Abstract
The presence of Acinetobacter baumannii outside hospitals is still a controversial issue. The objective of our study was to explore the extrahospital epidemiology of A. baumannii in Lebanon. From February 2012 to October 2013, a total of 73 water samples, 51 soil samples, 37 raw cow milk samples, 50 cow meat samples, 7 raw cheese samples, and 379 animal samples were analyzed by cultural methods for the presence of A. baumannii. Species identification was performed by rpoB gene sequencing. Antibiotic susceptibility was investigated, and the A. baumannii population was studied by two genotyping approaches: multilocus sequence typing (MLST) and blaOXA-51 sequence-based typing (SBT). A. baumannii was detected in 6.9% of water samples, 2.7% of milk samples, 8.0% of meat samples, 14.3% of cheese samples, and 7.7% of animal samples. All isolates showed a susceptible phenotype against most of the antibiotics tested and lacked carbapenemase-encoding genes, except one that harbored a blaOXA-143 gene. MLST analysis revealed the presence of 36 sequence types (STs), among which 24 were novel STs reported for the first time in this study. blaOXA-51 SBT showed the presence of 34 variants, among which 21 were novel and all were isolated from animal origins. Finally, 30 isolates had new partial rpoB sequences and were considered putative new Acinetobacter species. In conclusion, animals can be a potential reservoir for A. baumannii and the dissemination of new emerging carbapenemases. The roles of the novel animal clones identified in community-acquired infections should be investigated.
INTRODUCTION
Acinetobacter baumannii is an opportunistic pathogen involved in a large number of hospital-acquired infections and associated with increased mortality and morbidity (1). One of the main reasons for the current increased interest in A. baumannii is its remarkable ability to acquire mechanisms of resistance to almost all available antimicrobial agents, including carbapenems (1–3). Genotyping approaches have attributed its global spread to a limited number of successful clones responsible for the majority of the worldwide nosocomial outbreaks (4–6). Among them, international clones 1 and 2 have been extensively disseminated in more than 30 countries (2).
Despite the fact that the hospital ecology of the bacterium has been intensively studied, its ecology outside hospitals remains unclear and is the subject of great debate (7–9). Difficulties regarding A. baumannii identification methods enhance this ambiguity (9). A. baumannii can cause severe community-acquired pneumonia occurring mainly during the warm and humid months in tropical and subtropical zones (10, 11). In addition, A. baumannii isolates have been recovered from wounds of survivors of natural disasters (12, 13), as well as from soldiers (14) and civilians (15) during warfare. Reports studying A. baumannii human carriage in the community are rare, and the prevalence has varied according to the countries and the identification methods used, from 0.5% to 3% in Europe (16–18), 4% in Hong Kong (19), and 5.4% in Senegal (20) to 10.4% in the United States (21). A. baumannii was also found in environments such as soil (22, 23) and water (24, 25) and in food products, such as vegetables (22, 26), fish, meat (22), and raw bulk tank milk (27). Finally, in animals, the bacterium has been described as an emerging pathogen in veterinary clinics in Germany (28) and Switzerland (29). It was also involved in asymptomatic carriage in some animals (20, 30, 31). Moreover, its presence was reported worldwide in human body and head lice (20, 32–34), as well as in arthropods (35).
Unlike clinical strains, there are limited reports using genotyping methods to explore the extrahospital epidemiology of A. baumannii (20–22, 30, 31, 33, 36, 37). In Lebanon, only strains belonging to a clinical context have been studied (15, 38–40), and no data concerning the occurrence of A. baumannii outside Lebanese hospitals are available. The aim of this study was to look for the presence of A. baumannii in different environments, to study its susceptibility to antibiotics, and to characterize the predominant community genotypes by using two genotyping approaches: multilocus sequence typing (MLST) and blaOXA-51 sequence-based typing (SBT).
MATERIALS AND METHODS
Sample collection and cultivation.
Sampling was performed from February 2012 to October 2013 from soil, animals, and food products in different regions of Lebanon (Fig. 1). The majority of samples were taken from two regions in North Lebanon: Tripoli, the second-largest city of the country after Beirut, and Akkar, which is a rural district.
FIG 1.
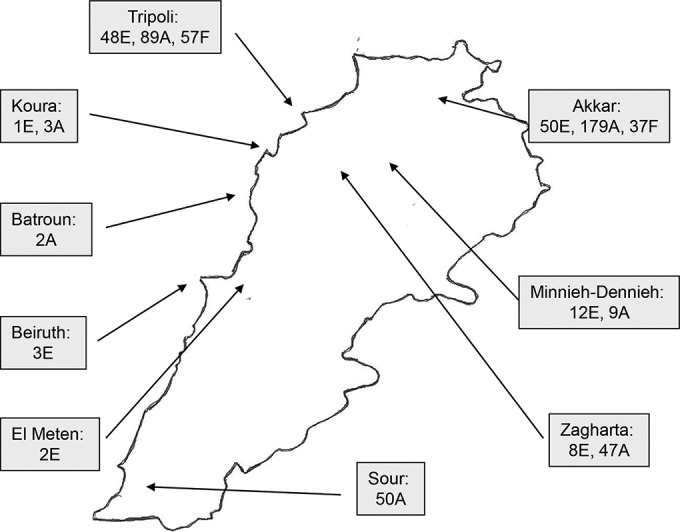
Map of Lebanon showing the distribution of epidemiological samples between districts. E, environmental samples (soil and water); A, animal samples; F, food samples (meat, milk, and cheese).
Fifty-one soil samples were collected in urban and agricultural zones. They were suspended in water at 10%, vortexed for 15 min to fully homogenize the suspension, and then decanted for 30 min. Fifty minced meat and seven raw cheese samples, purchased from butchers and shops, were cut aseptically into very small pieces and homogenized by using a stomacher bag (Interscience, Saint Nom, France) and then suspended at 10% in sterile water. Seventy-three water samples and 37 raw cow milk samples were also collected. Finally, 379 samples from different animals recovered from fecal specimens or from rectum and/or mouth swabbing were collected. Feces were collected directly after defecation or from the cow anus to limit contamination with soil or any other sources and suspended in water at 10%. Consent was given orally by the farmers.
For all the samples except the rectal swabs, 5 ml was added to 20 ml of Baumann medium (41), a minimum enrichment medium with acetate as the sole carbon source. The swabs were directly discharged in 20 ml of Baumann medium. Samples were then mixed on a rotor shaker at 200 rpm for 48 h at 37°C, and cultures were streaked on MacConkey agar with cephradine (40 mg/liter), amoxicillin (10 mg/liter), fosfomycin (30 mg/liter), and cycloheximide (400 g/liter) and incubated at 37°C for 48 h. In addition, 12 isolates identified as Acinetobacter calcoaceticus-Acinetobacter baumannii complex and stored in the collection of the AZM Center for Research in Biotechnology and Its Application were incorporated in this study. They were recovered from raw cheese and lettuce.
Bacterial identification.
Uncolored colonies grown on MacConkey agar were selected for further identification. Genus Acinetobacter identification was presumptively performed on the basis of Gram staining, a negative oxidase test, and a Vitek MS (bioMérieux, Marcy l'Étoile, France) test. Identification was further confirmed by partial sequencing of the rpoB gene (42).
Susceptibility testing and Investigation of carbapenemase-encoding genes.
Antibiotic susceptibility testing was performed by the disc diffusion method according to the guidelines of the French Comité de l'Antibiogramme de la Société Française de Microbiologie (http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFM_EUCAST_V1_0_2014(1).pdf). The antibiotics tested were ticarcillin, piperacillin plus tazobactam, ceftazidime, imipenem, ciprofloxacin, amikacin, gentamicin, tobramycin, co-trimoxazole, colistin, netilmicin, doxycycline, and rifampin. Resistance to carbapenem was confirmed by determining the MICs of imipenem, meropenem, and doripenem with Etest strips (bioMérieux, Marcy l'Étoile, France). All identified A. baumannii isolates were investigated by PCR assays for the presence of the carbapenemase-encoding genes blaOXA-23 (43), blaOXA-24 (43), blaOXA-58 (43), and blaOXA-143 (44) and the insertion sequence ISAba1 (45).
Genotyping.
Genotyping by MLST and blaOXA-51 SBT was performed on all identified A. baumannii isolates. MLST was done according to the Pasteur scheme (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html). The bacterial population was analyzed with eBURST (46) (on data available as of 8 July 2014), and when possible, isolates were assigned to their clonal complexes (CC), which are defined as the founder sequence type (ST) and its single-locus variants (SLV) (5). The full length of the blaOXA-51 gene (825 bp) was amplified and sequenced with the external primers OXA-69A/OXA-69B as described by Hamouda et al. (47). The sequences were compared to those of all variants present in BLAST. New variants were submitted to GenBank and the Lahey database for beta-lactamase classification (http://www.lahey.org/studies/webt.asp). Moreover, 24 new STs have been assigned by MLST Pasteur: ST286 to ST296 and ST464 to ST476.
Nucleotide sequence accession numbers.
Twenty-one nucleotide sequences of blaOXA-51 genes were submitted to GenBank with the following accession numbers: KF048909 to KF048919 and KJ584916 to KJ584925.
RESULTS
Bacterial identification.
A total of 597 samples were analyzed. The 12 Acinetobacter strains stored in the collection of the AZM Center were also incorporated. Overall, 161 Acinetobacter species isolates were isolated, and among them, 42 were identified as A. baumannii by rpoB gene sequencing. Table 1 shows the distribution of the identified Acinetobacter species according to the sources of the samples. Briefly, no A. baumannii isolate was identified in soil samples. Most of the isolates were isolated from animals. Moreover, 30 isolates had new partial rpoB sequences and were considered putative new Acinetobacter species.
TABLE 1.
Distribution of the identified Acinetobacter species according to the sources of samples
| Acinetobacter species isolated | Total no. of isolates | Environmental isolates |
Food isolates |
Animal isolates |
|||
|---|---|---|---|---|---|---|---|
| Source | No. of isolates found | Source | No. of isolates found | Source | No. of isolates found | ||
| A. baumannii | 42 | Water | 5 | Cheese | 2 | Cow | 17 |
| Meat | 4 | Cat | 2 | ||||
| Milk | 1 | Horse | 1 | ||||
| Goat | 3 | ||||||
| Dog | 3 | ||||||
| Rabbit | 1 | ||||||
| Donkey | 1 | ||||||
| Mule | 1 | ||||||
| Chicken | 1 | ||||||
| A. pittii | 61 | Water | 8 | Cheese | 6 | Cow | 14 |
| Soil | 7 | Lettuce | 4 | Horse | 4 | ||
| Meat | 6 | Goat | 2 | ||||
| Dog | 6 | ||||||
| Sheep | 2 | ||||||
| Rabbit | 1 | ||||||
| Chicken | 1 | ||||||
| A. calcoaceticus | 4 | Soil | 1 | Lettuce | 1 | Goat | 2 |
| A. bereziniae | 10 | Meat | 3 | Cow | 4 | ||
| Horse | 1 | ||||||
| Dog | 1 | ||||||
| Pigeon | 1 | ||||||
| A. johnsonii | 1 | Rabbit | 1 | ||||
| A. lwoffii | 1 | Cat | 1 | ||||
| A. schindleri | 3 | Cat | 3 | ||||
| A. radioresistens | 1 | Cat | 1 | ||||
| A. beijerinckii | 1 | Cow | 1 | ||||
| A. junii | 1 | Cat | 1 | ||||
| A. soli | 1 | Lettuce | 1 | ||||
| A. gerneri | 1 | Goat | 1 | ||||
| Gen. sp. 15 TUa | 4 | Cow | 2 | ||||
| Cat | 1 | ||||||
| Dog | 1 | ||||||
| Putative novel Acinetobacter species | 30 | Soil | 1 | Meat | 1 | Cow | 20 |
| Horse | 2 | ||||||
| Dog | 1 | ||||||
| Sheep | 3 | ||||||
| Goose | 1 | ||||||
| Pig | 1 | ||||||
| Total | 161 | 22 | 29 | 110 | |||
Gen. sp., genomic species.
Antimicrobial susceptibility testing results for A. baumannii.
The 42 A. baumannii isolates identified showed a susceptible phenotype in response to most of the antibiotics tested. Two isolates were intermediate to rifampin, and one isolate was resistant to ciprofloxacin and doxycycline. Only one isolate showed resistance to carbapenems. It was isolated from a horse's mouth and was susceptible to imipenem (MIC = 2 mg/liter), intermediate to meropenem (MIC = 4 mg/liter), and resistant to doripenem (MIC = 4 mg/liter). The blaOXA-143 gene was detected by PCR and confirmed by sequencing. The insertion sequence ISAba1 was present in only two isolates from cats.
As for the other Acinetobacter sp. isolates, most were wild type, and few isolates exhibited resistance to co-trimoxazole, ciprofloxacin, doxycycline, and rifampin. Only one showed carbapenem resistance. It was identified as Acinetobacter pittii and was isolated from a rabbit's mouth. It showed high MICs of imipenem (16 mg/liter), meropenem (>32 mg/liter), and doripenem (>32 mg/liter) and was an OXA-24-producing isolate.
MLST analysis.
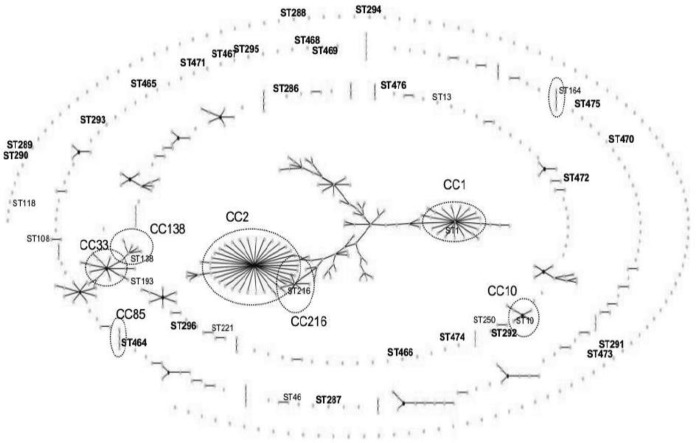
MLST was performed on all 42 identified A. baumannii isolates (Table 2). The isolates were grouped into 36 different STs, and among them, 30 were found in a single isolate. Twenty-four STs were new and reported for the first time in this study: 9 had new allelic combinations of previously known alleles (ST286 to ST288, ST293, ST464, and ST469 to ST472), and 15 had new alleles, leading to a new allelic profile (ST289 to ST292, ST294 to ST296, ST465 to ST468, and ST473 to ST476). The relationship between the STs in this study and the existing STs was studied by eBURST (Fig. 2). Some detected STs belonged to clonal complexes, such as ST1 (the founder of CC1), ST216 (the founder of CC216), ST10 (the founder of CC10), ST138 and ST193 (belonging to CC33), and ST464 (belonging to CC85). Other STs shared similarities with known STs: ST46 with ST149, ST108 with ST112, ST221 with ST133, ST250 with ST188, and ST472 with ST439. The remaining identified STs (ST13, ST286 to ST296, ST465 to ST471, and ST473 to ST476) were singletons, and no ST similar to them has been characterized yet.
TABLE 2.
Characteristics of the 42 A. baumannii isolates identified in this study
| No. of isolates | Sample | Origin | City | Sequence typea | OXA protein variantb |
|---|---|---|---|---|---|
| 1 | Water | Artesian well | Koura | 1 | OXA-69 |
| 1 | Feces | Cow | Akkar | 10 | OXA-68 |
| 1 | Meat | Cow | Tripoli | 13 | OXA-346 (KF048919) |
| 2 | Feces, water used by animals | Cow (feces) | Akkar | 46 | OXA-104 |
| 2 | Water | Sources | Akkar | 108 | OXA-132 |
| 1 | Feces | Cow | Sour | 118 | OXA-338 (KJ584925) |
| 1 | Rectum | Cat | Tripoli | 138 | OXA-64 |
| 1 | Mouth | Dog | Tripoli | 164 | OXA-91 |
| 1 | Milk | Cow | Akkar | 193 | OXA-120 |
| 1 | Cheese | Tripoli | 216 | OXA-51 | |
| 1 | Mouth | Rabbit | Tripoli | 221 | JX865392.1 |
| 1 | Mouth | Goat | Tripoli | 250 | OXA-407 (KJ584916) |
| 2 | Meat and mouth | Cow | Tripoli | 286 | OXA-338 (KF048909) |
| 1 | Meat | Cow | Tripoli | 287 | OXA-106 (KF048910) |
| 1 | Feces | Cow | Akkar | 288 | OXA-339 (KF048911) |
| 1 | Feces | Cow | Akkar | 289 | OXA-337 |
| 1 | Feces | Cow | Akkar | 290 | OXA-340 (KF048912) |
| 1 | Feces | Cow | Akkar | 291 | OXA-341 (KF048913) |
| 2 | Feces | Cow | Akkar | 292 | OXA-69 (KF048914) |
| 1 | Feces | Cow | Akkar | 293 | OXA-342 (KF048915) |
| 2 | Water, mouth | Artesian well (water) horse (mouth) | Zgharta, Tripoli | 294 | OXA-343 (KF048916) |
| 1 | Cheese | Tripoli | 295 | OXA-344 (KF048917) | |
| 1 | Meat | Cow | Tripoli | 296 | OXA-345 (KF048918) |
| 1 | Rectum | Chicken | Tripoli | 464 | OXA-94 |
| 1 | Rectum | Dog | Zgharta | 465 | OXA-408 (KJ584917) |
| 1 | Rectum | Cow | Zgharta | 466 | OXA-409 (KJ584918) |
| 1 | Rectum | Dog | Zgharta | 467 | OXA-410 (KJ584919) |
| 1 | Rectum | Cat | Tripoli | 468 | OXA-344 (KF048917) |
| 2 | Mouth | Cow and goat | Tripoli, El Denieh | 469 | OXA-71 |
| 1 | Mouth | Cow | Tripoli | 470 | OXA-51 |
| 1 | Mouth | Cow | Tripoli | 471 | OXA-411 (KJ584920) |
| 1 | Feces | Mule | Akkar | 472 | OXA67 |
| 1 | Feces | Cow | Akkar | 473 | OXA-412 (KJ584921) |
| 1 | Feces | Cow | Akkar | 474 | OXA-413 (KJ584922) |
| 1 | Feces | Donkey | Akkar | 475 | OXA-71 (KJ584923) |
| 1 | Feces | Goat | Akkar | 476 | OXA-65 (KJ584924) |
The novel STs found in this study are in boldface.
OXA-51 protein variants found for the first time in this study. GenBank accession numbers are provided in parentheses for new blaOXA-51 gene variants described for the first time in this study.
FIG 2.
Population snapshot determined by eBURST analysis of 587 sequences present in the MLST Pasteur database (last update, 8 July 2014). The dots represent STs. The STs identified in this study are shown next to their corresponding dots. Boldface indicates a new ST described in this study. The large circles indicate that our identified ST belonged to a clonal complex, whose name is shown next to the circle.
blaOXA-51 SBT analysis.
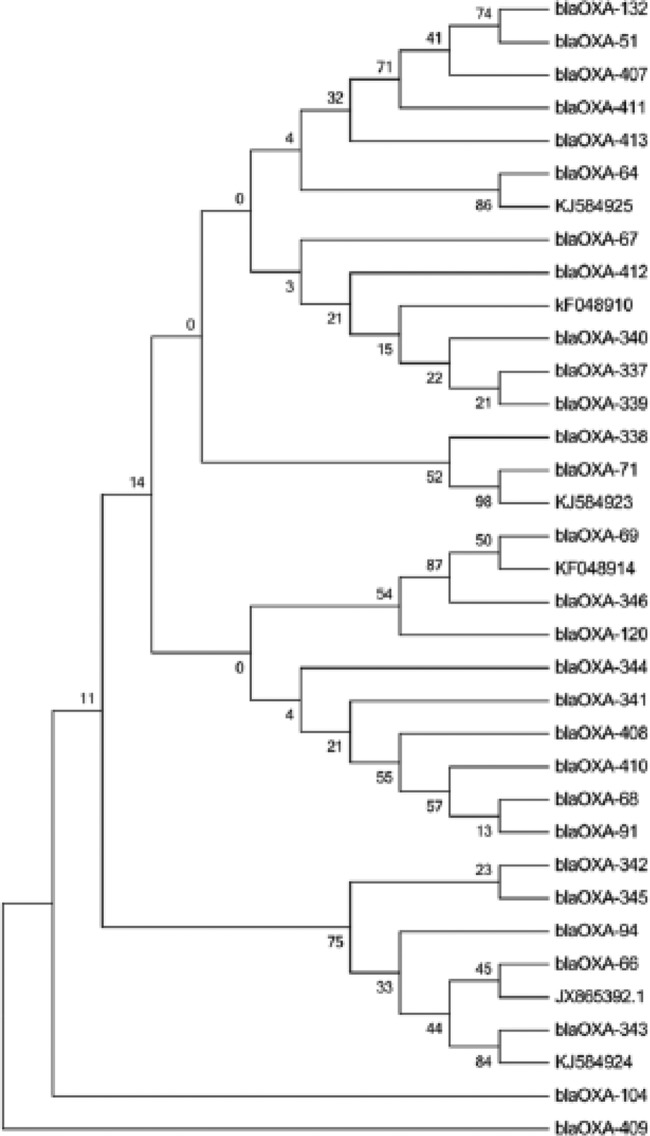
The full-length sequence analysis of the blaOXA-51 genes of the 42 A. baumannii isolates revealed the presence of 34 gene variants, and among them, 26 were singletons and 21 had not been described before (GenBank accession numbers KF048909 to KF048919 and KJ584916 to KJ584925; Lahey numbers OXA-338 to OXA-346 and OXA-407 to OXA-413) (Table 2 and Fig. 3). These 34 blaOXA-51 gene variants coded for 31 OXA protein variants, with 16 being new ones (Fig. 3). Figure 4 shows an alignment of the full amino acid sequences of these new enzymes. It should be noted that the OXA-410 protein (KJ584919) had an adenine insertion at bp 820, leading to the modification of the last amino acid (L274Y) and the addition of 4 supplementary amino acids. The OXA-409 protein (KJ584918) had an insertion of a cysteine at amino acid position 19. The new enzyme OXA-338 is encoded by two different nucleotide sequences (GenBank accession numbers KF048909 and KJ584925). Finally, the KJ584925 gene sequence differed from that of KF048909 by 6 synonymous mutations.
FIG 3.
Maximum-likelihood nucleotide tree of 35 blaOXA-51-like genes. These 35 genes correspond to 34 blaOXA-51 variants identified in this study and the blaOXA-66 gene (the blaOXA-51 representative of clonal complex 2). MEGA 6 was used to build the phylogenetic tree. Bootstrap values are shown at the nodes. One thousand replicates were used to calculate the bootstrap values.
FIG 4.
Amino acid sequence alignment of the 16 new OXA-51-like proteins detected in this study and of OXA-51 (the founding member of OXA-51-like beta-lactamases), OXA-66 (an OXA-51 representative variant of CC2), OXA-69 (an OXA-51 representative variant of CC1), and OXA-71 (an OXA-51 representative variant of CC3) (52). Protein accession numbers: OXA-51, WP_002033109.1; OXA-66, YP_001846219.1; OXA-69, YP_001713983.1; and OXA-71, WP_001021785.1.
Comparison between MLST and blaOXA-51 typing.
Most of the STs described in this study had a specific blaOXA-51-like gene variant (Table 2), with the exception of ST216 and ST470, both of which had the blaOXA-51 variant, and ST295 and ST468, both of which had the blaOXA-344 variant. Overall, each ST led to a specific OXA protein variant, except ST469 and ST475, both of which had the OXA-71 protein variant; ST1 and ST292, which had the OXA-69 protein variant; and ST118 and ST286, which had the OXA-338 protein variant (Table 2 and Fig. 3).
DISCUSSION
Although the ubiquitous existence of A. baumannii in nature has been considered a common misconception by some authors (7), several recent studies have undeniably highlighted the presence of extrahospital reservoirs (9, 22, 30, 31, 33). These observations have mainly been made through recent implementation of molecular methods, such as blaOXA-51-like PCR or rpoB gene sequencing, improving detection and identification of A. baumannii and other species of the genus Acinetobacter (42, 48, 49). In our study, we evidenced the extrahospital presence of A. baumannii in Lebanon. We showed that 8% of the animals studied carried A. baumannii in their flora. These animals lived on farms or were wild animals and had never been in contact with a hospital environment, such as veterinary clinics. A. baumannii has previously been documented as an animal colonizer with different prevalences in different countries: in Senegal, 5.1% (20); in Scotland, 1.2% (30); and on La Reunion Island, 6.5% (31). We have also found the bacterium in food produced from animals, such as cow meat, raw cheese, and raw milk, reinforcing the idea that animals could be a potential reservoir of A. baumannii. Additionally, we have isolated the bacterium from water samples, while all the soil samples tested were negative. Two hypotheses can arise from the detection of A. baumannii in water: that water is a normal habitat of A. baumannii or that the presence of the bacterium results from human or animal contamination. We cannot exclude either of these hypotheses, but the detection of a novel ST (ST294) in a horse's mouth, as well as in an artesian well, could support the second hypothesis.
We have studied the A. baumannii population structure by MLST typing, which is considered a gold standard and is intensively applied in the characterization of genotypes circulating in hospitals. The current MLST-based global population structure is formed by 26 clones divided into 18 international clones and 8 European- or Asian-restricted clones (2). However, it is evident that there are no sufficient data regarding genotypes of isolates isolated outside hospitals. Improving the MLST database with extrahospital A. baumannii genotypes could be important, since it may improve our understanding of the potential reservoirs, the origins of human infections, and the acquisition of resistance mechanisms in the species. In our study, we identified 36 STs of A. baumannii; some were identical to those isolated in human infections, and 24 were new genotypes never reported previously. They were all found in animals or in animal-derived food and showed huge diversity in population structures. This diversity was not in accordance with the findings of Hamouda et al. (30), who found only 4 different genotypes in the isolates recovered from cattle and pigs in their study, and the results of Belmonte et al. (31), who revealed the presence of a single pulsed-field gel electrophoresis (PFGE) genotype/ST25 in pets recovered from geographically distant veterinary clinics on La Reunion Island.
The blaOXA-51 gene is an intrinsic carbapenemase gene specific to A. baumannii and is regarded as a tool for A. baumannii identification (50). Analysis of our 42 A. baumannii isolates allowed the identification of 34 blaOXA-51 gene variants. Among them, 21 were new. These 21 sequences were all associated with animal origins, showing a potentially huge diversity in the A. baumannii population. These observations have previously been made by Hamouda et al., who reported three new blaOXA-148, blaOXA-149, and blaOXA-150 variants in cattle in Scotland (30). In addition, our results illustrate the usefulness of blaOXA-51 as a single-locus-based typing method (51). We observed good correlation between MLST and blaOXA-51 typing, since each blaOXA-51 like gene variant had its specific ST, with two exceptions: ST216 and ST470, which had the same blaOXA-51 gene, and ST295 and ST468, which had the same blaOXA-344 gene. Our blaOXA-51 gene-sequencing results were concordant with the worldwide MLST results reported in other studies, such as blaOXA-69 usually being associated with ST1 (51), blaOXA-120 with ST193 (52), blaOXA-68 with ST10 (3, 53), and blaOXA-94 with ST85 (15). Finally, some blaOXA-51 variants found in our new STs have previously been described in human STs. Thus, blaOXA-71, detected in our ST469 A. baumannii isolate, has been described in the international clone 3 (51). Similarly, blaOXA-64, previously reported in ST25 (51), has been found in ST138. It is interesting that ST138 is a trilocus variant of ST25.
Analysis of the antibiotic susceptibility results showed that a susceptible A. baumannii population prevailed outside hospitals in Lebanon. The majority of the isolates (40/42) lacked ISAba1, an insertion sequence that is considered the first step in resistance evolution in A. baumannii (54). However, we identified a blaOXA-143 gene in an A. baumannii isolate from a horse, as well as a blaOXA-24 gene in an A. pittii isolate from a rabbit. These results highlight the potential presence of reservoirs of resistance genes in the environment. The blaOXA-143 gene has recently been detected and reported only in Brazil and South Korea (50). Until now, most of the A. baumannii populations detected outside hospitals were susceptible to antibiotics (21, 27, 30–32). However, there have been growing concerns after the description of a blaOXA-23 gene from human stool and lice in Senegal (20); NDM-1-producing A. baumannii tk;4from a pig in China (55); and other carbapenemase-producing Acinetobacter spp. from pets, food, and their environments (56).
One other important finding in our study is the identification of 30 isolates with low levels of nucleotide homology with all available described Acinetobacter species, which are assumed to be putative novel species. These observations show the species diversity of environmental isolates within the genus Acinetobacter. Several publications have described new isolates (22, 36, 57), which indicates that our knowledge of the genus Acinetobacter is still evolving.
In conclusion, our paper reports the occurrence of A. baumannii isolates outside Lebanese hospitals and is one of a limited number of worldwide studies exploring the population in the environment, food, and animals. Detection of successful human genotypes, such as international clones 1 and 10, in water and animals is a worrying issue for public health. Furthermore, the roles of newly identified animal clones and their involvement in human diseases, especially in community-acquired infections, should be investigated. Our findings suggest that animals could be a potential reservoir for A. baumannii and the spread of new, emerging carbapenemase genes, such as blaOXA-143, to humans. Additional large epidemiological studies are required to confirm the significance of our primary results and to determine the real distribution of these clones in Lebanon and the possible interactions between the different environments.
ACKNOWLEDGMENTS
We thank the team of curators of the Institut Pasteur MLST databases for curating the data and making them publicly available at http://bigsdb.web.pasteur.fr/. We also thank Taha Abdo, Mariam Yehya, Farah Obeid Charrouf, Imane Darwich, Husam Khaled, and Catherine Quinqueneau for their excellent technical assistance. We are very grateful to our colleagues Dima Safadi and Marwan Osman and the veterinary doctors Rabah Outour, Rami El Rifi, and Kamal Mesto for their assistance in sampling.
We have no conflicts of interest.
This work was supported by the AZM Research Center for Biotechnology and its Application, Lebanese University, Tripoli, Lebanon.
REFERENCES
- 1.Kempf M, Rolain J-M. 2012. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents 39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Karah N, Sundsfjord A, Towner K, Samuelsen Ø. 2012. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Updat 15:237–247. doi: 10.1016/j.drup.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Dijkshoorn L, Aucken H, Gerner-Smidt P, Janssen P, Kaufmann M, Garaizar J, Ursing J, Pitt T. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol 34:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins Dammhayn C, Hackel M, Seifert H. 2010. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 7.Towner KJ. 2009. Acinetobacter: an old friend, but a new enemy. J Hosp Infect 73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 9.Eveillard M, Kempf M, Belmonte O, Pailhoriès H, Joly-Guillou M-L. 2013. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis 17:e802–e805. doi: 10.1016/j.ijid.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. 2007. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis 26:857–868. doi: 10.1007/s10096-007-0365-6. [DOI] [PubMed] [Google Scholar]
- 11.Ong CWM, Lye DCB, Khoo KL, Chua GSW, Yeoh SF, Leo YS, Tambyah PA, Chua AC. 2009. Severe community-acquired Acinetobacter baumannii pneumonia: an emerging highly lethal infectious disease in the Asia-Pacific. Respirology 14:1200–1205. doi: 10.1111/j.1440-1843.2009.01630.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Hao P, Lu B, Yu H, Huang W, Hou H, Dai K. 2010. Causes of infection after earthquake, China, 2008. Emerg Infect Dis 16:974–975. doi: 10.3201/eid1606.091523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uçkay I, Sax H, Harbarth S, Bernard L, Pittet D. 2008. Multi-resistant infections in repatriated patients after natural disasters: lessons learned from the 2004 tsunami for hospital infection control. J Hosp Infect 68:1–8. doi: 10.1016/j.jhin.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard FR, Keiser P, Craft DW, Gage F, Robson M, Brown TS, Petersen K, Sincock S, Kasper M, Hawksworth J, Tadaki D, Davis TA, Stojadinovic A, Elster E. 2010. The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility. Am J Surg 200:489–495. doi: 10.1016/j.amjsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Rafei R, Dabboussi F, Hamze M, Eveillard M, Lemarié C, Mallat H, Rolain J-M, Joly-Guillou M-L, Kempf M. 2014. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int J Infect Dis 21:21–23. doi: 10.1016/j.ijid.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol 35:2819–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berlau J, Aucken H, Malnick H, Pitt T. 1999. Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis 18:179–183. doi: 10.1007/s100960050254. [DOI] [PubMed] [Google Scholar]
- 18.Dijkshoorn L, van Aken E, Shunburne L, van der Reijden TJK, Bernards AT, Nemec A, Towner KJ. 2005. Prevalence of Acinetobacter baumannii and other Acinetobacter spp. in faecal samples from non-hospitalised individuals. Clin Microbiol Infect 11:329–332. doi: 10.1111/j.1469-0691.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- 19.Chu YW, Leung CM, Houang ET, Ng KC, Leung CB, Leung HY, Cheng AF. 1999. Skin carriage of acinetobacters in Hong Kong. J Clin Microbiol 37:2962–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempf M, Rolain J-M, Diatta G, Azza S, Samb B, Mediannikov O, Gassama Sow A, Diene SM, Fenollar F, Raoult D. 2012. Carbapenem resistance and Acinetobacter baumannii in Senegal: the paradigm of a common phenomenon in natural reservoirs. PLoS One 7:e39495. doi: 10.1371/journal.pone.0039495. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zeana C, Larson E, Sahni J, Bayuga SJ, Wu F, Della-Latta P. 2003. The epidemiology of multidrug-resistant Acinetobacter baumannii: does the community represent a reservoir? Infect Control Hosp Epidemiol 24:275–279. doi: 10.1086/502209. [DOI] [PubMed] [Google Scholar]
- 22.Houang ET, Chu YW, Leung CM, Chu KY, Berlau J, Ng KC, Cheng AF. 2001. Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. J Clin Microbiol 39:228–234. doi: 10.1128/JCM.39.1.228-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vangnai AS, Petchkroh W. 2007. Biodegradation of 4-chloroaniline by bacteria enriched from soil. FEMS Microbiol Lett 268:209–216. doi: 10.1111/j.1574-6968.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 24.Guardabassi L, Dalsgaard A, Olsen JE. 1999. Phenotypic characterization and antibiotic resistance of Acinetobacter spp. isolated from aquatic sources. J Appl Microbiol 87:659–667. doi: 10.1046/j.1365-2672.1999.00905.x. [DOI] [PubMed] [Google Scholar]
- 25.Huys G, Bartie K, Cnockaert M, Hoang Oanh DT, Phuong NT, Somsiri T, Chinabut S, Yusoff FM, Shariff M, Giacomini M, Teale A, Swings J. 2007. Biodiversity of chloramphenicol-resistant mesophilic heterotrophs from Southeast Asian aquaculture environments. Res Microbiol 158:228–235. doi: 10.1016/j.resmic.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Berlau J, Aucken H, Houang E, Pitt TL. 1999. Isolation of Acinetobacter spp including A. baumannii from vegetables: implications for hospital-acquired infections. J Hosp Infect 42:201–204. doi: 10.1053/jhin.1999.0602. [DOI] [PubMed] [Google Scholar]
- 27.Gurung M, Nam HM, Tamang MD, Chae MH, Jang GC, Jung SC, Lim SK. 2013. Prevalence and antimicrobial susceptibility of Acinetobacter from raw bulk tank milk in Korea. J Dairy Sci 96:1997–2002. doi: 10.3168/jds.2012-5965. [DOI] [PubMed] [Google Scholar]
- 28.Zordan S, Prenger-Berninghoff E, Weiss R, van der Reijden T, van den Broek P, Baljer G, Dijkshoorn L. 2011. Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg Infect Dis 17:1751–1754. doi: 10.3201/eid1709.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endimiani A, Hujer KM, Hujer AM, Bertschy I, Rossano A, Koch C, Gerber V, Francey T, Bonomo RA, Perreten V. 2011. Acinetobacter baumannii isolates from pets and horses in Switzerland: molecular characterization and clinical data. J Antimicrob Chemother 66:2248–2254. doi: 10.1093/jac/dkr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamouda A, Findlay J, Al Hassan L, Amyes SGB. 2011. Epidemiology of Acinetobacter baumannii of animal origin. Int J Antimicrob Agents 38:314–318. doi: 10.1016/j.ijantimicag.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Belmonte O, Pailhoriès H, Kempf M, Gaultier MP, Lemarié C, Ramont C, Joly-Guillou ML, Eveillard M. 2014. High prevalence of closely-related Acinetobacter baumannii in pets according to a multicentre study in veterinary clinics, Reunion Island. Vet Microbiol 170:446–450. doi: 10.1016/j.vetmic.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 32.La Scola B, Raoult D. 2004. Acinetobacter baumannii in human body louse. Emerg Infect Dis 10:1671–1673. doi: 10.3201/eid1009.040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempf M, Abdissa A, Diatta G, Trape J-F, Angelakis E, Mediannikov O, La Scola B, Raoult D. 2012. Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int J Infect Dis 16:e680–e683. doi: 10.1016/j.ijid.2012.05.1024. [DOI] [PubMed] [Google Scholar]
- 34.Bouvresse S, Socolovshi C, Berdjane Z, Durand R, Izri A, Raoult D, Chosidow O, Brouqui P. 2011. No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp Immunol Microbiol Infect Dis 34:475–477. doi: 10.1016/j.cimid.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Gouveia C, Asensi MD, Zahner V, Rangel EF, Oliveira SM. 2008. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera: Psychodidae). Neotrop Entomol 37:597–601. doi: 10.1590/S1519-566X2008000500016. [DOI] [PubMed] [Google Scholar]
- 36.Choi J-Y, Kim Y, Ko EA, Park YK, Jheong W-H, Ko G, Ko KS. 2012. Acinetobacter species isolates from a range of environments: species survey and observations of antimicrobial resistance. Diagn Microbiol Infect Dis 74:177–180. doi: 10.1016/j.diagmicrobio.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Rose M, Landman D, Quale J. 2014. Are community environmental surfaces near hospitals reservoirs for gram-negative nosocomial pathogens? Am J Infect Control 42:346–348. doi: 10.1016/j.ajic.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, Khan AU, Afif C, Triassi M. 2008. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob Agents Chemother 52:4115–4120. doi: 10.1128/AAC.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. 2011. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin Microbiol Infect 17:197–201. doi: 10.1111/j.1469-0691.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 40.Giannouli M, Tomasone F, Agodi A, Vahaboglu H, Daoud Z, Triassi M, Tsakris A, Zarrilli R. 2009. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii strains in intensive care units of multiple Mediterranean hospitals. J Antimicrob Chemother 63:828–830. doi: 10.1093/jac/dkp032. [DOI] [PubMed] [Google Scholar]
- 41.Baumann P. 1968. Isolation of Acinetobacter from soil and water. J Bacteriol 96:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gundi VAKB, Dijkshoorn L, Burignat S, Raoult D, La Scola B. 2009. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology 155:2333–2341. doi: 10.1099/mic.0.026054-0. [DOI] [PubMed] [Google Scholar]
- 43.Mesli E, Berrazeg M, Drissi M, Bekkhoucha SN, Rolain JM. 2013. Prevalence of carbapenemase-encoding genes including New Delhi metallo-β-lactamase in Acinetobacter species, Algeria. Int J Infect Dis 17:e739–e743. doi: 10.1016/j.ijid.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Higgins PG, Lehmann M, Seifert H. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 35:305–314. doi: 10.1016/j.ijantimicag.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz M, Marti S, Fernandez-Cuenca F, Pascual A, Vila J. 2007. Prevalence of IS(Aba1) in epidemiologically unrelated Acinetobacter baumannii clinical isolates. Clin Microbiol Infect 13:1192–1198. doi: 10.1111/j.1469-0691.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- 46.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamouda A, Evans BA, Towner KJ, Amyes SGB. 2010. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of blaOXA-51-like genes. J Clin Microbiol 48:2476–2483. doi: 10.1128/JCM.02431-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Scola B, Gundi VA, Khamis A, Raoult D. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol 44:827–832. doi: 10.1128/JCM.44.3.827-832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol 44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans BA, Amyes SGB. 2014. OXA β-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pournaras S, Gogou V, Giannouli M, Dimitroulia E, Dafopoulou K, Tsakris A, Zarrilli R. 2014. Single locus sequence-based typing of blaOXA-51-like gene for rapid classification of Acinetobacter baumannii clinical isolates to international clones. J Clin Microbiol 52:1653–1657. doi: 10.1128/JCM.03565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izdebski R, Fiett J, Hryniewicz W, Gniadkowski M. 2012. Molecular analysis of Acinetobacter baumannii isolates from invasive infections in 2009 in Poland. J Clin Microbiol 50:3813–3815. doi: 10.1128/JCM.02271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zander E, Nemec A, Seifert H, Higgins PG. 2012. Association between β-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J Clin Microbiol 50:1900–1904. doi: 10.1128/JCM.06462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W-J, Lu Z, Schwarz S, Zhang R-M, Wang X-M, Si W, Yu S, Chen L, Liu S. 2013. Complete sequence of the bla(NDM-1)-carrying plasmid pNDM-AB from Acinetobacter baumannii of food animal origin. J Antimicrob Chemother 68:1681–1682. doi: 10.1093/jac/dkt066. [DOI] [PubMed] [Google Scholar]
- 56.Guerra B, Fischer J, Helmuth R. 2014. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol 171:290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Kumsa B, Socolovschi C, Parola P, Rolain J-M, Raoult D. 2012. Molecular detection of Acinetobacter species in lice and keds of domestic animals in Oromia Regional State, Ethiopia. PLoS One 7:e52377. doi: 10.1371/journal.pone.0052377. [DOI] [PMC free article] [PubMed] [Google Scholar]