from 1995 to 2010 obtained from the NHIRD. The LHID consisted of one million beneficiaries randomly sampled from the original NHI beneficiaries.
This study was exempt from full review by the Institutional Review Board of Taipei Veterans General Hospital because the datasets consisted of de-identified secondary data released to the public for research purposes.
Study Sample
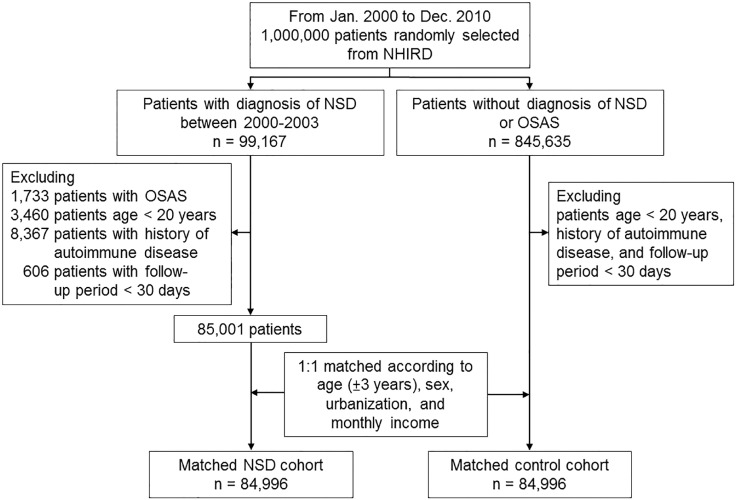
We performed a nationwide study to assess the association between incidence of NSD and autoimmune disease. Two cohorts, the NSD cohort and matched control cohort, were enrolled (as shown in Figure 1). The NSD cohort comprised all adult patients (age ≥ 20 years) who were newly diagnosed with sleep disorders (ICD-9-CM codes, 307.4 and 780.5x, except for sleep apnea [ICD-9-CM codes, 780.51, 780.53, and 780.57]) from inpatient and outpatient claims data of LHID for the period of January 2000 to December 2003.8 The control cohort was selected from the remaining participants in the LHID and matched for age (± 3 years), gender, income, and urbanization.
Figure 1.
Study profile. NHIRD, National Health Insurance Research Database; NSD, non-apnea sleep disorders; OSAS, obstructive sleep apnea syndrome.
The date of enrollment was defined as the date on which sleep disorders were initially diagnosed. In both cohorts, participants had a past medical history of any autoimmune disease before enrollment (ICD-9-CM code 714.0, 720, 720.0, 710.0, 370.33, 710.2, 710.1, 710.3, 710.4, 710.9, 242, 242.0, 242.00, 242.01, 245.2, 571.42, 696, 696.0, 696.1, 136.1, 556.9, 446.7, 446.5, 446.0, 446.4, 287.0, 713.6, 273.2, and 710.x) were excluded. The participants with a follow-up period less than 30 days were also excluded.
Charlson comorbidity index (CCI) scores were calculated to represent global comorbidity severity at baseline.9
Outcome
The primary outcome was the occurrence of autoimmune diseases, including rheumatoid arthritis (RA) (ICD-9-CM code 714.0), ankylosing spondylitis (AS) (ICD-9-CM codes 720 or 720.0), and SLE (ICD-9-CM code 710.0), Sjögren's syndrome (SS) (ICD-9-CM codes 370.33 or 710.2), and systemic sclerosis (SSc) (ICD-9-CM codes 710.1). Both cohorts were followed from the date of enrollment until the occurrence of primary outcome, death, or the end of the study (December 31, 2010).
Statistical Analysis
Pearson χ2 tests were used for comparisons between categorical variables, whereas independent t-tests were utilized for parametric continuous variables. The incidences were compared based on the assumption of Poisson distribution with 95% confidence interval (CI) expressed. A Cox regression model was employed for multivariate adjustment, including baseline comorbid diseases and CCI score. The cumulative incidences of overall and individual autoimmune diseases were compared by Kaplan-Meier method and log-rank test.
Microsoft SQL Server 2008 R2 (Microsoft Corp., Redmond, WA, USA) was used for data linkage, processing, and sampling. All statistical analyses were conducted using STATA statistical software (version 12.0; StataCorp, TX, USA). Statistical significance was defined as a P value < 0.05.
RESULTS
Baseline Characteristics of Non-Apnea Sleep Disorders and Comparison Cohort
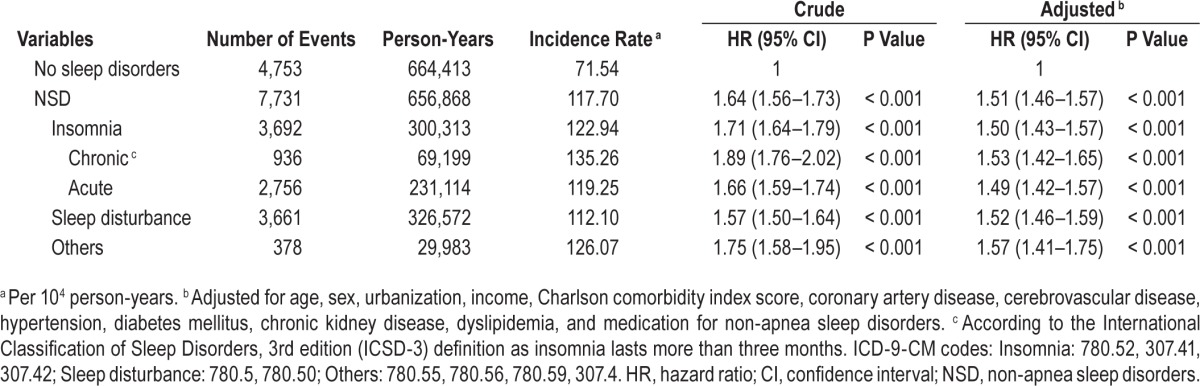
Table 1 shows the demographic characteristics for all sampled participants, including 84,996 NSD patients and 84,996 controls. NSD included insomnia (ICD-9-CM code 780.52, 41.12%), sleep disturbance (780.5, 780.50, 46.92%), unspecified disruptions of 24-h sleep-wake cycle (780.55, 0.006%), dysfunctions associated with sleep stage or arousal from sleep (780.56, 0.06%), and others (780.59 & 307.4, 11.89%).
Table 1.
Demographic and clinical characteristics of our study subjects.
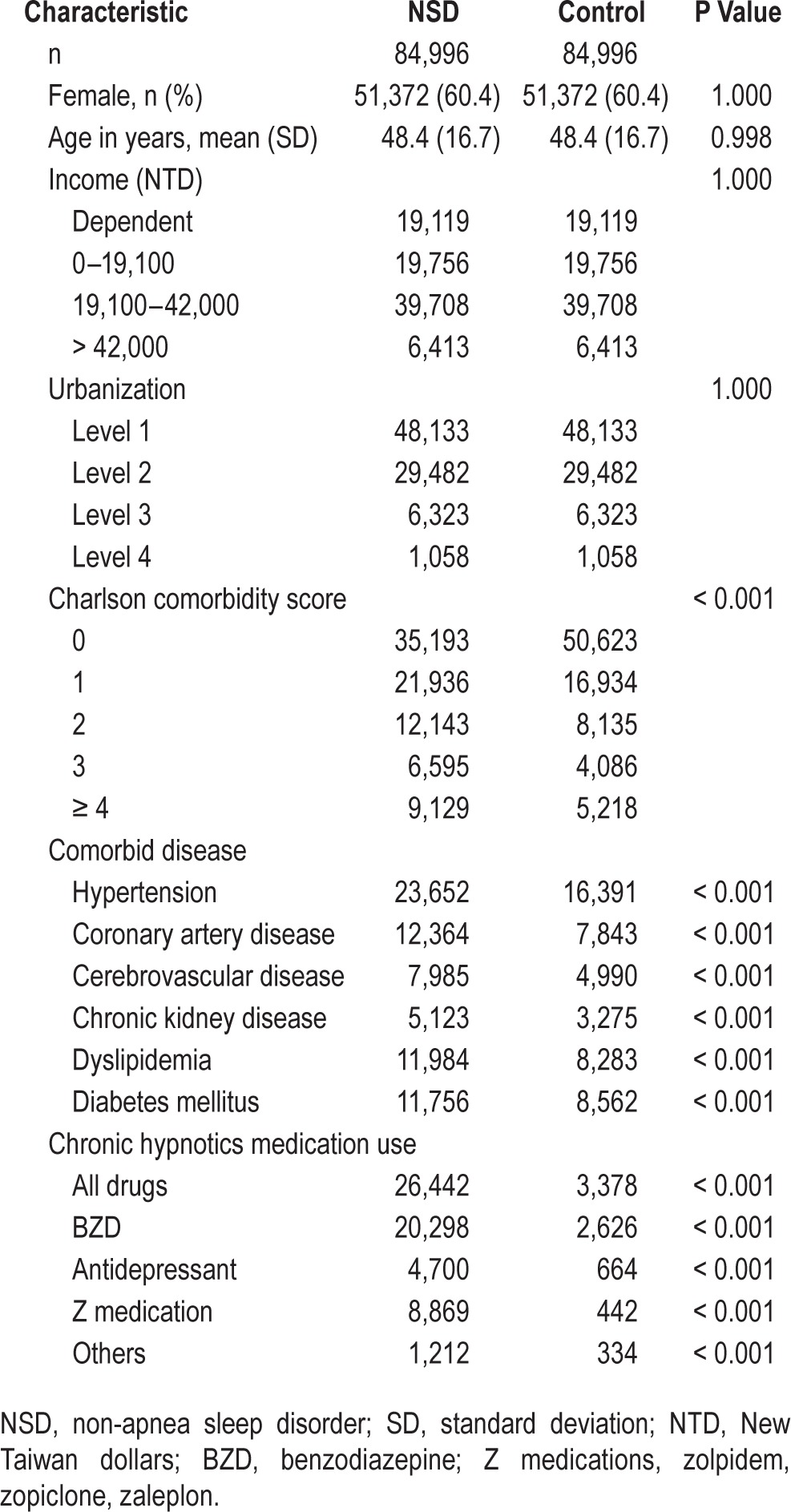
The controls were well matched for age, gender, income, and urbanization level (all P > 0.9). The mean age was 48.4 (standard deviation [SD] ± 16.7) years, and the majority of participants in two cohorts were female (60.4%). The follow-up period was 7.77 years on average. Patients with NSD had higher CCI score, more comorbid diseases, and more hypnotics medication use than controls (Table 1).
Higher Incidence of Incident Autoimmune Diseases among Non-Apnea Sleep Disorders Patients
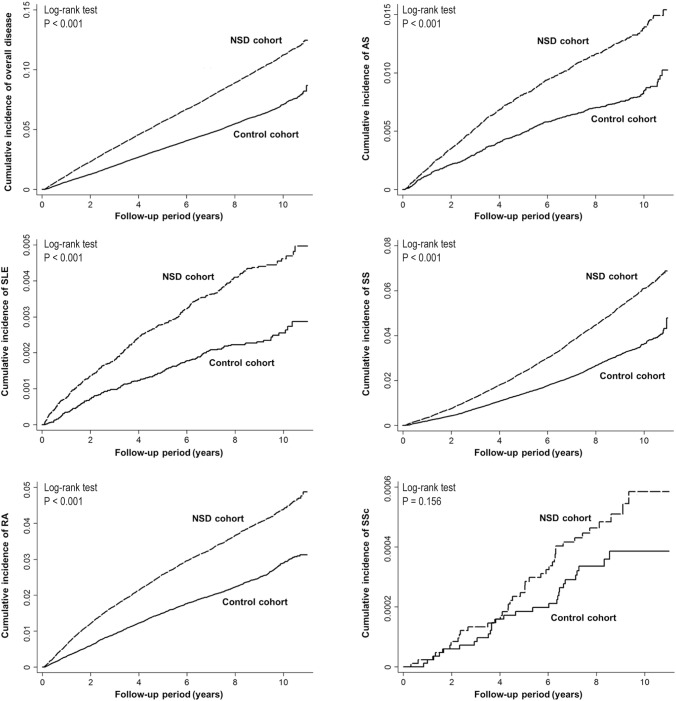
There were 7,731 new autoimmune diseases cases among the NSD cohort and 4,753 among the matched-control cohort during the follow-up of 1,321,281 person-years (Table 2). For NSD patients, the crude and adjusted hazard ratios (HR) for all autoimmune diseases were 1.64 (95% confidence interval [CI], 1.59–1.70; P < 0.001) and 1.47 (95% CI, 1.41–1.53; P < 0.001), respectively (Table 2). With regard to individual types of autoimmune disease, the risks for developing SLE, RA, AS and SS among patients with NSD were also significantly higher than in subjects without NSD (adjusted HR [95% CI] = 1.81 [1.50–2.18] for SLE; 1.45 [1.36–1.54] for RA; 1.53 [1.38–1.70] for AS; 1.51 [1.43–1.60] for SS), whereas the increased risk for SSc did not reach statistical significance (adjusted HR = 1.36 [95% CI: 0.82–2.26], P = 0.234). Kaplan-Meier analyses also revealed NSD patients had a higher risk for autoimmune diseases (log-rank test, P < 0.001, Figure 2). Further association between risk for developing autoimmune diseases and different types of NSD was also examined. The adjusted HRs (95% CI) were 1.49 (1.42–1.57) for acute insomnia; 1.53 (1.42–1.65) for chronic insomnia; 1.52 (1.46–1.59) for sleep disturbance; and 1.57 (1.41–1.75) for other NSD (Table 3).
Table 2.
Incidence of autoimmune diseases among patients with non-apnea sleep disorders and controls.
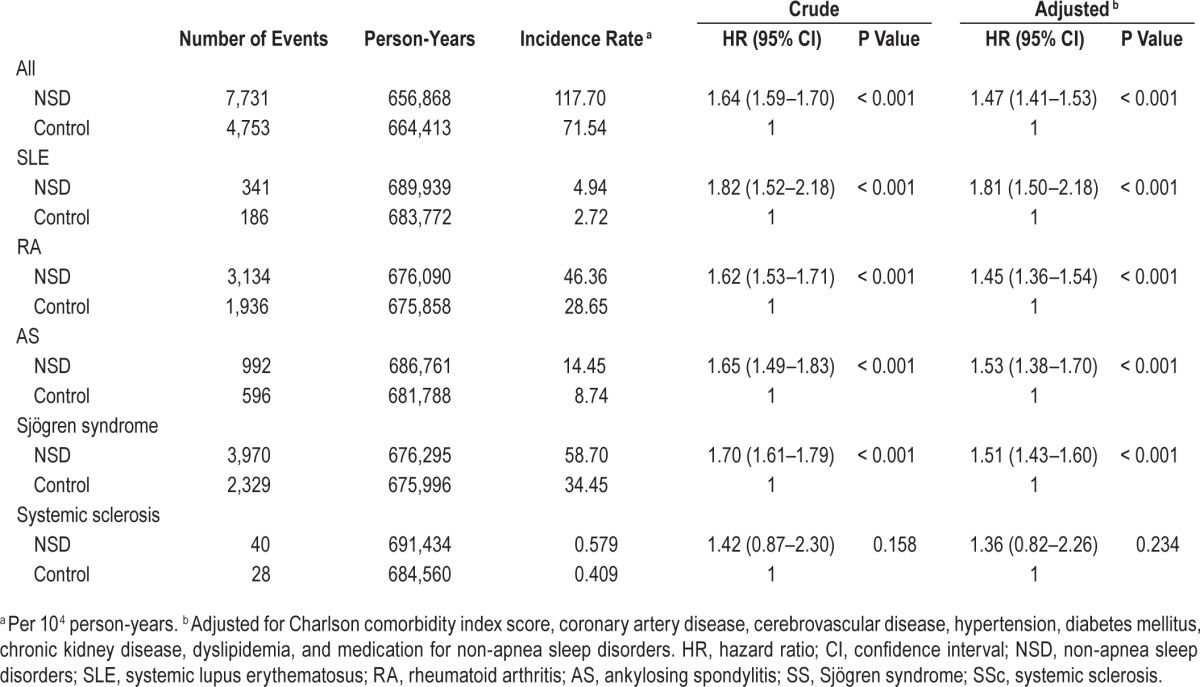
Figure 2.
Kaplan-Meier plots of cumulative incidence of autoimmune diseases. NSD, non-apnea sleep disorders; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; AS, ankylosing spondylitis; SS, Sjögren's syndrome; SSc, systemic sclerosis.
Table 3.
Incidence and hazard ratios of autoimmune diseases in patients with different types of non-apnea sleep disorders.
DISCUSSION
It has long been known that sleep has a great impact on immune function, although little evidence has been explored and translated to clinical practice. This study is the first to demonstrate the linkage of sleep disorders to subsequent occurrence of autoimmune diseases, including SLE, RA, AS, and SS, by using a large nationwide database. The increased risk of incident SSc did not reach statistical significance, which might be due to the relative lower incidence of SSc in our (Asian) cohorts.10 Although the etiology of autoimmune diseases is not well understood, our results suggested that disturbed sleep may be a trigger or a risk factor for such diseases.
Published literature has focused on the sleep quality of patients with autoimmune diseases, showing lower sleep quality is common and independently associated with higher disease activity.11,12 Whether or not individuals with sleep disorders are susceptible to autoimmune diseases remains unanswered. Our data disclosed sleep disorders confer a higher risk for autoimmune diseases, supporting a bidirectional relationship between sleep disorders and autoimmune diseases.
Our conclusion was reinforced by several animal and human studies. In an experimental model of SLE, mice subjected to sleep deprivation exhibited a hastened development of the disease, as reflected by an increased number of anti-nuclear antibodies.4 In addition, the function of regulatory T cells (Treg) in patients with autoimmune diseases appeared to be impaired.13 Treg are the key players to suppress inappropriate immune response, maintaining self-tolerance.14 Breakdown of self-tolerance is central to the pathogenesis of most autoimmune diseases. In experimentally sleep-deprived healthy persons, the suppressive activity of Treg is reduced, providing a link between sleep disorder and autoimmune diseases.15
Furthermore, sleep deprivation resulted in elevation of several proinflammatory cytokines, including IL (interleukin)-1, IL-1β, IL-6, IL-17, and TNF-α. Of note, IL-17 remained elevated despite recovery for 7 days following sleep deprivation, indicating the pivotal role IL-17 played in coordinating the inflammation.16 IL-17 has been associated with several autoimmune diseases including SLE, RA, inflammatory bowel disease, and multiple sclerosis.17 Overt production of IL-17 may amplify systemic inflammation through activating auto-antibody production and enhancing the immune response at inflammation locations.18,19 Taken together, disordered sleep may induce systemic inflammation and activate the function of IL-17-secreting (Th17) cells, thereby predisposing to the occurrence of autoimmune diseases.16,17,20
One particular strength of this study is its matched-control cohort study design. Since the NHI is a single-payer, mandatory insurance program, the majority of events could be traced with a minimum of referral bias. Also, the large sample size of our studied subjects allowed a powerful conclusion to be drawn.
Our study has several limitations. Firstly, diagnoses of sleep disorders and autoimmune diseases that rely on administrative claims data could be less accurate than diagnoses made by standardized criteria, which may be an inherent weakness for all database research. Furthermore, our database lacks some personal information, including body mass index, smoking status, alcohol consumption, and family history. Even though the etiology of most autoimmune diseases is largely unknown, cigarette smoking, for instance, has been reported to predis-pose to the occurrence of RA and SLE.21 Lastly, sleep disorders are complex and heterogeneous despite disruption of sleep being their main feature. More details regarding evaluation of sleep, such as sleep duration, sleep depth, or the extent of sleep disruption are not included in this study, which may call for further research to elucidate their differential influence on the development of autoimmunity.
CONCLUSION
Patients with NSD were associated with a higher risk of developing autoimmune diseases. Further investigation to elucidate the mechanism underlying this link is required.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. All authors had access to the data and played a role in writing this paper. IRB: Waived by the Institutional Review Board of Taipei Veterans General Hospital.
ACKNOWLEDGMENTS
Yi-Han Hsiao and Yung-Tai Chen contributed equally to this work. The authors thank the Taiwan National Health Research Institute for providing the insurance claims data. Author Contributions: Kun-Ta Chou: study conception and design, data analysis and interpretation, drafting of manuscript; Yi-Han Hsiao: study design, data analysis, drafting of manuscript; Yung-Tai Chen: data acquisition and analysis; Ching-Min Tseng: data analysis and interpretation and statistical analysis; Li-An Wu: data analysis and statistical analysis; Wei-Chen Lin: data analysis and interpretation; Vincent Yi-Fong Su: data analysis and statistical analysis; Diahn-Warng Perng: data analysis and interpretation; Shi-Chuan Chang: data analysis and interpretation; Yuh-Min Chen: data analysis and interpretation, statistical analysis; Tzeng-Ji Chen: acquisition of data and technical support; Yu-Chin Lee: study design, interpretation and statistical analysis, and critical revision of the manuscript for important intellectual content.
REFERENCES
- 1.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2000;278:R905–16. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–7. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma BD, Gabriel A, Jr., Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1527–32. doi: 10.1152/ajpregu.00186.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kang JH, Lin HC. Obstructive sleep apnea and the risk of autoimmune diseases: a longitudinal population-based study. Sleep Med. 2012;13:583–8. doi: 10.1016/j.sleep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.National Health Research Institutes. Introduction to the National Health Insurance Research Database (updated 2007) [Accessed April 1, 2014]. Available at: http://w3.nhri.org.tw/nhird//date_01.html.
- 7.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 8.Chung WS, Lin CL, Chen YF, et al. Sleep disorders and increased risk of subsequent acute coronary syndrome in individuals without sleep apnea: a nationwide population-based cohort study. Sleep. 2013;36:1963–8. doi: 10.5665/sleep.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol. 2010;6:468–76. doi: 10.1038/nrrheum.2010.86. [DOI] [PubMed] [Google Scholar]
- 11.Karadag O, Nakas D, Kalyoncu U, Akdogan A, Kiraz S, Ertenli I. Effect of anti-TNF treatment on sleep problems in ankylosing spondylitis. Rheumatol Int. 2012;32:1909–13. doi: 10.1007/s00296-011-1907-x. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasekhara PK, Jayachandran NV, Rajasekhar L, Thomas J, Narsimulu G. The prevalence and associations of sleep disturbances in patients with systemic lupus erythematosus. Mod Rheumatol. 2009;19:407–15. doi: 10.1007/s10165-009-0185-x. [DOI] [PubMed] [Google Scholar]
- 13.Carbone F, De Rosa V, Carrieri PB, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155:231–8. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. Int J Infereron Cytokine Mediator Res. 2009;29:393–8. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 17.Volin MV, Shahrara S. Role of TH-17 cells in rheumatic and other autoimmune diseases. Rheumatology (Sunnyvale) 2011;1(104) doi: 10.4172/2161-1149.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doreau A, Belot A, Bastid J, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 19.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–14. [PubMed] [Google Scholar]
- 20.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best practice & research. Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]