Abstract
Hematopoietic stem cells (HSCs) are an important target cell population for gene therapy since they can reconstitute the entire hematopoietic system. HSC-enriched cell populations can be recognized based on cell surface marker expression, such as CD34, which is broadly expressed on immature and partially differentiated cells. In mice, co-expression of CD34 and CD105 was previously shown to be relatively more specific for the most immature, long-term repopulating HSCs. Here, we evaluated whether CD105, which is expressed on 30%–80% of CD34+ cells, is a marker also for human long-term repopulating HSCs. Therefore, we tracked the mature progeny of CD34+ cells transduced with the CD105-targeted lentiviral vector CD105-LV in xenotolerant mice. Transduction was blocked with soluble CD105 protein confirming specificity. Importantly, CD105-LV transduced human CD34+ cells engrafted in NOD-scid IL2Rγ−/− mice with up to 20% reporter gene-positive cells detected long term in all human hematopoietic lineages in bone marrow (BM), spleen, and blood. In addition, competitive repopulation experiments in mice showed a superior engraftment of CD105-LV transduced CD34+ cells in BM and spleen compared with cells transduced with a conventional nontargeted lentiviral vector. Thus, human CD34+/CD105+ cells are enriched for early HSCs with high repopulating capacity. Targeting this cell population with CD105-LV offers a novel gene transfer strategy to reach high engraftment rates of transduced cells and highlights the applicability of receptor-targeted vectors to trace cell subsets offering an alternative to prospective isolation by surface markers.
Introduction
Hematopoietic stem cells (HSCs) serve as an important target cell population for gene therapy since they can reconstitute the entire hematopoietic system. Ex vivo gene modified HSCs were already used in several phase I/II clinical trials for the treatment of monogenetic hematological disorders like X-linked severe combined immunodeficiency, adenosine deaminase-deficient severe combined immunodeficiency, Wiskott-Aldrich syndrome, or X-linked chronic granulomatous disease [1–3]. For a successful therapy it is fundamental that the gene corrected cells engraft in the patient, especially when positive selective pressure on the transduced cells in vivo is missing [4]. Therefore, the relevant cells to treat hematological disorders and to achieve sustained gene correction are the most immature HSCs with best long-term repopulating and high self-renewal capacities. Usually, granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood CD34+ cells are used for genetic modification. This cell population is heterogeneous and contains progenitors with short-term engraftment properties and more differentiated lineage-restricted progenitors with low or no engraftment capabilities. Only a few cells are primitive long-term repopulating HSCs among the CD34+ population [5,6].
Typically lentiviral or retroviral vectors are used for gene modification of HSCs as these vectors mediate stable integration of the transgene into the target cell genome allowing dissemination of the gene to their differentiated progeny and, due to their long-term survival, potentially life-long generation of gene-corrected progeny. Most commonly lentiviral vectors are pseudotyped with the envelope protein G of the vesicular stomatitis virus (VSV), which mediates cell entry into a broad variety of human cell types via the ubiquitously expressed LDL-receptor [7]. Although vesicular stomatitis virus glycoprotein pseudotyped lentiviral vectors (VSVG-LV) allow efficient transduction of nondividing cells they do not provide substantial transduction of unstimulated, quiescent T lymphocytes, B lymphocytes, and HSCs [8].
Alternative to VSVG protein, engineered glycoproteins from other viruses have been incorporated into LV particles using cell surface receptors that are expressed less broadly. We have taken this a step further by engineering envelope glycoproteins such that the receptor used for cell entry can be predetermined through the choice of a single-chain antibody (scFv) recognizing a cell surface antigen selectively expressed on the target cell population of interest. This system relies on the glycoproteins of measles virus, namely hemagglutinin (H), which is responsible for receptor recognition and fusion (F) protein mediating fusion of the virus particle and the host cell membrane. Recognition of the measles virus receptors is abolished by mutating the H protein at four residues in its ectodomain [9]. The desired cell specificity is provided by displaying a scFv specific for the target receptor on the mutated H protein. This way, in vitro and in vivo gene transfer selective for a large variety of cell types such as B lymphocytes, T lymphocytes, dendritic cells, HSCs, neurons, endothelial cells, and tumor cells has been achieved [9–16].
Among these vectors, CD105-LV uses CD105/endoglin as receptor, which is a component of the transforming growth factor-β (TGF-β) receptor complex and is abundantly expressed on endothelial cells [17,18]. The specificity of CD105-LV was confirmed in vitro and in vivo, for example, by demonstrating exclusive gene transfer into liver sinusoidal endothelial cells in mice reconstituted with human liver cells upon systemic administration [14]. In vitro and in vivo data indicate that CD105 is a marker for long-term repopulating HSCs in mice [19–21]. As a co-factor in the TGF-β signaling pathway, CD105 is conserved in sequence and expression profile between human and mouse [17,18]. Human CD34+ cells contain a fraction of CD105+ cells, which was found to have a significantly higher long-term culture-initiating cell frequency than the CD105− fraction [22]. While these data indicated that CD105 might be a marker also for primitive human HSCs, in vivo data including transplantation and repopulation studies for human CD34+/CD105+ cells are not available [22,23]. Here, we evaluated, using CD105-LV, whether CD105 is a suitable target to facilitate selective gene transfer into human long-term repopulating HSCs with high engraftment potential.
We found that CD105-LV is highly efficient in transducing human CD34-purified cells. Transduction was blocked by preincubation of the vectors with soluble CD105 protein demonstrating specificity. Up to 20% transgene-positive cells (GFP+) were detected among the human cell population in bone marrow (BM), spleen, and blood of NOD-scid IL2Rγ−/− (NSG) mice 7–18 weeks after engraftment with human CD34+ cells that were transduced by CD105-LV. This percentage was stable over time. Moreover, we observed no lineage bias of GFP+ cells (CD3+, CD19+, CD33+, and CD34+) indicating that very early HSCs have been transduced. In addition, competitive repopulation experiments in NSG mice showed a superior engraftment of CD105-LV transduced HSCs in BM and spleen compared with cells transduced with nontargeted VSVG-LV.
Materials and Methods
Vector particle production and titration
Vector particles were produced by transient transfection of HEK 293-T cells by polyethylenimine as described previously [24]. For CD105-LV 1.3 μg pCG-Hmut-αCD105 [13], 4.1 μg pCG-FcΔ30 [9], 14.6 μg pCMVΔR8.9 packaging plasmid [25], and 15 μg transfer vector plasmid pSEW [26] or pSEW-blue fluorescent protein (BFP) encoding the green fluorescent protein (GFP) or the BFP were co-transfected (pSEW-BFP was constructed by exchange of the gfp gene in pSEW with the bfp gene using appropriate restriction enzymes). Vector particles pseudotyped with VSVG were produced by co-transfection of 6.13 μg pMD2.G (Tronolab, Lausanne, Switzerland), 11.4 μg pCMVΔR8.9, and 17.5 μg pSEW or pSEW-BFP. Forty-eight hours after transfection the supernatant was collected and filtered through a 0.45 μm filter. Vector particles were concentrated by centrifugation either at 100,000 g for 3 h at 4°C through a 20% sucrose cushion or at 4,400 g for 24 h at 4°C. Vector particles were titrated on HT1080 cells except for the competitive repopulation experiment where titration was performed on freshly isolated primary human CD34+ cells.
Primary cells and transduction
G-CSF mobilized peripheral blood was obtained from stem cell donations with written consent of the donors and in accordance with the ethical standards of the responsible committee on human experimentation (IRB permit 329/10). CD34+ cells were isolated by positive selection using anti-CD34 microbeads (Miltenyi, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. The purity was assessed by flow cytometry and accounts at least 95% in each experiment. CD34+ cells were cultivated in StemSpan serum-free expansion medium (Stemcell Technologies, Cologne, Germany) supplemented with 0.5% penicillin-streptomycin-fungizone mix (PromoCell, Heidelberg, Germany) and 2 mM glutamine. 5×104 CD34+ cells per well were transduced in 96-well plates by spinfection as described previously [13]. Cells were either transduced immediately after MACS purification (unstimulated) or after overnight stimulation with medium supplemented with StemSpan CC100 cytokine cocktail (Stemcell Technologies) and 2 μg/mL TPO (Peprotech, Rocky Hill, NJ). When unstimulated cells were transduced, cytokine-free medium was replaced by cytokine containing medium 24 h after vector particles had been added. When prestimulated cells were transduced, vector particles were added in presence of cytokines.
Colony forming assay
1.5×103 transduced or untransduced primary human CD34+ cells were transferred into 3 mL MethoCult GF H4434 medium (Stemcell Technologies) and plated in triplicates. After 10 days in an incubator at 37°C and 95% humidity total and GFP+ colonies were enumerated and morphologically classified by fluorescence microscopy.
In vivo repopulation experiments
Animal experiments were performed according to the German animal protection law and are approved by the responsible institutional ethical committee. Six-weeks-old NSG mice were sublethally irradiated (1.8 or 2 Gy). Four hours after conditioning 0.5–1.7×106 transduced or untransduced CD34+ cells were injected via the tail vein. Seven to 18 weeks after transplantation mice were sacrificed and blood, BM, and spleen cells were analyzed by flow cytometry for the presence of total and gene marked human cells.
Results
CD105 is expressed on unstimulated and stimulated human CD34+ cells
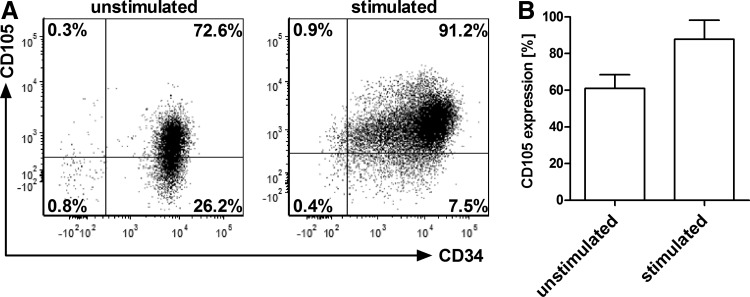
First, we analyzed the expression of CD105 on human cells that were purified by magnetic activated cell sorting either from cord blood cells or from fresh G-CSF mobilized peripheral blood using the common HSC marker CD34. In addition, frozen CD34+ cells from BM (Lonza, Cologne, Germany) were used. We found that CD105 surface expression on unstimulated CD34+ cells donor dependently varied between 30% and 80% (mean=60%). After overnight stimulation of the cells with cytokines CD105 expression increased to up to 97% (Fig. 1). There was no difference in CD105 expression between CD34+ cells purified from cord blood, G-CSF mobilized peripheral blood, or BM (data not shown). This shows that CD105, commonly recognized as marker for endothelial cells, is also expressed on human CD34+ cells and further induced upon cytokine stimulation.
FIG. 1.
Surface expression of CD105 on human CD34+ cells. After purification from G-CSF mobilized peripheral blood using CD34 MACS beads, CD34+ cells were analyzed by flow cytometry for their CD105 surface expression either immediately or after stimulation for 24 h with Flt-3 ligand, stem cell factor, IL-3, IL-6, and TPO. (A) Representative FACS plots of CD34+/CD105+ cells before and after stimulation. (B) Mean±SD of CD105 expression on CD34+ cells from seven individual donors. G-CSF, granulocyte-colony stimulating factor; SD, standard deviation.
Specific transduction of human CD105+/CD34+ cells by CD105-LV
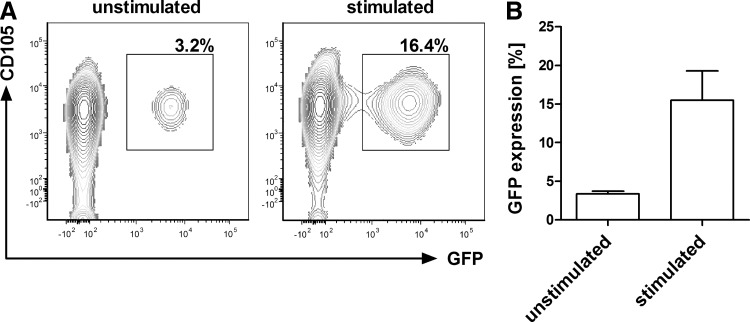
We next used CD105-LV to genetically label CD105+ cells and to follow their long-term repopulating potential. First, the ability of the vector to transduce human CD105+/CD34+ cells was analyzed. For this purpose, CD105-LV transferring the gfp gene (CD105-LVGFP) was incubated with human CD34-purified cells. The transduction efficiency was determined 72 h later by GFP expression using flow cytometry. We detected about 3%–5% GFP+ cells in unstimulated CD34+ cells and up to 20% GFP+ cells in CD34+ cells that had been stimulated for 24 h before transduction (Fig. 2). In agreement with previous data all GFP+ cells were also CD105+ [13,14] (Fig. 2A). Furthermore, the transduced cells were largely positive for the HSC markers CD133 (ca. 90%) and CD90 (ca. 60%), and mainly negative for CD38, a marker of more committed progenitors (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
FIG. 2.
Transduction of unstimulated and stimulated human CD105+/CD34+ cells by CD105-LVGFP. Unstimulated or stimulated CD34+ cells isolated from G-CSF mobilized peripheral blood were transduced by CD105-LVGFP at MOI of 1. Seventy-two hours after transduction the cells were analyzed by flow cytometry for CD105 and GFP expression. (A) Representative FACS plots of GFP+ cells transduced with or without previous stimulation of cells. (B) Mean±SD of GFP+ cells of seven independent experiments. GFP, green fluorescent protein; MOI, multiplicity of infection.
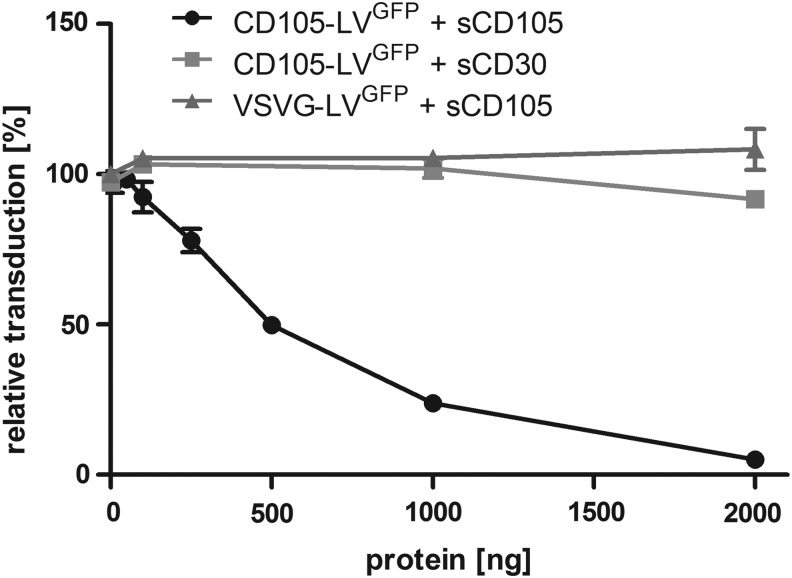
To further demonstrate the specificity of the vector for CD105 we expressed and purified the extracellular domain of CD105 fused to a Fc-Tag (sCD105) and preincubated CD105-LVGFP [CD105 (endoglin) targeted lentiviral vector transferring the gene for the green fluorescent protein] and VSVG-LVGFP (vesicular stomatitis virus glycoprotein pseudotyped lentiviral vector transferring the gene for the green fluorescent protein) as control with the protein before transduction of CD34-purified cells. There was an obvious correlation between the reduction in transduction of the cells by CD105-LVGFP and the amount of sCD105 applied. Incubation with 2 μg of sCD105 reduced the transduction by 95%. In contrast, an Fc-tagged control protein (sCD30) had no significant influence on CD105-LV mediated transduction and sCD105 did not affect the transduction by VSVG-LVGFP (Fig. 3). Furthermore, transduction by CD105-LVGFP was also blocked if either magnetic or fluorophore-labeled anti-CD105 antibodies (Miltenyi) were bound to the cells (data not shown). These data clearly demonstrate that CD105-LVGFP mediated transduction of CD34-purified cells depends on the expected mechanism, that is, binding of the vector particles to CD105 on the cell surface.
FIG. 3.
Transduction of CD34-purified cells by CD105-LVGFP is blocked by soluble CD105. The Fc-tagged extracellular part of CD105 (sCD105) or that of CD30 were added to CD105-LVGFP or VSVG-LVGFP in the indicated amounts. After 30 min incubation at 37°C the vector-protein mixtures were added to primary human CD34-purified cells at a MOI of 1 for CD105-LVGFP and a MOI of 5 for VSVG-LVGFP. Seventy-two hours later, the number of GFP-positive cells was determined by flow cytometry and the transduction efficiencies were calculated in relation to those obtained in absence of the soluble proteins. n=2; mean±SD.
Transgene expression in HSCs is stable over time and transduced cells differentiate into different lineages in vitro and in vivo
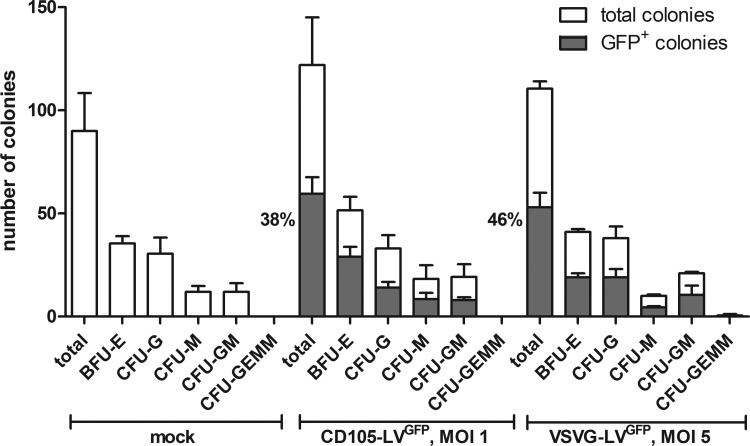
We analyzed the long-term gene expression and the multilineage potential of CD105-LVGFP transduced cells in vitro and in vivo. First, colony forming assays (CFA) with CD105-LVGFP or VSVG-LVGFP transduced CD34+ cells were performed. The fractions of GFP+ cells were determined before and after colony formation. Interestingly, the percentage of GFP+ colonies was substantially higher than the percentage of GFP+ cells before the CFA (Supplementary Fig. S2 and Fig. 4). This increase was especially pronounced for CD105-LVGFP transduced cells, which showed an average increase of 89.7% [n=3; standard deviation (SD)±14.6] compared to 29.3% (n=3; SD±13.5) for VSVG-LVGFP. In addition, CD105-LVGFP transduced cells differentiated into all hematopoietic lineages assessable by this assay. The lineage distribution was similar to that observed for mock or VSVG-LV transduced cells. These data demonstrate that CD105-LVGFP targets a HSC population with extensive proliferative and multipotent colony forming capacities as compared to mock or VSVG-LV transduced cells in vitro.
FIG. 4.
Long-term expression of the transgene in CD105-LVGFP transduced human HSCs in vitro. A colony forming assay was performed with CD34+ cells purified from G-CSF mobilized peripheral blood that were either transduced with CD105-LVGFP (MOI 1), the unspecific vector VSVG-LVGFP (MOI 5) or medium (mock). After 10 days incubation, the percentages of the indicated hematopoietic lineages were determined by fluorescence microscopy. Mean distribution±SD of all colonies is shown in white, mean distribution±SD of GFP+ colonies is shown in gray. Also, the percentages of GFP+ colonies in relation to the total colonies are indicated. One representative experiment out of three is shown. BFU-E, burst-forming unit-erythroid; CFU-G, colony-forming unit-granulocyte; CFU-GEMM, colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, colony-forming unit-granulocyte, macrophage; CFU-M, colony-forming unit-macrophage; HSCs, hematopoietic stem cells.
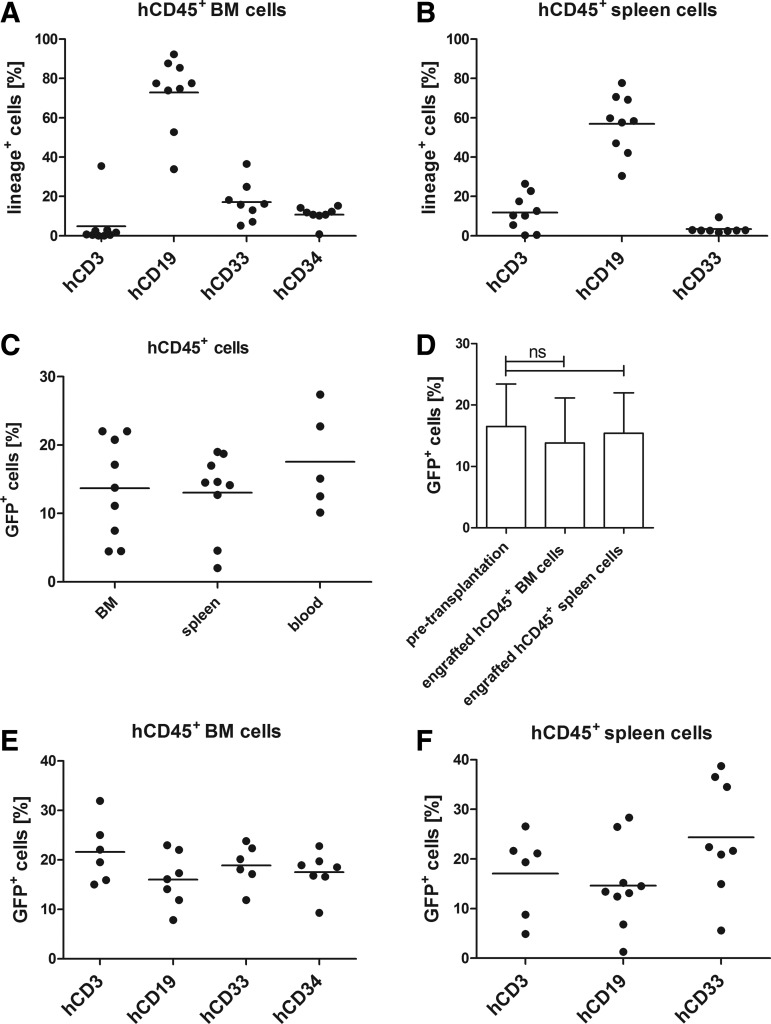
Next, we analyzed the repopulation capacity of CD105-LVGFP transduced HSCs in transplanted NSG mice over a prolonged period of time. Before transduction the cells were incubated overnight with stimulating cytokines. Twenty-four hours after transduction, the cells were washed and intravenously injected into radiation-conditioned mice. Blood, BM, and spleen cells were isolated and human CD45+ cells were analyzed for GFP+ expression and lineage distribution 7–18 weeks after transplantation. The engraftment rate, as determined by the percentage of human CD45+ cells among all CD45+ cells, varied from 13% to 69% in BM and spleen and between 5% and 25% in blood (Supplementary Fig. S3). The vast majority of human cells in all mice were CD19+ as expected from this type of humanized mouse model [27] (Fig. 5A, B). Besides, human CD3+ and CD33+ cells were detected in spleen and BM, and 10%–15% human CD34+ cells in BM indicating a capacity for self-renewal of the transplanted cells (Fig. 5A). Overall, 5%–22% of the engrafted human cells were GFP+ (Fig. 5C). This number corresponds well to the percentage of GFP+ cells initially present in the transplanted cells (Fig. 5D) demonstrating that the GFP expression remained stable over a period of up to 18 weeks and that transduced cells efficiently engrafted in mice. Importantly, the fractions of GFP+ cells in each analyzed lineage were similar indicating that they originated from transduced HSCs and that the capacity for differentiation was preserved in CD105-LV transduced cells (Fig. 5E, F).
FIG. 5.
Stable engraftment and repopulation of CD105-LVGFP transduced human HSCs in vivo. Six-weeks-old NSG mice (n=9) were irradiated at 1.8 or 2 Gy. Four hours after conditioning 1.2–1.7×106 CD105-LVGFP transduced human CD34+ cells that were isolated from G-CSF mobilized peripheral blood were transplanted intravenously. Seven to 18 weeks post-transplantation cells were isolated from BM, spleen, and blood and analyzed by flow cytometry. (A) Lineage distribution of human CD45+ cells in the BM and (B) spleen. (C) Percentages of GFP+ cells within the engrafted human CD45+ cell population in BM, spleen, and blood. (D) GFP expression in human CD34-purified cells 72 h post-transduction (pretransplantation) and in engrafted human CD45+ cells (7–18 weeks post-transplantation) in BM and spleen; ns, not significant. Comparable percentages of GFP+ cells in each lineage in human CD45+ engrafted (E) BM and (F) spleen cells; differences not significant according to one-way ANOVA analysis: P=0.2752 (BM), P=0.1254 (spleen). BM, bone marrow; h, human; NSG, NOD-scid IL2Rγ−/−.
Enhanced engraftment and repopulation capacity of CD105-LV transduced HSCs in competitive repopulation experiments
Next, we directly compared the repopulation capacities of CD105-LV and VSVG-LV transduced cells in vitro and in vivo. Therefore, two different transfer vectors encoding for GFP or BFP, respectively, were used for vector particle generation leading to four different vector types (CD105-LVGFP, CD105-LVBFP, VSVG-LVGFP, and VSVG-LVBFP). Transduction was performed without any preactivation of the cells. Vector particles were added to the cells in cytokine-containing medium and the vector input was adjusted to achieve matching transduction efficiencies of around 5% transduced cells.
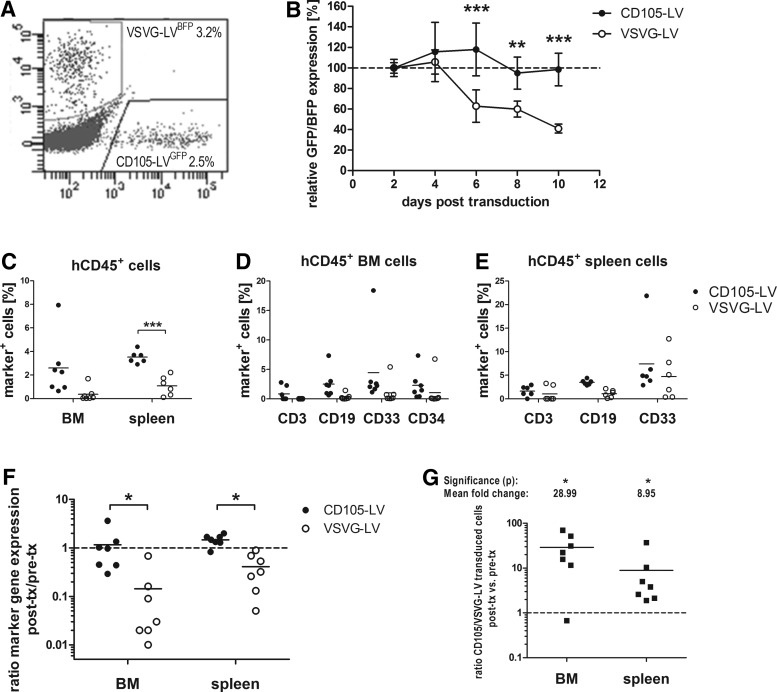
On the next day CD105-LVGFP and VSVG-LVBFP transduced cells and CD105-LVBFP and VSVG-LVGFP transduced cells were pooled in a 1:1 ratio resulting in two different populations, each consisting of three subpopulations, GFP-expressing, BFP-expressing, and untransduced. Out of each of the two populations three small aliquots were taken for prolonged in vitro culture to determine the stability of transgene expression while the majority of cells was transplanted into sublethally irradiated NSG mice (0.5–1.0×106 mobilized CD34+ cells per mouse, n=7). Forty-eight hours after transduction the percentage of marker gene expression was determined in vitro by flow cytometry revealing ∼2.5%–3% transduced cells for either vector type (Fig. 6A). This was in accordance with the anticipated transduction efficiency of 5%. In both combinations a slightly higher percentage of gene marking was observed for cells transduced by VSVG-LV as indicated by ratios of 1.3 and 1.1 (%VSVG-LV transduced cells/%CD105-LV transduced cells). Expression was monitored every other day until 10 days post-transduction by flow cytometry revealing no change in percentage of GFP/BFP-positive cells for CD105-LV. In contrast, a loss of 60% of GFP/BFP-positive cells was observed for VSVG-LV over time (Fig. 6B). Accordingly, CD105-LV facilitated transduction of cells that are maintained and/or proliferate throughout prolonged cell culture. No significant differences in cell toxicity were observed between both vector preparations (Supplementary Fig. S4), ruling out that an enhanced toxicity of VSVG-LV was responsible for the loss of transduced cells over time.
FIG. 6.
Competitive repopulating capacity of CD105-LV versus VSVG-LV transduced HSCs. CD34+ cells purified from G-CSF mobilized peripheral blood were transduced with CD105-LVGFP, CD105-LVBFP, VSVG-LVGFP, or VSVG-LVBFP. The next day VSVG-LVBFP and CD105-LVGFP and vice versa transduced cells were mixed to equal parts. (A) Representative FACS plot after pooling of the cells 48 h post-transduction. (B) In vitro monitoring of transgene expression in triplicates of both pseudotype and transgene combinations normalized to the percentage of transduced cells 48 h post-transduction; mean±SD; differences are significant on day 6 (P=0.004), day 8 (P=0.0024), and day 10 (P=0.0004). (C–E) 0.5–0.6×106 cells per mouse were injected intravenously. Eight weeks post-transplantation cells were isolated from BM and spleen and analyzed by flow cytometry for expression of human lineage markers and transgene expression (GFP+ or BFP+, marker+) in human CD45+ cells; filled circles, CD105-LV; open circles, VSVG-LV; (C) engraftment of CD105-LV transduced cells is significantly enhanced in spleen (P=0.009) compared with VSVG-LV transduced cells; no significant differences in the distribution of marker-positive cells in each lineage of human CD45+ engrafted BM (D) and spleen (E) cells [one-way ANOVA analysis: P=0.3326 (BM, CD105-LV), 0.5484 (BM, VSVG-LV), P=0.0816 (spleen, CD105-LV), P=0.0871 (spleen, VSVG-LV)]. (F) Cells transduced with CD105-LV showed stable expression of the transgene reflected by the ratio of gene-marked cells at final analysis compared to the initial transduction efficiency. The respective ratios for VSVG-LV were significantly lower as analyzed with the Wilcoxon matched-pairs signed rank test; *P<0.05 [P=0.0313 (BM); P=0.0156 (spleen)]. (G) The relative ratio was obtained by dividing the ratio of the different pseudotype transduced cells (CD105-LV/VSVG-LV) within one individual animal (in vivo) by the corresponding ratio obtained 48 h post-transduction (in vitro). Values above 1 indicate superior performance of CD105-LV compared with VSVG-LV. Mean fold change in BM and spleen are specified. Significance according to Wilcoxon matched-pairs signed rank test; *P<0.05 [P=0.0313 (BM); P=0.0156 (spleen)] is shown. BFP, blue fluorescent protein; h, human; tx, transplantation.
Eight weeks after transplantation, BM and spleen of all seven mice were analyzed and showed engraftment of human cells at moderate to high levels [%humanCD45+/%totalCD45+: 41.1±11.8% (BM); 30.0±6.6% (spleen), Supplementary Fig. S5]. Similarly as described above (Fig. 5A, B), most of the human cells were CD19+ (Supplementary Fig. S5). CD105-LV transduction resulted in transgene expression in all hematopoietic lineages with no significant differences (mean values of 2%–4%), which reflected or even exceeded the initial percentage of marker positive cells that were transplanted (Fig. 6A, C–E). In contrast, percentages of VSVG-LV transduced cells were below 2% and thus, reduced compared to the input, with the exception of CD33+ splenocytes (Fig. 6E). This difference in performance between the two vector types became evident also when described as a ratio of gene-marking retrieved in vivo (post-transplantation) to marker positive cells pretransplantation (Fig. 6F). CD105-LV achieved ratios approximating 1 in BM and spleen whereas the ratios for VSVG-LV were significantly lower.
The observed difference between the two vector types was further analyzed by performing the following calculation: the ratio between the CD105-LV transduced cells (GFP+ or BFP+) and the VSVG-LV transduced cells (GFP+ or BFP+) within each individual animal was calculated and divided by the corresponding ratio of the input cell population as determined on day 2 post-transduction. Accordingly, values above one indicate a repopulation advantage of cells transduced by CD105-LV. Cells targeted by CD105-LV revealed a 29- and 9-fold superior engraftment in BM and spleen, respectively, than cells transduced with VSVG-LV (Fig. 6G). Hence, CD105-LV provides transduction of unstimulated CD34+ HSCs that are able to repopulate NSG mice at sustained levels. These results implicate that within the CD34+ population, unstimulated CD34+/CD105+ cells are enriched for early stem cells with high repopulating capacity in NSG mice and confirming that CD105 can serve as a phenotypic marker of early human HSCs.
Discussion
CD105, also known as endoglin, has been described as a component of the TGF-β receptor complex. It binds TGF-β1 and TGF-β3 with high affinity (dissociation constant of about 60 pM) by associating with the constitutively active TGF-β receptor II [28]. The role of CD105 in the TGF-β signaling pathway is still poorly understood. However, it is suggested that both CD105 and CD34 are positively regulated by TGF-β1 on hematopoietic cells. This regulation appears to be a mechanism through which the more immature hematopoietic compartment maintains its primitive functions. TGF-β1 represents the main soluble effector that determines the maintenance of an immature phenotype, and a molecular state associated with prolonged survival and preserved proliferating capacity [23]. Previous studies have shown that TGF-β1 exerts its action on primitive hematopoiesis by inhibiting cell cycle progression of primitive precursors [29]. In summary, CD105 modulates the effects of TGF-β1 as part of the TGF-β receptor complex and thereby promotes the primitive state of HSCs. In line with this theory, several publications confirmed CD105 as marker for murine primitive HSCs in vitro and in vivo [19–21]. For human HSCs, Pierelli et al. [22,23] also indicate that CD105 expression on HSCs correlates with the stemness of the cells, however, in vivo analyses of CD34+/CD105+ HSCs are missing.
Applying a CD105-specific lentiviral vector we selectively gene marked CD105+/CD34+ HSCs, which enabled us to track the transduced cells and their progeny after intravenous injection into NSG mice. Thereby, we could demonstrate that the transduced cells (GFP+) repopulate the mice and differentiate into all hematopoietic lineages. Post-transplantation 5%–22% of the engrafted human cells were GFP+ (Fig. 5C), in accordance with the percentages of GFP+ cells that were initially transplanted (Fig. 5D) demonstrating that the GFP expression remained stable up to 18 weeks and that transduced cells efficiently engrafted in mice. Furthermore, there was no significant difference in the percentages of GFP+ cells between the different human lineages present in these mice (Fig. 5E, F), suggesting that multipotent HSCs rather than more differentiated and hence lineage-restricted progenitors had been transduced. This is in line with the results obtained in vitro by CFA (Fig. 4). There is ample evidence available for the high specificity of CD105-LVGFP for CD105+ cells: Here, we demonstrated that preincubation of CD105-LVGFP with soluble CD105 protein blocks the transduction of CD34-purified cells (Fig. 3). Furthermore, neither in mixed cultures of human CD105− peripheral blood mononuclear cells and CD105+ endothelial cells, nor when intravenously injected into mice transplanted with human CD105+ cells, any evidence for off-target transduction mediated by CD105-LV was found [13,14]. There is therefore a high if not absolute certainty that the GFP+ cells detected in the colonies formed or in the transplanted mice must have been derived from CD105+ HSCs.
Lentiviral vectors equipped with the VSVG envelope protein are today's standard for clinical trials with patients suffering from genetic diseases that can be treated by HSC-based gene therapy [30–32]. While this demonstrates that VSVG-LV transduces long-term repopulating HSCs, its broad tropism supports gene transfer into all cell types present within the CD34+ cell population making a high vector dose necessary to achieve sufficient transduction of the minor fraction of long-term repopulating HSCs present within the CD34+ cell population [33–35]. To follow the long-term repopulating potential of CD105-LV and VSVG-LV transduced cells in vivo, we labeled the vectors with different fluorescent reporter genes enabling us to apply both vector types to the same donor cells implanted into the same individual mouse. CD34+ cells transduced by CD105-LV revealed a 29- and 9-fold superior engraftment in BM and spleen, respectively, than CD34+ cells transduced with VSVG-LV (Fig. 6G). Furthermore, in all assessed lineages the fraction of CD105-LV transduced cells was similar or even higher than the initial percentage of marker gene positive cells present in the transplant (Fig. 6A, C–E). In contrast, the percentage of VSVG-LV transduced cells was reduced compared to the input. This reduction of gene marked cells over time in vivo and in vitro (Fig. 6B) indicates that VSVG-LV transduced the vast excess of multilineage progenitors with short-term engraftment properties and more differentiated lineage-restricted progenitors with low or no engraftment capabilities.
In our experiment we chose the same low multiplicity of infection (MOI) for CD105-LV and VSVG-LV to gain comparable transduction efficiencies. For clinical applications, VSVG-LV is used at high MOIs, which likely facilitates the transduction of the small amount of primitive HSCs in the CD34+ population [33–35], but may be disadvantageous in respect to vector amount needed and the risk of insertional mutagenesis due to multiple integrations [36]. Although experimental evidence for reduced vector copy numbers with CD105-LV remains to be collected, it is well conceivable that the high MOI and the broad tropism of VSVG-LV lead to the transduction of all cell populations in the CD34+ population, including CD105− precursors, which may further increase the risk of insertional mutagenesis compared with CD105-LV that selectively transduces a smaller cell population.
Moreover, we used unstimulated HSCs for transduction in the in vivo competition experiment. It is well established that transduction of these cells by VSVG-LV is strongly impaired which is likely due to the lack of the LDL receptor on quiescent HSCs [8]. However, quiescent HSCs have a higher long-term engraftment capacity than cytokine-stimulated HSCs [37,38]. It is therefore noteworthy that CD105-LV is able to transduce unstimulated HSCs and that these cells repopulated NSG mice at sustained levels. These results imply that unstimulated CD34+/CD105+ cells represent an early stem cell population and confirm that CD105 marks early human HSCs. In line with this, high CD105 levels have recently been identified to discriminate between long-term and short-term hematopoietic progenitor cells arising in the primitive aorta [21].
CD105-LV adds to a growing list of lentiviral vectors carrying other envelopes than VSVG for the transduction of HSCs [39–43]. Among these, CD105-LV is after CD133-LV the second vector rationally engineered to use a HSC surface marker as entry receptor. While both vectors transduce unstimulated HSCs and the transduced CD34+ cells show a long-term repopulation advantage over VSVG-LV transduced cells, CD105-LV particles mediate about 2-fold higher transduction rates on HSCs than CD133-LV [13,44]. In the absence of a vector specific for CD34, which turned out difficult to generate, possibly due to the small and highly glycosylated extracellular part of CD34 (Schneider and Buchholz, unpublished data), CD105-LV represents the only tool for the selective transduction of long-term repopulating HSCs besides CD133-LV [44].
As CD105 is also expressed on other cell types present in the BM such as mesenchymal stem cells [45], activated endothelial cells, and progenitor endothelial cells [28], the application of CD105-LV for HSC gene transfer may be limited to ex vivo approaches. Although clinical applications may need further optimization of the CD105-LV yield (about 35-fold lower than VSVG-LV [13]) targeting the CD105+/CD34+ population with CD105-LV offers a novel gene transfer strategy to reach high long-term hematopoietic engraftment rates of transduced cells using low vector doses. Moreover, our results nicely illustrate that receptor-targeted lentiviral vectors can be applied to identify stem cell and lineage markers and to trace lineage-fate decisions. The flexibility in receptor-targeting given [13], this approach can easily be transferred to other surface proteins to assess their potential as stem cell or progenitor marker.
Supplementary Material
Acknowledgments
The authors would like to thank Kay-Martin Hanschmann for his help with the statistical analysis. This work was supported by grants from the LOEWE Center for Cell and Gene Therapy Frankfurt funded by the Hessisches Ministerium für Wissenschaft und Kunst [HMWK; funding reference number: III L 4-518/17.004 (2013)] to M.G., H.B., C.J.B., and S.K. and by the Deutsche Forschungsgemeinschaft (DFG) Graduate Program GK1172-Biologicals to K.B.K. and C.B. The Georg-Speyer-Haus is supported by the Bundesministerium für Gesundheit and the Hessisches Ministerium für Wissenschaft und Kunst.
Prior conference presentation of the submitted material: XX. Annual Meeting of the German Society for Gene Therapy, Ulm, Germany. 17th Annual Meeting of the American Society of Gene- and Cell therapy, Washington D.C.
Author Disclosure Statement
C.J.B. and S.K. are listed as inventors on patent applications about receptor-targeted lentiviral vectors that have been out-licensed. All other authors declare that they have no conflicts of interest.
References
- 1.Aiuti A. and Roncarolo MG. (2009). Ten years of gene therapy for primary immune deficiencies. Hematology Am Soc Hematol Educ Program 682–689 DOI: 10.1182/asheducation-2009.1.682 [DOI] [PubMed] [Google Scholar]
- 2.Naldini L. (2011). Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 12:301–315 [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann KB, Büning H, Galy A, Schambach A. and Grez M. (2013). Gene therapy on the move. EMBO Mol Med 5:1642–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grez M, Reichenbach J, Schwäble J, Seger R, Dinauer MC. and Thrasher AJ. (2011). Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther 19:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guenechea G, Gan OI, Dorrell C. and Dick JE. (2001). Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol 2:75–82 [DOI] [PubMed] [Google Scholar]
- 6.McKenzie JL, Gan OI, Doedens M, Wang JC. and Dick JE. (2006). Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol 7:1225–1233 [DOI] [PubMed] [Google Scholar]
- 7.Finkelshtein D, Werman A, Novick D, Barak S. and Rubinstein M. (2013). LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 110:7306–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amirache F, Lévy C, Costa C, Mangeot P, Torbett BE, Wang CX, Nègre D, Cosset F. and Verhoeyen E. (2014). Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 123:1422–1424 [DOI] [PubMed] [Google Scholar]
- 9.Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R, Cichutek K. and Buchholz CJ. (2008). Targeted cell entry of lentiviral vectors. Mol Ther 16:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kneissl S, Zhou Q, Schwenkert M, Cosset F, Verhoeyen E. and Buchholz CJ. (2013). CD19 and CD20 targeted vectors induce minimal activation of resting B lymphocytes. PLoS One 8:e79047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Schneider IC, Edes I, Honegger A, Bach P, Schönfeld K, Schambach A, Wels WS, Kneissl S, Uckert W. and Buchholz CJ. (2012). T-cell receptor gene transfer exclusively to human CD8(+) cells enhances tumor cell killing. Blood 120:4334–4342 [DOI] [PubMed] [Google Scholar]
- 12.Ageichik A, Buchholz CJ. and Collins MK. (2011). Lentiviral vectors targeted to MHC II are effective in immunization. Hum Gene Ther 22:1249–1254 [DOI] [PubMed] [Google Scholar]
- 13.Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J, Schneider IC, Münch RC, Petznek H, et al. (2010). Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods 7:929–935 [DOI] [PubMed] [Google Scholar]
- 14.Abel T, El Filali E, Waern J, Schneider IC, Yuan Q, Münch RC, Hick M, Warnecke G, Madrahimov N, et al. (2013). Specific gene delivery to liver sinusoidal and artery endothelial cells. Blood 122:2030–2038 [DOI] [PubMed] [Google Scholar]
- 15.Münch RC, Mühlebach MD, Schaser T, Kneissl S, Jost C, Plückthun A, Cichutek K. and Buchholz CJ. (2011). DARPins: an efficient targeting domain for lentiviral vectors. Mol Ther 19:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchholz CJ, Mühlebach MD. and Cichutek K. (2009). Lentiviral vectors with measles virus glycoproteins—dream team for gene transfer?. Trends Biotechnol 27:259–265 [DOI] [PubMed] [Google Scholar]
- 17.St-Jacques S, Cymerman U, Pece N. and Letarte M. (1994). Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells. Endocrinology 134:2645–2657 [DOI] [PubMed] [Google Scholar]
- 18.Warrington K, Hillarby MC, Li C, Letarte M. and Kumar S. (2005). Functional role of CD105 in TGF-beta1 signalling in murine and human endothelial cells. Anticancer Res 25:1851–1864 [PubMed] [Google Scholar]
- 19.Chen C, Li M, de Graaf D, Monti S, Göttgens B, Sanchez M, Lander ES, Golub TR, Green AR. and Lodish HF. (2002). Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci U S A 99:15468–15473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Li L, Li M. and Lodish HF. (2003). The endoglin(positive) sca-1(positive) rhodamine(low) phenotype defines a near-homogeneous population of long-term repopulating hematopoietic stem cells. Immunity 19:525–533 [DOI] [PubMed] [Google Scholar]
- 21.Roques M, Durand C, Gautier R, Canto P, Petit-Cocault L, Yvernogeau L, Dunon D, Souyri M. and Jaffredo T. (2012). Endoglin expression level discriminates long-term hematopoietic from short-term clonogenic progenitor cells in the aorta. Haematologica 97:975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierelli L, Scambia G, Bonanno G, Rutella S, Puggioni P, Battaglia A, Mozzetti S, Marone M, Menichella G, et al. (2000). CD34+/CD105+ cells are enriched in primitive circulating progenitors residing in the G0 phase of the cell cycle and contain all bone marrow and cord blood CD34+/CD38low/- precursors. Br J Haematol 108:610–620 [DOI] [PubMed] [Google Scholar]
- 23.Pierelli L, Bonanno G, Rutella S, Marone M, Scambia G. and Leone G. (2001). CD105 (endoglin) expression on hematopoietic stem/progenitor cells. Leuk Lymphoma 42:1195–1206 [DOI] [PubMed] [Google Scholar]
- 24.Kneissl S, Abel T, Rasbach A, Brynza J, Schneider-Schaulies J. and Buchholz CJ. (2012). Measles virus glycoprotein-based lentiviral targeting vectors that avoid neutralizing antibodies. PLoS One 7:e46667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zufferey R, Nagy D, Mandel RJ, Naldini L. and Trono D. (1997). Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15:871–875 [DOI] [PubMed] [Google Scholar]
- 26.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M. and Thrasher AJ. (2002). High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 13:803–813 [DOI] [PubMed] [Google Scholar]
- 27.Brehm MA, Shultz LD, Luban J. and Greiner DL. (2013). Overcoming current limitations in humanized mouse research. J Infect Dis 208 (Suppl 2):S125–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K. and Lloyd RV. (2011). Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res 31:2283–2290 [PubMed] [Google Scholar]
- 29.Hatzfeld J, Li ML, Brown EL, Sookdeo H, Levesque JP, O'Toole T, Gurney C, Clark SC. and Hatzfeld A. (1991). Release of early human hematopoietic progenitors from quiescence by antisense transforming growth factor beta 1 or Rb oligonucleotides. J Exp Med 174:925–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341:123–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, et al. (2013). Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341:1233158. [DOI] [PubMed] [Google Scholar]
- 32.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, et al. (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326:818–823 [DOI] [PubMed] [Google Scholar]
- 33.Géronimi F, Richard E, Redonnet-Vernhet I, Lamrissi-Garcia I, Lalanne M, Ged C, Moreau-Gaudry F. and de Verneuil H. (2003). Highly efficient lentiviral gene transfer in CD34+ and CD34+/38-/lin- cells from mobilized peripheral blood after cytokine prestimulation. Stem Cells 21:472–480 [DOI] [PubMed] [Google Scholar]
- 34.Ailles L, Schmidt M, Santoni de Sio FR, Glimm H, Cavalieri S, Bruno S, Piacibello W, von Kalle C. and Naldini L. (2002). Molecular evidence of lentiviral vector-mediated gene transfer into human self-renewing, multi-potent, long-term NOD/SCID repopulating hematopoietic cells. Mol Ther 6:615–626 [PubMed] [Google Scholar]
- 35.Amsellem S, Ravet E, Fichelson S, Pflumio F. and Dubart-Kupperschmitt A. (2002). Maximal lentivirus-mediated gene transfer and sustained transgene expression in human hematopoietic primitive cells and their progeny. Mol Ther 6:673–677 [PubMed] [Google Scholar]
- 36.Knight S, Collins M. and Takeuchi Y. (2013). Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr Gene Ther 13:211–227 [DOI] [PubMed] [Google Scholar]
- 37.Glimm H, Oh IH. and Eaves CJ. (2000). Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0). Blood 96:4185–4193 [PubMed] [Google Scholar]
- 38.Kittler EL, Peters SO, Crittenden RB, Debatis ME, Ramshaw HS, Stewart FM. and Quesenberry PJ. (1997). Cytokine-facilitated transduction leads to low-level engraftment in nonablated hosts. Blood 90:865–872 [PubMed] [Google Scholar]
- 39.Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P, Vanin EF. and Nienhuis AW. (2002). Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther 5:242–251 [DOI] [PubMed] [Google Scholar]
- 40.Bell AJ, Fegen D, Ward M. and Bank A. (2010). RD114 envelope proteins provide an effective and versatile approach to pseudotype lentiviral vectors. Exp Biol Med 235:1269–1276 [DOI] [PubMed] [Google Scholar]
- 41.Verhoeyen E, Wiznerowicz M, Olivier D, Izac B, Trono D, Dubart-Kupperschmitt A. and Cosset F. (2005). Novel lentiviral vectors displaying “early-acting cytokines”. selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood 106:3386–3395 [DOI] [PubMed] [Google Scholar]
- 42.Frecha C, Costa C, Nègre D, Amirache F, Trono D, Rio P, Bueren J, Cosset F. and Verhoeyen E. (2012). A novel lentiviral vector targets gene transfer into human hematopoietic stem cells in marrow from patients with bone marrow failure syndrome and in vivo in humanized mice. Blood 119:1139–1150 [DOI] [PubMed] [Google Scholar]
- 43.Girard-Gagnepain A, Amirache F, Costa C, Lévy C, Frecha C, Fusil F, Nègre D, Lavillette D, Cosset F. and Verhoeyen E. (2014). Baboon envelope pseudotyped lentiviral vectors outperform VSV-G pseudotyped lentiviral vectors for gene transfer into cytokine-stimulated and resting hematopoietic stem cells. Blood 124:1221–1231 [DOI] [PubMed] [Google Scholar]
- 44.Brendel C, Goebel B, Abriß D, Brugman M, Kneissl S, Schwäble J, Kaufmann KB, Müller-Kuller U, Kunkel H, et al. (2015). CD133-targeted gene transfer into long-term repopulating hematopoietic stem cells. Mol Ther 23:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin C, Xin Z, Dai J. and Lue TF. (2013). Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol 28:1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.