Abstract
Toward developing engineered cartilage for the treatment of cartilage defects, achieving relevant functional properties before implantation remains a significant challenge. Various chemical and mechanical stimuli have been used to enhance the functional properties of engineered musculoskeletal tissues. Recently, Ca2+-modulating agents have been used to enhance matrix synthesis and biomechanical properties of engineered cartilage. The objective of this study was to determine whether other known Ca2+ modulators, digoxin and adenosine triphosphate (ATP), can be employed as novel stimuli to increase collagen synthesis and functional properties of engineered cartilage. Neocartilage constructs were formed by scaffold-free self-assembling of primary bovine articular chondrocytes. Digoxin, ATP, or both agents were added to the culture medium for 1 h/day on days 10–14. After 4 weeks of culture, neocartilage properties were assessed for gross morphology, biochemical composition, and biomechanical properties. Digoxin and ATP were found to increase neocartilage collagen content by 52–110% over untreated controls, while maintaining proteoglycan content near native tissue values. Furthermore, digoxin and ATP increased the tensile modulus by 280% and 180%, respectively, while the application of both agents increased the modulus by 380%. The trends in tensile properties were found to correlate with the amount of collagen cross-linking. Live Ca2+ imaging experiments revealed that both digoxin and ATP were able to increase Ca2+ oscillations in monolayer-cultured chondrocytes. This study provides a novel approach toward directing neocartilage maturation and enhancing its functional properties using novel Ca2+ modulators.
Introduction
Arthritis is the second most common chronic disease in the United States. Affecting 46.4 million people annually, this disease has a tremendous socioeconomic impact.1 The current standard approach to treating end-stage degenerative joint disease is total joint replacement surgery. To avoid such highly invasive surgical procedures, newer cell-based methods are being implemented to treat symptomatic cartilage defects earlier with the goal of delaying, if not preventing, total joint deterioration. Although satisfactory short-term outcomes have been achieved with these techniques, they do not offer a permanent solution. Recently, advancements in cartilage tissue engineering offer much promise as long-term treatment options for repairing or replacing damaged cartilage. In spite of such progress, translation of such techniques into clinical practice has not yet been achieved, as many challenges remain to be resolved. Specifically, the functional properties of engineered tissues still remain far from native tissue values, leaving the neotissue incapable of withstanding the rigorous mechanical environment of articulating joints.2 Therefore, focusing on methods to enhance the maturation and biomechanical integrity of the neocartilage is crucial toward developing durable regenerative treatments.
Structure–function relationships dictate that a direct link exists between the extracellular matrix (ECM) content and mechanical integrity of a tissue. The classic structure–function paradigm correlates the compressive properties of cartilage with glycosaminoglycan (GAG) composition and the tensile modulus with collagen content.3 However, later studies have reported that the compressive and tensile moduli to each correlated with both collagen content and collagen cross-links in articular cartilage,4,5 introducing a more complex structure–function relationship between the biochemical composition and the biomechanical properties of the tissue. More recently, studies in engineered tissues have shown pyridinoline collagen cross-links to play a critical role in stabilizing the ECM of the neocartilage and to promote structural and functional maturation of neotissues.6,7 Thus, developing strategies that specifically target collagen synthesis and collagen cross-linking, two fundamental aspects of an engineered cartilage's matrix, is crucial toward developing functional neotissues.
Mechanical stimuli have been used to mimic the highly loaded environment of the native tissue as a means to enhance matrix synthesis and biomechanical properties of engineered cartilage. In vitro experiments have shown that bioreactors that apply dynamic compression8,9 and hydrostatic pressure10,11 can induce chondrocytes to increase collagen and proteoglycan synthesis. Various mechanical stimuli, such as fluid flow-induced shear,12 compression,13–15 and hydrostatic pressure,16 have also been shown to induce immediate increases in intracellular Ca2+. Thus, mechanotransduction pathways in chondrocytes have been widely linked to Ca2+-regulated signaling pathways. Furthermore, Ca2+ oscillations have been shown to play significant roles in chondrogenesis,17 chondrocyte matrix production,18–20 and, potentially, the development of functional biomechanical properties in engineered cartilage.
The complexity involved in using bioreactors to biomechanically stimulate engineered tissues, the potential confounding factors implicated in such processes, and the inconsistent results reported in the literature8,21,22 have shifted interest to more direct methods. Much emphasis has instead been placed into the investigation of chemical agents that modulate known Ca2+-regulated molecular pathways as a means to improve the neotissue's functional properties. For instance, adenosine triphosphate (ATP) is known to induce immediate Ca2+ oscillations in two-dimensional (2D)23 and three-dimensional24-cultured chondrocytes. These Ca2+ oscillations induced by ATP have been attributed to the activation of P2Y receptors and release of endoplasmic reticulum-stored Ca2+ through the IP3 pathway.24 Studies have proposed ATP to be an integral part of the chondrocyte mechanotransduction pathway.23–26 More recently, it has been shown that temporal application of ATP in chondrocyte pellet cultures27 and engineered cartilage constructs28 increases proteoglycan and collagen production. Thus, ATP could potentially be used as a means to modulate Ca2+-mediated pathways toward enhancing neocartilage functional properties.
Ouabain, a known cardiac glycoside, has similarly been shown to improve the functional properties of the neocartilage. Ouabain is a well-described inhibitor of the Na+/K+-ATPase. By disrupting membrane polarization, it can reverse the function of the Na+/Ca2+ exchanger (NCX), thereby raising intracellular Ca2+.29–31 Given its water-soluble nature and ability to selectively inhibit the Na+/K+-ATPase, ouabain has been used in biomedical research. Previous results have demonstrated that temporal application of ouabain during tissue culture (20 μM) increases neocartilage collagen content by 90% and tensile properties increase by 110% over controls.19 In spite of the benefits of ouabain toward enhancing the functional properties of the neocartilage, it is not a clinically approved agent, thus greatly hindering its clinical applicability.
The FDA-approved cardiac glycoside digoxin is another Ca2+ modulator that has been widely used for the treatment of cardiac pathologies. Although similar in structure to ouabain, digoxin's effects on engineered cartilage remain unknown. Despite the structural similarities between digoxin and ouabain, their intracellular effects are thought to be markedly different.32,33 Unlike ouabain, digoxin is able to pass through the cell membrane whereby it binds to ryanodine receptors.34 Once bound, Ca2+ is released from the sarcoplasmic reticulum. Digoxin is also capable of forming transmembrane calcium channels, resulting in increased intracellular Ca2+ concentrations35 that could potentially promote the collagen synthetic activity of chondrocytes and subsequently neotissue formation; especially considering the already approved clinical status of digoxin, the use of this agent in neocartilage tissue engineering merits investigation.
The objective of the present study was to determine whether digoxin and ATP could increase the functional properties of engineered neocartilage when applied individually or in combination. For a positive control, 20 μM of ouabain was used since its beneficial effects on chondrocytes through ion modulation have already been reported.19 KB-R7943, an inhibitor of the NCX, and suramin, an inhibitor of the P2Y receptor, were also used to probe the mechanisms of action of digoxin and ATP, respectively. All agents were applied on days 10–14 for 1 h/day, as this treatment window has been shown to work best for mechanical and chemical stimuli that similarly alter calcium transients.10 Overall, it was hypothesized that the simultaneous application of digoxin and ATP would additively or synergistically improve the biochemical and biomechanical properties of engineered cartilage.
Materials and Methods
Chondrocyte isolation
Superficial and middle/deep zone bovine articular chondrocytes were isolated from the femoral condyle and trochlear groove of eight calf legs (Research 87), as previously described.10 All tissue-engineered cartilage constructs were derived from cells of the same harvest.
Self-assembly of neocartilage constructs and growth
Disc-shaped neocartilage constructs were grown, as previously described.36 Briefly, 5.5×106 chondrocytes were seeded into 5-mm diameter 2% agarose wells. After 4 h, 400 μL of medium was carefully added. The medium formulation consisted of Dulbecco's Modified Eagle Medium (25 mM glucose with GlutaMAX™; Life Technologies), 100 nM dexamethasone (Sigma), 1% penicillin/streptomycin/fungizone (Lonza), 1% insulin-transferrin-selenium (BD Biosciences), 1% non-essential amino acids (Life Technologies), 100 μg/mL sodium pyruvate (Fischer Scientific), 50 μg/mL ascorbate-2-phosphate (Sigma), and 40 μg/mL L-proline (Sigma). Five hundred microliters of medium was changed every 24 h until day 10. On day 10, constructs were unconfined from the wells and placed into six-well plates where the medium was changed every 48 h. At 4 weeks, constructs were portioned for testing.
Application of digoxin, ouabain, ATP, KB-R7943, and suramin
From days 10 to 14, the constructs were treated daily with a low or high concentration of digoxin (200 nM and 20 μM; Sigma), ATP (250μM; Sigma), ouabain (20 μM; Sigma), or both digoxin (200 nM) and ATP. A concentration of 250 μM ATP has been previously shown to increase chondrocyte collagen synthesis.28 For the groups involving the use of inhibitors, constructs were first treated with either KB-R7943 (10 μM; Tocris Bioscience) or suramin (100 μM; Tocris Bioscience), alone for 30 min. The constructs were then incubated in a medium containing both the inhibitor and the respective agent for 1 h. After treatment, constructs were washed for 30 min and returned to normal culture conditions. Stock digoxin was prepared at 20 mM in dimethyl sulfoxide (DMSO; Sigma), KB-R7943 at 20 mM in DMSO, ouabain at 1 mM in phosphate-buffered saline, and suramin at 2 mM in culture medium. ATP was freshly prepared in the medium before application. Due to differing preparation methods for each drug, no vehicle control solution was used for the control group. The final concentration of DMSO exposed to the cells is well below the threshold observed to have an effect on cell cytotoxicity and signaling.37
Analysis of growth metrics
Pictures of gross construct morphology were taken and construct total wet weights (WWs) measured. Each construct was then sectioned into appropriately sized pieces for biochemical and biomechanical analysis. Water content was calculated with samples used for biochemical analysis.
Histology and immunohistochemistry
Samples were cryosectioned at 16 μm, fixed in formalin, and stained with Safranin O/Fast Green and Picrosirius Red. Collagen II and collagen I immunohistochemistry were conducted, as described previously.38 Briefly, cryosections were fixed in cold acetone and incubated with mouse anti-collagen I (Accurate) and rabbit anti-collagen II (Cedarlane Labs) primary antibodies. The secondary antibodies used were anti-mouse and anti-rabbit immunoglobulin antibodies, respectively, from the Vectastain ABC kit (Vector Labs). Color was developed using the Vectastain ABC reagents and DAB (Vectastain).
Biochemical analysis
Lyophilized portions of the constructs were digested in papain for 18 h at 60°C. Total collagen content was determined by measuring hydroxyproline content using a chloramine T assay.39 Total sulfated GAG content was measured using the Blyscan sGAG assay kit (Biocolor). DNA content was measured using the Quant-iT PicoGreen assay kit (Life Technologies) and normalized to cell number using a conversion of 7.7 ng DNA/cell. Pyridinoline cross-links were measured as previously described.40 Briefly, tissue samples were digested in 800 μL 6 N HCl at 100°C for 18 h, dried with a SpeedVac, resuspended in 50 μL of a solution containing 10 nmol pyridoxine/mL and 2.4 μmol homoarginine/mL (Sigma) in water, and analyzed with high performance liquid chromatography.
Biomechanical analysis
For compressive testing, 3-mm diameter punches were tested with a creep indentation apparatus, as previously described.19 Aggregate modulus, permeability, and Poisson's ratio were calculated using a semianalytical, seminumeric, linear biphasic model, and finite element analysis.41 For tensile testing, dog-bone-shaped samples were obtained, photographed, and subjected to uniaxial tension using an Instron Model 5565 (Instron), as previously described.19 A strain rate of 1% of the gauge length (1.45 mm) was used. Cross-sectional areas were calculated using the photographs and ImageJ software. The linear region of the stress–strain curve was used to determine the Young's modulus. The ultimate tensile strength (UTS) was determined as the maximum stress reached in the curve.
Ca2+ imaging of chondrocytes stimulated with ATP, digoxin, and ATP+digoxin
Chondrocytes were seeded on glass coverslips (No. 1, 25×25 mm) at 70,000 cells/cm2 and cultured overnight. Cells were loaded with 2.5 μM Fluo-4/AM (Life Technologies) for 1–2 h in the presence of 0.75 μM Pluronic F-127 (Life Technologies). Coverslips were placed into a custom-built drug perfusion system using gravity flow to perfuse the cells with fresh solution at a flow rate of ∼1.5 mL/min. Confocal images were obtained using an Olympus FluoView 1000 Confocal Microscope (inverted configuration) with a water immersion fluorescence objective UPlanSApo 60×, NA1.2, corrected for the thickness of the No. 1 glass coverslip that was used at the bottom of the perfusion chamber. Fluo-4 was excited with a 488 nm laser beam (laser power set to 5%), and the emitted fluorescence light was passed through a bandpass filter BA505-605 and collected with a photomultiplier tube (PMT). The PMT voltage, gain, and offset were set to avoid any saturation and to obtain high fidelity images.
Time-lapse images (6 s/frame) were acquired over 30 min in a field of view containing ∼20–30 cells under the 60×objective. During the first 15 min, the buffer solution (Tyrode's solution containing 1 mM Ca2+) was perfused onto the cells to capture baseline Ca2+ activity. During the next 15 min, the buffer solution containing either ATP (250 μM), digoxin (200 nM), or both agents was perfused onto the cells. Time-lapse were analyzed in ImageJ software. Regions of interest (ROI) were drawn around each cell, the fluorescence values were measured by counting the total fluorescence intensity in the ROI for each frame, and the values were used to index the Ca2+ concentration in the cell. MATLAB was used to count the number of Ca2+ oscillations, which were defined as peaks exceeding a threshold of 1.3 times (above the noise level) of the baseline fluorescence intensity. The frequency of Ca2+ oscillations was defined as the average number of oscillations per cell over 15 min. Each slide represented one sample (n= 4–6).
Statistics
All data sets were analyzed with one-factor analysis of variance (ANOVA), to determine the significance among groups, and Fischer's LSD post hoc test (p<0.05) using StatView software. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters. To test for synergistic or additive effects between digoxin and ATP, the interaction term of a two-factor ANOVA was used as previously described,42 where the factors assessed were digoxin and ATP. Univariate regression was run to correlate pyridinoline content with the tensile modulus.
Results
Gross morphology and histology
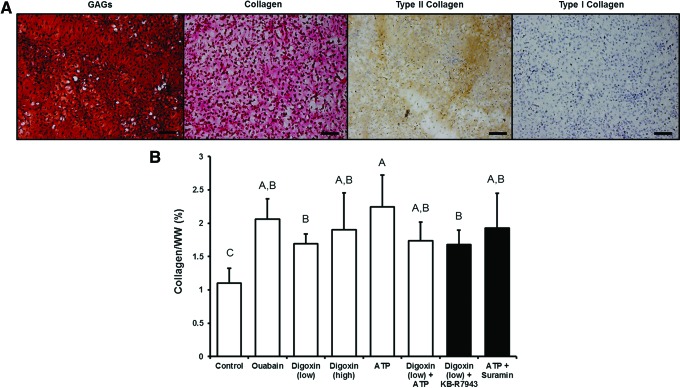
At the end of 4 weeks of culture, all neocartilage constructs had smooth surfaces and uniform morphology (Fig. 1). All neotissues stained uniformly for GAG and collagen with no gross differences in staining intensity among the groups (Fig. 2A). Histology images were taken of regions near the center of the constructs. No indication of necrosis in the center of the construct has been observed among all constructs. In addition, the neocartilage from all groups stained positively for type II collagen and negatively for type I collagen (Fig. 2A), indicating the formation of hyaline-like cartilage phenotype.
FIG. 1.
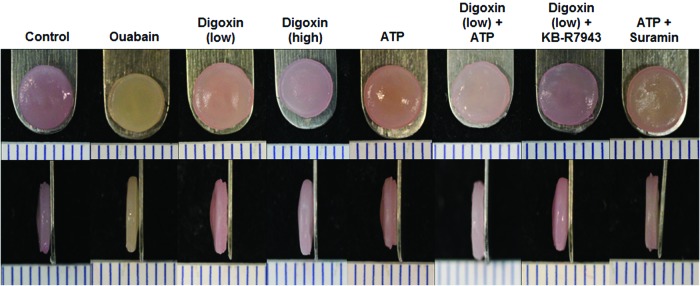
Representative images of self-assembled neocartilage constructs from each group at the end of 4 weeks of culture. All constructs appeared flat and disc-shaped with no gross morphological differences. ATP, adenosine triphosphate. Color images available online at www.liebertpub.com/tea
FIG. 2.
Neocartilage glycosaminoglycan (GAG) and collagen content at the end of 4 weeks of culture. (A) Histological staining showed the presence of GAGs (Safranin O/Fast green stain) and total collagen (Picrosirius red stain) throughout the construct. Immunohistochemistry indicated the presence of type II collagen and the absence of type I collagen. Scale bar: 50 μm. (B) Measurement of the collagen content indicated that all chemical agents increased collagen production over controls. Significant differences (p<0.05) exist between groups not sharing the same letters. Color images available online at www.liebertpub.com/tea
Biochemical properties
At the end of the 4-week culture, the biochemical components of the engineered tissues were quantitatively assessed. All digoxin- and ATP-treated constructs had significantly higher collagen per WW than untreated controls (52–110% increase) (Fig. 2B). Digoxin and ATP alone groups presented the highest amount of collagen/WW. In line with previous results,19 ouabain increased collagen/WW by ∼90% over controls. Application of inhibitors, KB-R7943 and suramin, did not abolish the action of digoxin and ATP in terms of collagen content. These results indicate that digoxin and ATP promote collagen synthesis in engineered neocartilage.
Assessing the pyridinoline cross-linking content in the engineered tissue revealed that digoxin (low) and digoxin (low)+ATP groups had a higher amount of pyridinoline per collagen (PYR/collagen) than the ouabain, digoxin (high), and ATP groups (Table 1). Univariate regression analysis revealed that PYR/collagen was statistically correlated with the Young's modulus (R2=0.70) (Fig. 3), indicating that the formation of collagen cross-links was a major contributor to neocartilage biomechanical properties.
Table 1.
Neocartilage Wet Weight, Water Content, Glycosaminoglycan Content, Cells/Construct, and Pyridinoline Per Collagen Content at the End of 4 Weeks of Culture
| Group | Wet weight (mg) | Water content (%) | GAG/WW (%) | Cells/construct (millions) | PYR/collagen (nmol/mg) |
|---|---|---|---|---|---|
| Control | 38.5±1.5c | 85.1±1.5ab | 4.1±0.6a | 5.8±0.2ab | 1.36±0.24a |
| Ouabain | 32.6±1.0d | 87.1±3.2a | 4.7±1.2a | 5.1±0.6bc | 0.83±0.15cd |
| Digoxin (low) | 41.6±3.5ab | 84.9±1.6ab | 3.5±0.4bc | 6.1±0.5a | 1.07±0.21b |
| Digoxin (high) | 33.1±3.2d | 86.4±2.0a | 2.5±0.2c | 4.5±0.4c | 0.81±0.20cd |
| ATP | 41.7±1.7a | 85.0±1.4ab | 4.0±0.8a | 5.8±0.4ab | 0.68±0.11d |
| Digoxin (low)+ATP | 40.0±2.5abc | 86.6±1.2a | 3.3±0.6bc | 5.7±0.7ab | 1.06±0.21b |
| Digoxin (low)+KB-R7943 | 38.9±2.5bc | 83.5±2.2b | 3.9±0.6ab | 6.1±0.7a | 0.75±0.18cd |
| ATP+suramin | 40.5±1.7abc | 83.3±1.3b | 4.2±1.0a | 5.5±0.8ab | 0.83±0.23bcd |
Significant differences (p<0.05) exist between groups not sharing the same letters.
PYR/collagen, pyridinoline per collagen; ATP, adenosine triphosphate.
FIG. 3.
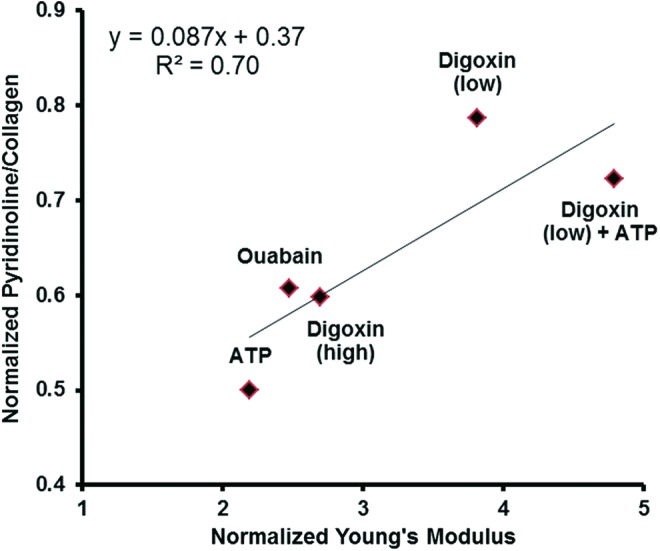
Pyridinoline per collagen plotted with Young's modulus (both normalized to control) indicated a strong correlation between collagen cross-links and tensile properties. Color images available online at www.liebertpub.com/tea
Ouabain and ATP did not affect GAG content over controls. However, all digoxin-treated groups had lower GAG content (GAG/WW) (Table 1). Pretreatment with the NCX inhibitor KB-R7943 abolished digoxin-induced changes in the GAG content of neotissue. Finally, the number of cells per construct was significantly decreased in the digoxin (high) group compared with controls, while no significant differences were observed in the other groups (Table 1).
Biomechanical properties
Compressive and tensile testing was conducted to determine the biomechanical properties of the neocartilage constructs. In terms of compressive properties, no significant differences were detected among the groups (Table 2). For the tensile properties, digoxin (low), digoxin (high), ATP, and ouabain significantly increased the neotissue's Young's modulus over untreated controls by 282%, 170%, 148%, and 120%, respectively (Fig. 4A). A low dose of digoxin (200 nM) was more effective in enhancing the tensile properties of the neocartilage compared with the higher dose of digoxin (20 μM) and ouabain (20 μM). Combined treatment of digoxin (low) and ATP resulted in the highest increase in the tensile modulus (∼380% increase over controls). Pretreatment with the NCX inhibitor KB-R7943 only partially abolished digoxin-induced effects, while pretreatment with suramin completely abolished ATP-induced effects. Similar trends were shown in the UTS of the constructs (Fig. 4B). Results in this study show that digoxin and ATP significantly enhanced the tensile properties of the constructs, while their combined treatment resulted in an additive increase.
Table 2.
Neocartilage Compressive Properties at the End of 4 Weeks of Culture
| Group | Aggregate modulus (kPa) | Permeability (10−15 m4/N s) | Poisson's ratio |
|---|---|---|---|
| Control | 125±45 | 46±26a | 0.12±0.06 |
| Ouabain | 111±40 | 20±12b | 0.08±0.05 |
| Digoxin (low) | 139±49 | 28±12ab | 0.10±0.05 |
| Digoxin (high) | 120±35 | 21±14b | 0.11±0.08 |
| ATP | 90±23 | 29±22ab | 0.11±0.07 |
| Digoxin (low)+ATP | 123±36 | 17±8b | 0.14±0.05 |
| Digoxin (low)+KB-R7943 | 93±36 | 33±19ab | 0.09±0.07 |
| ATP+suramin | 123±19 | 38±22ab | 0.13±0.10 |
Significant differences (p<0.05) exist between groups not sharing the same letters.
FIG. 4.
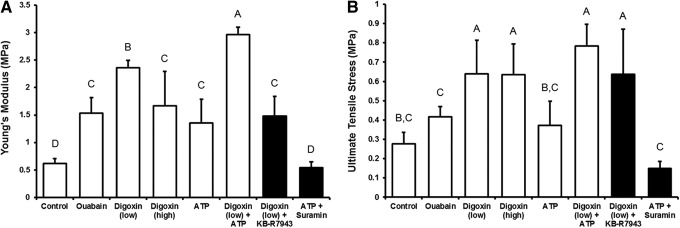
Neocartilage Young's modulus (A) and ultimate tensile strength (B) after 4 weeks of culture. Digoxin and adenosine triphosphate (ATP), either alone or combined, were able to increase the tensile properties of the neocartilage compared with controls. Significant differences (p<0.05) exist between groups not sharing the same letters.
Ca2+ oscillations in 2D-cultured chondrocytes
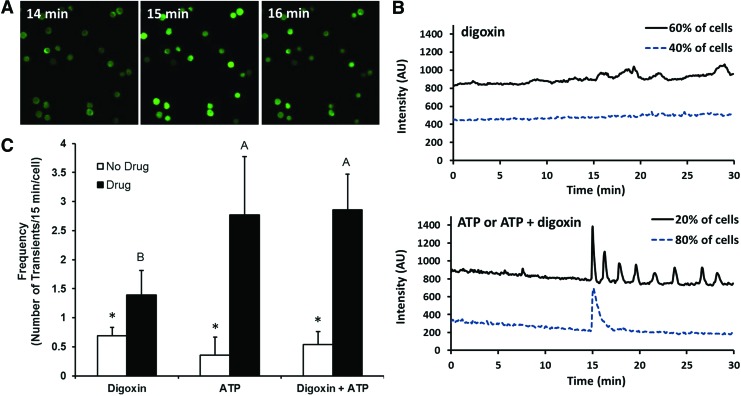
To probe the mechanisms underlying the effects of digoxin and ATP toward enhancing the above properties of the neocartilage, we examined the effects of these agents on modulating Ca2+ signaling in chondrocytes. Without any stimulation, chondrocytes are known to exhibit a basal level of Ca2+ oscillations, a signaling process that has been linked to the regulation of matrix production following mechanical stimulation. Application of digoxin (200 nM) and ATP (250 μM) to monolayer-cultured chondrocytes significantly increased the frequency of intracellular Ca2+ oscillations over basal levels (Fig. 5C). When comparing between treatments, ATP induced a higher frequency of Ca2+ oscillations than digoxin, while the combination of the two produced a frequency similar to that of ATP alone.
FIG. 5.
Intracellular Ca2+ signaling in two-dimensional-cultured articular chondrocytes stimulated with digoxin and ATP. (A) Representative images of chondrocytes cultured on glass slides showing an increase in intracellular Ca2+ upon induction with ATP at 15 min. (B) Representative Ca2+ traces induced by each agent. ATP and digoxin+ATP induced similar responses. Traces were shifted on the y-axis for better visualization. Downward drifts were due to photobleaching. (C) All agents increased the frequency of Ca2+ oscillations over baseline controls, as determined by a paired t-test (significance denoted by an asterisk). ATP and digoxin+ATP induced more Ca2+ oscillations than digoxin alone, as determined by a one-way analysis of variance comparing the different drugs (black bars). Significant differences (p<0.05) exist between groups not sharing the same letters. Color images available online at www.liebertpub.com/tea
The digoxin- and ATP-induced Ca2+ oscillations exhibited different characteristics as seen in the representative traces in Figure 5B. It was also noted that chondrocyte Ca2+ activities were heterogeneous among individual cells in a population of cells cultured and treated under identical conditions. Digoxin-induced Ca2+ oscillations showed little periodicity, were generally small in amplitude, and had a rolling shape (Fig. 5B, top panel). Such a response to digoxin was observed in ∼60% of the cells, while the other 40% did not show any discernable response. ATP, on the other hand, induced a single large Ca2+ peak immediately after drug application in more than 95% of the cells. This initial peak was followed by trains of periodic oscillations (3–8 peaks over 15 min) in ∼20% of the cells (Fig. 5B, bottom panel). In the other ∼80% of the cells, only 0–2 peaks were observed immediately after the initial peak (Fig. 5B, bottom panel). The combination of digoxin and ATP generated Ca2+ oscillations that were similar to those generated by ATP alone. Collectively, these results demonstrate the ability of digoxin and ATP to increase the frequency of Ca2+ oscillations in chondrocytes. The differential effects of digoxin and ATP on modulating the Ca2+ signaling in chondrocytes shed light into their different effects on modifying the biochemical and biomechanical properties of the neocartilage. More extensive mechanistic studies are needed to fully understand these differences, but are out of the scope of this project.
Discussion
Tissue engineering holds great potential toward treating articular cartilage defects resulting from trauma or degenerative diseases, such as osteoarthritis. However, current methods to develop the neocartilage in vitro result in functionally inadequate matrices possessing poor biochemical and subsequently inferior biomechanical properties compared with native tissue. To address this problem, mechanical stimuli, such as static loading, dynamic compression, or hydrostatic pressure, have often been used to increase chondrocyte collagen production.10,43,44 Although the mechanism of chondrocyte mechanotransduction is not fully understood, previous studies suggest that ATP signaling, ion channels, and the primary cilia are likely involved in transducing extracellular biomechanical signals to both intracellular chemical and electrical signals.28,45,46 Given calcium's role in the mechanotransduction pathway, the application of Ca2+-modulating agents to developing neocartilage presents as a potential strategy to improve the ECM of neotissues and enhance their functional properties.
The results of this study demonstrate that digoxin or ATP can improve the biomechanical properties of the neocartilage through ECM component modification. Specifically, application of either digoxin (200 nM and 20 μM) or ATP (250 μM) significantly increased the collagen content of neocartilage by ∼1.5- to 2-fold over controls and the tensile modulus by ∼2- to 4-fold over controls. When digoxin (200 nM) and ATP (250 μM) were applied together, they additively increased the tensile modulus by ∼5-fold over controls reaching a Young's modulus of ∼3 MPa. In comparison with native tissue values, juvenile bovine articular cartilage tested under the same methods possessed a Young's modulus of ∼4–5 MPa.47 When further matured over the long term in an in vivo environment, self-assembled cartilage constructs are envisioned to have the potential to approach native tissue properties. In addition to the characterization of neocartilage properties, live Ca2+ imaging of chondrocytes supports the ability of ATP, digoxin, or ATP+ digoxin to increase the frequency of intracellular Ca2+ oscillations in these cells. Collectively, this study demonstrates that modulation of intracellular Ca2+ in articular chondrocytes through digoxin and ATP is a viable strategy toward enhancing neocartilage functional properties.
A pilot study initially investigated the effects of six different doses (0.002, 0.02, 0.2, 2, 20, and 200 μM) of digoxin on the viability and metabolic activity of articular chondrocytes (data not shown). Based on these results, a high dose of digoxin (20 μM) was applied to investigate its effect on the engineered neotissue; a low dose of digoxin (200 nM) was tested to explore a lower therapeutic dose. The 200 nM (low) dose of digoxin was found to have a better effect on construct properties than the 20 μM (high) dose of digoxin. Both doses significantly increased collagen content to similar values (54–73% increase over controls), but the low dose increased the tensile modulus more than the high dose. The lower tensile properties of the digoxin (high) group can be attributed to its lower collagen cross-linking content. It is also possible that the 20 μM dose of digoxin promoted a cytotoxic effect on the cells, as potentially indicated by the lower DNA content. Therefore, 200 nM of digoxin was determined to be an effective dose that enhances both collagen content and tensile properties of neocartilage constructs without having significant adverse effects.
When compared with 20 μM ouabain, digoxin (low and high) was found to have a more potent effect. Specifically, digoxin (low) significantly increased the Young's modulus by 54% and digoxin (low and high) significantly increased the UTS by ∼50% over that of ouabain. The current data also suggest that these differences can be attributed to digoxin's ability to induce more collagen cross-links than ouabain. In addition, differences in the amount of collagen between the two treatments may be attributed to the fact that these agents act in different cellular spaces.48 Interestingly, previous reports have reported different mechanisms of action of digoxin and ouabain. For example, digoxin and ouabain can induce different hemodynamic effects in rats,49 indicating the two drugs may have different cellular effects. Digoxin not only affects the Na+/K+-ATPase but may also affect intracellular receptors and transmembrane calcium channels differently than ouabain. Although these molecular mechanisms are still under investigation, this study supports that digoxin has a more potent effect over ouabain in enhancing neocartilage properties.
Although digoxin and ATP increased neocartilage collagen content and tensile properties, these two properties did not correlate as typically expected.50 Previous attempts to correlate mechanical properties with matrix components, such as collagen, GAG, and mineralization, have been met with inconsistent results.50–53 In one study, a significant correlation was found between tensile strength and collagen content of engineered cartilage,54 while another study reported no significant correlation between the Young's modulus and collagen content.51 A tissue's tensile properties most likely arise from the interactions among multiple matrix components. Some of these have received little attention, such as pyridinoline cross-links within the collagen network. Pyridinoline cross-links of the collagen fibers of the ECM have been shown to significantly contribute to the tensile properties of bovine articular cartilage.55 In an analysis of rat tendon, the pyridinoline cross-link was even shown to be a better indicator of the ultimate tensile stress than collagen content.56 Therefore, both collagen content and cross-linking can contribute significantly to the tensile properties of musculoskeletal tissues. In the present study, regression analysis revealed that for the treated groups, tensile properties were found to correlate (R2=0.70) with the amount of pyridinoline cross-links normalized to collagen (Fig. 4). Interestingly, the digoxin (low) group exhibited the highest amount of PYR/collagen, showing for the first time that digoxin can increase collagen cross-links in articular cartilage.
Although the control group contained a higher cross-link-to-collagen ratio compared with all treated groups, its tensile properties were lower. This paradox may be explained by the timing of treatment application. In self-assembled neocartilage, the collagen content plateaus at roughly 10 days, which is then followed by tissue maturation.57 Treatment with the agents on days 10–14 may have induced additional collagen synthesis that may not have had sufficient time to form cross-links, as formation of mature (trifunctional) pyridinoline cross-links requires 7–28 days.58 Therefore, the control group did not exhibit any correlation between the Young's modulus and PYR/collagen content, as did the other groups.
The compressive properties of the neocartilage remained indifferent to treatment, despite observed changes in the GAG content. All digoxin-treated groups significantly lowered neocartilage GAG content, while ouabain and ATP had no effects on GAG. However, digoxin was found to promote greater collagen cross-links, which may result in a more functional collagen matrix better able to handle both tensile and compressive loads, despite decreased GAG levels. Furthermore, while most articular cartilage engineering approaches result in relatively low levels of collagen, they are able to attain quantities of GAG on par with native tissue.38 Therefore, it is of interest to target approaches that increase the collagen content and tensile properties of engineered cartilage without necessarily further increasing GAG content. Intracellular ion modulation with digoxin and ATP provides a method for increasing tensile properties and collagen content independent of affecting the compressive modulus.
Time-lapse confocal microscopy supports that digoxin and ATP can modulate intracellular Ca2+ signaling in 2D-cultured chondrocytes. Effects of digitalis glycosides on nonexcitable cells, such as chondrocytes, have not been previously studied. In excitable cells, such as cardiomyocytes, digoxin can induce spontaneous Ca2+ transients from overloaded and unstable intracellular Ca2+ stores.59 The increased frequency of Ca2+ oscillations in digoxin-treated chondrocytes may be a result of similar mechanisms. On the other hand, ATP has been well known to induce trains of Ca2+ oscillations in chondrocytes60 and other cell types. In chondrocytes, Ca2+ oscillations induced by ATP have been attributed to the activation of P2Y receptors and release of endoplasmic reticulum-stored Ca2+ through the IP3 pathway.24 It is unclear why ATP induces periodic trains of Ca2+ oscillations in only a proportion of the cells (20%), as reported in this study and in previous studies,60 but the heterogeneity of the chondrocyte population may be a factor. Treatment with the combination of digoxin and ATP induced Ca2+ oscillations of a similar frequency, as in ATP-treated cells, despite the two groups resulting in significantly different neocartilage properties. Further studies will be required to explore the nuances of Ca2+ oscillations or parallel signaling pathways that could lead to such differences. The data in this study support that digoxin and ATP can modulate intracellular Ca2+ signaling in chondrocytes and that these signaling cascades are likely linked to observed changes in neocartilage properties.
The mechanisms of action of digoxin and ATP were also explored using the inhibitors KB-R7943 and suramin, respectively. KB-R7943 binds to and inhibits the reverse mode of the NCX and has been shown to prevent digoxin-induced rises in intracellular Ca2+. In the present study, addition of KB-R7943 partially abolished digoxin (low)-induced changes in Young's modulus and GAG content, but did not abolish digoxin-induced changes in other construct properties. Therefore, digoxin may act by pathways independent of the NCX. Inhibition of the Na+/K+-ATPase may alter cell resting potential, cell swelling, and MAPK activation61 among other effects. Interestingly, these cellular changes are also known to occur in chondrocytes during mechanical compression of cartilage.62–64 Suramin, on the other hand, a reported inhibitor of the P2Y receptor, abolished ATP-induced changes in the Young's modulus, but did not affect ATP-induced changes in the collagen content. These results indicate that ATP can act by pathways independent of the P2Y receptor. ATP is also known to bind cell surface P2X receptors, ligand-gated ion channels that can affect intracellular ion concentrations, and cell signaling. In addition, chondrocyte surface ectonucleotidases can convert extracellular ATP into adenosine,65 a widely studied extracellular signaling molecule in cartilage biology. Depletion of extracellular adenosine has been shown to promote cartilage degradation,66 indicating a role of adenosine in cartilage matrix homeostasis. Therefore, digoxin and ATP may have multiple effects on the chondrocyte, which require further study. These Ca2+-independent pathways may also contribute to the additive effects seen by digoxin and ATP, which could not be explained by the Ca2+ signaling characteristics.
In conclusion, the present study supports that modulating intracellular Ca2+ concentration through exogenous application of the chemical agents, digoxin and ATP, can alter the development of engineered articular cartilage. Application of 200 nM digoxin and 250 μM ATP individually increased the tensile modulus and collagen content of the engineered cartilage, while their combination resulted in additive increases in both the biochemical and functional properties. Overall, this study provides evidence that agents known to modulate intracellular Ca2+ concentration are effective in developing more robust neocartilage through increasing collagen content and collagen cross-links. Additionally, the ability of these agents to be applied exogenously raises the potential for direct in vivo applications of these drugs, for example, through intra-articular injections, to treat degenerative cartilage pathologies such as osteoarthritis.
Acknowledgments
This work was funded in part by NIH grants R01-AR053286 and R01-AR047839. The funding sources did not have a role in the collection, analysis, and interpretation of the data. The authors acknowledge the Cardiac Signaling Laboratory for use of the confocal microscope and reagents, the Proteonomics Core Facility for use of sample preparation equipment, and Derek Cissell for statistical discussions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hootman J.M., and Helmick C.G.Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 54,226, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Makris E.A., Hadidi P., and Athanasiou K.A.The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32,7411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X.L., and Mow V.C.Biomechanics of articular cartilage and determination of material properties. Med Sci Sports Exerc 40,193, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Julkunen P., Harjula T., Iivarinen J., Marjanen J., Seppanen K., Narhi T., Arokoski J., Lammi M.J., Brama P.A., Jurvelin J.S., and Helminen H.J.Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthritis Cartilage 17,1628, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ficklin T., Thomas G., Barthel J.C., Asanbaeva A., Thonar E.J., Masuda K., Chen A.C., Sah R.L., Davol A., and Klisch S.M.Articular cartilage mechanical and biochemical property relations before and after in vitro growth. J Biomech 40,3607, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makris E.A., MacBarb R.F., Paschos N.K., Hu J.C., and Athanasiou K.A.Combined use of chondroitinase-ABC, TGF-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials 35,6787, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makris E.A., MacBarb R.F., Responte D.J., Hu J.C., and Athanasiou K.A.A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. FASEB J 27,2421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nebelung S., Gavenis K., Luring C., Zhou B., Mueller-Rath R., Stoffel M., Tingart M., and Rath B.Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann Anat 194,351, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Jeon J.E., Schrobback K., Hutmacher D.W., and Klein T.J.Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis Cartilage 20,906, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Elder B.D., and Athanasiou K.A.Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A 15,1151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno S., and Ogawa R.Using changes in hydrostatic and osmotic pressure to manipulate metabolic function in chondrocytes. Am J Physiol Cell Physiol 300,C1234, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Degala S., Williams R., Zipfel W., and Bonassar L.J.Calcium signaling in response to fluid flow by chondrocytes in 3D alginate culture. J Orthopaed Res 30,793, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pingguan-Murphy B., El-Azzeh M., Bader D.L., and Knight M.M.Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol 209,389, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Han S.K., Wouters W., Clark A., and Herzog W.Mechanically induced calcium signaling in chondrocytes in situ. J Orthopaed Res 30,475, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Waldman S.D., Couto D.C., Grynpas M.D., Pilliar R.M., and Kandel R.A.A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage 14,323, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Mizuno S.A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am J Physiol Cell Physiol 288,C329, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fodor J., Matta C., Olah T., Juhasz T., Takacs R., Toth A., Dienes B., Csernoch L., and Zakany R.Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium 54,1, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Parvizi J., Parpura V., Greenleaf J.F., and Bolander M.E.Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. J Orthopaed Res 20,51, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Natoli R.M., Skaalure S., Bijlani S., Chen K.X., Hu J., and Athanasiou K.A.Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 62,1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Conor C.J., Leddy H.A., Benefield H.C., Liedtke W.B., and Guilak F.TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A 111,1316, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton M.L., Chen J., Guilak F., and Setton L.A.Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthopaed Res 21,963, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Fink C., Fermor B., Weinberg J.B., Pisetsky D.S., Misukonis M.A., and Guilak F.The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage 9,481, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kono T., Nishikori T., Kataoka H., Uchio Y., Ochi M., and Enomoto K.Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct 24,103, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Elfervig M.K., Graff R.D., Lee G.M., Kelley S.S., Sood A., and Banes A.J.ATP induces Ca2+ signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage 9,518, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Garcia M., and Knight M.M.Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res 28,510, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wann A.K., Zuo N., Haycraft C.J., Jenson C.G., Poole C.A., McGlashan S.R., and Knight M.M.The primary cilium orchestrates chondrocyte mechanotransduction. Osteoarthritis Cartilage 20, Supplement 1,S241, 2012 [Google Scholar]
- 27.Croucher L.J., Crawford A., Hatton P.V., Russell R.G., and Buttle D.J.Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochim Biophys Acta 1502,297, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Waldman S.D., Usprech J., Flynn L.E., and Khan A.A.Harnessing the purinergic receptor pathway to develop functional engineered cartilage constructs. Osteoarthritis Cartilage 18,864, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Swift F., Tovsrud N., Sjaastad I., Sejersted O.M., Niggli E., and Egger M.Functional coupling of alpha(2)-isoform Na+/K+-ATPase and Ca2+extrusion through the Na+/Ca2+-exchanger in cardiomyocytes. Cell Calcium 48,54, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Aperia A.New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med 261,44, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tian J., and Xie Z.J.The Na-K-ATPase and calcium-signaling microdomains. Physiology 23,205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvela M., Rosen H., Feldmann T., Nesher M., and Lichtstein D.Diverse biological responses to different cardiotonic steroids. Pathophysiology 14,159, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Fuerstenwerth H.On the differences between ouabain and digitalis glycosides. Am J Therapeut 21,35, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Nunez-Duran H., and Fernandez P.Evidence for an intracellular site of action in the heart for two hydrophobic cardiac steroids. Life Sci 74,1337, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Arispe N., Diaz J.C., Simakova O., and Pollard H.B.Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc Natl Acad Sci U S A 105,2610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J.C., and Athanasiou K.A.A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12,969, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Santos N.C., Figueira-Coelho J., Martins-Silva J., and Saldanha C.Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol 65,1035, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Natoli R.M., Revell C.M., and Athanasiou K.A.Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 15,3119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woessner J.F., Jr.The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93,440, 1961 [DOI] [PubMed] [Google Scholar]
- 40.Makris E.A., Hu J.C., and Athanasiou K.A.Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21,634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mow V.C., Gibbs M.C., Lai W.M., Zhu W.B., and Athanasiou K.A.Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J Biomech 22,853, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Slinker B.K.The statistics of synergism. J Mol Cell Cardiol 30,723, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Sah R.L., Kim Y.J., Doong J.Y., Grodzinsky A.J., Plaas A.H., and Sandy J.D.Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7,619, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Ballyns J.J., and Bonassar L.J.Dynamic compressive loading of image-guided tissue engineered meniscal constructs. J Biomech 44,509, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Wann A.K., Zuo N., Haycraft C.J., Jensen C.G., Poole C.A., McGlashan S.R., and Knight M.M.Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+signaling in compressed chondrocytes. FASEB J 26,1663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan M.N., Leddy H.A., Votta B.J., Kumar S., Levy D.S., Lipshutz D.B., Lee S.H., Liedtke W., and Guilak F.Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 60,3028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paschos N.K., Makris E.A., Hu J.C., and Athanasiou K.A. Topographic variations in biomechanical and biochemical properties in the ankle joint: an in vitro bovine study evaluating native and engineered cartilage. Arthroscopy 2014 [DOI] [PubMed] [Google Scholar]
- 48.Wang H., Yuan W., and Lu Z.Effects of ouabain and digoxin on gene expression of sodium pump alpha-subunit isoforms in rat myocardium. Chin Med J 114,1055, 2001 [PubMed] [Google Scholar]
- 49.Manunta P., Hamilton J., Rogowski A.C., Hamilton B.P., and Hamlyn J.M.Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens Res 23Suppl,S77, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Kelly D.J., Crawford A., Dickinson S.C., Sims T.J., Mundy J., Hollander A.P., Prendergast P.J., and Hatton P.V.Biochemical markers of the mechanical quality of engineered hyaline cartilage. J Mater Sci Mater Med 18,273, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Mauck R.L., Martinez-Diaz G.J., Yuan X., and Tuan R.S.Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec (Hoboken) 290,48, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Vunjak-Novakovic G., Martin I., Obradovic B., Treppo S., Grodzinsky A.J., Langer R., and Freed L.E.Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res 17,130, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Responte D.J., Arzi B., Natoli R.M., Hu J.C., and Athanasiou K.A.Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials 33,3187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mauck R.L., Seyhan S.L., Ateshian G.A., and Hung C.T.Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 30,1046, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Eleswarapu S.V., Responte D.J., and Athanasiou K.A.Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PloS One 6,e26178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan B.P., Fu S.C., Qin L., Rolf C., and Chan K.M.Pyridinoline in relation to ultimate stress of the patellar tendon during healing: an animal study. J Orthopaed Res 16,597, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Ofek G., Revell C.M., Hu J.C., Allison D.D., Grande-Allen K.J., and Athanasiou K.A.Matrix development in self-assembly of articular cartilage. PloS One 3,e2795, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahsan T., Harwood F., McGowan K.B., Amiel D., and Sah R.L.Kinetics of collagen crosslinking in adult bovine articular cartilage. Osteoarthritis Cartilage 13,709, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Alemanni M., Rocchetti M., Re D., and Zaza A.Role and mechanism of subcellular Ca2+distribution in the action of two inotropic agents with different toxicity. J Mol Cell Cardiol 50,910, 2011 [DOI] [PubMed] [Google Scholar]
- 60.DAndrea P., and Vittur F.Ca2+oscillations and intercellular Ca2+waves in ATP-stimulated articular chondrocytes. J Bone Miner Res 11,946, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Chen J.Q., Contreras R.G., Wang R., Fernandez S.V., Shoshani L., Russo I.H., Cereijido M., and Russo J.Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti-breast cancer drugs? Breast Cancer Res Treat 96,1, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Bougault C., Aubert-Foucher E., Paumier A., Perrier-Groult E., Huot L., Hot D., Duterque-Coquillaud M., and Mallein-Gerin F.Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS One 7,e36964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chao P.H., West A.C., and Hung C.T.Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol 291,C718, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Wright M.O., Nishida K., Bavington C., Godolphin J.L., Dunne E., Walmsley S., Jobanputra P., Nuki G., and Salter D.M.Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: evidence of a role for alpha 5 beta 1 integrin as a chondrocyte mechanoreceptor. J Orthop Res 15,742, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Corriden R., and Insel P.A.Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3,re1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tesch A.M., MacDonald M.H., Kollias-Baker C., and Benton H.P.Endogenously produced adenosine regulates articular cartilage matrix homeostasis: enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthritis Cartilage 12,349, 2004 [DOI] [PubMed] [Google Scholar]