Abstract
Extrapulmonary and, in particular, spinal tuberculosis (TB) constitutes a minor but significant part of the total TB incidence. In spite of this, almost no studies on the genetic diversity and drug resistance of Mycobacterium tuberculosis isolates from spinal TB patients have been published to date. Here, we report results of the first Russian and globally largest molecular study of M. tuberculosis isolates recovered from patients with tuberculous spondylitis (TBS). The majority of 107 isolates were assigned to the Beijing genotype (n = 80); the other main families were T (n = 11), Ural (n = 7), and LAM (n = 4). Multidrug resistance (MDR) was more frequently found among Beijing (90.5%) and, intriguingly, Ural (71.4%) isolates than other genotypes (5%; P < 0.001). The extremely drug-resistant (XDR) phenotype was exclusively found in the Beijing isolates (n = 7). A notable prevalence of the rpoB531 and katG315 mutations in Beijing strains that were similarly high in both TBS (this study) and published pulmonary TB (PTB) samples from Russia shows that TBS and PTB Beijing strains follow the same paradigm of acquisition of rifampin (RIF) and isoniazid (INH) resistance. The 24-locus mycobacterial interspersed repetitive unit–variable-number tandem-repeat (MIRU-VNTR) subtyping of 80 Beijing isolates further discriminated them into 24 types (Hunter Gaston index [HGI] = 0.83); types 100-32 and 94-32 represented the largest groups. A genotype of Russian successful clone B0/W148 was identified in 30 of 80 Beijing isolates. In conclusion, this study highlighted a crucial impact of the Beijing genotype and the especially prominent role of its MDR-associated successful clone B0/W148 cluster in the development of spinal MDR-TB in Russian patients.
INTRODUCTION
Extrapulmonary tuberculosis (EPTB) remains a major health problem due to a significantly high rate of morbidity and mortality in both developing and developed countries and constitutes a significant part of the total TB incidence (1, 2). In Russia, the rate of EPTB decreased from 9.4% in 1992 to 3.4% in 2011, and the EPTB incidence constituted 2.4/100,000 in 2011 (3, 4). At the same time, the rate of tuberculous spondylitis (TBS) among all new EPTB cases increased in Russia from 23% in 2006 to 33% in 2010, which is higher than TB rate for of all other sites of the disease (3, 5).
Tuberculous spondylitis constitutes about half of bone and joint TB cases and thus represents the most severe orthopedic disease, frequently leading to irreversible neurological disorders and disability and constituting a serious social and economic problem (6–8). TBS is developed as a result of blood-born dissemination of Mycobacterium tuberculosis. Bacteriological diagnosis of bone and joint TB is a most challenging task, and findings of drug resistance in these isolates may be crucial for adequate management of such cases. Delayed initial diagnosis and confirmation (from 3 months to 10 years after onset of the disease) and the increasing incidence of the multidrug-resistant TBS with spondyloarthropathy in adults require improvement of the etiological diagnostics, taking into account biological and molecular properties of the pathogen (4, 6, 7).
M. tuberculosis has a clonal population structure, and it has been demonstrated that not only its large genetic families but also their variants or subgenotypes may play a special role in the disease progression. Russian strain Beijing B0/W148, initially defined by IS6110-restriction fragment length polymorphism (RFLP) analysis (9), presents a remarkable example of a successful clone highly associated with multidrug resistance (MDR) (10). Correlating different typing schemes, 24-locus mycobacterial interspersed repetitive unit–variable-number tandem-repeat (MIRU-VNTR) type 100-32 makes a major component of the B0/W148 cluster in the European part of Russia (10).
While genetic diversity of pulmonary TB strains has been the subject of many publications worldwide, M. tuberculosis isolates from spinal TB cases have been characterized in a very limited number of studies (6–8, 11). The present study aimed to assess the genetic diversity and drug susceptibility profiles of M. tuberculosis isolates from TBS patients in Russia. Along with being the first such study in Russia, the sample size makes it the largest study of this kind to date globally.
MATERIALS AND METHODS
M. tuberculosis strains and drug susceptibility testing.
The existing system of providing surgical care in few specialized hospitals to patients with spinal TB in the Russian Federation was built in the middle of the 1970s. Historically, about 60% of all patients with spinal TB from all regions in Russia are treated in the clinics of St. Petersburg Research Institute of Phthisiopulmonology. The mycobacteriology laboratory in this institute serves as a reference center for northwestern and northern Russia; additionally, the laboratory receives strains from other regions across Russia. The laboratory is externally quality assured by the Federal System for External Quality Assessment in Laboratory Medicine of Russian Federation.
This study enrolled all TBS-diagnosed adult patients admitted within the survey period from January 2008 to December 2011 at the clinics of bone and joint TB of the St. Petersburg Research Institute of Phthisiopulmonology. They came from different regions across Russia and could not be linked on the basis of standard epidemiological investigations. The patients were initially referred from local hospitals to the clinics of the St. Petersburg Research Institute of Phthisiopulmonology on the basis of clinical and X-ray examination. The histological and bacteriological (Loewenstein-Jensen culture) methods were used for final confirmation of the TBS diagnoses in the St. Petersburg clinics described above. In more detail, blind prospective microbiological analysis was performed on surgical material from spinal lytic lesion. Further, on the basis of pathomorphological investigation, a group of patients with infectious spinal lesion (n = 359) was selected from the entire cohort of patients treated for tuberculous spondylitis.
Specimens (surgical material from lytic lesions: pus, granulation, and fragments of intervertebral disks and bones) from 144 patients were found culture positive and Ziehl-Neelsen (ZN) microscopy positive for the presence of M. tuberculosis. All 144 specimens were confirmed as M. tuberculosis by IS6110-based real-time PCR analysis using an Amplitub-RV diagnostic kit (Syntol, Russia) and iCycleriQ5 apparatus (Bio-Rad, USA).
Analysis of M. tuberculosis susceptibility to the first-line (isoniazid [INH], rifampin [RIF], pyrazinamide, ethambutol, and streptomycin) and second-line (ofloxacin, cycloserine, amikacin, kanamycin, capreomycin, and ethionamide) anti-TB drugs was done using the method of absolute concentrations according to the guidelines of the Russian Ministry of Health (order no. 109 of 21 March 2003) and/or a Bactec MGIT 960 system according to the manufacturer's recommendations.
The study was approved by Ethical Board of St. Petersburg Research Institute of Phthisiopulmonology.
Genotyping.
DNA was extracted from bacterial cultures using a recommended method (12). Of 144 M. tuberculosis isolates, 107 (74.3%) were available and/or had enough quality and quantity of extracted DNA for genotyping of all targeted loci. While this situation makes the sample both a convenience sample and a systematic sample, the long (4-year) time span of sampling, exhaustive enrollment of patients, good representation of regions across Russia, and, on the whole, high proportion (74.3%) of the isolates available for genotyping made the collection representative enough to get an adequate image of the molecular structure of the population of spinal M. tuberculosis in Russia.
DNA was used for M. tuberculosis species identification and detection of mutations in the katG315, inhA promoter, and oxyR-ahpC regions associated with isoniazid (INH) resistance and mutations in the rpoB rifampin resistance-determining region (RRDR; rpoB codons 507 to 533) associated with RIF resistance by using a TB-Biochip kit (Biochip-IMB, Moscow, Russia). In addition, some isolates with discrepant phenotype versus genotype results were externally retested using an improved expanded biochip assay (Egor Shitikov, Research Institute of Physical and Chemical Medicine, Moscow, Russia).
Genotyping was performed using spoligotyping, IS6110-RFLP typing, and 24-locus MIRU-VNTR typing as recommended (12–14). The spoligotyping profiles were entered into Excel spreadsheets and compared with entries in SITVIT2, an international spoligotype database in Institut Pasteur de Guadeloupe, which is the most recent update of the published SITVIT_WEB database (15). Genetic families and subtypes were assigned to the genotyping profiles using SITVIT_WEB. In addition, LAM family status was verified by testing LAM-specific single nucleotide polymorphism (SNP) Rv0129c 309G>A (16, 17) since the specific population structure of this family in Russia makes the application of the spoligotype-based decision rules unreliable (17).
The MIRU-VNTR data of the Beijing isolates were compared to the MIRU-VNTRplus database (http://www.miru-vntrplus.org) and a proprietary database of the Beijing genotype (18, 19). The Beijing B0/W148 cluster was detected using a multiplex PCR assay (20).
The MIRU-VNTRplus online tool was used to build the unweighted-pair group method using average linkages (UPGMA) dendrogram of the MIRU-VNTR digital profiles. A cluster is usually defined based on the similarity or identity of genetic profiles (in cases in which high-resolution typing was done) and is assumed to have been the result of recent transmission. Here, all isolates were from patients without proven epidemiological links and coming from different regions across Russia, which is why this definition of a cluster should be regarded with caution, and the term “cluster” is used here solely to define genetically related strains without the implication of recent transmission. The objective of this study (and hence its methodology) was to reconstruct the population structure of the spinal TB isolates in Russia rather than to trace particular epidemiological links between patients.
The Hunter-Gaston index (HGI) was calculated at http://www.hpa-bioinformatics.org.uk/cgi-bin/DICI/DICI.pl and was used to evaluate the diversity of a marker/population. A 2-by-2 χ2 test was used to detect any significant difference between the two groups. Yates-corrected χ2 and P values were calculated with a 95% confidence interval using EpiCalc 2000 version 1.02 software. Fisher's P value was calculated in the case of limited numbers (n < 10) of samples.
RESULTS
The mean age of 107 patients was 39.5 ± 13.5 years. The patients included 71 males (age range, 18 to 73; mean age, 36 years) and 36 females (age range, 22 to 77; mean age, 46 years). Patients represented all macroregions (“federal districts”) of Russia, although most of them came from the European northwestern (n = 47) and European (n = 83) parts of Russia as a whole (see Fig. S1 and data set S1 in the supplemental material).
Spoligotyping of 107 M. tuberculosis isolates from TBS patients revealed 19 variants, including 6 shared types (2 to 76 isolates) and 13 singletons (Table 1). The Beijing genotype (SIT1, SIT265, and SIT269) included 80 (75%) isolates. Three isolates created three new types in SITVIT_WEB and were assigned respective numbers SIT3783, SIT3784, and SIT3785 (Table 1).
TABLE 1.
Spoligotyping profiles and drug resistance of M. tuberculosis isolates from Russian TBS patientsa
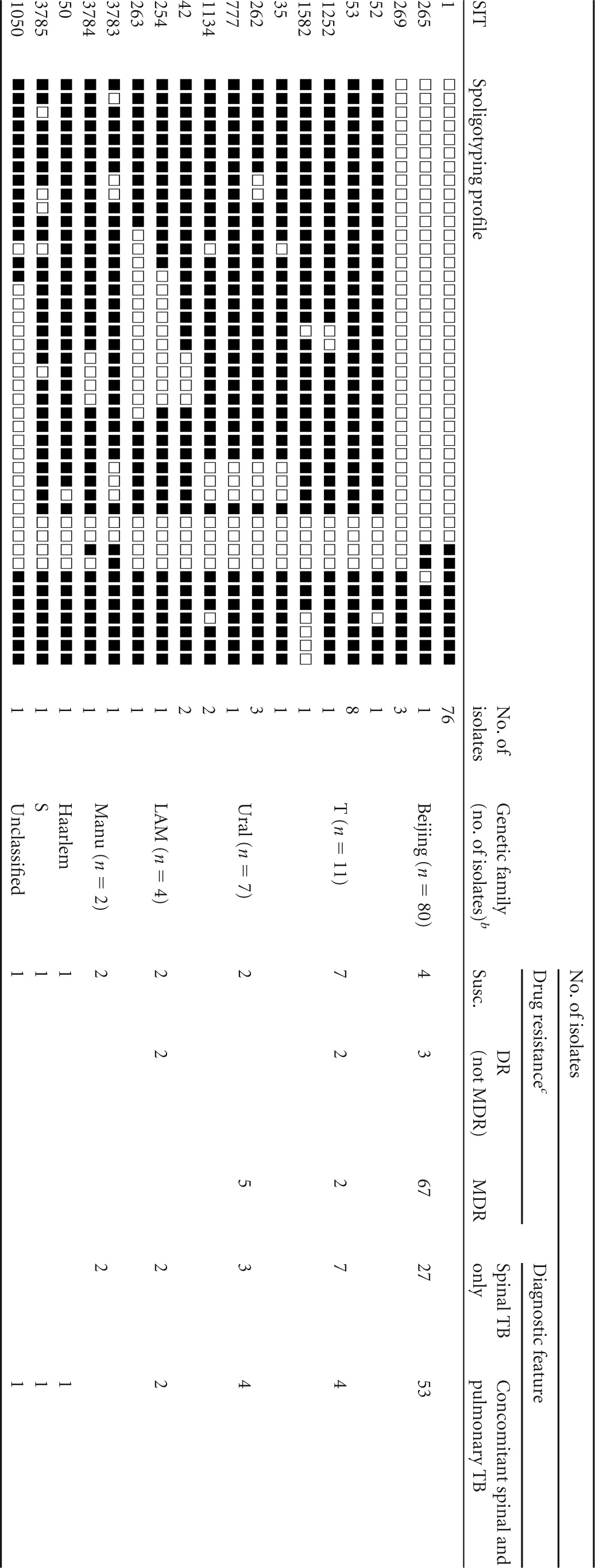
Abbreviations: SIT, spoligotype international type (SITVIT_WEB); DR, drug resistant; MDR, multidrug resistant; Susc., susceptible.
Based on comparison with SITVIT_WEB and MIRU-VNTRplus databases and additional molecular tests.
Drug resistance data do not include 6 phenotypically susceptible isolates with drug resistance mutations.
The isolates were assigned to the families following comparisons to both the SITVIT_WEB and the MIRU-VNTRplus online databases. Due to the uncertainty of the spoligotype-based decision rules for some of the families, the other family-specific molecular markers were also taken into consideration or directly tested. Based on the MIRU-VNTRplus decision rules, some Haarlem family isolates were redefined as members of the Ural family. SIT254 and SIT263 isolates defined as T in SITVIT were reassigned to the LAM family on the basis of detection of the LAM-specific SNP in Rv0129c.
To sum up, non-Beijing genotypes were represented by several families (T, Ural, LAM, Manu, Haarlem, and S) whereas the T superfamily (n = 11) and Ural (n = 7) families were the largest.
Drug resistance properties of the isolates were evaluated using phenotypic and genotypic tests. The latter concerned biochip analysis of gene mutations associated with RIF and INH resistance. Both (the method and the mutations) were previously shown to be specific and sensitive tools in Russian settings (21, 22).
The mutation in rpoB531 was found most frequently among Beijing isolates (60 of 72 isolates with rpoB mutation) and was found in other genotypes as well, although in a lower number of samples (3 of 7 isolates with rpoB mutation) (Table 2). Different mutations in other rpoB codons were found in single isolates. Five isolates (four of which were Beijing) showed a double or triple mutation in rpoB (four of them harbored the rpoB531 mutation); none represented a mix of different isolates, as confirmed by 24-locus VNTR typing.
TABLE 2.
Distribution of rpoB, katG, and inhA alleles in different families and genotypesa
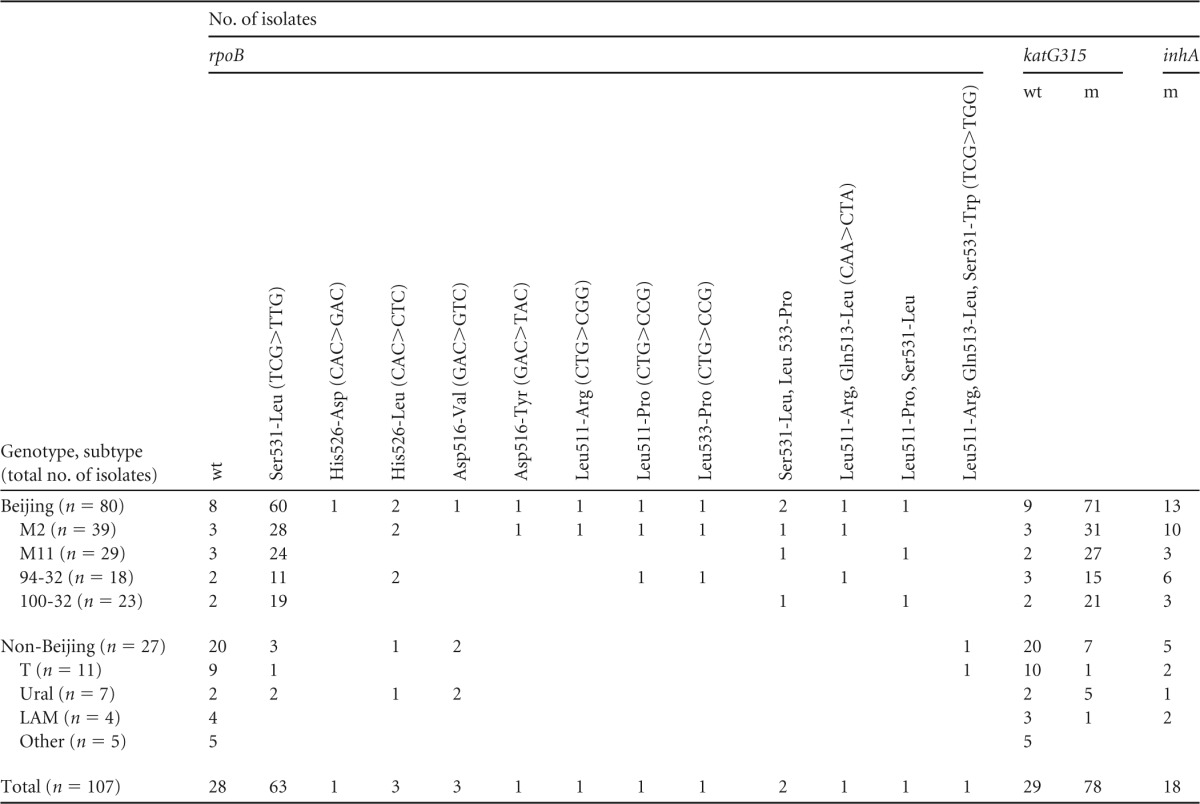
wt, wild type; m, mutation. Only major variants within the Beijing genotype are shown (M2 and M11 were defined by the 12-MIRU scheme and 94-32 and 100-32 were defined by the 24-MIRU scheme).
Regarding INH resistance mutations, the katG315 mutation was the most frequent among Beijing isolates (n = 71), whereas non-Beijing isolates were marked with equal and independent representation of katG315 mutations (n = 7) and inhA mutations (n = 5) (Table 2). Finally, a comutation (katG315AGC>ACC and inhA-15C>T) was identified in 12 Beijing and 3 non-Beijing isolates. No mutations in the oxyR-ahpC region were found.
Comparison of the results of phenotypic and genotypic analyses performed to detect RIF and INH drug resistance identified a number of discrepancies. First, 4 phenotypically resistant isolates had no mutation in either of the targeted genes; thus, their INH and/or RIF resistance may be mediated by a mutation in another gene target not covered by the biochip assay. Second, 6 isolates with drug resistance mutations were phenotypically reported as susceptible to RIF or INH or both; this was confirmed by retesting in a Bactec system. Their DNA samples were externally retested at the Research Institute of Physical and Chemical Medicine, and our initial genotypic results were confirmed. Of them, all 6 INH-susceptible isolates harbored the katG315 mutation. The 5 RIF-susceptible isolates included those with the rpoB531 mutation (n = 3) and mutations in rpoB codons 511 and 533 (1 isolate each). Some rpoB mutations cause low-level resistance: indeed, mutations in rpoB codons 511 and 533 in isolates with low-level RIF resistance were previously reported in Germany (23). Ultimately, these 6 isolates were excluded from further comparison of genotypes and families.
Analysis of the drug resistance properties across the families (Table 1; see also data set S1 in the supplemental material) showed that 20 (19.8%) of 101 isolates were susceptible to all tested drugs. MDR was found in 74 (73.3%) isolates (including 15 of 25 from HIV-positive patients). The MDR isolates mainly belonged to the Beijing genotype (90.5%), while the drug-susceptible subgroup was dominated by isolates of non-Beijing genotypes (80%). In other words, MDR was more prevalent among Beijing genotype isolates (67 of 74) than among the isolates of all other families pooled (7 of 27; P < 0.0001). However, it should be noted that a closer look at the particular non-Beijing families revealed that 5 of 7 Ural strains and 2 of 20 other strains were MDR (P = 0.005; Table 1). These results were unbiased in terms of geographic region (patient origin).
Analysis of phenotypic drug resistance to the second-line drugs permitted identification of 7 extremely drug-resistant (XDR) isolates; XDR was determined according to the WHO definition (24). All XDR isolates belonged to the Beijing genotype.
Further analysis of the allelic diversity of the Beijing genotype isolates was performed using 24-locus MIRU-VNTR typing. Particular MIRU-VNTR loci showed different and variable levels of polymorphism. QUB26 and MIRU26 were the most heterogeneous (Hunter-Gaston discrimination index [HGDI] values, 0.68 and 0.51), while 11 loci were monomorphic (Table 3).
TABLE 3.
Allelic diversity of the 13 polymorphic MIRU-VNTR loci in 80 M. tuberculosis Beijing genotype isolatesa
| MIRU-VNTR locus | MIRU-VNTR alleles (copy no. in particular loci) |
HGDI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| QUB26 | 17 | 31 | 29 | 3 | 0.680 | |||||
| MIRU26 | 41 | 39 | 0.506 | |||||||
| Mtub21 | 1 | 7 | 71 | 1 | 0.207 | |||||
| Mtub04 | 3 | 3 | 74 | 0.143 | ||||||
| MIRU10 | 5 | 75 | 0.119 | |||||||
| QUB11b | 1 | 3 | 76 | 0.097 | ||||||
| Mtub39 | 3 | 76 | 1 | 0.097 | ||||||
| ETR-A | 4 | 76 | 0.096 | |||||||
| MIRU31 | 4 | 76 | 0.096 | |||||||
| MIRU39 | 3 | 77 | 0.073 | |||||||
| QUB 4156 | 77 | 3 | 0.073 | |||||||
| Mtub29 | 1 | 1 | 78 | 0.050 | ||||||
| MIRU40 | 78 | 2 | 0.049 | |||||||
Another 11 loci of the 24-MIRU-VNTR scheme (MIRU2, MIRU4, MIRU16, MIRU20, MIRU23, MIRU24, MIRU27, Mtub30, Mtub34, ETR-B, and ETR-C) were monomorphic (HGDI = 0).
As a first step, the 12-locus MIRU-VNTR scheme was applied to the Beijing isolates. While this approach lacks discriminatory power and is not suitable for epidemiological typing, it is phylogenetically helpful to assign isolates to their subtypes according to the available large databases compiled since 2000. This differentiated 80 isolates into 9 variants; the largest groups were M2/MIT16 and M11/MIT17, which included 39 and 29 isolates, respectively. The global prevalence of these types according to our proprietary database is shown in Table S1 in the supplemental material.
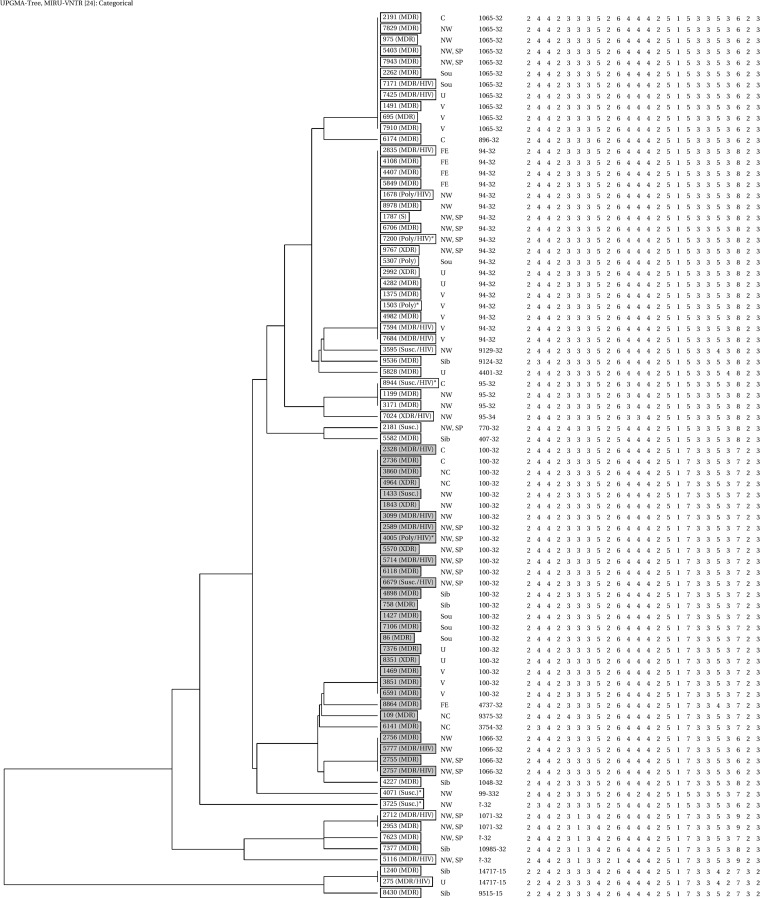
Use of the complete 24-locus MIRU-VNTR scheme revealed 24 variants of the Beijing genotype (HGI = 0.83), including 7 clusters (Fig. 1). The largest clusters with digital profiles 244233352644425173353723 (code 100-32, MIRU-VNTRplus) and 244233352644425153353823 (code 94-32) included 23 and 18 isolates, respectively; the order of loci is clockwise. The largest 24-locus MIRU cluster, 100-32, included 29% of the Beijing strains, and 87% of them were MDR.
FIG 1.
UPGMA dendrogram of 80 M. tuberculosis Beijing genotype isolates from Russian TBS patients based on 24-locus MIRU-VNTR loci (the Mlva15-9 codes are according to the MIRU-VNTRplus database, but the order of loci is clockwise, starting with VNTR154). B0/W148 isolates are shaded in gray. The asterisks depict 6 phenotypically RIF- and/or INH-susceptible but genotypically resistant isolates. Abbreviations: susc., susceptible; poly, polyresistant, i.e., resistant to more than one drug but not MDR; HIV, isolates from HIV-positive patients. Russian regions: NW, northwest, SP, St. Petersburg, C, central, V, Volga region, Sou, South, NC, North Caucasus (those regions constitute the European part of Russia), U, Ural, Sib, Siberia, FE, Far East.
Thirty (37.5%) of 80 Beijing genotype isolates belonged to the B0/W148 cluster defined by specific IS6110 insertion (20). Correlating this information to that obtained by MIRU typing showed that 23 B0/W148 isolates belonged to 24-locus MIRU type 100-32, 4 were of type 1066-32, and others were unique types (Fig. 1). Most (27 of 30) of the B0/W148 isolates were MDR, and only 2 were susceptible. The XDR phenotype was found in 3 isolates of the B0/W148 cluster and in 4 isolates of other Beijing variants.
A closer look at HIV coinfection across different patient/strain genotypes showed no association: 21 of 80 Beijing isolates and 4 of 27 non-Beijing isolates were from HIV-positive patients (P = 0.2). Further, the Beijing B0/W148 cluster showed a rate of HIV-positive patients similar to the rate seen with other Beijing variants: 7 (23.3%) of 30 versus 14 (28%) of 50. On the whole, the isolates from HIV-positive patients were evenly distributed across the dendrogram of the Beijing family (Fig. 1).
Based on the diagnosis and site of disease, the patients were subdivided into two groups: (i) spinal TB only and (ii) concomitant spinal TB and pulmonary TB (here defined as “concomitant TB” solely for the purposes of discussion). These two groups were compared for distributions of the genotypes and drug resistance of the strains (Table 1 and Table 4; see also data set S1 in the supplemental material). The concomitant TB sample was dominated by the Beijing genotype more than the spinal TB sample: 50 (79.4%) of 63 versus 24 (63.2%) of 38 (P = 0.07). Furthermore, Beijing strains in the concomitant TB group were almost always MDR/polydrug resistant (polyDR) compared to non-Beijing strains in the same group: 49 of 50 versus 6 of 13 (P = 0.0005). In the case of the spinal TB group, the rate of MDR/polyDR among Beijing versus non-Beijing isolates was 19 of 24 versus 4 of 14 (P = 0.0004). The rate of MDR/polyDR was higher in concomitant TB isolates than in spinal TB isolates (55/63 versus 24/38, P = 0.006), and this increase was mainly due to MDR Beijing genotype strains (Table 4). For the Beijing group, the MDR rate was significantly higher in concomitant TB isolates than in spinal TB isolates (49/50 versus 193/24, P = 0.02). For the non-Beijing group, this was not the case, since the MDR rates determined for those isolates did not differ between the two clinical groups (P = 0.3). Taken together, these statistically robust differences highlight a crucial capacity of the MDR-associated Beijing genotype strains to disseminate.
TABLE 4.
Distribution of M. tuberculosis genotypes and drug resistance in different diagnosis groups
| M. tuberculosis strain diagnostic feature | No. of isolates with indicated: |
|||
|---|---|---|---|---|
| Genotype |
Drug resistanceb |
|||
| Beijing | Non-Beijing | MDR + polyDR | Monoresistant + susceptible | |
| Concomitant spinal and pulmonary TB; n = 66 | 50 (49 MDRa, including 6 XDR) | 13 (6 MDR) | 55 (49 Beijing) | 8 (1 Beijing) |
| Spinal TB only; n = 41 | 24 (19 MDR, including 1 XDR) | 14 (4 MDR) | 24 (20 Beijing) | 14 (4 Beijing) |
MDR data represent MDR plus polydrug resistance (polyDR).
Drug resistance data do not include 6 phenotypically susceptible isolates with drug resistance mutations.
DISCUSSION
Global view of M. tuberculosis genotypes from TBS patients.
Culture of M. tuberculosis from clinical specimens of spinal TB is admittedly a serious challenge. This situation explains why almost no studies on genetic diversity of spinal TB strains have been published to date.
The present Russian study demonstrated a high (75%) prevalence of the Beijing genotype in the studied M. tuberculosis population from TBS patients, which is higher than the 40% to 50% average rate of this genotype across Russia in almost exclusively PTB patients (reviewed in reference 10). A higher rate of TBS than PTB (28% versus 6%) was described in north India (11, 25, 26) and a decreased rate of TBS versus PTB (23% versus 32%) in central Taiwan (8, 27).
Different variables may influence an increase or a decline of the rate of the Beijing genotype in the TBS versus PTB groups: the total burden of TB as a whole and that caused by the Beijing genotype, strain drug resistance, and/or hypervirulence; host factors such as age and host genetics; and environmental factors. At present, most of them cannot be comprehensively addressed by analysis of the published information. Still, some speculations are tempting. Comparing EPTB and spinal TB subsamples in the settings described above, one may note that the Russian and Indian patients were of young or middle age compared to the older age of the Taiwan patients. Weng et al. (8) suggested that the older age range of their spinal TB group was due to the high life expectancy in Taiwan. Besides, it may be that TB in elderly people reflects reactivation of a long-term infection whereas active spinal TB in young people may be due to active progression of the extrapulmonary disease. This implies a role of different host immune factors. On the other hand, considering pathogen-related factors, a capacity to rapidly acquire drug resistance, i.e., the MDR rate, is one of them. Both this Russian study and a previous Indian study (11), in spite of differences in their M. tuberculosis population structures, showed a high rate of MDR isolates as well as a higher rate of MDR among Beijing genotype strains. This is in contrast to the isolates in the Taiwan study, which were marked with lower rate of MDR and similar rates of MDR among Beijing and other genotypes (27). Thus, the relative increase of the Beijing genotype prevalence in the TBS group versus the PTB group among Russians and north Indians may be explained by the similar manifestations of host- and pathogen-related factors and, furthermore, has likely been aggravated by insufficient TB control. Future studies are warranted to test these speculations.
M. tuberculosis genotypes, drug resistance, and clinical issues.
Two observations immediately emerge from a closer look at the distribution of drug resistance across large genotype families (Table 1). First, and not unexpectedly (for Russia), the Beijing genotype is associated with MDR. Second, and intriguingly, the isolates in the Ural family were also found to be mainly MDR. This result is unusual, as the Ural genotype has been considered of low virulence and low transmissibility and was associated with MDR only in some studies across the former Soviet Union (reviewed in reference 28). Genomic studies of the larger and more diverse collections of the Ural family isolates from Eastern Europe and Russia are needed to clarify these findings.
Looking at the large populations of 12-locus MIRU subtypes within the most abundant Beijing family, neither was overwhelmingly prevalent in the studied TBS collection. However, a subsequent in-depth analysis permitted us some interesting insights. In this TB spondylitis study, the Beijing B0/W148 cluster and other Beijing genotypes were found in 30 (28%) and 50 (47%) of all 107 isolates. According to the recent comprehensive review (10), the mean prevalence rate of B0/W148 constitutes 10% to 15% and that of other Beijing strains 35% to 40% in total, mainly, or exclusively pulmonary TB populations across Russia. Thus, the rate of Beijing B0/W148 in the TBS group versus the PTB group shows a 2-fold (100%) increase compared to the 20% increase seen for other Beijing variants.
In analyses of the genetic basis of RIF and INH resistance, our results were mainly confirmatory with regard to pulmonary TB and Beijing studies. Notable prevalences of the rpoB531 and katG315 mutations in Beijing strains that were similarly high in the TBS and the PTB samples show that TBS and PTB Beijing strains follow the same paradigm of acquisition of RIF and INH resistance.
Last but not least, looking closer at the gender distribution, stratification by genotype showed that the Beijing subsample was more biased toward males. Beijing genotype-infected patients included 57 males and 23 females compared to the patients infected with strains of the other genotypes (14 males and 13 females). Even though this difference is not significant (P = 0.1) (odds ratio, 2.30; 95% confidence interval [CI], 0.94 to 5.64), it may suggest a trend of the capacity of the Beijing genotype to cause TB spondylitis in men rather than in women; future studies on this effect are warranted.
In summary, this study demonstrated the impact of the M. tuberculosis Beijing genotype and, first of all, the most prominent role of its B0/W148 Russian clonal group in the development of multidrug-resistant spinal tuberculosis in Russia. Although the hypervirulence of the Beijing genotype is more like a cliché, indeed, some studies demonstrated its increased capacity to cause disseminated and extrapulmonary disease (29, 30). The present study highlighted a role of the MDR-associated Beijing strains in development of both spinal TB and concomitant spinal and pulmonary TB. One may assume that an association of a genotype with MDR leads to prolonged treatment, giving more time for a strain to disseminate. Indeed, results of this study suggest that this property of the Beijing genotype, and especially of the Beijing B0/W148 variant, is the force driving their very high rate among spinal TB cases in Russia.
Interestingly, an insight into the spinal TB M. tuberculosis population offers an additional and less apparent clue for ancient DNA studies. It is known that it is mainly or almost exclusively spinal (and not pulmonary) TB that is revealed in paleopathological specimens (31, 32). Apparently, these osteopathological cases do not exactly reflect the past population structure of the entire M. tuberculosis population. Thus, understanding the molecular population structure of spinal versus pulmonary TB in extant populations may help us to interpret the spinal TB genotyping data of the past populations more adequately.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Nalin Rastogi and David Couvin for comparison of the spoligotype and VNTR data with the SITVIT2 database of Pasteur Institute of Guadeloupe and assignment of the SIT/MIT numbers and genetic lineages.
We acknowledge partial support from the Russian Science Foundation (grant no. 14-14-00292—subtyping experiments).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04221-14.
REFERENCES
- 1.World Health Organization (WHO). 2012. Global tuberculosis control: WHO report. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Solovic I, Jonsson J, Korzeniewska-Koseła M, Chiotan DI, Pace-Asciak A, Slump E, Rumetshofer R, Abubakar I, Kos S, Svetina-Sorli P, Haas W, Bauer T, Sandgren A, van der Werf MJ. 2013. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 18:pii=20432 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20432. [PubMed] [Google Scholar]
- 3.Anonymous. 2012. Infectious morbidity in the subjects of Russian Federation in 2010–2011. In Information Proceedings of Statistical and Analytical Data, part 3. Federal Center of Hygiene and Epidemiology, Moscow, Russia: (In Russian.) [Google Scholar]
- 4.Levashev YN, Mushkin AY, Grishko AN. 2006. Extrapulmonary tuberculosis in Russia: official statistics and reality. Probl Tuberk Bolezn Legk 2006:3–6. (In Russian.) [PubMed] [Google Scholar]
- 5.Kartavykh AA, Borisov SE, Matveeva MV. 2009. Tuberculosis of extrapulmonary localization based on personal registers of newly diagnosed patients. Tuberk Bolezn Legk 2009:17–26. (In Russian.) [Google Scholar]
- 6.Garg R, Somvanshi D. 2011. Spinal tuberculosis: a review. J Spinal Cord Med 34:440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasouli M, Mirkoohi M, Vaccaro A, Yarandi KK, Rahimi-Movaghar V. 2012. Spinal tuberculosis: diagnosis and management. Asian Spine J 6:294–308. doi: 10.4184/asj.2012.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng C, Ho C, Dou HY, Ho MW, Lin HS, Chang HL, Li JY, Lin TH, Tien N, Lu JJ. 2013. Molecular typing of Mycobacterium tuberculosis isolated from adult patients with tubercular spondylitis. J Microbiol Immunol Infect 46:19–23. doi: 10.1016/j.jmii.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Narvskaia O, Mokrousov I, Otten TF, Vyshnevskiy BI. 1999. Genetic marking of polyresistant Mycobacterium tuberculosis strains isolated in the North-West of Russia. Probl Tuberk 1999:39–41. (In Russian.) [PubMed] [Google Scholar]
- 10.Mokrousov I. 2013. Insights into the origin, emergence, and current spread of a successful Russian clone of Mycobacterium tuberculosis. Clin Microbiol Rev 26:342–360. doi: 10.1128/CMR.00087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankar MM, Singh J, Diana SC, Singh S. 2013. Molecular characterization of Mycobacterium tuberculosis isolates from North Indian patients with extrapulmonary tuberculosis. Tuberculosis (Edinb) 93:75–83. doi: 10.1016/j.tube.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 12.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small P. 1993. Strain identification on Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van Soolingen D. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit–variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demay C, Liens B, Burguière T, Hill V, Couvin D, Millet J, Mokrousov I, Sola C, Zozio T, Rastogi N. 2012. SITVITWEB – a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol 12:755–766. doi: 10.1016/j.meegid.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Gibson AL, Huard RC, Gey van Pittius NC, Lazzarini LC, Driscoll J, Kurepina N, Zozio T, Sola C, Spindola SM, Kritski AL, Fitzgerald D, Kremer K, Mardassi H, Chitale P, Brinkworth J, Garcia de Viedma D, Gicquel B, Pape JW, van Soolingen D, Kreiswirth BN, Warren RM, van Helden PD, Rastogi N, Suffys PN, Lapa e Silva J, Ho JL. 2008. Application of sensitive and specific molecular methods to uncover global dissemination of the major RDRio sublineage of the Latin American-Mediterranean Mycobacterium tuberculosis spoligotype family. J Clin Microbiol 46:1259–1267. doi: 10.1128/JCM.02231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokrousov I, Vyazovaya A, Narvskaya O. 2014. Mycobacterium tuberculosis Latin American-Mediterranean family and its sublineages in the light of robust evolutionary markers. J Bacteriol 196:1833–1841. doi: 10.1128/JB.01485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokrousov I. 2008. Genetic geography of Mycobacterium tuberculosis Beijing genotype: a multifacet mirror of human history? Infect Genet Evol 8:777–785. doi: 10.1016/j.meegid.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Mokrousov I. 2012. Human migratory history: through the looking-glass of genetic geography of Mycobacterium tuberculosis, p 317–341. In Crawford MH, Campbell B (ed), Causes and consequences of human migration. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 20.Mokrousov I, Narvskaya O, Vyazovaya A, Otten T, Jiao WW, Gomes LL, Suffys PN, Shen AD, Vishnevsky B. 2012. Russian ‘successful’ clone B0/W148 of Mycobacterium tuberculosis Beijing genotype: multiplex PCR assay for rapid detection and global screening. J Clin Microbiol 50:3757–3759. doi: 10.1128/JCM.02001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gryadunov D, Mikhailovich V, Lapa S, Roudinskii N, Donnikov M, Pan'kov S, Markova O, Kuz'min A, Chernousova L, Skotnikova O, Moroz A, Zasedatelev A, Mirzabekov A. 2005. Evaluation of hybridisation on oligonucleotide microarrays for analysis of drug-resistant Mycobacterium tuberculosis. Clin Microbiol Infect 11:531–539. doi: 10.1111/j.1469-0691.2005.01183.x. [DOI] [PubMed] [Google Scholar]
- 22.Caoili JC, Mayorova A, Sikes D, Hickman L, Plikaytis BB, Shinnick TM. 2006. Evaluation of the TB-Biochip oligonucleotide microarray system for rapid detection of rifampin resistance in Mycobacterium tuberculosis. J Clin Microbiol 44:2378–2381. doi: 10.1128/JCM.00439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuerriegel S, Oberhauser B, George AG, Dafae F, Richter E, Rüsch-Gerdes S, Niemann S. 2012. Sequence analysis for detection of first-line drug resistance in Mycobacterium tuberculosis strains from a high-incidence setting. BMC Microbiol 12:90. doi: 10.1186/1471-2180-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). 2006. Extensively drug-resistant tuberculosis (XDR.TB): recommendations for prevention and control. Wkly Epidemiol Rec 81:430–432. [PubMed] [Google Scholar]
- 25.Varma-Basil M, Kumar S, Arora J, Angrup A, Zozio T, Banavaliker JN, Singh UB, Rastogi N, Bose M. 2011. Comparison of spoligotyping, mycobacterial interspersed repetitive units typing and IS6110-RFLP in a study of genotypic diversity of Mycobacterium tuberculosis in Delhi, North India. Mem Inst Oswaldo Cruz 106:524–535. doi: 10.1590/S0074-02762011000500002. [DOI] [PubMed] [Google Scholar]
- 26.Stavrum R, Myneedu VP, Arora VK, Ahmed N, Grewal HM. 2009. In-depth molecular characterization of Mycobacterium tuberculosis from New Delhi–predominance of drug resistant isolates of the ‘modern’ (TbD1) type. PLoS One 4:e4540. doi: 10.1371/journal.pone.0004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jou R, Chen HY, Chiang CY, Yu MC, Su IJ. 2005. Genetic diversity of multidrug-resistant Mycobacterium tuberculosis isolates and identification of 11 novel rpoB alleles in Taiwan. J Clin Microbiol 43:1390–1394. doi: 10.1128/JCM.43.3.1390-1394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mokrousov I. 2012. The quiet and controversial: Ural family of Mycobacterium tuberculosis. Infect Genet Evol 12:619–629. doi: 10.1016/j.meegid.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 29.López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133:30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thwaites G, Caws M, Chau TT, D'Sa A, Lan NT, Huyen MN, Gagneux S, Anh PT, Tho DQ, Torok E, Nhu NT, Duyen NT, Duy PM, Richenberg J, Simmons C, Hien TT, Farrar J. 2008. Relationship between Mycobacterium tuberculosis genotype and the clinical phenotype of pulmonary and meningeal tuberculosis. J Clin Microbiol 46:1363–1368. doi: 10.1128/JCM.02180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crubézy E, Legal L, Fabas G, Dabernat H, Ludes B. 2006. Pathogeny of archaic mycobacteria at the emergence of urban life in Egypt (3400 BC). Infect Genet Evol 6:13–21. doi: 10.1016/j.meegid.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Jaeger LH, Leles D, dos Santos Lima V, da Piedade da Silva L, Dias O, Iñiguez AM. 2012. Mycobacterium tuberculosis complex detection in human remains: tuberculosis spread since the 17th century in Rio de Janeiro, Brazil. Infect Genet Evol 12:642–648. doi: 10.1016/j.meegid.2011.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.