Abstract
The dysfunction of the primary cilium, a complex, evolutionarily conserved, organelle playing an important role in sensing and transducing cell signals, is the unifying pathogenetic mechanism of a growing number of diseases collectively termed “ciliopathies”, typically characterized by multiorgan involvement. Developmental defects of the central nervous system (CNS) characterize a subset of ciliopathies showing clinical and genetic overlap, such as Joubert syndrome (JS) and Meckel syndrome (MS). Although several knock-out mice lacking a variety of ciliary proteins have shown the importance of primary cilia in the development of the brain and CNS-derived structures, developmental in vitro studies, extremely useful to unravel the role of primary cilia along the course of neural differentiation, are still missing.
Mouse embryonic stem cells (mESCs) have been recently proven to mimic brain development, giving the unique opportunity to dissect the CNS differentiation process along its sequential steps. In the present study we show that mESCs express the ciliary proteins Meckelin and Jouberin in a developmentally-regulated manner, and that these proteins co-localize with acetylated tubulin labeled cilia located at the outer embryonic layer. Further, mESCs differentiating along the neuronal lineage activate the cilia-dependent sonic hedgehog signaling machinery, which is impaired in Meckelin knockout cells but results unaffected in Jouberin-deficient mESCs. However, both lose the ability to acquire a neuronal phenotype. Altogether, these results demonstrate a pivotal role of Meckelin and Jouberin during embryonic neural specification and indicate mESCs as a suitable tool to investigate the developmental impact of ciliary proteins dysfunction.
Keywords: Embryonic stem cells, Neural differentiation, Primary cilium
1. Introduction
Primary cilia are non-motile microtubule-based, dynamic, elon-gated structures extending from the membrane of non-proliferating and G1 phase cycling cells, that play a central role in sensing and transducing cell signals, in regulating developmental pathways and in maintaining tissue homeostasis (Marshall and Nonaka, 2006). A variety of signal transduction pathways depend on the primary cilium, especially during the embryonic development. In particular, the sonic hedgehog (shh), the non-canonical wnt/Planar Cell Polarity (PCP), and the Platelet Derived Growth Factor Receptor (PDGFR) pathways are all known to be regulated by primary cilia (Lancaster and Gleeson, 2009). Both positive and negative effects on the canonical wnt pathway have been reported (Lancaster et al., 2009; Ocbina et al., 2009) and it has recently been shown that primary cilia down-regulate the wnt/β-catenin signaling through a spatial mechanism involving compartimentalization of specific downstream signaling components (Lancaster et al., 2011a).
Mutations in several ciliary genes have been found to cause an expanding number of human disorders now grouped under the term “ciliopathies”. The wide genetic heterogeneity of these diseases is the consequence of the high complexity of the primary cilium, whose central structure is constituted by more than 1000 polypeptides (Gherman et al., 2006 and http://www.ciliaproteome.org); on the other hand, the broad phenotypic spectrum of ciliopathies, often involving distinct organs and tissues, well reflects the extensive distribution of cilia in all sorts of cell types, including renal podocytes, endothelial and smooth muscle cells, fibroblasts, retinal photoreceptors and neurons.
The involvement of the central nervous system (CNS) is found in a subset of ciliopathies, including Joubert syndrome (JS; MIM 213300) and Meckel syndrome (MS; MIM 249000), and is mainly characterized by an abnormal development of the mid-hindbrain structures, leading to cerebellar vermis hypoplasia and other posterior fossa abnormalities. The pathogenetic mechanism underlying these neurological phenotypes has not been fully elucidated. A perturbed shh signaling has been evoked as a major cause of primary cilia-dependent CNS developmental defects (Han et al., 2008; Louie and Gleeson, 2005); more recently, decreased wnt activity was found in the developing cerebellum of mice knock out for Ahi1, the first gene found mutated in JS patients, which encodes for the ciliary protein Jouberin (Jbn) (Lancaster et al., 2011b). The vast majority of these studies are based on the use of knock out animals, and related functional analyses have been largely performed in recipient cells overexpressing the ciliary protein(s) of interest, beyond any developmental context.
Mouse embryonic stem cells (mESCs) represent the prototype of pluripotent stem cells and are a useful tool to investigate developmental pathways in vitro. They maintain their self-renewal properties when cultured in the presence of the Leukemia Inhibitory Factor (LIF), ensuring the constant expression of a transcriptional network, whose members (mainly Oct4, Nanog and Sox2) are responsible for the epigenetic maintenance of mESC stemness (Boheler, 2009). Upon LIF deprivation, the expression of these factors begins to “oscillate” to decline when differentiation occurs Boheler, 2009; Spallotta et al., 2010). Under proper culture conditions and addition of specific morphogens, mESCs may give origin to virtually any cell type. A great advantage is represented by the opportunity to culture these cells both as adherent cultures and three-dimensional embryoid bodies (EBs), which mimic at the best the early stages of in vivo embryonic differentiation, as soon as they form the three embryonic germ layers (ectoderm, mesoderm and endoderm) (Kurosawa, 2007). Currently, at least 200 somatic cell types have been obtained by using mESCs, including those belonging to the neuronal lineage. Indeed, using differentiation protocols based on the administration of defined cocktails of growth factors and/or morphogens within precise temporal windows, neuronal population from forebrain, mid-hindbrain and spinal cord have been obtained together with glial cells, following sequential differentiation steps that closely resemble those occurring in vivo (Gaspard and Vanderhaeghen, 2010). These results have given the unique opportunity to study complex developmental pathways in a relatively simple in vitro cell model.
Intriguingly, human ESCs possess primary cilia and cilia-dependent signaling machinery, and it has been recently demonstrated that also mESCs are provided with primary cilia (Hunkapiller et al., 2011; Kiprilov et al., 2008). Further, primary cilia are required for the formation of neural progenitors (Spassky et al., 2008). In the present study, we demonstrate that two ciliary proteins implicated in the pathogenesis of MS and JS, Meckelin (Baala et al., 2007; Brancati et al., 2010; Smith et al., 2006) and Jouberin (Louie et al., 2010), influence mESCs neuronal differentiation. Specifically, we found that Meckelin and Jouberin localized to cilia found at the outer ectodermal embryonic layer when mESCs were cultured as EBs, in a neural differentiation medium allowing the generation of mESC-derived neurons and, to a lesser extent, astrocytes. Moreover, Meckelin and Jouberin silencing (siMeckelin and siJbn) severely impaired the onset of specific early neural markers in mESC, but showed a differential effect on shh and wnt ligands production. In fact, siMeckelin blocked retinoic acid-dependent expression of shh (Chang et al., 1997), and consistently, the production of wnt3a was enhanced in siMeckelin mESCs with respect to control cells (Corbit et al., 2008). On the contrary, siJbn cells showed a marked decrease in wnt3a protein, while shh expression levels remained comparable in siJbn and scrambled transfected mESCs.
Altogether, these results provide new insights about the role of primary cilia and ciliary proteins during embryonic neurogenesis and, more importantly, suggest mESCs as a suitable model to study primary cilia-related molecular mechanisms, that may be implicated in embryonic development and in the pathogenesis of human ciliopathies.
2. Materials and methods
2.1. Cell culture, treatments and embryoid bodies formation
mESCs (ESD3, LGC Promochem, London, UK) were cultured as described (Illi et al., 2005). Briefly, mESCs were adapted in culture without feeder layer and grown in Dulbecco's modified eagle medium (DMEM, Life Technologies, Carlsbad, CA, USA) supplemented with 20 ng/ml Leukemia Inhibitory Factor (LIF) (Euroclone, Milan, Italy), 0.1 mM β-mercaptoethanol, 10% ES-tested Fetal Bovine Serum (FBS, Euroclone, Milan, Italy), 20 mM glutamine and penicillin/streptomycin (Life Technologies, Carlsbad, CA, USA). EBs were obtained in hanging drops as described (Kurosawa, 2007). mESCs were dissociated with trypsin and diluted to a concentration of 2.5 × 104 cells/ml. 20 μl of the cell suspension were used to obtained drops on the cover plate of a Petri dish (about 20 drops/dish for at least 80–100 total drops to be analyzed later) previously filled with sterile PBS. Cells were allowed to form EBs for 48 h at 37 °C/5% CO2. Thereafter, only well formed EBs were used for experimental procedures. For neural induction, mESCs, either as EBs or as adherent cells, were plated in culture medium without LIF onto 0.1% gelatin-coated dishes and treated, immediately after plating, with 10 nM RA or control DMSO for 7 days. Fresh RA was added every 2 days. After 1 week, mESCs were shifted to Neurobasal medium supplemented with N2 cocktail (100 μg/ml human transferrin, 5 μg/ml insulin recombinant full chain, 6 ng/ml progesterone, 16 μg/ml putrescine, 5.2 ng/ml selenite) (Life Technologies, Carlsbad, CA, USA), glutamine and penicillin/streptomycin (hereafter named NB+N2) for another week. Cell density at plating was as follow: for earlier time points (i.e. 5 and 7 days) cells were at 60% confluence; for the latest time point (i.e. 14 days) cells were at 30% confluence. Control cells were maintained in mESCs culture medium (Illi et al., 2005) deprived of LIF. HEK293T cells were cultured in DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 10% Fetal Bovine Serum (FBS, Life Technologies, Carlsbad, CA, USA), 20 mM glutamine and penicillin/streptomycin (Life Technologies, Carlsbad, CA, USA).
2.2. Reverse transcription and real time PCR
Total RNA was extracted with TRIZOL reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was produced by using SuperScript III (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. mRNA levels were analyzed using the QuantiTect SYBR Green PCR Kit (QIAGEN, Hilden, Germany) and quantified by the ABI Prism 7000 SDS analyzer (Life Technologies, Carlsbad, CA, USA). The RPL13 gene was used for normalization. The fold changes of each target mRNA expression relative to RPL13 under experimental and control conditions were calculated based on the threshold cycle (CT) as r=2 –Δ (ΔCT), where Δ=CT CT (target)–CT (RPL13) and Δ (ΔCT) Δ=CT (experimental) –ΔCT (control). Primers (PAGE purified) were:
Meckelin Fw: 5’-ggagccccatgattgattta-3’; Meckelin Rev: 5’-acattggccagatcaccag-3’
Jouberin Fw: 5’-ggattatggacctgcggata-3’; Jouberin Rev: 5’-ctcacggtaatttgctgcac-3’
Math1 Fw: 5’-tgcgatctccgagtgagag-3’; Math1 Rev: 5’-ctcttctgcaaggtctgattttt-3’
Ptf1a Fw: 5’-gggacgagcaagcagaagta-3’; Ptf1a Rev: 5’-cgcggtagcagtattcgtg-3’
Gbx2 Fw: 5’-gctgctcgctttctctgc-3’; Gbx2 Rev: 5’-gctgtaatccacatcgctctc-3’
En2 Fw: 5’-gaccggccttcttcaggt-3’; En2 Rev: 5’-cctgttggtctgaaactcagc-3’
RPL13 Fw: 5’-ctcggccgttctcctgtat-3’; RPL13 Rev: 5’-gtggagtggggcttcagta-3’
2.3. Cell extracts, nuclear extracts and western blot
Cells were lysed in a buffer containing 100 mM Tris (pH 6.8), 20% glycerol, and 4% sodium dodecyl sulfate, phosphatase and protease inhibitors cocktails (Life Technologies, Carlsbad, CA, USA). Nuclear extracts were performed as described (Illi et al., 2008). Protein amount was determined by BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Lysates were boiled for 5 min after the addition of 200 mM DTT (Magenta et al., 2008) and loaded onto NuPAGE Novex Bis-Tris 4–12% polyacrylamide gels (Life Technologies, Carlsbad, CA, USA). Western blots were performed according to standard procedures. Briefly, protein were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) O/N at +4 °C at 80 mA. The day after, membranes were blocked with 5% milk in PBS/0.1% Tween-20 (SIGMA, St. Louis, MO, USA) (PBST), 1 h at room temperature (RT). Primary antibodies were incubated for 2 h at RT. After 3 washes in PBST (5’ each), secondary antibodies were incubated for 1 h at RT. Membranes were washed 3 times with PBST (10’ each wash) and proteins revealed with SuperSignal WestPico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's instruction. Red Ponceau staining or H3 histone were used to normalize protein loading. Meckelin (code: ab76786), Jouberin (code: ab93386), Math1 (code: ab137534), Gli2 (code: ab26056), Gbx2 (code: ab58576), and H3 (code: ab1791) antibodies were from Abcam (Cambridge, UK); En2 (code: sc66878), Ptf1a, (code: sc98612) shh (code: sc9024), β-catenin (code: sc7199), wnt3a (code: sc28824), Vangl2 (code: sc67136) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All primary antibodies were used at a dilution of 1:1000 in PBST 2.5% milk. Secondary antibodies were from Millipore (Darmstadt, Germany) and used at a dilution of 1:5000 (anti-rabbitHRP) and 1:2000 (anti-mouseHRP) in PBST 2.5% milk.
2.4. Densitometric analysis
Densitometric analyses were performed by using the ImageJ software (http://rsbweb.nih.gov/ij/index.html). Normalized areas’ values were reported as Relative Area Density (R.A.D.).
2.5. Plasmids and transfections
pEGFP-Jbn and pCMV-HA-Meckelin expression vectors were courtesy of Prof. J.G. Gleeson and C.A. Johnson, respectively. 4 μg of plasmid DNA (or control empty pEGFP and pcDNA3 plasmids) were transfected in HEK293T cells, plated the day before at 80% confluence onto 60 mm culture plates, by using Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. After 48 h, cells were harvested, lysed and analyzed for Jouberin and Meckelin expression by western blot, as described (see previous section).
2.6. Immunofluorescence and confocal analyses
Immunofluorescence experiments were performed as described (Illi et al., 2005). Briefly, whole EBs were fixed with 4% paraformaldheyde 10’, at RT, washed 3 times with PBS and blocked with 10% Bovine Serum Albumin (BSA, SIGMA, St. Louis, MO, USA) in PBS for 1 h, at RT. Primary antibodies (1:100 dilution in PBS/1% BSA) were allowed to incubate O/N at 4 °C. The day after, three washes in PBS were performed and secondary antibodies ( anti-rabbit AlexaFluor555 and anti-mouse AlexaFluor488, Molecular Probes, Life technologies, Carlsbad, CA, USA; 1:100 dilution in PBS/ 1%BSA), were incubated 1 h, at RT, in dark. After three washes in PBS, nuclei were stained with Hoechst (1 μg/ml, Life Technologies, Carlsbad, CA, USA), 15’, at RT. After additional three washes, a total of 20 μl 70% glycerol buffered in PBS were used to mount cover-slips. Confocal analysis for the detection of Jouberin and Tmem67 co-localization with acetylated-tubulin labeled cilia was performed as described (Illi et al., 2008). Lasers’ power, beam splitters, filter settings, pinhole diameters, and scan mode were the same for all examined field of each sample. Fields reported in the figures are representative of all examined fields. Both immunofluorescence and confocal images were captured with a PCM Eclipse TE300 (Nikon Instruments, Tokyo, Japan). Merged images were obtained with the NIS Element software. Meckelin (ab76786), Jouberin (ab93386), β-tubulin III (ab18207) antibodies were from Abcam; GFAP (sc9065) and Nestin (sc21248) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); O4 (O7139), acetylated-α-tubulin (T7451) and γ-tubulin (T5326) antibodies were from SIGMA (St. Louis, MO, USA). All the antibodies were used according to the manufacturer's instructions.
2.7. Immunoprecipitation
Immunoprecipitation experiments were performed as described (Spallotta et al., 2010). Briefly, proteins were extracted in a lysis buffer containing 150 mM NaCl, 50 mM Tris pH 7.4, 0.5% Nonidet P-40, protease and phosphatase inhibitors (Eley et al., 2008), centrifuged at 10,000g for 20’ at 4 °C and the supernatant was precleared with protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 1 h at RT. At the same time, 4 μg of either Jouberin or Tmem67 specific antibody or control rabbit and mouse IgG were conjugated with 50 μl of protein A/G agarose in 500 μl of PBS at 4 °C. Precleared extracts (about 0.5 mg) were allowed to incubate with protein A/G agarose-conjugated antibodies O/N, at 4 °C. The day after, samples were washed three times with lysis buffer and once with PBS. After addition of Laemmli sample buffer, samples were boiled and resolved onto a NuPAGE Novex Bis-Tris 4–12% polyacrylamide gel (Life Technologies, Carlsbad, CA, USA) and processed in western blot analysis.
2.8. Count of cilia
EBs, about 150 μm in diameter the smallest and 500 μm the largest, were observed at the immunofluorescence microscope at 40× magnification on a gridded area of four 0.04 mm2 and 0.16 mm2 squares respectively and acetylated-α-tubulin and γ-tubulin positive cilia were manually counted within. The average of the four squares was considered as the number of cilia for each EB.
2.9. Small interfering RNA-mediated gene silencing
Small interfering RNA-mediated gene silencing was performed as described (Spallotta et al., 2010). Briefly, cells were plated in Pen-Strep free medium; the next day were transfected using siRNA Transfection Reagent (sc29528, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 40% confluence (750,000 in 60 mm), in serum-free medium. siRNAs final concentration was 0.2 μM. After 12 h, cells were re-fed with fresh medium The day after transfection, cells were treated with RA or control solvent and experiments were performed 5 days later. Jouberin (sc72466), Meckelin (sc149460) and control (FITC-conjugate)-A (sc36869) siRNAs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.10. Statistical analysis
Variables were analyzed by both Student's t test and one-way analysis of variance, and a value of p<0.05 was deemed statistically significant. Values are expressed as standard error (s.e.).
3. Results
3.1. mESCs express mid-hindbrain and cerebellar markers when cultured in specific conditions
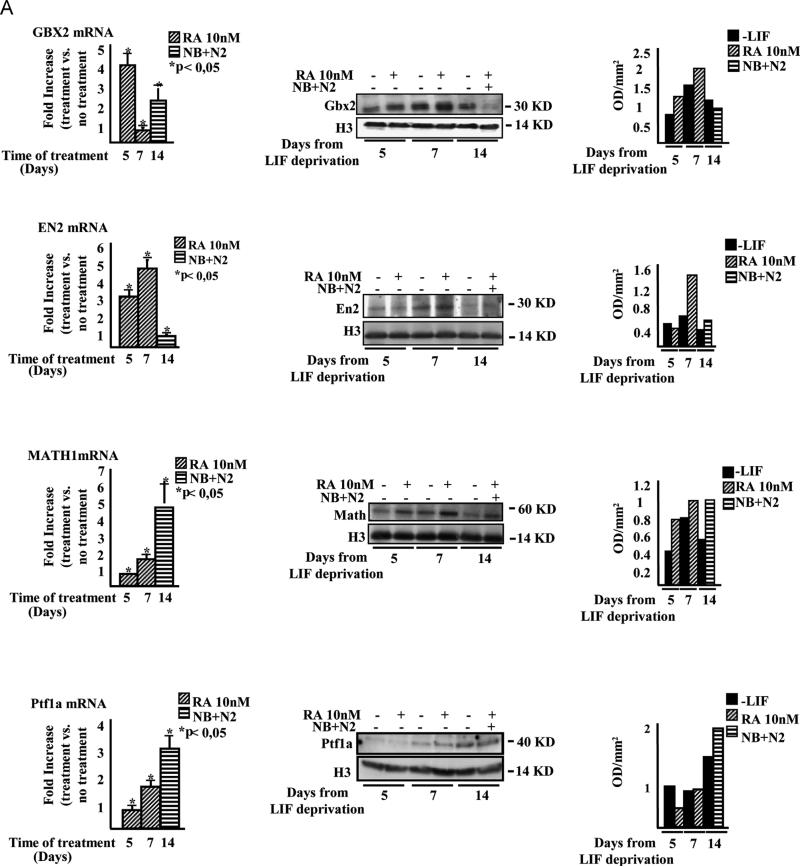
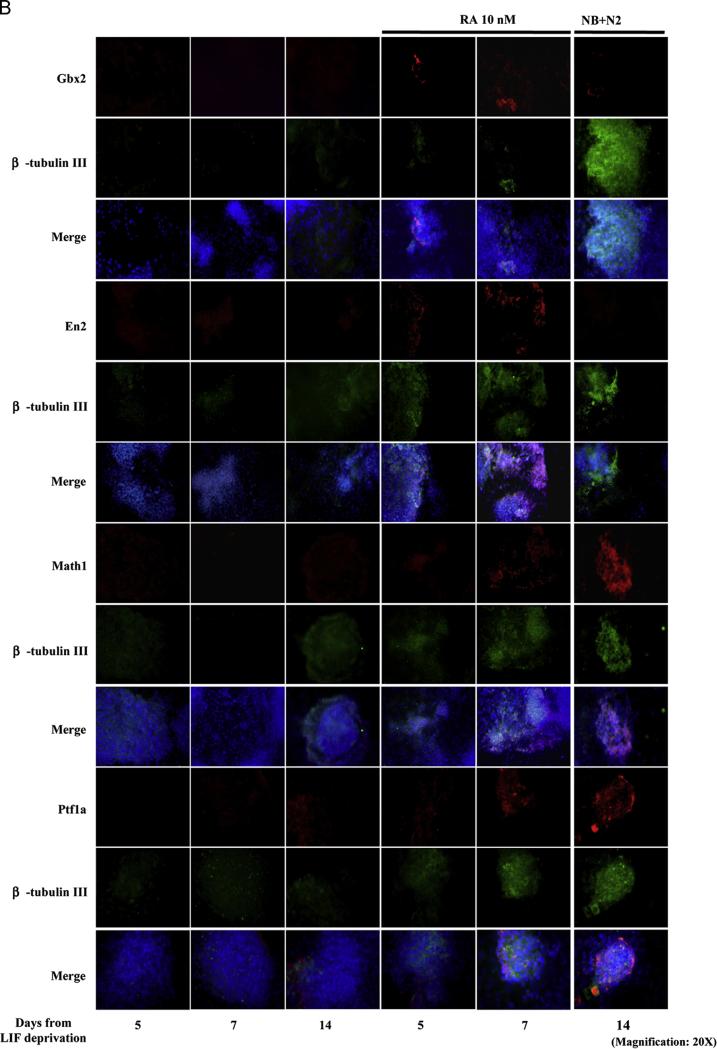
Along a time course between 5 and 14 days, by using a low dose retinoic acid (RA)-based differentiation protocol (see Section 2 and Guan et al., 2001), we were able to obtain both mature β-tubulin III-positive neurons and astrocytes marked by the Glial Fibrillary Acidic Protein (GFAP), together with nestin-positive neural and glial progenitors. Oligodendrocytes, specified by the O4 protein, were observed only at days 5 and 7 indicating that, in our experimental conditions, mESCs preferentially differentiate into mature neurons and astrocytes (Supplementary Fig. S1). By real time-PCR, western blot and immunofluorescence analyses we also detected the expression of genes typical of the developing mid-hindbrain and cerebellar structures (Fig. 1A and B), which were induced in temporal windows compatible to those occurring in vivo. Gbx2, marking the anterior hindbrain at E7.75 of in vivo development, appeared at day 5, followed at day 7 by En2, which in vivo marks at E8.5 the hindbrain territory (Simeone, 2000). At day 7, we also observed the expression of Math1 and Ptf1a transcription factors, which in vivo at day E10.5 mark the rhombic lip and the ventricular zone from which the cerebellum structure will result (Ten Donkelaar and Lammens, 2009), with further enhancement till day 14.
Fig. 1.
mESCs express mid-hindbrain and cerebellar markers when cultured in RA-enriched and neurobasal plus N2 culture medium. (A) Picture depicts the kinetics of expression of Gbx2, En2, Math1 and Ptf1a. Left panels show real time-PCR experiments; middle panels show representative western blot analyses and right panels show the densitometric analyses relative to western blots. These results are representative of three independent experiments. (B) Immunofluorescence experiments showing the expression of mid-hindbrain (Gbx2 and En2) and cerebellar markers (Math1 and Ptf1a) in differentiating mEBs and their co-localization with β-tubulin III. Consistently with real-time PCR and western blot data, Gbx2 and En2 expression anticipates Math1 and Ptf1a onset. Nuclei are shown in blue. These results are representative of three independent experiments. Uncropped western blots are shown in Supplementary Fig. 2. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. mESCs possess primary cilia and activate the shh pathway while repressing the canonical wnt signaling
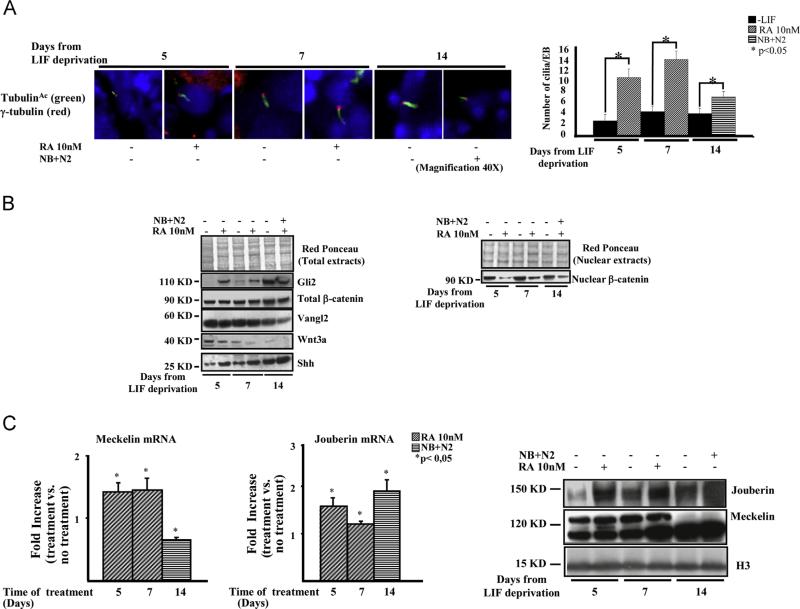
A low number of primary cilia, marked by acetylated-α and γ-tubulin, labeling the ciliary axoneme and basal bodies respectively, were found at the mEB ectodermal layer, either in the presence or absence of RA, at each time point of differentiation (Fig. 2A, left), indicating that cilia formation occurred independently of specific culture conditions (Kiprilov et al., 2008). However, the number of cilia detected upon LIF deprivation remained constant along the whole time course, while RA induced a significant increase of the number of cilia, which then diminished at day 14 upon NB+N2 culture (Fig. 2B, right).
Fig. 2.
mESCs possess primary cilia, differentially regulate the Shh and Wnt pathways and express Meckelin and Jouberin. (A) Left panel. Representative immunofluorescence experiments performed by using anti-acetylated-α-tubulin (green) and γ-tubulin (red) are shown. Right panel. The graph shows the number of cilia counted on mEBs undergoing neural differentiation. These results are representative of two independent experiment performed in triplicate. (B) Regulation of shh and wnt pathways. Left panel. A RA-dependent induction of shh-related molecules can be observed along the course of mESCs neural commitment. On the contrary, the wnt3a ligand shows a marked reduction in RA-treated mESCs. β-catenin and Vangl2 are constantly expressed, although Vangl2 slightly diminishes at the latest time points, regardless of RA presence. Right panel. Western blot on mESC nuclear extracts shows the decrease in nuclear β-catenin protein levels. These results are representative of three independent experiments. (C) Real time-PCR experiments (left panel) and western blot analysis (right panel) show the modulation of Meckelin and Jouberin mRNA and protein expression upon RA treatment and NB+N2 culture. Uncropped western blots are shown in Supplementary Fig. S4. Densitometric analyses relative to westrern blots shown in this figure are in Supplementary Fig. S5. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The primary cilium-mediated shh pathway plays a key role in the commitment of stem cells towards neuroectodermal differentiation (Maye et al., 2004). On the contrary, the canonical wnt pathway is antagonized both during embryonic neural differentiation (Aubert et al., 2002 and Yoshikawa et al., 1997) and in response to primary cilia stimulation which, in turn, may activate the non-canonical wnt/PCP pathway (Dale et al., 2009). To evaluate how these signaling pathways were activated and/or repressed in our experimental model, western blot analyses were performed. As shown in Fig. 2B (left), shh and its effector Gli2, were constantly induced upon RA treatment till day 7, to remain elevated in NB+N2 culture condition; conversely, the expression of wnt3a was down-regulated. The protein levels of β-catenin remained constant along the whole time course; however, the amount of nuclear β-catenin decreased in RA and NB+N2-treated samples (Fig. 2B right). The Van Gogh-like protein 2 (Vangl2) protein, belonging to the wnt/PCP pathway, remained unmodified in treated vs. untreated cells and decreased at the latest time point of differentiation. These results demonstrate that, in our model, mESCs possess primary cilia, activate the shh signaling pathway as soon as neural differentiation occurs, while repressing the canonical wnt pathway.
3.3. Meckelin and Jouberin are expressed in mESCs and localized at the primary cilium
We next explored the expression of endogenous ciliary proteins in our neural differentiation model. We focused on Jouberin and Meckelin (encoded by the Ahi1 and Tmem67 genes, respectively), implicated in the pathogenesis of JS and MS (Smith et al., 2006; Valente et al., 2006).
Real-time PCR and western blot experiments were performed to evaluate the expression of both genes at the mRNA and protein levels (Fig. 2C). In our experimental conditions, Meckelin mRNA was rapidly induced at day 5 and 7, to decline shortly after (Fig. 2C, left); at the protein level, two bands were detected, of which the one with the lower electrophoretic mobility, and showing the same expression kinetics of Meckelin mRNA, was consistent with Meckelin molecular weight (Dawe et al., 2009). These results are in line with the increased number of cilia detected in mEBs between day 5 and 7, with subsequent decrease at day 14 (see Fig. 2A). The expression of Jouberin was also well evident, with both mRNA and protein levels sustained up to 14 days of differentiation (Fig. 2C).
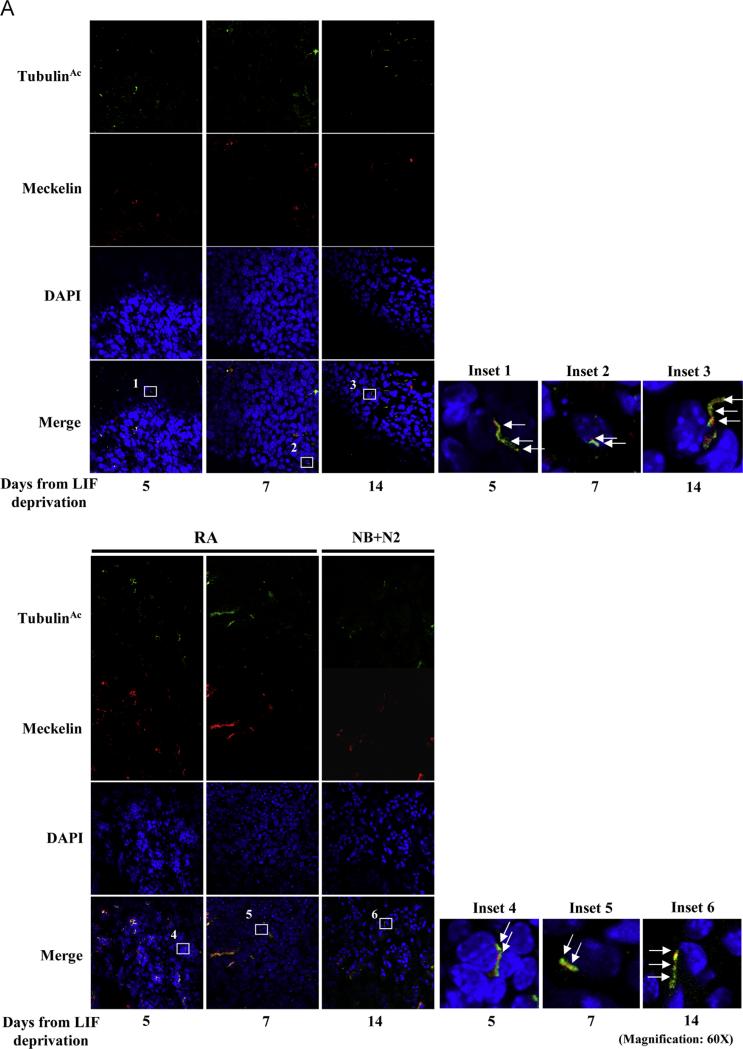
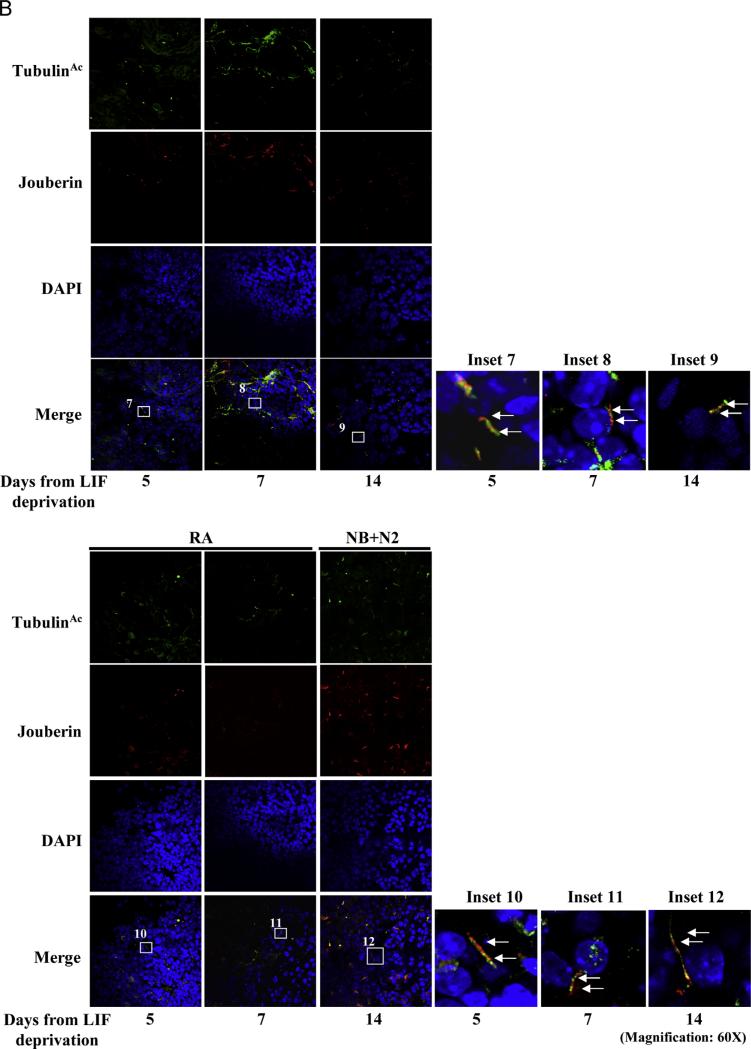
Confocal analyses were performed to detect Meckelin and Jouberin localization in the context of differentiating mEBs. In untreated and differentiated EBs, Meckelin was clearly expressed (Supplementary Fig. S6A) and co-localized with the low number of acetylated tubulin-labeled primary cilia at the outer layer of spontaneously differentiating mEBs (Fig. 3A). Jouberin was found at low levels in LIF-deprived mESCs, while upon differentiation its expression was much more intense both in the mEB cores and at the ectodermal layer (Supplementary Fig. S6B). In both conditions, we observed co-localization of Jouberin with acetylated tubulin-labeled primary cilia (Fig. 3B).
Fig. 3.
Meckelin and Jouberin localizes to primary cilia in mESCs. (A) Confocal analysis. Whole EBs were stained with anti-acetylated-α-tubulin and anti-Meckelin antibodies. Meckelin is expressed along the whole time course in mEBs. In both undifferentiated and differentiated cells, few primary cilia labeled both by acetylated-α-tubulin and Meckelin are protruding from cells localized at the ectodermal EB layer (insets 1–6). All results are representative of at least three independent experiments. (B) Whole EBs were stained with anti-acetylated-α-tubulin and anti-Jouberin antibodies. Co-localization between Jouberin and acetylated tubulin-labeled cilia is evident at the ectodermal layer of mEBs (insets 7–12). All results are representative of at least three independent experiments.
3.4. Meckelin and Jouberin depletion impairs the onset of neural markers in mESCs
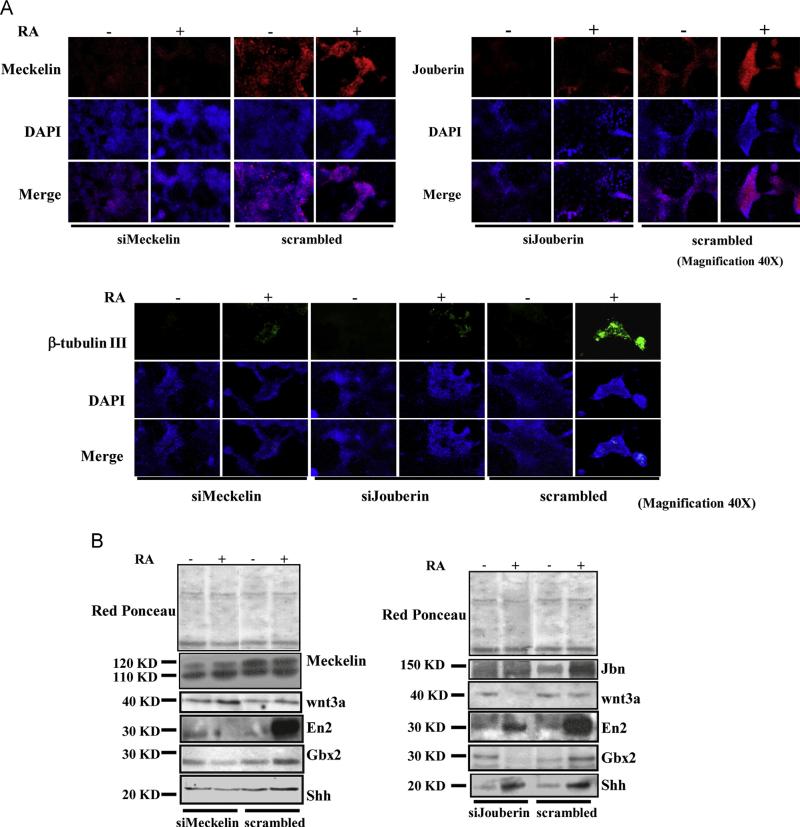
To assess the validity of our model in exploring the role of ciliary proteins during mESCs neural differentiation, we performed short interfering RNA (siRNA) experiments. Given the transient expression of both Meckelin and Jouberin siRNAs (siMeckelin and siJouberin), we analyzed interfered mESCs at 5 days of differentiation, when neural specific markers, such as Gbx2, En2 and β-tubulin III, were already well expressed at the protein level (see Fig. 1A and B and Supplementary Fig. S1), consistently with shh activation (see Fig. 2B). As a control we used a FITC-conjugate control scrambled siRNA which allowed us to monitor siRNAs expression till day 6 (Supplementary Fig. S7C). Transfection of siMeckelin and siJouberin produced a significant decrease of both endogenous proteins at day 6 from transfection, as revealed by immunofluorescence (Fig. 4A, top). In the presence of RA, low levels of β-tubulin III were observed in both silenced cells compared to controls (Fig. 4A, bottom). Similarly, western blot analyses showed a severe impairment of Gbx2 and En2 RA-dependent expression, when compared to scramble-transfected mESCs (Fig. 4B), suggesting a failure of mESCs to differentiate towards the neural lineage.
Fig. 4.
The absence of Meckelin and Jouberin impairs mESCs response to retinoic acid. mESCs were transfected with siMeckelin and siJbn in the absence of serum and antibiotics and in the presence of LIF. After 12 h, mESCs were re-fed with fresh LIF-containing medium. After additional 24 h, cells were shifted to LIF-deprived medium and treated with RA or control solvent for 5 days. (A) Upper panel. Immunofluorescence showing the reduction in Jouberin and Meckelin expression. Lower panel. Immunofluorescence showing the dramatic decrement of β-tubulin III protein in siMeckelin and siJbn mESCs. (B) Western blot analyses showing both the down-regulation of Meckelin and Jbn in siRNA-transfected cells, the modulation of shh and wnt pathways and the effect on the expression of the transcription factors Gbx2 and En2. These results are representative of three independent experiments. Uncropped western blots are shown in Supplementary Fig. S7A and B.
Accordingly, siMeckelin-transfected cells failed to activate shh in response to RA, while inducing wnt3a (Fig. 4, lower panel, left); on the contrary, and consistently with previously published data (Lancaster et al., 2009), wnt3a was completely lost in siJbntransfected mESCs, but shh was still present (Fig. 4, lower panel, right).
3.5. Meckelin and Jouberin associate during RA-dependent neural differentiation of mESCs
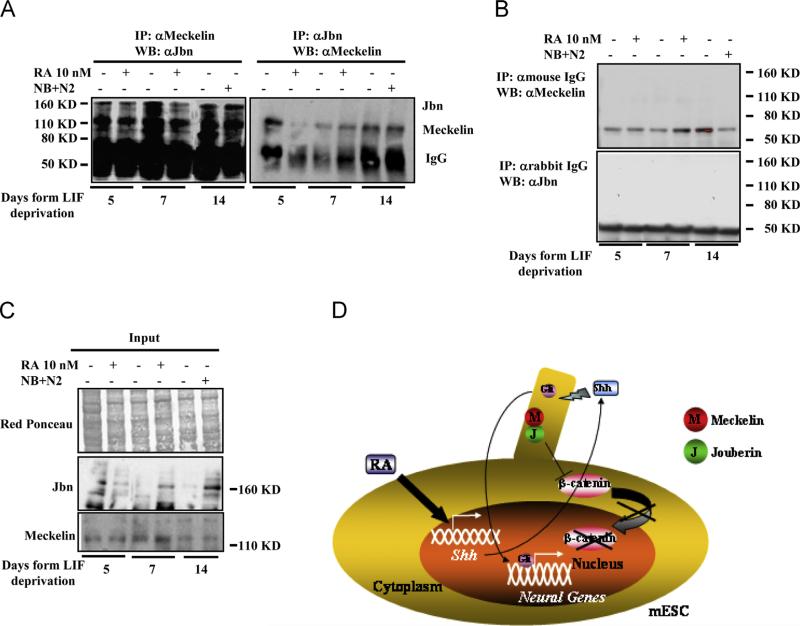
The downregulation of shh expression in siMeckelin cells, paralleled by wnt3a production, and the role of primary cilium in sequestering Jouberin from the cytoplasm (Lancaster et al., 2011a), led us to postulate a possible interaction between Meckelin and Jouberin. To verify this hypothesis, reciprocal coimmunoprecipitation experiments were performed from mESCs cell extracts, obtained at the different time points of RA and NB+N2-dependent differentiation. As shown in Fig. 5A, the two endogenous proteins were effectively found in the same protein complex, being their interaction even stronger as terminal differentiation occurs. In control experiments we did not detect either Meckelin or Jouberin proteins in the immunoprecipitate (Fig. 5B). In light of these evidences, an active role for Meckelin in retaining Jouberin into the primary cilium may be suggested.
Fig. 5.
Meckelin and Jouberin associate in mESCs. (A) Co-immunoprecipitation experiment. Cell extracts (0.5 mg) were immunoprecipitated with 4 μg of anti-Meckelin (left) or anti-Jouberin (right) antibodies. Immunoprecipitated proteins were loaded onto a 4–12% polyacrylamide gel, blotted O/N at +4 °C and probed with anti-Jbn (left) and anti-Meckelin (right) antibodies. These results are representative of three independent experiments. (B) Control immunoprecipitation experiments by using anti-mouse and anti-rabbit IgG. Neither Meckelin nor Jouberin proteins were detected. (C) Western blot analysis showing Jouberin and Meckelin expression in whole cell extracts before immunoprecipitation. (D) A model for primary cilia-dependent neural differentiation of mESCs. In mESCs, retinoic acid induces the expression of shh which, acting through primary cilia, induces the expression of neural genes. Meckelin, playing a pivotal role in primary cilium formation and possibly in sequestering Jouberin, may have a major role in this process, allowing the activation of shh cascade and restraining the canonical wnt pathway.
4. Discussion
The neural defects occurring in JS and MS patients are illustrative of the importance of primary cilia in the embryonic formation of the CNS. In JS, the paradigmatic “molar tooth sign” (MTS) is given by the association of cerebellar vermis hypodysplasia, lack of decussation and abnormal positioning of superior cerebellar peduncles and pyramidal tracts, and a deepened interpeduncular fossa. Patients with MS typically present with occipital encephalocele, that is often associated with other posterior fossa abnormalities. Frequent neuropatho-logical findings are a cleft foramen magnum, micropolygyria and heterotopia of the cerebral cortex, hypoplasia of the vermis and central white matter of the cerebellum, diffuse heterotopia of Purkinje. Brain morphology defects in JBTS patients with extensive cerebral malformation include absence of the vermis and corpus callosum, ventricles enlargement, dentate nuclei fragmentation into islands, dysplasia of the inferior olives and an almost complete absence of the pyramidal decussation. Many nodular heterotopias of the cerebral cortex and of the basal ganglia, the amygdala and the diencephalon may be observed (Ten Donkelaar et al., 2006). in vivo studies on mice knock-out for the Ahi1 gene, mutated in JS, have clearly shown a defect in cerebellar midline fusion that is dependent on an impaired Wnt signaling pathway (Lancaster et al., 2011b). Very recently, Tmem67 targeted knock out mouse models revealed phenotypic characteristics typical either of MS (exencephaly, occipital encephalocele) or JS (cerebellar hypoplasia, molar tooth like aspect), with abnormal cilia and deregulation of shh and canonical wnt pathways (Abdelhamed et al., 2013). In particular, MKS-like E11.5 Tmem67−/− KO embryos show variable cerebral defects including an occipital meningocele, midbrain-hind brain exencephaly, with a failure of fusion of the neural folds, as a result of an anterior neuropore closure defect. Prosencephalon dysgenesis is a consistent finding in all E11.5 Tmem67−/− KO embryos. At later developmental stages (from E13.5), frontal encephalocele, from a mild defect (slight protrusion in the frontal area) to a gross encephalocele/meningocele-like anomaly, is observed. At later developmental stages, prosencephalon dysgenesis manifest as semi-lobar holoprosencephaly, with fusion of the two lateral ventricles and an absence of some midline structures (e.g. the anterior commissar and variable degrees of corpus callosum dysgenesis). The ‘MKS-like’ group of animals shows also an enlargement of the hippocampus and basal ganglia. All JBTS-like Tmem67−/− embryos have a reduced anteroposterior axis of the developing forebrain, a small hindbrain region, microcephaly or other overt facial dysmorphologies. Some of them have a deep interpenduncular fossa with a reduced anterioposterior axis of the midbrain tegmentum at the level of the isthmus, associated with cerebellar vermis hypoplasia or aplasia. These phenotypic manifestation comprise the MTS that is pathognomonic for JBTS in humans. Complex posterior fossa defects and features compatible with the Dandy–Walker malformation are also observed (Abdelhamed et al., 2013).
Primary cilia are required for cerebellar development in the embryo (Spassky et al., 2008) and for the generation of adult neural stem cells (Han et al., 2008). To date, a major limitation in studying primary cilia developmental function in vitro remains the lack of suitable cellular models that could recapitulate as accurately as possible the developmental steps observed in vivo. Such models would represent a key tool to rapidly analyze the impact of distinct ciliary proteins on the wide variety of differentiation modules regulated by primary cilia. To this end, ESCs represent an ideal cell system to study developmental processes, due to their high plasticity, the ability to differentiate into many lineages, and the availability of differentiation protocols to obtain tissue-specific cells. Interestingly, it has been recently demonstrated that both human and mouse ESCs possess primary cilia, which play a key role in the activation of shh and wnt pathways and in neurogenesis (Hunkapiller et al., 2011; Kiprilov et al., 2008).
This observation led us to optimize a mESC-based model of neural differentiation to analyze developmental defects related to the depletion of specific ciliary proteins. The added values of this model are represented by the lack of exogenous morphogens, including shh, that usually force the appearance of neural cell subtypes from mESCs, and by the choice not to overexpress the ciliary proteins of interest, thus simulating at the best the physiological conditions occurring during neural development. By using a specific RA-based neural differentiation protocol, we obtained a heterogeneous population of cell subtypes expressing markers of neural and glial progenitors (Nestin), mature neurons (β-tubulin III), astrocytes (GFAP), oligodendrocytes (O4), along with the expression of markers that are indicative of midhindbrain development (Gbx2, En2, and then Ptf1a and Math1). In our experimental conditions, the expression of all these neural-specific proteins was regulated in a time-dependent fashion, resembling those processes occurring in vivo during embryonic neurogenesis (Fig. 1A and B and Supplementary Fig. S1). Moreover, we also observed a time-dependent modulation of both shh and canonical wnt pathways, that are known to play a major role in CNS development (Fig. 2B).
In this model, we decided to analyze the expression and localization of Jouberin and Meckelin, implicated in the pathogenesis of JS and MS. We showed that mESCs committed to the neural fate expressed in a time-dependent manner both Meckelin and Jouberin (Fig. 2C), which localized to acetylated-tubulin labeled cilia detected at the mEBs outer layer (Fig. 3A and B). The low number of cilia observed in our experimental conditions may be related to the three-dimensional structure of mEBs. Therefore, only cilia projected outside the most external layer are detected with conventional immunofluorescence techniques, while cilia protruding inside the inner mass escape the observation. In our model, the kinetics of expression especially of Meckelin is consistent with the increase in the number of cilia detected between day 5 and 7, and the subsequent decrease at day 14 of differentiation (Fig. 2A). Indeed, it has been shown that Tmem67 ablation leads to a defective primary cilium formation (Dawe et al., 2007), while Ahi1 is not necessary for axonemal development (Louie et al., 2010) but seems to play a relevant role in ciliary-mediated signaling (Lancaster et al., 2011a).
We also noticed a diffuse expression of Meckelin and Jouberin also in the mEB core, suggesting possible additional functions for these proteins during mESC differentiation (Supplementary Fig. S4). For instance, it is known that in renal cells, Jouberin is expressed also at the cell–cell junctions (Eley et al., 2008); while in non- ciliated cells it is found in the nuclear compartment (Lancaster et al., 2011b) and it acts as an oncogene in cutaneous T-cell lymphomas and Sezary cells (Kennah et al., 2009).
The observed time-dependent production of shh, which is indispensable for the differentiation of cells belonging to the neuroectodermal lineage (Maye et al., 2004), supports the hypothesis that shh deficiency represents a major determinant of the neurological defects observed in patients with CNS-related ciliopathies (Cardenas-Rodriguez and Badano, 2009). In parallel with shh activation, we also observed a progressive reduction of wnt3a and a decrease of the nuclear quota of β-catenin in RA-treated mESCs. These decrements perfectly paralleled the increase of Jouberin and the appearance of cilia in mEB, in line with the known role of the primary cilium in limiting β-catenin nuclear entry once Jouberin has been sequestered (Lancaster et al., 2011b). Therefore, our in vitro model well recapitulates the cilia-dependent activation of shh pathway and constraint of wnt canonical signaling occurring during embryonic development (Corbit et al., 2008). To assess whether this model could represent a useful tool to explore the function of specific ciliary proteins in neural differentiation of mESCs, we knocked down Jouberin and Meckelin expression by use of siRNAs (Fig. 4). Silenced cells failed to induce early neural genes in response to RA, as shown by the low expression levels of the shh-responsive Gbx2 and En2 genes (Kobayashi et al., 2002; Szabo et al., 2009), as well as to terminally differentiate into neurons, as illustrated by the markedly lower number of β-tubulin III positive cells compared to controls. Thus, both proteins appear to be required for early neural differentiation of mESCs. These in vitro findings are in agreement with the results obtained in mouse embryos, either wild type or knock out for Ahi1 and Tmem67 genes. The Ahi1−/− mouse features cerebellar hypoplasia and defects in the vermis-midline fusion (Lancaster et al., 2011b), while targeted Tmem67 KO mice show a variable range of early (E11.5–E12.5) neural tube and mid-hindbrain defects (Abdelhamed et al., 2013; Logan et al., 2011). Further, we observed that Meckelin silencing impaired RA-dependent shh production in mESCs, while enhancing wnt3a signaling. Interestingly, Jouberin silencing had an opposite effect, as demonstrated by the complete loss of wnt3a in RA-treated mESCs, while shh expression was not affected at all. Till now, the loss of ciliary proteins has been mostly related to both the reduction or absence of shh receptors and dysregulation of β-catenin expression levels and/or subcellular localization (Mahjoub and Stearns, 2012; Abdelhamed et al., 2013; Lancaster et al., 2011a). However, very recently it has been demonstrated that Tmem67−/− KO embryos have reduced levels of shh protein at the floor plate and notochord (Abdelhamed et al., 2013), supporting our RA-based in vitro model as a reliable tool to study primary cilia-related neurodevelopmental defects. Further, our results fit perfectly with the known role of Jouberin in stimulating wnt signaling (Lancaster et al., 2009) and with the recent finding that another ciliary protein mutated in MS, MKS1, is linked to hedgehog signaling, while Jouberin does not (Sang et al., 2011). The results obtained in siMeckelin and siJbn mESCs and the recent finding that Jouberin is sequestered as soon as the cilium is formed, to constrain wnt signaling, led us to postulate a possible interaction between Meckelin and Jouberin, suggesting a potential additional role for the transmembrane protein Meckelin, in retaining Jouberin into the cilium. To answer this question, we performed immunoprecipitation experiments in mESCs directed to the neural lineage, in which the two endogenous proteins were effectively detected in the same protein complex (Fig. 5A). Therefore, in the presence of Meckelin, shh signaling may prevail, while in its absence, Jouberin may be released from the cilium and wnt signaling may be activated. In light of these evidences we suggest a model mESCs in which RA-dependent neural commitment rely on shh production which, through primary cilia, activates a transcriptional program necessary for the acquisition of the neuronal phenotype (Fig. 5C). The ciliary protein Meckelin, which is required for primary cilium formation (Dawe et al., 2007) and possibly sequestering Jouberin to primary cilia, seems to have a major role in this process.
Overall, this study enlightens the great potential of mESCs to study ciliary-related developmental disorders. In fact, the molecular mechanisms underlying the multiorgan defects in human ciliopathies may be investigated in a wide variety of differentiation modules by simply culturing mESCs in the presence of appropriate morphogens. Further, to our knowledge, our data represent the first evidence of a developmental role of Meckelin and Jouberin in regulating the onset of neuronal lineages from mESCs.
Supplementary Material
Acknowledgments
We thank prof. Joseph G. Gleeson and prof. Colin A. Johnson for pEGFP-Jbn and pCMV-HA-Meckelin expression vectors, respectively and for helpuful criticisms and discussion. This work has been partially supported by: Italian Telethon Foundation (grant GGP13146 to EMV), Italian Ministry of Health (Ricerca Corrente 2012-2013, Ricerca Finalizzata 2009 Malattie Rare to EMV), European Research Council (Starting Grant nr. 260888 to EMV), National Institute of Health (grant R01NS048453 to JGG).
Abbreviations
- JS
Joubert syndrome
- MS
Meckel syndrome
- Jbn
Jouberin
- NB
Neurobasal medium
Footnotes
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.diff.2014.02.005.
References
- Abdelhamed ZA, Wheway G, Szymanska K, Natarajan S, Toomes C, Inglehearn C, Johnson CA. Variable expressivity of ciliopathy neurological pheno-types that encompass Meckel–Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects. Hum. Mol. Genet. 2013;22:1358–1372. doi: 10.1093/hmg/dds546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. The Meckel–Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am. J. Hum. Genet. 2007;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boheler KR. Stem cell pluripotency: a cellular trait that depends on transcription factors, chromatin state and a checkpoint deficient cell cycle. J. Cell. Physiol. 2009;221:10–17. doi: 10.1002/jcp.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancati F, Dallapiccola B, Valente EM. Joubert Syndrome and related disorders. Orphanet J. Rare Dis. 2010;5:20. doi: 10.1186/1750-1172-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am. J. Med. Genet. C: Semin. Med. Genet. 2009;151C:263–280. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- Chang BE, Blader P, Fischer N, Ingham PW, Strähle U. Axial (HNF3beta) and retinoic acid receptors are regulators of the zebrafish sonic hedgehog promoter. EMBO J. 1997;16:3955–3964. doi: 10.1093/emboj/16.13.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Dale RM, Sisson BE, Topczewski J. The emerging role of Wnt/PCP signaling in organ formation. Zebrafish. 2009;6:9–14. doi: 10.1089/zeb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, et al. The Meckel–Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J. Cell Sci. 2009;122:2716–2726. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley L, Gabrielides C, Adams M, Johnson CA, Hildebrandt F, Sayer J. Jouberin localizes to collecting ducts and interacts with nephrocystin-1. Kidney Int. 2008;74:1139–1149. doi: 10.1038/ki.2008.377. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Vanderhaeghen P. Mechanisms of neural specification from embryonic stem cells. Curr. Opin. Neurobiol. 2010;20:37–43. doi: 10.1016/j.conb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for genetic and fucntional dissection of cilia. Nat. Genet. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hunkapiller J, Singla V, Seol A, Reiter JF. The ciliogenic protein Oral– Facial–Digital 1 regulates the neuronal differentiation of embryonic stem cells. Stem Cells Dev. 2011;20:831–841. doi: 10.1089/scd.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ. Res. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- Illi B, Dello Russo C, Colussi C, Rosati J, Pallaoro M, Spallotta F, Rotili D, Valente S, Ragone G, Martelli F, et al. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class IIa histone deacetylases nuclear shuttling. Circ. Res. 2008;102:51–58. doi: 10.1161/CIRCRESAHA.107.157305. [DOI] [PubMed] [Google Scholar]
- Kennah E, Ringrose A, Zhou LL, Esmailzadeh S, Qian H, Su MW, Zhou Y, Jiang X. Identification of tyrosine kinase, HCK, and tumor suppressor, BIN1, as potential mediators of AHI-1 oncogene in primary and transformed CTCL cells. Blood. 2009;113:4646–4655. doi: 10.1182/blood-2008-08-174037. [DOI] [PubMed] [Google Scholar]
- Kiprilov EN, Awan A, Desprat R, Velho M, Clement CA, Byskov AG, Andersen CY, Satir P, Bouhassira EE, Christensen ST, Hirsch RE. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J. Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 2009;19:220–229. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat. Cell Biol. 2011a;13:700–707. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM, Thacker BE, Williams Y, Zaki MS, Gleeson JG. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat. Med. 2011b;17:726–731. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CV, Abdel-Hamed Z, Johnson CA. Molecular genetics and pathogenic mechanisms for the severe ciliopathies: insights into neurodevelopment and pathogenesis of neural tube defects. Mol. Neurobiol. 2011;43:12–26. doi: 10.1007/s12035-010-8154-0. [DOI] [PubMed] [Google Scholar]
- Louie CM, Gleeson JG. Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum. Mol. Genet. 2005;14:R235–242.. doi: 10.1093/hmg/ddi264. [DOI] [PubMed] [Google Scholar]
- Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schloss-man AM, Otto EA, Leitges M, Gröne HJ, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat. Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenta A, Fasanaro P, Romani S, Di Stefano V, Capogrossi MC, Martelli F. Protein phosphatase 2A subunit PR70 interacts with pRb and mediates its dephosphorylation. Mol. Cell. Biol. 2008;28:873–882. doi: 10.1128/MCB.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub MR, Stearns T. Supernumerary centrosomes nucleate extra cilia and compromise primary cilium signaling. Curr. Biol. 2012;22:1628–1634. doi: 10.1016/j.cub.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Nonaka S. Cilia: tuning in to the cell's antenna. Curr. Biol. 2006;16:R604–614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Maye P, Becker S, Siemen H, Thorne J, Byrd N, Carpentino J, Grabel L. Hedgehog signaling is required for the differentiation of ES cells into neurectoderm. Dev. Biol. 2004;265:276–290. doi: 10.1016/j.ydbio.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP–JBTS–MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A. Positioning the isthmic organizer: where Otx2 and Gbx2 meet. Trends Genet. 2000;16:237–240. doi: 10.1016/s0168-9525(00)02000-x. [DOI] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel–Gruber syndrome and the wpk rat. Nat. Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- Spallotta F, Rosati J, Straino S, Nanni S, Grasselli A, Ambrosino V, Rotili D, Valente S, Farsetti A, Mai A, et al. Nitric oxide determines mesodermic differentiation of mouse embryonic stem cells by activating class IIa histone deacetylases: potential therapeutic implications in a mouse model of hindlimb ischemia. Stem Cells. 2010;28:431–442. doi: 10.1002/stem.300. [DOI] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo NE, Zhao T, Zhou X, Alvarez-Bolado G. The role of Sonic hedgehog of neural origin in thalamic differentiation in the mouse. J. Neurosci. 2009;29:2453–2466. doi: 10.1523/JNEUROSCI.4524-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Donkelaar HJ, Lammens M, Hori A. Clinical Neuroembriology. Springer-Verlag; Berlin Heidelberg: 2006. [Google Scholar]
- Ten Donkelaar HJ, Lammens M. Development of the human cerebellum and its disorders. Clin. Perinatol. 2009;36:513–530. doi: 10.1016/j.clp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, Barrano G, Bestini E, Boltshauser E, Zaki MS, Abdel-Aleem A, et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann. Neurol. 2006;59:527–534. doi: 10.1002/ana.20749. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev. Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.