Abstract
The Cl- secretory pathway that is defective in cystic fibrosis (CF) can be bypassed by an alternative pathway for Cl- transport that is activated by extracellular nucleotides. Accordingly, the P2 receptor that mediates this effect is a therapeutic target for improving Cl- secretion in CF patients. In this paper, we report the sequence and functional expression of a cDNA cloned from human airway epithelial (CF/T43) cells that encodes a protein with properties of a P2U nucleotide receptor. With a retrovirus system, the human airway clone was stably expressed in 1321N1 astrocytoma cells, a human cell line unresponsive to extracellular nucleotides. Studies of inositol phosphate accumulation and intracellular Ca2+ mobilization induced by extracellular nucleotides in 1321N1 cells expressing the receptor identified this clone as the target receptor in human airway epithelia. In addition, we independently isolated an identical cDNA from human colonic epithelial (HT-29) cells, indicating that this is the same P2U receptor that has been functionally identified in other human tissues. Expression of the human P2U receptor (HP2U) in 1321N1 cells revealed evidence for autocrine ATP release and stimulation of transduced receptors. Thus, HP2U expression in the 1321N1 cell line will be useful for studying autocrine regulatory mechanisms and in screening of potential therapeutic drugs.
Full text
PDF

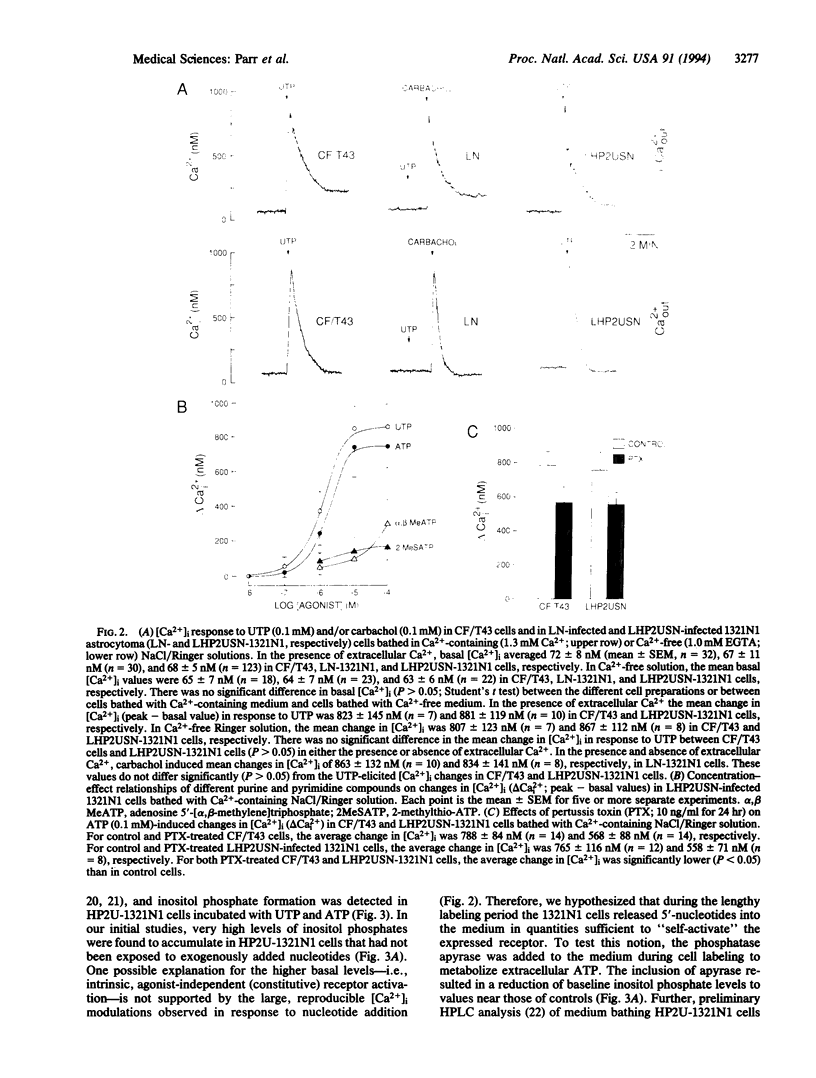
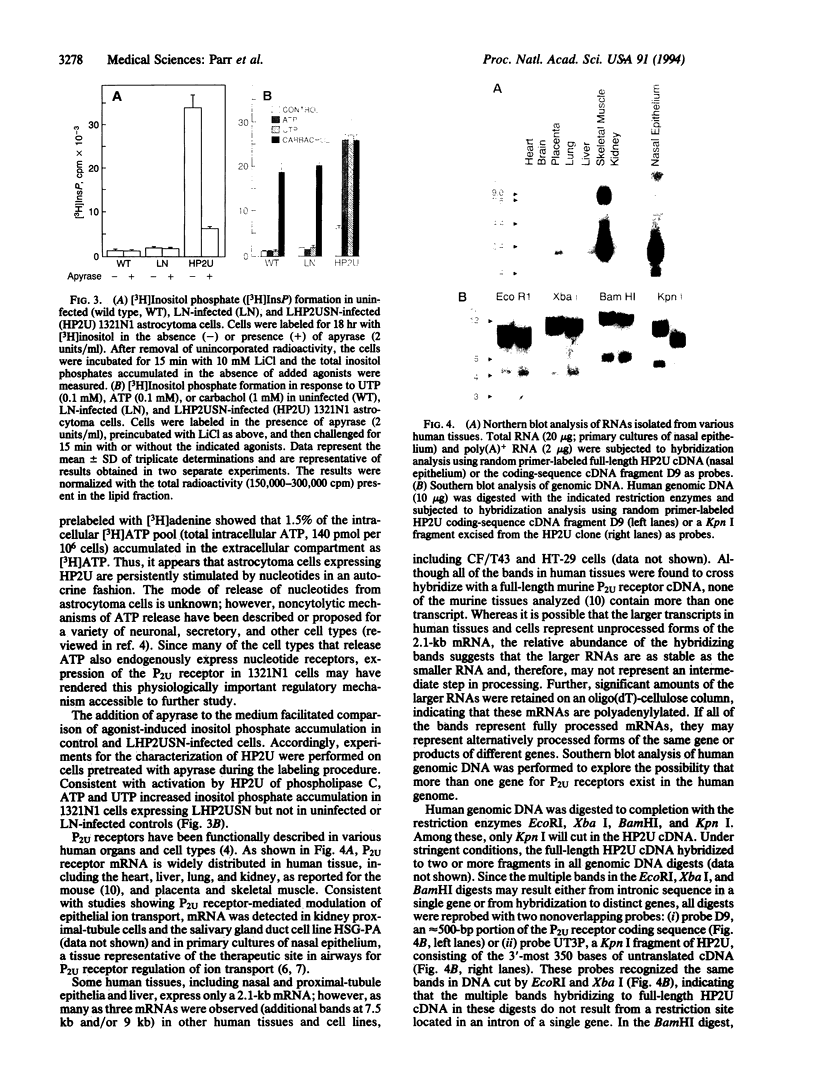

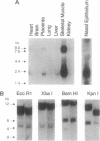
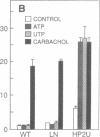
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. A., Lazarowski E. R., Boucher R. C., Harden T. K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5'-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991 Nov;40(5):648–655. [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Clarke L. L., Grubb B. R., Yankaskas J. R., Cotton C. U., McKenzie A., Boucher R. C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. W., Dowell M. L., Lethem M., Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am J Physiol. 1992 May;262(5 Pt 1):C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Erb L., Lustig K. D., Sullivan D. M., Turner J. T., Weisman G. A. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Mann J. S., Church M. K., Cushley M. J. Mechanisms and significance of adenosine-induced bronchoconstriction in asthma. Allergy. 1987 Oct;42(7):481–484. doi: 10.1111/j.1398-9995.1987.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Clarke L. L., Boucher R. C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991 Aug 22;325(8):533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- Lazarowski E. R., Mason S. J., Clarke L., Harden T. K., Boucher R. C. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br J Pharmacol. 1992 Aug;106(4):774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethem M. I., Dowell M. L., Van Scott M., Yankaskas J. R., Egan T., Boucher R. C., Davis C. W. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol. 1993 Sep;9(3):315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- Lustig K. D., Erb L., Landis D. M., Hicks-Taylor C. S., Zhang X., Sportiello M. G., Weisman G. A. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992 Feb 19;1134(1):61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason S. J., Paradiso A. M., Boucher R. C. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991 Jul;103(3):1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Characterization of phagocyte P2 nucleotide receptors expressed in Xenopus oocytes. J Biol Chem. 1990 Jul 15;265(20):11615–11621. [PubMed] [Google Scholar]
- Ryll T., Wagner R. Improved ion-pair high-performance liquid chromatographic method for the quantification of a wide variety of nucleotides and sugar-nucleotides in animal cells. J Chromatogr. 1991 Sep 18;570(1):77–88. doi: 10.1016/0378-4347(91)80202-n. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Wu H., Franklin C. C., Kim H. D., Turner J. T. Regulation of calcium-activated potassium efflux by neurotensin and other agents in HT-29 cells. Am J Physiol. 1991 Jan;260(1 Pt 1):C35–C42. doi: 10.1152/ajpcell.1991.260.1.C35. [DOI] [PubMed] [Google Scholar]
- Yu H. X., Turner J. T. Functional studies in the human submandibular duct cell line, HSG-PA, suggest a second salivary gland receptor subtype for nucleotides. J Pharmacol Exp Ther. 1991 Dec;259(3):1344–1350. [PubMed] [Google Scholar]