Abstract
Caspases are proteases with a well-defined role in apoptosis. However, increasing evidence indicates multiple functions of caspases outside apoptosis. Caspase-1 and caspase-11 have roles in inflammation and mediating inflammatory cell death by pyroptosis. Similarly, caspase-8 has dual role in cell death, mediating both receptor-mediated apoptosis and in its absence, necroptosis. Caspase-8 also functions in maintenance and homeostasis of the adult T-cell population. Caspase-3 has important roles in tissue differentiation, regeneration and neural development in ways that are distinct and do not involve any apoptotic activity. Several other caspases have demonstrated anti-tumor roles. Notable among them are caspase-2, -8 and -14. However, increased caspase-2 and -8 expression in certain types of tumor has also been linked to promoting tumorigenesis. Increased levels of caspase-3 in tumor cells causes apoptosis and secretion of paracrine factors that promotes compensatory proliferation in surrounding normal tissues, tumor cell repopulation and presents a barrier for effective therapeutic strategies. Besides this caspase-2 has emerged as a unique caspase with potential roles in maintaining genomic stability, metabolism, autophagy and aging. The present review focuses on some of these less studied and emerging functions of mammalian caspases.
Facts
Caspases are involved in cell death mediated by apoptosis, pyroptosis, necroptosis and autophagy.
Caspase function is not limited to cell death.
Non-apoptotic roles of caspases include proliferation, tumor suppression, differentiation, neural development and axon guidance and aging.
Open Questions
What are the mechanisms by which a caspase can mediate both cell death and non-cell death functions?
In vivo validation of some of the proposed functions of caspases remains to be addressed.
Are there other as yet undiscovered physiological roles for caspases?
Cell death is a fundamental process that maintains tissue homeostasis, removes unwanted or damaged cells and ensures recycling of cellular constituents promoting further growth and differentiation. Based on the morphology of dying cells two distinct modes of cell death commonly studied include apoptosis and necrosis, however, other types of cell death types have been recently described.1, 2, 3
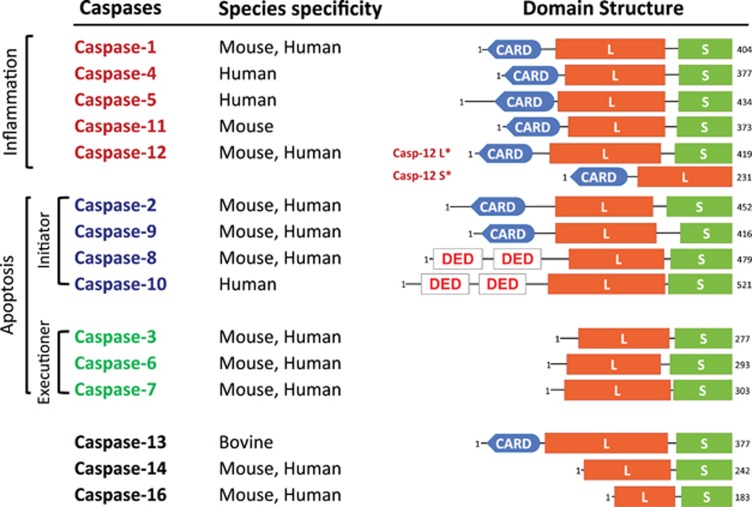
Caspases (cysteine-aspartic proteases) are proteolytic enzymes largely known for their role in controlling cell death and inflammation. The function of caspases in cell death was identified more than two decades ago with the discovery of ced-3 as an executioner of cell death during development of the nematode Caenorhabditis elegans.4 The first mammalian ced-3 homologs included interleukin-1-beta-converting enzyme (ICE), later renamed caspase-1,5 and Nedd2, renamed caspase-2.6, 7 Owing to inconsistencies in naming caspases, 18 mammalian caspases are known.8 However, newly identified caspases-15, -17 and -18 are absent in placental mammals with the exception of caspase-16 (Figure 1).8 It is also important to note that caspase-5 is not present in mice and caspase-11 and -13 are the murine and bovine orthologues of caspase-4, respectively.8, 9 Caspase-12 exists in both truncated and full-length alleles in humans and as a full-length caspase in rodents.10 Caspase-14 is expressed in the epidermis and has a primary role in cornification and protection of underlying layers of skin.11
Figure 1.
Domain structure and functional classification of placental mammalian caspases. Caspase-1, -4, -5, -11 and -12 are inflammatory caspases. Apoptotic caspase-2, -8, -9 and -10 are initiators, while caspase-3, -6 and -7 are key executioner caspases. CARD, caspase recruitment domain; DED, death effector domain; L, large subunit; S, small subunit; S*, short form; L*, long form
Based on their function, mammalian caspase-2, -3, -7, -8, -9 and -10 are apoptotic caspases, where as caspase-1, -4, -5, -11 and -12 are involved in inflammation. The apoptotic caspases are subdivided into the initiators and the effectors based on the presence or absence of specific-protein interaction domains toward the N-terminus (Figure 1). Initiator caspases comprise death effector domains (DED; caspase-8 and -10) or caspase-recruitment domains (CARD; caspase-2, -9, -1 and -11), which mediate their dimerization and/or recruitment into larger complexes to facilitate their activation.12
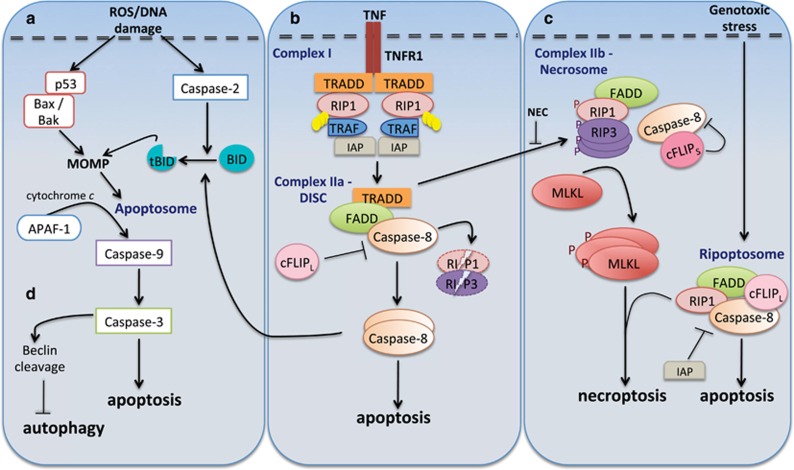
Initiator caspase activation during apoptosis is mediated by two main pathways; the mitochondrial or Bcl-2-regulated (intrinsic) pathway and the death receptor (extrinsic) pathway (Figure 2). The intrinsic pathway is activated in response to cellular stress (e.g., cytotoxic drugs, DNA damage) and is regulated by the Bcl-2 family of proteins. This pathway involves activation of the pro-apoptotic effectors BAX and BAK, which induce mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release. Apaf-1 (apoptotic protease-activating factor 1) associates with cytochrome c into a large multimeric complex called the apoptosome to activate caspase-9.13 Death receptor-mediated apoptosis is initiated following ligand-binding and activation of the death-domain-containing tumor necrosis receptor superfamily (e.g., TNFR, Fas, TRAIL).14 This mediates recruitment and activation of caspase-8 or -10, through the death-inducing signaling complex (DISC) comprising FAS-associated death domain protein (FADD) and/or TNFR-associated death domain protein (TRADD) and other components. Caspase-8 also cleaves BID to a truncated form (tBID), which engages the mitochondrial pathway to amplify the apoptotic response.15 Once initiator caspases are activated through the extrinsic or intrinsic apoptosis pathways, they mediate activation of effector caspases-3, -6 and -7.
Figure 2.
Caspases in cell death pathways. Caspases have been implicated in apoptosis, necroptosis and autophagy. Apoptosis can proceed in two ways: intrinsic or extrinsic. (a) Intrinsic apoptosis is initiated by a death-inducing stimulus, which causes activation of p53 and pro-apoptotic Bcl-2 proteins such as Bax and Bak. This leads to MOMP and activation of a death-inducing platform called the apoptosome. Following this caspase-9 is cleaved and activates other effector caspases such as caspase-3. Alternatively, caspase-2 gets activated following DNA damage and causes MOMP by cleaving Bid. (b) Extrinsic or receptor-mediated apoptosis involves complex formation by death-domain-containing proteins (called DISC), activation of caspase-8, which initiates apoptosis by directly activating effector caspases. Caspase-8 also cleaves Bid and this causes cytochrome c release, further amplifying apoptosis via the mitochondrial/intrinsic pathway. Depending on ligands (TNF and Fas) different complexes are formed. TNF stimulates TNFR1 that recruits TRADD, RIP1, TRAFs and IAPs into a complex called TRADD-dependent complex I. Subsequent removal of polyubiquitin on RIP1, dissociates this complex and allows it to interact with TRADD, procaspase-8, FADD and cFLIP as complex IIa/DISC. This results in caspase-8 activation, leading to apoptosis. (c) In certain conditions when caspase activity is blocked, RIP1, FADD and the FLIPL–caspase-8 heterodimer, form a large multiprotein complex called the 'ripoptosome' following genotoxic stress. If caspase activity is lost, RIP1 and RIP3 are stabilized and associate in microfilament-like complexes called necrosomes (complex IIb). Recruitment of MLKL complex at the plasma membrane occurs, leading to pore formation and necroptotic cell death. Alternatively, RIPK1 and RIPK3 are inactivated by active caspase-8 and apoptosis occurs. Necroptosis can also occur following stimulation of TNF receptor or activation of Toll-like receptors (TLRs). (d) Some caspases such as caspase-3, mediate cleavage of crucial autophagic proteins and thus indirectly control autophagy. FADD, FAS-associated death domain; TNFR1, TNF receptor 1; TRADD, TNF receptor-associated death domain; cIAP1, cellular inhibitor of apoptosis protein 1; RIP1, receptor-interacting protein 1; MLKL, mixed lineage kinase domain-like; cFLIP, cellular FLICE-like inhibitory proteins; MOMP, mitochondrial outer membrane permeabilization
Caspase-2 is unique, in that it can be activated both upstream of MOMP and/or downstream of MOMP following various apoptotic stimuli. The activation of caspase-2 is primarily via CARD-mediated homo-dimerization and auto-proteolysis.16, 17 Caspase-2 is also recruited to a large multiprotein complex called the PIDDosome, comprising proteins RAIDD and PIDD,18 and can also interact with RAIDD independent of PIDD. However, RAIDD and PIDD are not required for caspase-2 activation in vivo, which can occur normally in RAIDD- and PIDD-deficient mice.19 Caspase-2 has been implicated in cell death induced by metabolic imbalance in oocytes, in mitotic catastrophe and in functions outside apoptosis.20
In recent years, it has become evident that the role of many caspases is not limited to apoptosis and that they function in other modes of cell death (necrosis, autophagy and pyroptosis). Furthermore, several other non-cell death functions have emerged. We begin by first addressing the role of caspases in cell death pathways other than apoptosis.
Caspases in Non-Apoptotic Cell Death
Necroptosis
Cell death by necroptosis is typically induced when caspase activity is blocked, and is morphologically characterized by an increase in cell volume, swelling of organelles and rupture of the plasma membrane.21 Necroptosis can be initiated following stimulation of the Toll-like receptor TLR3, genotoxic stress or ‘pathogen-associated molecular patterns (PAMPs).22 However, the best-studied model is TNF-induced necroptosis.23 Upon stimulation by TNF, TNFR1 recruits TRADD, RIP1, TRAFs and IAPs into a complex called TRADD-dependent complex I. Formation of this complex ensures activation of the canonical NFκB signaling pathway. Subsequently removal of polyubiquitin chains from RIP1, allows RIP1 to dissociate from the plasma membrane and interact with TRADD, FADD, procaspase-8 and cFLIPL as a TRADD-dependent complex IIa (also called DISC; Figure 2). Caspase-8 then cleaves RIP1 and RIP3 and initiates apoptosis. Alternatively, by chemical or viral inhibition of caspase-8, or in the absence of cIAPs, another multiprotein complex called the ripoptosome is formed, which includes RIP1, FADD, caspase-8 and cFLIPL. Ripoptosome formation is dependent on RIP1, which can then mediate either apoptosis or necroptosis depending on the presence and levels of cFLIP proteins and caspase-8 activity.23 When caspase activity is inhibited, RIP1 and RIP3 are stabilized, undergo auto and trans-phosphorylation and recruit MLKL into a complex called the necrosome (complex IIb), that initiates necroptosis. Alternatively, caspase-8 activation leads to cleavage of RIP1 and RIP3, this prevents necroptosis but apoptosis can still occur (Figure 2).23, 24 RIP1 is thus a master regulator of cell fate that acts to regulate caspase-8-mediated apoptosis or promote RIPK3-MLKL-dependent necroptosis signaling pathway when apoptosis is blocked.25, 26 Blocking the activity of RIP1 completely inhibits necroptosis.27 Interestingly, RIP1 can also inhibit necroptosis to mediate pro-survival and inflammatory signaling via TLR complexes and NFκB signaling.25, 26 Furthermore, recent studies have demonstrated that RIP1-caspase-8 signaling functions as the critical cell fate decision point that drives either cell survival or cell death. RIP1 functions to protect cells from excessive caspase-8-mediated apoptosis and TNF-driven inflammation to maintain cell homeostasis in barrier tissues such as the gut epithelium and skin.28, 29
Pyroptosis
Cell death by pyroptosis was first observed in macrophages following infection with Shigella flexneri or Salmonella typhimurium.30, 31 This cell death was later characterized as a novel programmed cell death process, distinct from apoptosis. 32, 33 Pyroptosis is a caspase-1- and -11-dependent cell death process and is considered an immune response to dispose of microbe-laden macrophages and clear intracellular pathogens in response to acute bacterial or viral infection or exposure to bacterial toxins. The process involves activation of these inflammatory caspases, resulting in plasma membrane pore formation allowing water influx, cell swelling and osmotic lysis, followed by release of proinflammatory intracellular contents.32, 34 This demarcates it clearly from apoptosis where no loss of membrane integrity is observed. At the same time cytokines are secreted either through the newly formed plasma membrane pores or through microvesicles- or lysosome-mediated exocytosis.35 The pyroptosis of macrophages also releases the bacteria that can be taken up by neutrophils as a secondary mechanism of phagocytic clearance and destroyed through the NADPH oxidase system.36 Chromosomal DNA cleavage and nuclear condensation occurs in pyroptosis but in the absence of oligonucleosomal DNA fragmentation or cleavage of typical caspase substrates such as PARP and ICAD that are characteristic of apoptosis.34, 37
Autophagy
Cross talk between autophagy and apoptosis is well established.38 Initiation of apoptosis by inhibiting autophagy is beneficial for execution of cell death since autophagy is often considered a pro-survival mechanism. Several human ATG proteins can be cleaved by calpain or caspases. hATG3 has been shown to be cleaved by caspase-3, -6 and -8.39 Beclin-1/ATG-6 is a caspase-3 target40 and cleaved beclin-1 reduced autophagy and promoted apoptosis in HeLa cells.41 Caspase-6 and -8 also cleave p62 that serves to inhibit autophagy.42 Other studies have shown that hATG3 is a direct target of caspase-8, and cleavage of ATG3 blocked autophagy.43 Recently, the Drosophila effector-caspase Dcp-1 was found to localize to mitochondria following starvation-induced stress and functions to positively regulate autophagy.44
A recent study suggests caspase-2 as a repressor of autophagy.45 Cells/tissues lacking caspase-2 show upregulation of autophagy, which provides them with a survival advantage. Importantly, caspase-2-induced autophagy in a mTOR-AMPK dependent pathway with accompanying MAPK1/3 activation and MAPK14 repression.45 Atg4 and AMBRA1 are examples of other autophagic proteins that are caspase targets.46, 47
Mitotic catastrophe
Mitotic catastrophe is a cell death mechanism that occurs during mitosis in cells that have accumulated DNA damage due to dysregulated checkpoint mechanisms.3 Such cells would normally arrest cell-cycle progression into mitosis and hence suppress catastrophic events until DNA repair has been achieved.48 Although cell-cycle kinase such as cyclin B1-dependent kinase Cdk1, polo-like kinases and aurora kinases and other cell-cycle checkpoint proteins, are key players in regulating this process, cell death occurs via caspase activation, including caspase-2.48
Caspases in Inflammation and Immunity
The first indication of a role for caspases in inflammation came from the identification of caspase-1 function in the processing and maturation of interleukin (IL) cytokine family members IL-1β and IL-18, critical mediators of inflammation.49, 50 Mice deficient for caspase-1 exhibit defective maturation of pro-IL-1β and pro-IL-18, are resistant to LPS-induced endotoxic shock50, 51, 52 and are protected from experimental mucosal inflammation.53
Additional studies in Drosophila unexpectedly identified the ortholog of caspase-8, Dredd, in a genetic screen as a regulator of the innate immune response.54 In particular, Dredd interacts with a Drosophila NF-κB protein, Relish, in the immune deficiency (IMD) pathway, leading to Relish cleavage and activation, following exposure to Gram-negative bacteria.54, 55 Loss-of-function dredd mutants exhibit high susceptibility to Gram-negative bacteria, and defective production of antibacterial peptides, such as drosomycin54 further substantiating an important role for this Drosophila caspase in immune regulation.
In mammals, there are now several caspases, which have been identified as important mediators of the innate immune response, termed the ‘inflammatory caspases' and include human caspase-1, -4, -5, -12 and mouse caspase-1, -11 and -12.34, 56 Caspase-1 is the prototypical member of the inflammatory caspase family, while; the functions of caspase-4 and -5 are not well defined (caspase-5 is absent in mice and mouse caspase-11 is a paralogue of human caspase-4). Caspase-11 has been suggested to be an upstream activator of caspase-1 but unlike caspase-1, caspase-11 expression is induced by LPS at the transcriptional level.57 Indeed, caspase-4, -5, and -11 act as cytoplasmic LPS receptors and have the ability to directly bind LPS via CARD domain, causing oligomerization and cell death. This represents a novel mechanism for inflammasome activation that is distinct from LPS sensing and activation of caspase-1.58
In contrast to the roles of other inflammatory cytokines, caspase-12 can abrogate the inflammatory response in mice by inhibiting caspase-1, in a protease-independent function.59 As a result, mice deficient for caspase-12 show increased bacterial clearance and resistance to sepsis.60 Interestingly, full-length human caspase-12 protein is expressed only in ~20% of sub-Saharan African descendants, who as a consequence exhibit weakened inflammatory and innate immune responses and increased susceptibility to sepsis.61
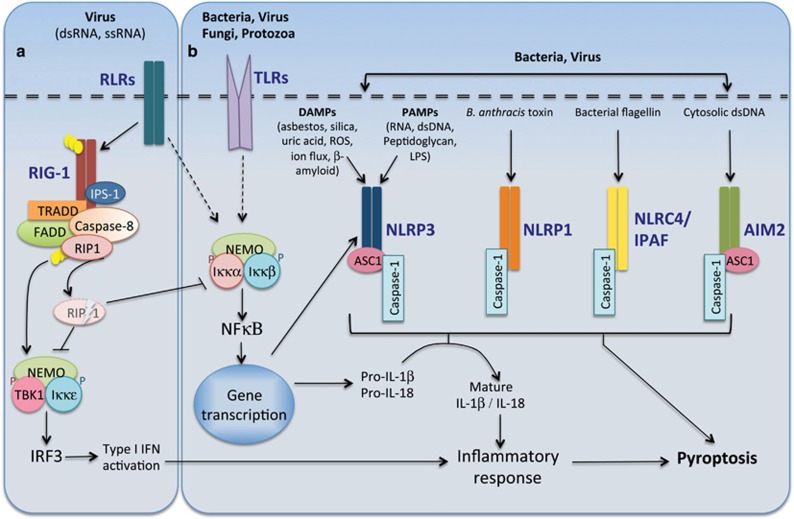
The activation of inflammatory caspases is mediated through their recruitment to large multiprotein complexes known as ‘inflammasomes'.62, 63 In response to specific internal or external cellular insults (viral or bacterial infection, toxin exposure, environmental irritants and metabolites), the specific activation of different pattern-recognition receptors (PRRs), leads to formation of distinct inflammasome complexes. There are three main classes of PRRs, activated in response to specific pathogenic stimuli, including (i) TLRs that can detect PAMPs derived from bacteria, viruses and fungi; (ii) retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), which detect virus infection; and (iii) the family of bacterial-sensing NOD-like receptor proteins (NLRs) or nucleotide-binding domain, leucine-rich repeat-containing proteins.64
Caspase-1 is activated by recruitment to multiple NLR-driven inflammasomes depending on the specific stimuli (Figure 3). There are currently four major, well-characterized inflammasomes: (i) NLRP1 inflammasome activated in response to the anthrax lethal toxin (LT)62; (ii) NLRP3/NALP3/cryopyrin inflammasome is activated by a wide range of PAMPs as well as whole pathogens, including fungi56, 62; (iii) the NLRC4/IPAF inflammasome, which senses bacterial flagellin and various secreted proteins from Gram-negative bacteria65; and (iv) AIM2 inflammasome, an atypical NLR, activates caspase-1 in response to cytosolic dsDNA.66 NLRP1 and NLRC4 can bind and activate caspase-1 directly via a CARD-CARD-mediated interaction. In contrast, NALP3 and AIM2 do not comprise a CARD domain and require, the CARD/pyrin-containing inflammasome adapter, apoptosis-associated speck-like protein (ASC), to recruit caspase-1 to these inflammasomes. The NLRP1 inflammasome can also recruit caspase-5 via the CARD of the inflammasome adapter protein, CARDINAL,62, 67 but the requirement of CARDINAL in caspase-5 activation by NALP1 is unclear.
Figure 3.
Caspase-mediated inflammatory responses. (a) Caspase-8 functions to restrict RIG-1 signaling. Activation of the retinoic-acid-like receptors (RLRs) are critical for anti-viral immunity. Following viral infection the caspase-8 DISC complex comprising FADD, TRADD and ubiquitin-conjugated RIP1 (Ub-RIP1) are recruited to the RIG-1 complex. Although Ub-RIP1 can enhance the phosphorylation of IRF3, it is also cleaved by activated caspase-8. Cleaved RIP1 inhibits IRF3 and NFκB thereby reducing the type I interferon (IFN) and IL-1β-/IL-18-mediated inflammatory responses. (b) Inflammasome complex formation is triggered by specific pathogen-associated molecular patterns (PAMPs) or by damage-associated molecular patterns (DAMPs). Activation of Toll-like receptors (TLRs) following pathogen exposure induces NFκB-mediated transcription of IL-1β, IL-18 and also NLRP3, and this is an important step in inflammasome priming and activation. Caspase-1 activation is mediated by its recruitment to different inflammasome complexes (NLRP3, NLRP1, NLRC4 and AIM2) in an ASC-dependent or independent manner. Activated caspase-1 mediates the cleavage and maturation of pro-inflammatory cytokines IL-1β and IL-18. Following acute infection and an acute inflammatory response, caspase-1 induces cell death by pyroptosis
The first step toward an inflammasome-mediated immune response requires a process known as inflammasome priming, where stimulation of TLR or RIG leads to transcriptional activation of NALP3 and inflammatory cytokines (pro-IL-1β, IFN). Activation of the NALP3 inflammasome only occurs following detection of PAMPs as a second stimulus, and contrary to previous studies,68 does not involve mitochondrial-mediated apoptosis signaling.69 Recruitment and activation of caspase-1 by the inflammasome complex is a critical second step in the cleavage and activation of pro-inflammatory cytokines, IL-1β and IL-18, facilitating their secretion and promotion of innate immune responses. Although caspase-1 activation is required to activate inflammatory cytokines, it induces pyroptosis-mediated pro-inflammatory cell death following acute infection (Figure 3).
A novel role for caspase-8 in the regulation of inflammasome activation and maturation of pro-inflammatory cytokines has recently been revealed. Cleavage of pro-IL-1β can be mediated by both caspase-1 and caspase-8.70, 71 Interestingly, deletion of caspase-8 in RIP3 knockout bone-marrow-derived macrophages (BMDMs) results in significant reduction of pro-IL-1β and TNF transcription levels, and reduced NALP3 activation following TLR stimulation. Importantly, this role for caspase-8 in the regulation of inflammasome priming is independent of apoptosis69 and highlights an unexpected role for this caspase in immune homeostasis. Previous studies have also reported an inflammasome-independent role for caspase-8 in inhibiting extensive inflammation in vivo.72, 73 Conditional deletion of caspase-8 in hepatocytes, augments Listeria infection in liver and chronic inflammation following hepatectomy.73 Caspase-8 deficiency also protects against inflammation-related hepatocarcinogenesis, while inducing necrosis-mediated liver injury.74 In addition, conditional deletion of caspase-8 in epidermal keratinocytes, is associated with chronic skin inflammatory disease associated with constitutive IRF3 signaling.72 Interestingly, this role for caspase-8 in suppressing inflammation is independent of signaling mediated through TNF, IL-1β or TLR receptors and independent of NFκβ signaling. Instead, caspase-8 regulates the activation of the IκK-related, TANK-binding kinase 1 (TBK1) and IRF3 to downregulate the inflammatory response.72 Later reports demonstrated that caspase-8 suppresses inflammation following viral RNA-induced RIG-1 signaling75 by interaction with the RIG-I signaling complex, RIP1 cleavage and inhibition of IRF3-mediated activation of interferons (Figure 3).75
Mutations in NALP3 and defective inflammasomes are associated with innate-immune-mediated diseases, and aberrant IL-1 processing has been reported in numerous auto-inflammatory diseases including gout, diabetes and juvenile-onset arthritis.76 Mutations in caspase-8 (and caspase-10) are also associated with autoimmune lymphoproliferative syndrome (ALPS).9 Detailed understanding of the regulation of caspase activation during inflammation and secretion of pro-inflammatory cytokines will potentially provide new avenues for treatment of inflammatory and infectious diseases.
Caspases in Cell-Fate Determination and Differentiation
Early studies in Drosophila were instrumental in delineating a role for caspases in cell fate and differentiation. Loss-of-function mutation of the Drosophila Apaf-1 ortholog, dark, reduces activation of the initiator caspase, Dronc and abolishes developmental apoptosis.77, 78 Interestingly, dark-mutant flies expressing a dominant-negative form of Dronc, have defects in development with extra sensory organ precursor (SOP) cells.79 These studies were the first to highlight an apoptosis-independent role for a caspase in regulating neural precursor cell formation in Drosophila. This process involves Dronc-mediated cleavage of Shaggy (Sgg46), which negatively regulates Wingless signaling required for SOP formation.80 In turn, Dronc activity is positively regulated by Drosophila IκK, DmIKK, which degrades the caspase inhibitor protein DIAP1, to control SOP development.81
In mammals, caspase function in terminal differentiation has also been described in various cell types (Table 1), including keratinocytes, megakaryocytes, erythrocytes and lens cells.82 A non-apoptotic role for the key effector caspase, caspase-3, was identified in lens fiber formation.83 The process of differentiation of lens epithelial cell into a lens fiber involves enucleation (loss of nuclei), which was found to be caspase-3 dependent.83 Other cells that demonstrate enucletion during differentiation include RBCs and keratinocytes. Several caspases, including caspase-3, -2, and -9 are transiently activated prior to enucleation during erythroid differentiation and involved cleavage of several proteins such as PARP, acinus and lamin B.84 Expression of active caspase-3 was shown to be associated with cell proliferation and migration during neuronal differentiation.85 Furthermore, in a mouse model of stroke, cleaved caspase-3 levels were increased in neuronal precursor cells (NPCs) during stroke recovery, without any associated increase in apoptosis.86 Inhibition of caspase-3 activity significantly promoted the proliferation and migration of NPCs indicating that caspase-3 limits self-renewal capacity of regenerating neurons. Mechanistically, this was attributed to the ability of caspase-3 to reduce phosphorylation of Akt.86
Table 1. Differentiation of many cell types involves caspases.
| Progenitor cells | Caspase activation | Mechanism | Reference (s) |
|---|---|---|---|
| Erythroid cells | Caspase-2, -3 and -9 | Cleavage of lamin B and acinus | 84 |
| Keratinocytes | Caspase-3 | Terminal differentiation of embryonic cells involving Casp3-Notch1 mechanism | 102 |
| Lens cells | Caspase-14 | Caspase-14 induction and processing during differentiation of keratinocytes | 98, 99 |
| Caspase-3 | Lens fiber formation | 83 | |
| Muscle progenitor cell | Caspase-3 and -9 | Muscle fiber formation | 87, 88 |
| Bone marrow stromal stem cells | Caspase-3 | Osteoblasts and bone formation involving TGFβ signaling | 89 |
| Osteoblasts | Caspase-2, -3 and -8 | Activation of caspases in differentiating cells | 90 |
| Odontoblasts | Caspase-7 | Activation of caspase-7 in bone-forming cells | 91 |
| Monocytes (bone marrow) | Caspase-3, -8 and -9 | Macrophage formation | 94 |
| Neurons | Caspase-3 | Neuronal differentiation, migration and plasticity | 85 |
| Embryonic stem cells | Caspase-3 | Caspase-3-mediated cleavage of Nanog | 92 |
| Haematopoietic stem cell | Caspase-3 | Maintenance of stem-cell quiescence and response to external stimuli | 93 |
Differentiation of muscle progenitor cells into myotubes and myofibres also requires caspase-3.87 Later studies demonstrated the involvement of caspase-9 in this process. It was observed that depletion of caspase-9 or overexpression of Bcl-xL reduced caspase-3 activation and this had a limiting effect on the differentiation of myoblasts.88
Osteogenic differentiation of mesenchymal stem cells involves caspase-3 activity, and caspase-3-deficient mice exhibit attenuated differentiation of the bone marrow stromal stem cells and consequently decreased bone mineral density.89 It is noteworthy that the process of normal osteoblast differentiation requires the transient and potent activation of other caspases, including caspase-8 and -2, without any increase in osteoblast apoptotic cell death.90 Caspase-7 has an additional role in proper functioning of odontoblasts as caspase-7 knockout mice show reduced dental mineralization.91
A role for caspase-3 in differentiation of embryonic stem cells (ESCs) and hematopoietic stem cells (HSCs) has also been reported,92, 93 with caspase-3deficiency-impeding differentiation of these stem cell populations. Caspase activity is also required for macrophage colony-stimulating factor (M-CSF)-mediated differentiation of human peripheral blood monocytes.94 Deletion of caspase-8 in bone-marrow cells abrogates monocyte differentiation into macrophages.95 In addition, caspase-8-mediated cleavage of RIP1, is required for downregulation of NF-κβ during macrophage differentiation.96
Caspase-14, unlike other caspases, is present only in terrestrial mammals97 and its expression is limited to epidermal tissue and epithelia, particularly the cornifying epithelia of the skin.98, 99 In the skin caspase-14 is expression is restricted to differentiating keratinocytes and absent in proliferating keratinocytes.99, 100 Caspase-14 was found to be involved in cornification, hydration and protection against UVB by correct processing and degradation of (pro)filaggrin.101 In addition to caspase-14, caspase-3 functions in the commitment stage of embryonic keratinocytes to terminal differentiation.102 During keratinocyte differentiation, caspase-3 activity is induced following Notch1 signaling and mediates cleavage and activation of PKC-δ, a positive regulator of keratinocyte differentiation.102 In support of this role, mice deficient for caspase-3 exhibit increased keratinocyte proliferation and deceased keratinocyte differentiation during embryogenesis.102 Altered caspase-14 has also been observed in inflammatory skin disorders such as psoriasis.103
Caspases in Cell Proliferation and Tissue Regeneration
Apoptosis is closely linked to the process of regeneration that is evolutionary conserved. Invertebrates such as Hydra104 and amphibians such as Xenopus show increased apoptosis during regeneration.105 MAPK signaling in dying cells activates Wnt-ligand secretion that drives this compensatory proliferation in surrounding cells.104, 106
The process of ‘compensatory proliferation' was originally defined in Drosophila-larval-imaginal tissues, which carry extensive regenerative capacity and cell proliferation to compensate for cell loss through damage or injury, and thereby maintain tissue size and shape.107 In the absence of effector-caspase activity these cells express high levels of activated Dronc but fail to die. Instead, Dronc activity is required for compensatory proliferation and functions to promote expression of the mitogens Wingless (Wg) and Decapentaplegic (Dpp) to promote proliferation of neighboring cells.108, 109, 110 The effector caspases Drice and Dcp-1 can also induce compensatory proliferation in differentiating eye tissue, by activation of the Hedgehog signaling pathway.106 These studies demonstrated critical roles for caspases in coordinating both cell death and proliferation during development and regeneration, and provided the first in vivo evidence for a non-apoptotic function for an initiator-apoptotic caspase.109
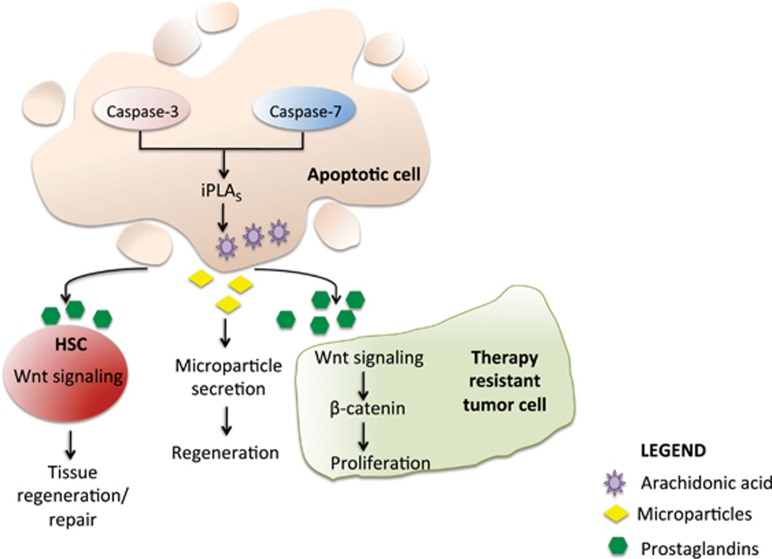
Mammals have greatly reduced regenerative ability but some tissues, such as liver and skin undergo extensive regeneration. Both caspase-3- and -7-deficient mice have impaired liver regeneration and wound healing.111 Mechanistically, this was attributed to calcium-independent phospholipase A2 activity (iPLA2). Both caspase-3 and -7 cleave iPLA2 which increases secretion of arachidonic acid and lysophosphocholine that further induce the secretion of prostaglandin E2 (PGE2), which then promotes stem cell proliferation, tissue regeneration and repair (Figure 4).111 PGE2 also has been shown to interact with the Wnt pathway at the level of β-catenin destruction to control HSC formation in vivo.112 This highlights that caspases are capable of cell repair by modulating the secretion of paracrine factors independent of their cell death function. Further evidence for indirect control of cell growth and repair comes from another study involving conditional caspase-8 knockout mice. Mice with hepatocyte-specific deletion of caspase-8 (Casp8Δhepa) show greatly improved liver regeneration due to increased NFκB signaling113 and wound healing. Caspase-7 has another important role in osteogenesis. Adult caspase-7 knockout mice display reduced bone mineral density in endochondronal (long bones of limbs) and reduced bone volume (intramembraneous bones such as mandibular and alveolar bone). This is attributed to reduced gene expression of key bone-forming genes, Smad1 and Msx1.114
Figure 4.
Caspase function in tissue regeneration and tumor cell proliferation. Activation of caspase-3 and -7 during radiotherapy or during tissue injury causes cleavage and activation of iPLA2. This results in AA synthesis and subsequent PGE2 activation. The secreted PGE2 promotes proliferation in surrounding tumor cells by activation of Wnt/β-catenin signaling. This facilitates tumor cell repopulation and acts as a limiting factor of effective therapy. Shedding of microparticles by apoptosing cells (pancreatic beta cells) stimulates proliferation/differentiation of neighboring cells through activation of regenerating (reg) genes. PGE2 also interacts with Wnt signaling pathway to regulate stem cell/progenitor formation and function. iPLA2, calcium-independent phospholipase A2; AA, arachidonic acid; PGE2, prostaglandin E2; HSC, hematopoietic stem cells
There is increasing evidence that mammalian caspases influence cell proliferation in a cell autonomous manner. Studies using caspase inhibitors, implicate a role for caspase-8 in regulating lymphocyte proliferation.115, 116, 117 Caspase-8 deletion in T cells causes normal thymocyte development, but causes a substantial reduction in peripheral T cells and impaired T-cell response to stimuli.118 Further with advancing age, these mice develop a lymphoproliferative phenotype characterized by lymphoadenopathy, splenomegaly and T-cell infiltration in several organs.119 These findings implicate an essential role for caspase-8 in T-cell homeostasis and immunity. In support of this role, patients with caspase-8 homozygous mutations have defects in lymphocytes and natural killer cell activation.120 FADD, is also essential for cytokine-induced proliferation of hematopoietic progenitor cells.121 These results, at least in part, could suggest that that loss of caspase-8 or FADD makes cells undergo necroptosis, rather than caspase-8 or FADD only having a direct effect on cell proliferation. However, the DISC complex comprising Fas/FADD/c-FLIPL/caspase-8 also controls cell-cycle progression of hepatocytes following EGF stimulation.122 Like caspase-8, caspase-1 and -6 have also been shown to regulate B-cell proliferation and maintain lymphocyte homeostasis.123, 124
In a contrasting role, caspase-3-deficient B cells show hyperproliferation that is dependent on p21, a cyclin-dependent kinase inhibitor.125 Many caspase-3 substrates are cell-cycle regulators, including cyclin-dependent kinase (CDK) inhibitor p27, a mediator of lymphoid cell proliferation.126 Caspase-3 also cleaves p21 and this blocks p21 interaction with proliferating-cell nuclear antigen (PCNA), and consequently prevents cell proliferation.
Caspases in Tumorigenesis
The phenomena of cell growth and cell death are closely linked. Basically, tumors arise due to unregulated increase in proliferative activity although the cause for this is multifactorial. The most likely reason for increased cell number would be loss of cell death control. Several genes with well-established roles in cell death have tumor suppressor function. Activation of Bcl-2 due to chromosomal translocation and gene amplification, blocks caspase activation and is frequently detected in non-Hodgkin's lymphoma (NHL) and small cell lung cancers.127, 128 Several clinical trials targeting Bcl-2 proteins as cancer therapeutics are ongoing. The cell death-inhibitor proteins, IAPs, are also promising targets for cancer research, as they suppress apoptosis either by inhibiting caspase activity or by modulating growth by targeting NFκB signaling pathways.129 Caspases promote cell death and it would be expected therefore that loss of caspases promotes tumor development. They, however, are also involved in tumorigenesis through non-apoptotic pathways by affecting proliferation, invasion and migration.130
Caspase-1
Caspase-1 tumor-suppressor function has been widely demonstrated. Overexpression of caspase-1 enhances the sensitivity of androgen-independent prostate cancer cell lines, to irradiation-induced cell death.131 It is also known that caspase-1 is frequently downregulated in human cancers, particularly prostate cancer.132, 133 When caspase-1-overexpressing renal cancer cells were injected into flanks of syngenic mice, not all mice developed solid tumors, while all control mice did.134 This was attributed to increased cell death following caspase-1 overexpression. It was also shown that while caspase-1 expression in renal cancer cell lines varies, tumor cells frequently have low caspase-1 levels, and inducing gene expression by demethylation increases apoptosis and could prevent tumor growth. Further evidence for its role as a tumor suppressor comes from a mouse model of colitis-associated colorectal cancer.135 Caspase-1-deficient mice showed enhanced tumor formation, characterized by early stage increase in epithelial cell proliferation in the colon and reduced apoptosis during advanced disease stage.135 Recent studies have shown that originally generated caspase-1-deficient mice also harbor a mutation in the caspase-11 locus, which mediates LPS-induced lethality in these mice.136
Caspase-2
A tumor suppressor function for caspase-2 was first proposed based on an evidence that the human CASP2 gene region on chromosome 7q is commonly deleted in leukemia.137 Although somatic mutations of CASP2 are not common in acute leukemia or solid cancers,138 the reduced expression of caspase-2 is correlated with poor prognosis in some cancers,139, 140 and high caspase-2 levels are associated with remission and survival in adults with ALL and AML.141 Furthermore, reduced expression and somatic missense mutations in CASP2 have been identified in some gastric and colorectal cancers.142
Studies using caspase-2 knockout mice and cells generated from these mice provided the first direct evidence that loss of caspase-2 enhances oncogene-induced cellular transformation and tumorigenic potential.143 Loss of caspase-2 augments tumorigenesis in several different mouse tumor models, including EμMyc-driven lymphomagenesis,143 MMTV/c-neu mammary carcinogenesis144 and ATM−/−-mediated thymic lymphomagenesis.145 Although the mechanism by which caspase-2 functions as a tumor suppressor is still unclear, it does not appear to be a PIDDosome dependent.146 Loss of caspase-2 has been shown to impede undefined radiation (IR)-induced DNA damage response in primary murine embryonic fibroblasts by significantly reducing p53 activation and function.147 Recently, a tumor suppressor role for caspase-2 was demonstrated in a mouse model of KRas-driven lung cancer. Tumors lacking caspase-2 responded better to chemotherapy, however, were more prone to relapse due to increased proliferation. It was observed that following DNA damage-mediated PIDD activation, caspase-2 cleaves mdm2, thus stabilizing p53 and preventing tumorigenesis.148 These studies suggest that caspase-2 cooperates with established tumor suppressors such as p53. However, caspase-2 deficiency does not influence IR-induced thymoma development in mice or affect spontaneous or IR-mediated thymoma in p53-null mice.146 In addition, loss of caspase-2 does not influence MCA-driven fibrosarcomas in mice.149 Interestingly, while caspase-2 acts as a tumor suppressor in cells with high c-myc expression, loss of Pidd had only slightly delayed tumor onset and had no additive effect. 149 In contrast to a tumor suppressor function, caspase-2-deficient mice showed significantly delayed onset and development of MYCN-mediated neuroblastoma, and high caspase-2 levels are associated with poor survival outcome in human neuroblastoma patients.150 Together these studies indicate that caspase-2 role as a tumor suppressor is tissue and context specific.
Caspase-3
Recently, Human Genome Epidemiology review and meta-analysis polymorphisms of CASP3 and CASP7 genes were found to be linked to cancer risk.151 Although somatic CASP3 mutations are rare in most tumors,152 CASP3-variant alleles have been detected in squamous cell carcinomas of the head and neck (SCCHN) and associated with increased risk of SCCHN.153 In addition, CASP3-variant alleles are associated with risk of endometrial cancer 154 and NHL 155 and multiple myeloma.156 CASP3 polymorphisms in some lung cancer patients also show significantly decreased risk of disease.157 Although it was reported that caspase-3 expression is significantly reduced in breast cancer cells, cancer tissue and even in the normal parenchyma surrounding tumors,158 several groups have demonstrated the converse. Higher levels of caspase-3 protein were found in malignant, compared to nonmalignant breast tissue, and coincided with increased apoptosis.159 Indeed, it was observed that caspase-3 is activated in dying tumor cells and causes increased secretion of PGE2 that results in tumor cell repopulation and resistance to radiotherapy.160 Further support to this study comes from human trials where two different cohorts of head and neck cancer patients and breast cancer patients, had high expression of active caspase-3 (cleaved caspase-3) and associated increased recurrence of the disease. This suggests that apoptosis is an essential aspect of tumor growth, and paracrine signals released by dying cells act to promote repopulation of these tumor cells and aids further growth.
Caspase-6 and -7
Mutations in CASP6 and CASP7 are rare in most human cancers.161 CASP6 mutations associated with reduced caspase-6 expression have been detected in colonic and gastric cancers.142 Somatic mutations in CASP7 are associated with reduced apoptosis161 and have been detected in colon carcinoma, esophageal carcinomas and head/neck carcinomas, but not stomach, urinary bladder or lung cancers.161 CASP7 polymorphisms can promote susceptibility to lung cancer and endometrial cancer.162
Caspase-8
The connection between caspase-8 and carcinogenesis has been extensively studied. A comprehensive review on caspase-8 expression and different types of tumors is available in the Human Protein Atlas.163 The studies demonstrate that in most epithelial-derived cancers, such as colon carcinoma, gastric cancer and breast cancer that progress through multiple stages, the loss of caspase-8 is a rare event and loss of expression is not detected. On the contrary, CASP8 mutations have been detected in 5% of invasive colorectal carcinomas (but not adenomas), and are associated with dominant-negative caspase-8 function and decreased apoptosis.164 Somatic mutation of CASP8 has also been detected in hepatocellular carcinomas (HCCs)165 and advanced gastric cancers,166 and are associated with decreased cell death.165 Interestingly, a CASP8 polymorphism (D302H) is associated with significantly reduced overall risk breast cancer.167 In addition, malignant neuroendocrine tumors frequently show loss of caspase-8, such as neuroblastoma,168 primitive neuroectoderm tumors,169 medulloblastoma,170 relapsing glioblastoma171 and small cell lung carcinoma.172 Loss of caspase-8 renders neuroblastoma cell lines resistant to apoptosis173 and enhances metastatic potential in chick embryos.174 In a mouse MYCN neuroblastoma model, deletion of caspase-8 promotes bone marrow metastasis.175 Increased caspase-8 expression has been observed in many advanced-stage tumors suggesting that caspase-8 might promote metastasis.176 Further evidence in support of its metastatic role comes from the fact that caspase-8 has been shown to interact with the focal adhesion complex,177 thereby promote cell migration. The complex observations on caspase-8 can be attributed to the fact that this caspase is involved in apoptosis and also protected against necroptosis. Hepatocyte-specific deletion of caspase-8 (Casp8Δhepa) was found to be protective against hepatocarcinogenesis in NEMOΔhepa mice that develop spontaneous HCC.74 Thus caspase-8-induced apoptosis and compensatory proliferation is important in HCC progression, but the increased necrosis and liver damage observed in the absence of caspase-8, is insufficient for tumor progression.
Caspase-10
The absence of caspase-10 in mice has perhaps resulted in delay in understanding potential roles of this caspase. Nonetheless, inactivating CASP10 mutations have been detected in NHL.178 CASP10 mutations have also been detected in T-cell acute lymphoblastic leukemia and multiple myeloma,179 as well as colon, breast, lung, HCCs180 and gastric cancers.181 In a very interesting study, it has been shown that myeloma cells require caspase-10 for survival. Caspase-10 dimerizes with cFLIP to inhibit autophagy by preventing cleavage of the Bcl-2-interacting protein BCLAF1.182
Caspase-14
Expression of caspase-14 is reduced in the colon, cervical and ovarian tumor tissue and associated with more advanced-stage cancers.183 However, ductal carcinomas showed increased caspase-14 levels compared to surrounding mammary tissue.183 Caspase-14 overexpression in a human salivary gland adenocarcinoma has been shown to promote growth inhibition and prevent tumorigenicity in athymic mice.184
Caspase Activation During Aging
Aging is often described as a process of progressive decline in function of organs/organism largely due to accumulation of damaged cells. Ever since caspases were discovered and apoptosis established as an intricate cell death mechanism, it was causally linked to organismal aging. Apoptosis was implicated in the aging process in two ways: by clearing multicellular organism of dysfunctional or unwanted cells, it maintains normal homeostasis; or by removing functionally important post-mitotic cells such as neurons or cardiac myocytes, it may hasten aging-associated pathologies.
Mutations in NALP3 gene, which increase caspase-1 activity and consequently IL-1β and IL-18, lead to systemic inflammatory diseases that increases during aging.185 Increase in oxidative stress and low-grade inflammation are the hallmarks of the aging process, which have been linked to caspase-1 activity.186 In another study, improvement in memory was observed in rats injected intracerebroventricularly with a caspase-1 inhibitor (Ac-YVAD-CMK),187 indicating that inhibition of caspase-1 can delay aging-associated pathologies.
Alzheimer's disease is an age-associated pathology and active caspase-6, which plays an important role in the etiology of this disease.188 Sarcopenia, age-related muscle loss, is associated with decreased differentiation of a population of stem cells, called satellite cells (SCs), into myofibrils. In a study, analyzing caspase activity in young and aged individuals, an increased rate of apoptosis and increased CASP2, CASP6, CASP7 and CASP9 levels in aged SCs, was observed, indicating an enhanced susceptibility of human SCs to undergo apoptosis with age.189 This would diminish the stem cell population and trigger increased muscle loss or damage.
Age-associated hearing loss occurs with accompanying cochlear degeneration and high levels of apoptosis. Calorie restriction, CR, which is known to delay aging in mice and other laboratory animals prevented apoptosis and hearing loss.190 The underlying mechanism is reduced ROS and mitochondrial damage during CR.191 Another quality control process that declines with aging is the unfolded protein response (UPR). Aged mice fail to employ UPR, especially when sleep deprived and simultaneously showed increased caspase-12 activity and apoptosis. This was not observed in young mice and suggests increased caspase activity to compensate a poor ER-stress-induced adaptive response.192
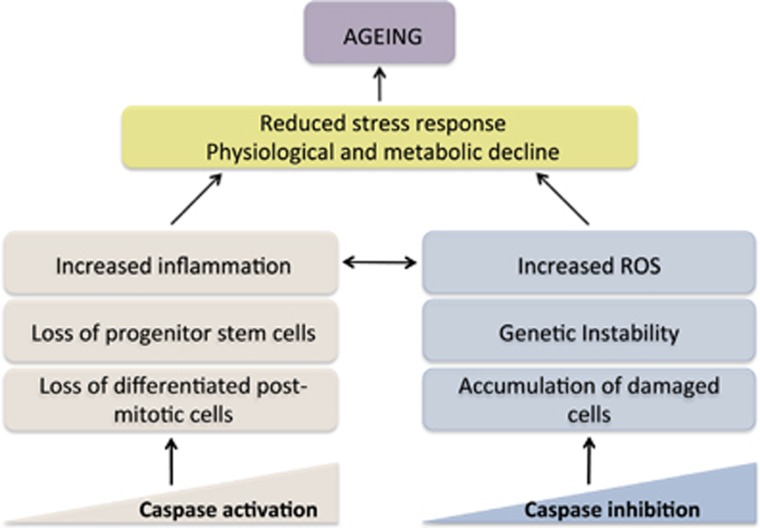
Zhang et al.193 first demonstrated a role for caspase-2 in delaying aging. Latter it was shown that caspase-2-deficient mice have reduced antioxidant ability and experience increased oxidative stress that accounts for their shorter lifespan.193 Aged caspase-2 knockout mice tissues also exhibit increased oxidative DNA damage and double-strand DNA breaks (γH2AX).194 Paraquat-induced pulmonary lesions in aged caspase-2 knockout mice were more severe and associated with hepatocyte karyomegaly.195 It was recently shown that the increase in mitochondrial ROS in aged caspase-2 knockout mice is associated with mitochondrial complex-III activity.196 The early-aging phenotype observed in caspase-2 knockout is distinct and involves non-apoptotic properties of this protease including its role in oxidative stress. A proposed model depicting how presence or absence of caspases can lead to aging is shown in Figure 5.
Figure 5.
Caspase activation occurs during aging, which is normally accompanied by a general decline in stress tolerance and functional strength. On one hand, this increased caspase activity, accelerates aging by increasing inflammation (e.g., caspase-1). Increased activity of caspases also results in removal of stem cells (e.g., loss of satellite cells by caspase-6), while in conditions such as stroke/ischemia, it causes loss of highly differentiated cells such as neurons and cardiomyocytes (e.g., caspase-9), causing functional decline and reduced regenerative ability. In another alternate scenario, loss of apoptosis may also hasten the aging process by preventing removal of damaged cells, increasing genetic instability and oxidative stress (e.g., caspase-2)
Caspases in Neural Development
Apoptosis plays a crucial role during neural development both in embryos and during adult life.197 Caspase-3, -9 and Apaf-1 are involved in forebrain development as mice lacking these proteins show exencephaly and neural overgrowth, attributed to increased cell death both in NPCs and developing neurons.197 However, caspases also affect neural development in non-apoptotic manner (Table 2).
Table 2. Non-apoptotic roles of caspases in neuronal function.
| Process | Caspase | Mechanism | Reference |
|---|---|---|---|
| Dendritic pruning | Caspase-2 | Activation of Rho/ROCK-II | 200 |
| Caspase-3 | Activation of caspase-3 | 199 | |
| Axon guidance and synaptogenesis | Caspase-3 and -6 are involved in axonal guidance and synapse formation | Mice lacking caspase-3 and -6 have developmental delay in pruning retinocollicular axons | 202 |
| Caspase-9 and Apaf regulate synapse formation | Casp9−/− and Apaf−/− mice have misrouted axons, impaired synapse and defects in olfaction. Cleavage of semaphorin 7A is vital for proper projection of axons | 201 | |
| Normal synaptic plasticity and function | Caspase-3 is important in LTD | Caspase- 3-mediated internalization of AMPA | 203 |
One such process is neuronal sculpting by dendrite pruning that results in removal of aberrant dendrites/axonal connections. Pioneering work in Drosophila revealed that Dronc is essential for dendrite pruning during larval development and the consequent formation of the adult nervous system.198 This process is dependent on ubiquitin-mediated degradation of DIAP1, to permit Dronc activation, and altered Dronc localization in axon and dendrites.198 Similar to apoptosis, the effector phase of pruning of dendrites involves cytoskeletal disruption, cell fragmentation and phagocytic clearance, indicating that Dronc activation may induce these morphological changes in the absence of cell death.
Recently, caspase-3 was found to control spine density and dendrite morphology without any observed increase in cell death.199 In support to these findings supernumerary spines have been reported in caspase-3 knockout mice.199 In a related study, caspase-2-deficient J20 APP transgenic mice did not show age-related reduction in cognitive function. This was attributed to an important role for caspse-2 in controlling dendrite spine density.200 Caspase-2 was found to activate the RhoA/ROCK-II signaling pathway, leading to collapse of dendritic spines.
Caspases are also involved in axonal guidance and synaptogenesis. Mice lacking caspase-9 and Apaf1 display misrouted axons, impaired synapse formation and defects in sensory neurons responsible for olfaction. However, no defects in neuronal cell death were observed.201 The underlying mechanism involves caspase-9-mediated cleavage of an essential protein, semaphorin 7A that is crucial for proper projection of axons.201 Caspase-3 and -6 have also been implicated in the process of axonal guidance.202
Normal brain functions depend on proper synaptic activity. Long-term potentiation (LTP) and long-term depression (LTD) are long-lasting modifications of synapses, in the hippocampus region. Caspase-3 is essential for LTD.203 During LTD, AMPA receptor in the post-synaptic membrane is internalized and the process is facilitated by caspase-3 and blocked by peptide inhibitors of caspase-3 and -9. It is again worth noting that during LTD, transient caspase-3 activation occurs without causing cell death.202
Conclusions
Ever since their discovery almost two decades ago, caspases have been shown to modulate cellular functions in more than one way. Although the mechanism and their activation platforms during cell death and apoptosis are well characterized, many of their non-apoptotic functions are poorly defined. Caspases mediate most of their activity by cleaving their target proteins and this substrate specificity guides cellular behavior. Several caspases are involved in tissue differentiation, cell proliferation, tumor formation and metastasis, determining immune response, synaptic plasticity, DNA damage and stress response, metabolism and aging. Although activation of caspases in some processes involves apoptosis/cell death, some others are independent of their apoptotic activity. Thus a better understanding of these functions may have much broader implications than previously thought. A better knowledge about caspase function may provide greatly improved strategies to design effective therapeutics in the area of inflammation, autoimmune disease and cancer.
Acknowledgments
Research work on caspase and cell death in our laboratory is supported by the National Health and Medical Research Council (NHMRC) of Australia project grants (1021456, 1041807 and 1043057), a Cancer Council Collaborative Research Fellowship to LD and a NHMRC Senior Principal Research Fellowship (1002863) to SK.
Glossary
- ROS
reactive oxygen species
- Apaf-1
apoptotic protease-activating factor 1
- CARD
caspase-recruitment domains
- cFLIP
cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein
- cIAP
cellular inhibitor of apoptosis protein
- DED
death effector domains
- DISC
death-inducing signaling complex
- HCC
Hepatocellular carcinoma
- NEMO
NF-κB essential modulator
- FADD
FAS-associated death domain protein
- MLKL
Mixed lineage kinase domain-like protein
- MOMP
mitochondrial outer membrane permeabilization
- PAMPs
pathogen-associated molecular patterns
- PIDD
p53-inducible protein with a death domain
- RAIDD
RIP-associated Ich-1/Ced-3 homologous protein with a death domain
- RIP1
Receptor-interacting protein 1
- RIP3
Receptor-interacting serine-threonine kinase 3
- TLR3
Toll-like receptor 3
- LTP
Long-term potentiation
- TNFR
tumor necrosis factor receptor
- TRADD
TNFR-associated death domain protein
- TRAF
TNF receptor-associated factors
- TRAIL
TNF-related apoptosis-inducing ligand
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015 Cell Death Differ 2014. e-pub ahead of print 19 September 2014; doi: 10.1038/cdd.2014.137 [DOI] [PMC free article] [PubMed]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Ballaun C, Hermann M, VandeBerg JL, Sipos W, Uthman A, et al. Identification of novel mammalian caspases reveals an important role of gene loss in shaping the human caspase repertoire. Mol Biol Evol. 2008;25:831–841. doi: 10.1093/molbev/msn012. [DOI] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293:722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–2241. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Baliga BC, Read SH, Kumar S. The biochemical mechanism of caspase-2 activation. Cell Death Differ. 2004;11:1234–1241. doi: 10.1038/sj.cdd.4401492. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Harvey NL, Parasivam G, Kumar S. Dimerization and autoprocessing of the Nedd2 (caspase-2) precursor requires both the prodomain and the carboxyl-terminal regions. J Biol Chem. 1998;273:6763–6768. doi: 10.1074/jbc.273.12.6763. [DOI] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F, et al. Caspase-2 activation in the absence of PIDDosome formation. J Cell Biol. 2009;185:291–303. doi: 10.1083/jcb.200811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more. Nat Rev Cancer. 2009;9:897–903. doi: 10.1038/nrc2745. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, et al. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1:468–477. doi: 10.1007/s13238-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JM, Cohen GM, Bampton ET. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6:1042–1056. doi: 10.4161/auto.6.8.13337. [DOI] [PubMed] [Google Scholar]
- Oral O, Oz-Arslan D, Itah Z, Naghavi A, Deveci R, Karacali S, et al. Cleavage of Atg3 protein by caspase-8 regulates autophagy during receptor-activated cell death. Apoptosis. 2012;17:810–820. doi: 10.1007/s10495-012-0735-0. [DOI] [PubMed] [Google Scholar]
- DeVorkin L, Gorski SM. A mitochondrial-associated link between an effector caspase and autophagic flux. Autophagy. 2014;10:1866–1867. doi: 10.4161/auto.32170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M, Sharma LK, Vanegas D, Callaway DA, Bai Y, Lechleiter JD, et al. A nonapoptotic role for CASP2/caspase 2: modulation of autophagy. Autophagy. 2014;10:1054–1070. doi: 10.4161/auto.28528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini V, Wirawan E, Romagnoli A, Ciccosanti F, Lisi G, Lippens S, et al. Proteolysis of Ambra1 during apoptosis has a role in the inhibition of the autophagic pro-survival response. Cell Death Differ. 2012;19:1495–1504. doi: 10.1038/cdd.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betin VM, Lane JD. Atg4D at the interface between autophagy and apoptosis. Autophagy. 2009;5:1057–1059. doi: 10.4161/auto.5.7.9684. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Valent A, Raslova H, Yakushijin K, et al. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene. 2004;23:4362–4370. doi: 10.1038/sj.onc.1207572. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Siegmund B. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64:1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, et al. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci USA. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Ip YT. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 2005;26:193–198. doi: 10.1016/j.it.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Scott AM, Saleh M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 2007;14:23–31. doi: 10.1038/sj.cdd.4402026. [DOI] [PubMed] [Google Scholar]
- Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- Bouchier-Hayes L, Conroy H, Egan H, Adrain C, Creagh EM, MacFarlane M, et al. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J Biol Chem. 2001;276:44069–44077. doi: 10.1074/jbc.M107373200. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Arditi M. Innate immune responses to Chlamydia pneumoniae infection: role of TLRs, NLRs, and the inflammasome. Microbes Infect. 2012;14:1301–1307. doi: 10.1016/j.micinf.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Moshe T, Barash H, Kang TB, Kim JC, Kovalenko A, Gross E, et al. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176–2187. doi: 10.1053/j.gastro.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Rajput A, Kang TB, Bogdanov K, Kim JC, Ben-Moshe T, Kovalenko A, et al. Anti-inflammatory functions of caspase-8. Adv Exp Med Biol. 2011;691:253–260. doi: 10.1007/978-1-4419-6612-4_25. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Mills K, Daish T, Harvey KF, Pfleger CM, Hariharan IK, Kumar S. The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. J Cell Biol. 2006;172:809–815. doi: 10.1083/jcb.200512126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daish TJ, Mills K, Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev Cell. 2004;7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Kanuka H, Sawamoto K, Inohara N, Matsuno K, Okano H, Miura M. Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4-related caspase activator. Mol Cell. 1999;4:757–769. doi: 10.1016/s1097-2765(00)80386-x. [DOI] [PubMed] [Google Scholar]
- Kanuka H, Kuranaga E, Takemoto K, Hiratou T, Okano H, Miura M. Drosophila caspase transduces Shaggy/GSK-3beta kinase activity in neural precursor development. EMBO J. 2005;24:3793–3806. doi: 10.1038/sj.emboj.7600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Tonoki A, Takemoto K, Tomioka T, Kobayashi M, et al. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell. 2006;126:583–596. doi: 10.1016/j.cell.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases leave the beaten track: caspase-mediated activation of NF-kappaB. J Cell Biol. 2006;173:165–171. doi: 10.1083/jcb.200509092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki Y, Jacobson MD, Raff MC. A role for caspases in lens fiber differentiation. J Cell Biol. 1998;140:153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Najbauer J, Woo CC, Dashtipour K, Ribak CE, Leon M. Expression of active caspase-3 in mitotic and postmitotic cells of the rat forebrain. J Comp Neurol. 2001;433:4–22. doi: 10.1002/cne.1121. [DOI] [PubMed] [Google Scholar]
- Fan W, Dai Y, Xu H, Zhu X, Cai P, Wang L, et al. Caspase-3 modulates regenerative response after stroke. Stem Cells. 2014;32:473–486. doi: 10.1002/stem.1503. [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TV, McMahon JM, Howley BA, Stanley A, Ritter T, Mohr A, et al. A non-apoptotic role for caspase-9 in muscle differentiation. J Cell Sci. 2008;121:3786–3793. doi: 10.1242/jcs.024547. [DOI] [PubMed] [Google Scholar]
- Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, Seo BM, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Togari A. Activation of caspases is required for osteoblastic differentiation. J Biol Chem. 2003;278:47477–47482. doi: 10.1074/jbc.M307055200. [DOI] [PubMed] [Google Scholar]
- Matalova E, Lesot H, Svandova E, Vanden Berghe T, Sharpe PT, Healy C, et al. Caspase-7 participates in differentiation of cells forming dental hard tissues. Dev Growth Differ. 2013;55:615–621. doi: 10.1111/dgd.12066. [DOI] [PubMed] [Google Scholar]
- Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, et al. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Fleming HE, Riedt T, Karlsson G, Riese MJ, Lo Celso C, et al. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell. 2008;2:584–594. doi: 10.1016/j.stem.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Rebe C, Cathelin S, Launay S, Filomenko R, Prevotat L, L'Ollivier C, et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007;109:1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, et al. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- Rendl M, Ban J, Mrass P, Mayer C, Lengauer B, Eckhart L, et al. Caspase-14 expression by epidermal keratinocytes is regulated by retinoids in a differentiation-associated manner. J Invest Dermatol. 2002;119:1150–1155. doi: 10.1046/j.1523-1747.2002.19532.x. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, et al. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004;6:551–562. doi: 10.1016/s1534-5807(04)00098-x. [DOI] [PubMed] [Google Scholar]
- Hsu S, Dickinson D, Borke J, Walsh DS, Wood J, Qin H, et al. Green tea polyphenol induces caspase 14 in epidermal keratinocytes via MAPK pathways and reduces psoriasiform lesions in the flaky skin mouse model. Exp Dermatol. 2007;16:678–684. doi: 10.1111/j.1600-0625.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, et al. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Adams DS, Qiu D, Koustubhan P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Dev Biol. 2007;301:62–69. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. Developmental parameters of cell death in the wing disc of Drosophila. Proc Natl Acad Sci USA. 1997;94:5691–5696. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the ‘phoenix rising' pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimuth J, Bangen JM, Lambertz D, Hu W, Nevzorova YA, Sonntag R, et al. Loss of caspase-8 in hepatocytes accelerates the onset of liver regeneration in mice through premature nuclear factor kappa B activation. Hepatology. 2013;58:1779–1789. doi: 10.1002/hep.26538. [DOI] [PubMed] [Google Scholar]
- Svandova E, Lesot H, Vanden Berghe T, Tucker AS, Sharpe PT, Vandenabeele P, et al. Non-apoptotic functions of caspase-7 during osteogenesis. Cell Death Dis. 2014;5:e1366. doi: 10.1038/cddis.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens SM, Kataoka T, Fortner KA, Tinel A, Ferrero I, MacDonald RH, et al. The caspase 8 inhibitor c-FLIP(L) modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol Cell Biol. 2002;22:5419–5433. doi: 10.1128/MCB.22.15.5419-5433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Bath S, Marsden VS, Huang DC, Metcalf D, Harris AW, et al. FADD and caspase-8 are required for cytokine-induced proliferation of hemopoietic progenitor cells. Blood. 2005;106:1581–1589. doi: 10.1182/blood-2005-01-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilot D, Serandour AL, Ilyin GP, Lagadic-Gossmann D, Loyer P, Corlu A, et al. A role for caspase-8 and c-FLIPL in proliferation and cell-cycle progression of primary hepatocytes. Carcinogenesis. 2005;26:2086–2094. doi: 10.1093/carcin/bgi187. [DOI] [PubMed] [Google Scholar]
- Beisner DR, Ch'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- Olson NE, Graves JD, Shu GL, Ryan EJ, Clark EA. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J Immunol. 2003;170:6065–6072. doi: 10.4049/jimmunol.170.12.6065. [DOI] [PubMed] [Google Scholar]
- Woo M, Hakem R, Furlonger C, Hakem A, Duncan GS, Sasaki T, et al. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol. 2003;4:1016–1022. doi: 10.1038/ni976. [DOI] [PubMed] [Google Scholar]
- Frost V, Al-Mehairi S, Sinclair AJ. Exploitation of a non-apoptotic caspase to regulate the abundance of the cdkI p27(KIP1) in transformed lymphoid cells. Oncogene. 2001;20:2737–2748. doi: 10.1038/sj.onc.1204367. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Monni O, Joensuu H, Franssila K, Klefstrom J, Alitalo K, Knuutila S. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90:1168–1174. [PubMed] [Google Scholar]
- Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- Jager R, Zwacka RM. The enigmatic roles of caspases in tumor development. Cancers (Basel) 2010;2:1952–1979. doi: 10.3390/cancers2041952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter RN, Rhee JG, Kyprianou N. Caspase-1 enhances the apoptotic response of prostate cancer cells to ionizing radiation. Anticancer Res. 2004;24:1377–1386. [PubMed] [Google Scholar]
- Ummanni R, Lehnigk U, Zimmermann U, Woenckhaus C, Walther R, Giebel J. Immunohistochemical expression of caspase-1 and -9, uncleaved caspase-3 and -6, cleaved caspase-3 and -6 as well as Bcl-2 in benign epithelium and cancer of the prostate. Exp Ther Med. 2010;1:47–52. doi: 10.3892/etm_00000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter RN, Kramer A, Borkowski A, Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001;61:1227–1232. [PubMed] [Google Scholar]
- Ueki T, Takeuchi T, Nishimatsu H, Kajiwara T, Moriyama N, Narita Y, et al. Silencing of the caspase-1 gene occurs in murine and human renal cancer cells and causes solid tumor growth in vivo. Int J Cancer. 2001;91:673–679. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kumar S, White DL, Takai S, Turczynowicz S, Juttner CA, Hughes TP. Apoptosis regulatory gene NEDD2 maps to human chromosome segment 7q34-35, a region frequently affected in haematological neoplasms. Hum Genet. 1995;95:641–644. doi: 10.1007/BF00209480. [DOI] [PubMed] [Google Scholar]
- Kim MS, Chung NG, Yoo NJ, Lee SH. Somatic mutation of proapoptotic caspase-2 gene is rare in acute leukemias and common solid cancers. Eur J Haematol. 2011;86:449–450. doi: 10.1111/j.1600-0609.2011.01591.x. [DOI] [PubMed] [Google Scholar]
- Hofmann WK, de Vos S, Tsukasaki K, Wachsman W, Pinkus GS, Said JW, et al. Altered apoptosis pathways in mantle cell lymphoma detected by oligonucleotide microarray. Blood. 2001;98:787–794. doi: 10.1182/blood.v98.3.787. [DOI] [PubMed] [Google Scholar]
- Holleman A, den Boer ML, Kazemier KM, Beverloo HB, von Bergh AR, Janka-Schaub GE, et al. Decreased PARP and procaspase-2 protein levels are associated with cellular drug resistance in childhood acute lymphoblastic leukemia. Blood. 2005;106:1817–1823. doi: 10.1182/blood-2004-11-4296. [DOI] [PubMed] [Google Scholar]
- Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M, et al. Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood. 1998;92:3090–3097. [PubMed] [Google Scholar]
- Yoo NJ, Lee JW, Kim YJ, Soung YH, Kim SY, Nam SW, et al. Loss of caspase-2, -6 and -7 expression in gastric cancers. APMIS. 2004;112:330–335. doi: 10.1111/j.1600-0463.2004.apm1120602.x. [DOI] [PubMed] [Google Scholar]
- Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA. 2009;106:5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, McCormick L, Janke L, Howard A, Bouchier-Hayes L, Green DR. Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ. 2013;20:1174–1182. doi: 10.1038/cdd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccini J, Shalini S, Voss AK, Gatei M, Wilson CH, Hiwase DK, et al. Loss of caspase-2 augments lymphomagenesis and enhances genomic instability in Atm-deficient mice. Proc Natl Acad Sci USA. 2013;110:19920–19925. doi: 10.1073/pnas.1311947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzl C, Fava LL, Krumschnabel G, Peintner L, Tanzer MC, Soratroi C, et al. Death of p53-defective cells triggered by forced mitotic entry in the presence of DNA damage is not uniquely dependent on Caspase-2 or the PIDDosome. Cell Death Dis. 2013;4:e942. doi: 10.1038/cddis.2013.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L, Puccini J, Wilson CH, Shalini S, Nicola M, Moore S, et al. Caspase-2 deficiency promotes aberrant DNA-damage response and genetic instability. Cell Death Differ. 2012;19:1288–1298. doi: 10.1038/cdd.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MR, Arya R, Mukhopadhyay A, Berrett KC, Clair PM, Witt B, et al. Caspase-2 impacts lung tumorigenesis and chemotherapy response in vivo Cell Death Differ 2014. e-pub ahead of print 10 October 2014; doi: 10.1038/cdd.2014.159 [DOI] [PMC free article] [PubMed]
- Manzl C, Peintner L, Krumschnabel G, Bock F, Labi V, Drach M, et al. PIDDosome-independent tumor suppression by caspase-2. Cell Death Differ. 2012;19:1722–1732. doi: 10.1038/cdd.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L, Puccini J, Nikolic A, Shalini S, Wilson CH, Norris MD, et al. An unexpected role for caspase-2 in neuroblastoma. Cell Death Dis. 2014;5:e1383. doi: 10.1038/cddis.2014.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Li YZ, Zhu XW, Liu CL, Wang P, Liu YL. HuGE systematic review and meta-analysis demonstrate association of CASP-3 and CASP-7 genetic polymorphisms with cancer risk. Genet Mol Res. 2013;12:1561–1573. doi: 10.4238/2013.May.13.10. [DOI] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, et al. Somatic mutations of CASP3 gene in human cancers. Hum Genet. 2004;115:112–115. doi: 10.1007/s00439-004-1129-3. [DOI] [PubMed] [Google Scholar]
- Chen K, Zhao H, Hu Z, Wang LE, Zhang W, Sturgis EM, et al. CASP3 polymorphisms and risk of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:6343–6349. doi: 10.1158/1078-0432.CCR-08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HL, Xu WH, Cai Q, Feng M, Long J, Zheng W, et al. Polymorphisms and haplotypes in the caspase-3, caspase-7, and caspase-8 genes and risk for endometrial cancer: a population-based, case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2009;18:2114–2122. doi: 10.1158/1055-9965.EPI-09-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Morton LM, Armstrong B, Hartge P, Menashe I, Zheng T, et al. Genetic variation in caspase genes and risk of non-Hodgkin lymphoma: a pooled analysis of 3 population-based case-control studies. Blood. 2009;114:264–267. doi: 10.1182/blood-2009-01-198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosgood HD, 3rd, Baris D, Zhang Y, Zhu Y, Zheng T, Yeager M, et al. Caspase polymorphisms and genetic susceptibility to multiple myeloma. Hematol Oncol. 2008;26:148–151. doi: 10.1002/hon.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JS, Kim KM, Choi JE, Cha SI, Kim CH, Lee WK, et al. Identification of polymorphisms in the caspase-3 gene and their association with lung cancer risk. Mol Carcinog. 2008;47:383–390. doi: 10.1002/mc.20397. [DOI] [PubMed] [Google Scholar]
- Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- O'Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O'Higgins N, et al. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9:738–742. [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Kim HS, Park WS, Kim SY, Lee JH, et al. Inactivating mutations of CASPASE-7 gene in human cancers. Oncogene. 2003;22:8048–8052. doi: 10.1038/sj.onc.1206727. [DOI] [PubMed] [Google Scholar]
- Lee WK, Kim JS, Kang HG, Cha SI, Kim DS, Hyun DS, et al. Polymorphisms in the Caspase7 gene and the risk of lung cancer. Lung Cancer. 2009;65:19–24. doi: 10.1016/j.lungcan.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas—a tool for pathology. J Pathol. 2008;216:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee JW, Soung YH, Park WS, Kim SY, Lee JH, et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003;125:708–715. doi: 10.1016/s0016-5085(03)01059-x. [DOI] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Kim SY, Sung YJ, Park WS, Nam SW, et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005;24:141–147. doi: 10.1038/sj.onc.1208244. [DOI] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Kim SY, Jang J, Park YG, Park WS, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005;65:815–821. [PubMed] [Google Scholar]
- MacPherson G, Healey CS, Teare MD, Balasubramanian SP, Reed MW, Pharoah PD, et al. Association of a common variant of the CASP8 gene with reduced risk of breast cancer. J Natl Cancer Inst. 2004;96:1866–1869. doi: 10.1093/jnci/dji001. [DOI] [PubMed] [Google Scholar]
- Teitz T, Lahti JM, Kidd VJ. Aggressive childhood neuroblastomas do not express caspase-8: an important component of programmed cell death. J Mol Med (Berl) 2001;79:428–436. doi: 10.1007/s001090100233. [DOI] [PubMed] [Google Scholar]
- Grotzer MA, Eggert A, Zuzak TJ, Janss AJ, Marwaha S, Wiewrodt BR, et al. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene. 2000;19:4604–4610. doi: 10.1038/sj.onc.1203816. [DOI] [PubMed] [Google Scholar]
- Ebinger M, Senf L, Wachowski O, Scheurlen W. Promoter methylation pattern of caspase-8, P16INK4A, MGMT, TIMP-3, and E-cadherin in medulloblastoma. Pathol Oncol Res. 2004;10:17–21. doi: 10.1007/BF02893403. [DOI] [PubMed] [Google Scholar]
- Martinez R, Setien F, Voelter C, Casado S, Quesada MP, Schackert G, et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis. 2007;28:1264–1268. doi: 10.1093/carcin/bgm014. [DOI] [PubMed] [Google Scholar]
- Shivapurkar N, Toyooka S, Eby MT, Huang CX, Sathyanarayana UG, Cunningham HT, et al. Differential inactivation of caspase-8 in lung cancers. Cancer Biol Ther. 2002;1:65–69. doi: 10.4161/cbt.1.1.45. [DOI] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, et al. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–99. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- Teitz T, Inoue M, Valentine MB, Zhu K, Rehg JE, Zhao W, et al. Th-MYCN mice with caspase-8 deficiency develop advanced neuroblastoma with bone marrow metastasis. Cancer Res. 2013;73:4086–4097. doi: 10.1158/0008-5472.CAN-12-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG. Caspase-8 as a therapeutic target in cancer. Cancer Lett. 2013;332:133–140. doi: 10.1016/j.canlet.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf RP, Keller N, Barbero S, Stupack D. Caspase-8 as a regulator of tumor cell motility. Curr Mol Med. 2014;14:246–254. doi: 10.2174/1566524014666140128111951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Kim HS, Kang CS, Park WS, Kim SY, Lee SN, et al. Inactivating mutations of CASP10 gene in non-Hodgkin lymphomas. Blood. 2002;99:4094–4099. doi: 10.1182/blood.v99.11.4094. [DOI] [PubMed] [Google Scholar]
- Kim MS, Oh JE, Min CK, Lee S, Chung NG, Yoo NJ, et al. Mutational analysis of CASP10 gene in acute leukaemias and multiple myelomas. Pathology. 2009;41:484–487. doi: 10.1080/00313020903041143. [DOI] [PubMed] [Google Scholar]
- Oh JE, Kim MS, Ahn CH, Kim SS, Han JY, Lee SH, et al. Mutational analysis of CASP10 gene in colon, breast, lung and hepatocellular carcinomas. Pathology. 2010;42:73–76. doi: 10.3109/00313020903434371. [DOI] [PubMed] [Google Scholar]
- Park WS, Lee JH, Shin MS, Park JY, Kim HS, Lee JH, et al. Inactivating mutations of the caspase-10 gene in gastric cancer. Oncogene. 2002;21:2919–2925. doi: 10.1038/sj.onc.1205394. [DOI] [PubMed] [Google Scholar]
- Lamy L, Ngo VN, Emre NC, Shaffer AL, 3rd, Yang Y, Tian E, et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell. 2013;23:435–449. doi: 10.1016/j.ccr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska M, Kim H, Shin E, Kennedy S, Duffy MJ, Wong YF, et al. Tumor-associated alterations in caspase-14 expression in epithelial malignancies. Clin Cancer Res. 2005;11:5462–5471. doi: 10.1158/1078-0432.CCR-04-2527. [DOI] [PubMed] [Google Scholar]
- Wu M, Kodani I, Dickinson D, Huff F, Ogbureke KU, Qin H, et al. Exogenous expression of caspase-14 induces tumor suppression in human salivary cancer cells by inhibiting tumor vascularization. Anticancer Res. 2009;29:3811–3818. [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc AC. Caspase-6 as a novel early target in the treatment of Alzheimer's disease. Eur J Neurosci. 2013;37:2005–2018. doi: 10.1111/ejn.12250. [DOI] [PubMed] [Google Scholar]
- Fulle S, Sancilio S, Mancinelli R, Gatta V, Di Pietro R. Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis. 2013;4:e955. doi: 10.1038/cddis.2013.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Bai U, Shirwany N, Quirk WS. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;21:161–167. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B, et al. Caspase-2 deficiency enhances aging-related traits in mice. Mech Ageing Dev. 2007;128:213–221. doi: 10.1016/j.mad.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Wilson C, Puccini J, Ho L, Kumar S. Impaired antioxidant defence and accumulation of oxidative stress in caspase-2-deficient mice. Cell Death Differ. 2012;19:1370–1380. doi: 10.1038/cdd.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini S, Puccini J, Wilson CH, Finnie J, Dorstyn L, Kumar S.Caspase-2 protects against oxidative stress in vivo Oncogene 2014. doi: 10.1038/onc.2014.413 [DOI] [PubMed]
- Lopez-Cruzan M, Herman B. Loss of caspase-2 accelerates age-dependent alterations in mitochondrial production of reactive oxygen species. Biogerontology. 2013;14:121–130. doi: 10.1007/s10522-013-9415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Erturk A, Wang Y, Sheng M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J Neurosci. 2014;34:1672–1688. doi: 10.1523/JNEUROSCI.3121-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Ribe EM, Troy CM, Arancio O, Shelanski M. Caspase-2 is required for dendritic spine and behavioural alterations in J20 APP transgenic mice. Nat Commun. 2013;4:1939. doi: 10.1038/ncomms2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Hamada S, Kuida K, Yoshida H, Igaki T, Miura M. Maturation of the olfactory sensory neurons by Apaf-1/caspase-9-mediated caspase activity. Proc Natl Acad Sci USA. 2010;107:13366–13371. doi: 10.1073/pnas.0910488107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, et al. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]