Background: The mechanisms whereby α5β1 integrin triggers osteogenesis are poorly understood.
Results: CRRETAWAC-mediated α5β1 integrin priming promotes osteoblast differentiation via PI3K/Wnt/β-catenin signaling independently of the RGD-like REET sequence. Systemic delivery of the α5β1 integrin-priming peptide improved long bone microarchitecture in senescent osteopenic mice.
Conclusion: Wnt signaling mediates osteoblast differentiation induced by peptide-mediated α5β1 integrin priming.
Significance: A novel mechanism underlying α5β1 integrin-mediated osteoblast differentiation is provided.
Keywords: Bone, Cell Differentiation, Integrin, Osteoblast, Wnt Signaling, CRRETAWAC, Osteopenic Mice
Abstract
The α5β1 integrin is a key fibronectin (FN) receptor that binds to RGD-containing peptides to mediate cell adhesion. We previously reported that α5β1 integrin promotes osteogenic differentiation in mesenchymal skeletal cells (MSCs), but the underlying mechanisms are not fully understood. In this study, we determined the signaling mechanisms induced by α5β1 integrin interaction with its high-affinity ligand CRRETAWAC in murine and human MSCs and in vivo. We show that cyclized CRRETAWAC fully displaced MSC adhesion to FN, whereas related peptides lacking the full RRET sequence produced a partial displacement, indicating that RRET acts as an RGD-like sequence that is required to antagonize FN-mediated cell adhesion. However, all peptides increased focal adhesion kinase phosphorylation, OSE2 transcriptional activity, osteoblast gene expression, and matrix mineralization in MSCs, indicating that peptide-induced α5β1 integrin priming can promote osteogenic differentiation independently of the RRET sequence. Biochemical analyses showed that peptide-induced α5β1 integrin priming transiently increased PI3K/Akt phosphorylation and promoted Wnt/β-catenin transcriptional activity independently of RRET. Consistently, pharmacological inhibition of PI3K activity reduced osteoblast differentiation and abolished Wnt regulatory gene expression induced by α5β1 integrin priming. In vivo, systemic delivery of cyclized GACRETAWACGA linked to (DSS)6 to allow delivery to bone-forming sites for 6 weeks increased serum osteocalcin levels and improved long bone mass and microarchitecture in SAMP-6 senescent osteopenic mice. The results support a mechanism whereby α5β1 integrin priming by high-affinity ligands integrates Wnt/β-catenin signaling to promote osteoblast differentiation independently of cell adhesion, which could be used to improve bone mass and microarchitecture in the aging skeleton.
Introduction
The maintenance of bone mass in adults involves the balance between bone resorption by osteoclasts and bone formation by osteoblasts (1). With aging, bone formation declines due to decreased osteoblast recruitment, function, and life span (2, 3). One important issue to prevent age-related bone loss is to identify tools to promote osteogenesis (4, 5). Bone-forming cells derive from mesenchymal skeletal cells (MSCs)2 that differentiate into osteoblasts under the control of multiple mechanisms (6). Osteoblast differentiation is characterized by expression of the osteoblast transcription factor RUNX2 and downstream osteoblast markers, such as alkaline phosphatase, and is typified by type I collagen (Col1A1), extracellular matrix (ECM) synthesis, and mineralization (7). Cell/ECM interactions involve integrins, a family of transmembrane αβ-heterodimer adhesion molecules (8, 9) that convey signals to and from the cytosol across the plasma membrane (10, 11). Integrin binding to the ECM leads to the recruitment and phosphorylation of focal adhesion kinase (FAK) and activation of several kinases (12–14). In bone, the ECM plays an important role in osteoblast function (15–17). Specifically, ECM/integrin interaction leads to activation of the MAPKs ERK1 and ERK2, resulting in increased RUNX2 phosphorylation and expression of osteoblast-specific genes (18, 19). Although osteoblasts express several integrins in vitro, few of them have been shown to play an essential role in bone formation in vivo (20). The α5β1 integrin, a cell-surface receptor for fibronectin (FN), controls osteoblast adhesion (21) and survival (15, 22, 23) and plays a critical role in MSC osteogenic differentiation and bone formation and repair (24–26). However, the molecular mechanisms whereby α5β1 integrin promotes osteogenic differentiation are not fully understood. The α5β1 integrin recognizes and binds RGD, a sequence that mediates cell adhesion on FN (27). A peptide ligand with high affinity for α5β1 integrin (CRRETAWAC) was found to act as a direct competitive inhibitor of RGD binding to α5β1 integrin (28–30). We previously showed that priming α5β1 integrin with CRRETAWAC is able to trigger MSC osteogenic differentiation in vitro and to promote osteogenesis in vivo (24, 26). The peptide is known to fit in the binding site of α5β1 integrin and is believed to interact with an overlapping binding site for RGD in the α5 integrin subunit propeller domain (29–31). However, how the peptide primes α5β1 integrin is unknown. In particular, whether the RRET sequence, which plays a similar role in cell adhesion as RGD (29), is required for α5β1 integrin-mediated osteogenic differentiation has not been determined.
The signaling pathways involved in α5β1 integrin-mediated osteogenesis are not fully understood. We previously reported that MSC osteoblast differentiation mediated by α5β1 integrin in part involves activation of FAK/ERK1/ERK2, PI3K (24), and IGF2/IGFBP2 (32) signaling, suggesting that several pathways may mediate the effect of the integrin. In bone, Wnt signaling is an important pathway that regulates osteoblastogenesis (33, 34). Binding of canonical Wnt proteins to Frizzled receptors and LDL5 and LDL6 co-receptors leads to the inhibition of glycogen synthase kinase-3β (GSK-3β), decreased degradation of β-catenin, and its translocation into the nucleus, where it interacts with the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factor to activate the expression of Wnt target genes (35). In osteoblast precursor cells, activation of Wnt/β-catenin signaling increases the expression of phenotypic osteoblast genes (36). Several mechanisms may link integrins to Wnt signaling. Notably, ligand binding to integrins may activate integrin-linked kinase (37), resulting in GSK-3β inactivation (38) and β-catenin/LEF transcriptional activity (39, 40). Consistently, integrin-linked kinase knockdown was found to reduce β-catenin/TCF/LEF-dependent transcription in osteoblastic cells (41, 42). In addition, integrin-mediated FAK activation may lead to PI3K/Akt activation and subsequent GSK-3β inhibition (43, 44). Whether α5β1 integrin activation can be linked to Wnt/β-catenin signaling to control osteoblastogenesis has not been determined.
In this study, we report a novel mechanism by which priming of α5β1 integrin in MSCs promotes osteoblast differentiation through activation of PI3K/Akt and Wnt/β-catenin signaling, a model that can translate into improved bone microarchitecture in senescent osteopenic mice.
EXPERIMENTAL PROCEDURES
Cells
Murine pluripotent mesenchymal C3H10T1/2 cells were obtained from American Type Culture Collection (Manassas, VA). Human primary MSCs derived from the bone marrow stroma were purchased from PromoCell (Heidelberg, Germany). Cells were cultured in DMEM (Invitrogen) supplemented with 10% heat-inactivated FCS, 1% l-glutamine, and penicillin/streptomycin. Wnt3a-conditioned medium (CM) was prepared as described previously (45).
Oligopeptides
CRRETAWAC interacts specifically with α5β1 integrin with high affinity and is a potent inhibitor of α5β1 integrin-mediated cell attachment to FN (29, 30). To determine the role of the RRET sequence, we used the related peptides CRRTAWAC and CRETAWAC, which lack the full RRET sequence. All peptides were synthesized and flanked by GA residues next to cysteines (GACRRETAWACGA, GACRRTAWACGA, and GACRETAWACGA) to allow for cyclization and stability (26). For the in vivo study, a selected cyclized peptide (GACRETAWACGA) was linked to six repetitive sequences of aspartate, serine, and serine ((DSS)6) to allow specific delivery to bone-forming sites (46). The corresponding cyclic peptide ((DSS)6-GACRETAWACGA) and the inactive control peptide ((DSS)6)-GREGSP) used as control (24) were synthesized on a CEM Liberty1 peptide synthesizer using standard automated continuous-flow solid-phase peptide synthesis methods. The completed peptides were cleaved from the resin and side chain-deprotected by treatment with the scavengers water/triisopropylsilane/dithiothreitol/phenol/trifluoroacetic acid (2.5:2.5:2.5:2.5:90, v/v/w/w/v) for 30 min under microwave radiation (Discover; temperature, 38 °C; power, 20 watts). The cyclization of (DSS)6-GACRETAWACGA was performed for 24 h at room temperature in water/acetonitrile/trifluoroacetic acid (50:50:0.1) with 1.2 eq of Aldrithiol-4 (Sigma-Aldrich) under conditions of high dilution (1.10−4 m) to avoid formation of the corresponding dimeric cyclopeptide. The corresponding cyclic peptide and (DSS)6-GRGESP were purified by reverse-phase HPLC using a Shimadzu preparative HPLC system with a reverse-phase HPLC column (Phenomenex C12 Jupiter Proteo, 90 Å, 21.2 × 250 mm) with mixture of aqueous 0.1% (v/v) TFA (system A) and 0.1% (v/v) TFA in acetonitrile (system B) as the mobile phase (flow rate of 15 ml/min) and employing UV detection at 220 nm. Characterizations of the peptides were performed by mass spectrometry on a Q-Tof Ultima GLOBAL hybrid quadrupole time-of-flight instrument. The predicted and observed high-resolution masses for (DSS)6-GRGESP (C83H129N27O52) were 2336.8358 and 2336.7764 Da, respectively. The predicted and observed high-resolution masses for (DSS)6-GACRETAWACGA (C108H162N34O58S2) were 2928.0292 and 2928.0332 Da, respectively.
Cell Adhesion
To determine cell adhesion in response to the peptides, human (hMSCs) and mouse (mMSCs) MSCs were resuspended in DMEM and 0.5% BSA and incubated with cyclized peptides or a specific anti-α5β1 integrin antibody (40 μg/ml; MAB1969, Millipore) for 30 min at 4 °C. Cell adhesion to a plate coated with 20 μg/ml human FN for 90 min at 37 °C and washed with 1% BSA was tested after 10 min at 37 °C. The plates were washed twice with DMEM, and cells were fixed with 4% paraformaldehyde and quantified using the crystal violet assay (Sigma).
FAK Assay
hMSCs and mMSCs were grown to 80% confluence, serum-starved for 4 h, treated for 30 min with 50 ng/ml PDGF (used as a positive control; R&D Systems) or the indicated peptide (70 μm), and fixed. Total and phosphorylated (Tyr-397) FAK levels were determined by ELISA (FACE FAK assay, Active Motif, La Hulpe, Belgium).
Transfections and Luciferase Activity
To test RUNX2 transcriptional activity, mMSCs were cotransfected with 0.3 μg/well p6OSE-Luc (a luciferase expression plasmid containing six RUNX2-binding sites), pControl-Luc plasmid (a luciferase expression plasmid without RUNX2 binding used as a control), or phRL-SV40 (a Renilla expression plasmid without a RUNX2-binding site used as an internal transfection control). Twenty-four hours after transfection, cells were treated with 70 μm peptides or 100 ng/ml BMP2 (bone morphogenetic protein-2; used as a positive control; R&D Systems) for 24 h, and luciferase and Renilla activities were determined sequentially using a Renilla luciferase reporter assay system (Promega). Luciferase activity was normalized to Renilla activity to avoid transfection variability and to pControl-Luc to normalize the luciferase background. Results are expressed as relative luciferase units. For the TOPFlash luciferase reporter assays, cells were cotransfected with 0.3 μg/well TOPFlash or FOPFlash and 10 ng/well phRL-SV40, and CM was used as a positive control (47).
Differentiation Assays
For in vitro osteogenic assay, the cell culture medium was supplemented with 50 μg/ml ascorbic acid and 3 mm Pi to allow matrix synthesis and mineralization (48). After 18 days of treatment with the indicated peptide (70 μm), cells were fixed in 70% ethanol at 4 °C. Matrix mineralization was evaluated by Alizarin Red staining and microphotographed using an Olympus microscope (48).
Western Blot Analysis
Murine cells were treated with the indicated peptide (70 μm) for 5 min or 24 h, and cell lysates were prepared as described (47). Protein concentrations were measured using a DC protein assay (Bio-Rad). Equal aliquots of cell lysates were resolved by 10% SDS-PAGE. Western blotting was performed using specific primary antibodies against total or phosphorylated PI3K and Akt (1:1000 dilution; Cell Signaling Technology). Relative levels are expressed as a ratio of treated to control.
RNA Extraction and Quantitative RT-PCR Analysis
mMSCs were treated with the indicated peptide (70 μm) in the presence of 10 μm wortmannin (Sigma) at the indicated time points, and total RNA was isolated using TRIzol reagent (Eurobio, Les Ulis, France). Three μg of total RNA from each samples were reverse-transcribed using the a high-capacity cDNA reverse transcription kit (Applied Biosystems) in a total volume of 30 μl at 37 °C for 2 h. Relative mRNA levels were evaluated by quantitative PCR (LightCycler, Roche Applied Science) using a SYBR Green PCR kit (ABgene, Courtaboeuf, France) and specific primers (24, 47, 49). Signals were normalized to hypoxanthine phosphoribosyltransferase as an internal control. The relative amount of RNA was calculated using the 2−ΔΔCt method.
Animals and Treatments
A pilot study was conducted to investigate whether the observed effects of peptide-mediated α5β1 integrin priming in vitro translate to beneficial effects in vivo. Toward this goal, one cyclized peptide (GACRETAWACGA) selected based on its osteogenic potential on mMSCs was linked to (DSS)6 to allow specific delivery to bone-forming sites in vivo (46). The compound was administered to SAMP-6 osteopenic senescent mice, an established model of age-related bone loss associated with decreased bone formation (50, 51). Nine-week-old SAMP-6 mice (Harlan Laboratories) were injected in the tail vein with purified cyclized (DSS)6-GACRETAWACGA or the inactive control peptide ((DSS)6)-GREGSP) at 760 nmol/kg of body weight/day for 5 days/week. Body weight was recorded every week. After 6 weeks, mice were killed by ketamine/xylazine injection, and blood samples were collected for determination of bone formation parameters. The main soft tissue organs (livers and kidneys) were collected and weighted. The femurs were removed for microstructure histomorphometric analysis. The protocol was conducted according to the guidelines of the local ethics committee (CEEALV/2011.11.01).
Histomorphometric Analysis
Femurs were embedded undecalcified in methyl methacrylate, and 5-μm sections were stained with Aniline Blue to analyze structural parameters (bone volume and trabecular number, thickness, and separation) as described (47).
Procollagen Type 1 N-terminal Propeptide (P1NP) and Osteocalcin Analysis
The serum levels of P1NP (IDS, Paris, France) and osteocalcin (Tecomedical, Rambouillet, France), which are established markers of bone formation, were determined by ELISA.
Statistical Analysis
Data are presented as the mean ± S.D. of repeated experiments with 3–12 replicates (in vitro), or data were obtained from seven mice per group. The data were analyzed using one-way analysis of variance or Student's t test, and a minimal level of p < 0.05 was considered significant.
RESULTS
RRET-dependent MSC Adhesion on FN
We first determined whether RGD and RRET in CRRETAWAC play equivalent roles in MSC adhesion on FN. Toward this goal, we used an optimal dose of the peptide that maximally binds to α5β1 integrin and fully inhibits α5β1 integrin-mediated cell attachment to FN (29). As expected, cell adhesion was fully abolished by the anti-α5β1 integrin antibody MAB1969 (Fig. 1A). Cell incubation with cyclized CRRETAWAC abolished hMSC adhesion on FN, indicating that the recognition of RGD in FN by α5β1 integrin was blocked by cyclized CRRETAWAC, which is consistent with its effect in fibroblasts (29). Interestingly, cyclized peptides lacking the full RRET sequence partially displaced adhesion on FN in both hMSCs (Fig. 1B) and mMSCs (Fig. 1C) at the same dosage. These results indicate that the RRET sequence acts as an RGD-like sequence that is required for CRRETAWAC to fully antagonize FN-mediated cell adhesion in MSCs.
FIGURE 1.
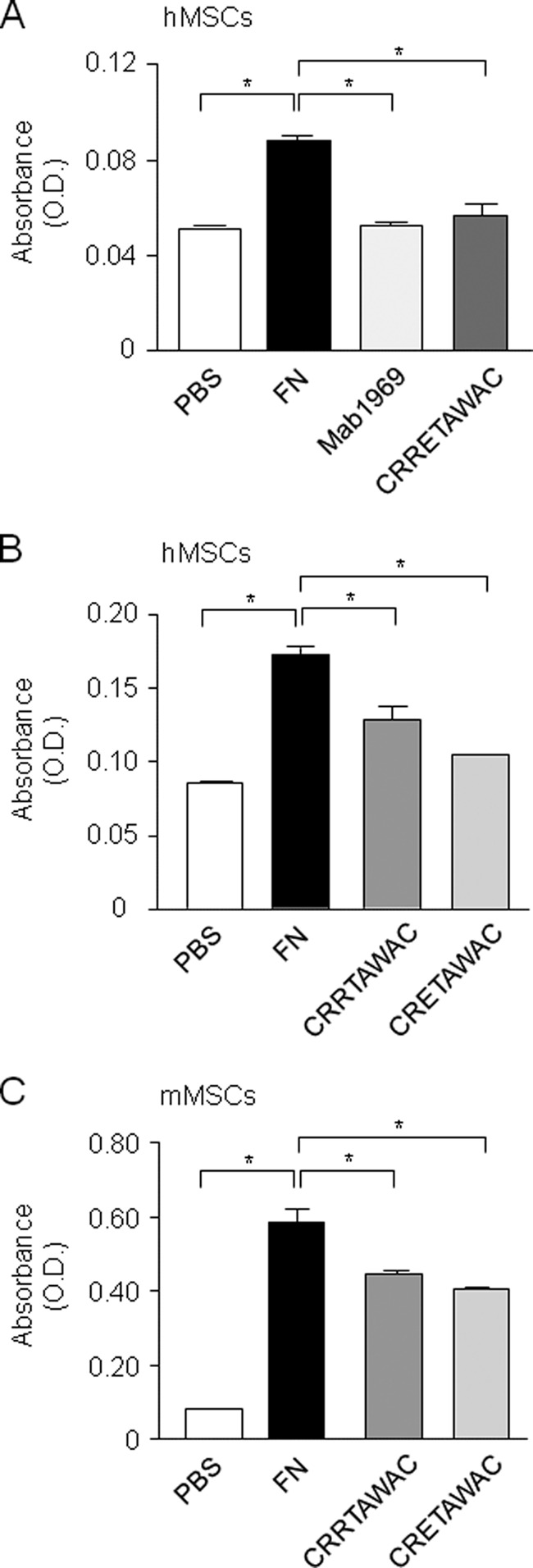
The RGD-like RRET sequence mediates MSC adhesion to FN. hMSCs (A and B) and mMSCs (C) were incubated with cyclized peptides (GACRRETAWACGA, GACRRTAWACGA, or GACRETAWACGA, used at the optimal dosage of 70 μm) that fully inhibit α5β1 integrin-mediated cell attachment to FN (29) or anti-α5β1 integrin antibody MAB1969 (used as a control), and cell adhesion to FN was determined after 10 min. Data are the mean ± S.D. *, significant difference (p < 0.05).
α5β1 Integrin-mediated FAK Signaling in MSCs Is RRET-independent
Because the RGD sequence is believed to mediate cell signaling triggered by α5β1 integrin in fibroblasts (29), we next determined the importance of the RGD-like RRET sequence in cell signaling induced by α5β1 integrin priming in MSCs. Biochemical determination of FAK activity measured at FAK Tyr-397 showed that cyclized CRRETAWAC, CRRTAWAC, and CRETAWAC increased phospho-FAK levels in hMSCs with no significant difference between peptides (Fig. 2A). All peptides also similarly increased phospho-FAK levels in mMSCs (Fig. 2B), confirming the data in hMSCs. Although the amplitude of the FAK response to the peptides was modest in mMSCs, the increase in FAK in these cells was close to that induced by PDGF, which was used as a positive control. The amplitude of the FAK response to the peptides is also consistent with the reported increase in FAK activity during osteoblast differentiation (52, 53). The finding that all peptides increased FAK levels in MSCs indicates that the full RRET sequence is not required for FAK activation evoked by peptide-induced α5β1 integrin priming in MSCs.
FIGURE 2.
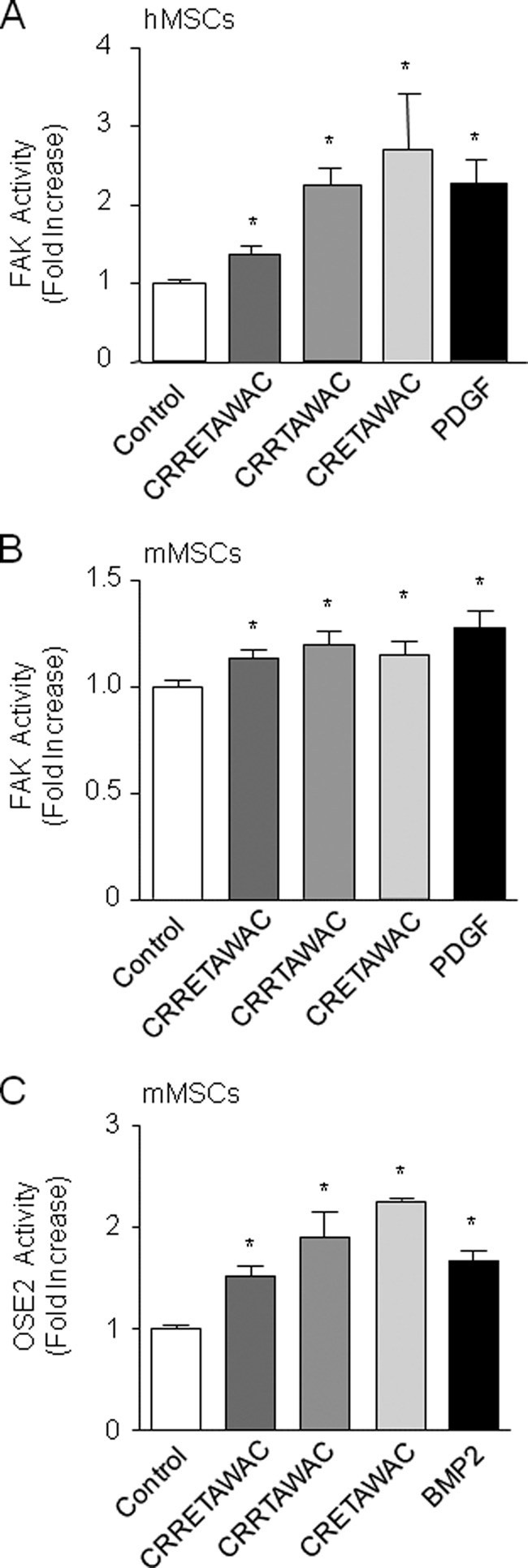
RRET-independent, peptide-mediated α5β1 integrin priming increases FAK signaling and OSE2 transcription in MSCs. Biochemical analysis showed that cyclized peptides (GACRRETAWACGA, GACRRTAWACGA, or GACRETAWACGA, 70 μm) increased FAK phosphorylation at Tyr-397 in hMSCs (A) and mMSCs (B and C). In this assay, PDGF (50 ng/ml) was used as a positive control. Transcriptional assay in mMSCs showed that OSE2 transcriptional activity was increased by the cyclized peptides lacking the full RGD-like RRET sequence. BMP2 (100 ng/ml) was used as a positive control. Data are the mean ± S.D. *, significant difference compared with control cells (p < 0.05).
α5β1 Integrin-induced Osteogenic Differentiation in MSCs Is RRET-independent
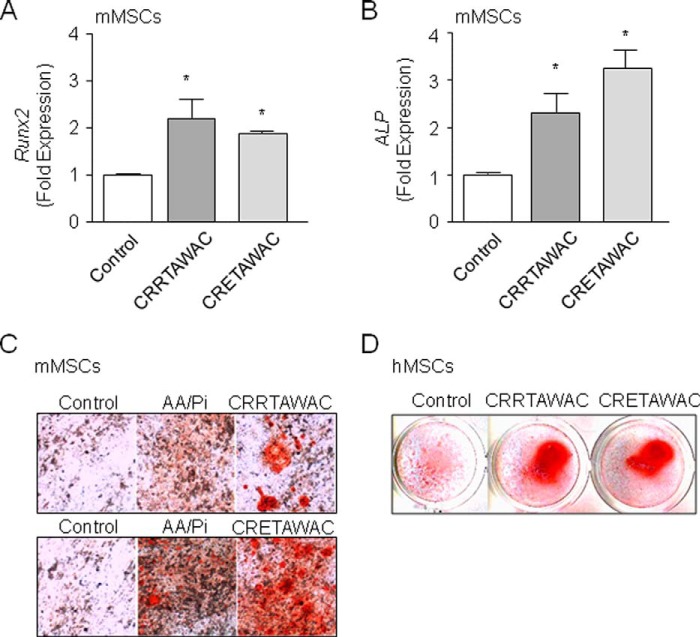
We next determined the importance of the RRET sequence in the molecular mechanisms that drive osteogenic differentiation in mMSCs. Toward this goal, we analyzed RUNX2 transcriptional activity, which is directed by RUNX2 phosphorylation and RUNX2 binding to OSE2 sequences in the promoters of target genes (54). As shown in Fig. 2C, all cyclized peptides increased OSE2 transcriptional activity to a similar extent as the positive control BMP2 in mMSCs. Consistent with this finding, cyclized peptides lacking the full RRET sequence increased Runx2 and alkaline phosphatase mRNA levels in mMSCs with no significant difference between peptides (Fig. 3, A and B). This indicates that the high-affinity ligand CRRETAWAC up-regulates RUNX2 transcriptional activity and osteoblast gene expression in mMSCs independently of the full RRET sequence. We also determined the functional role of the RRET sequence in matrix mineralization, a hallmark of mature osteoblasts. We found that cyclized peptides lacking the full RRET sequence increased matrix mineralization in mMSCs in long-term cultures (Fig. 3C). Similar effects were observed in hMSCs (Fig. 3D). Taken together, the data indicate that α5β1 integrin priming by the high-affinity ligand CRRETAWAC activates FAK and promotes RUNX2 transcriptional activity, RUNX2-dependent osteoblast gene expression, and matrix mineralization in MSCs independently of the full RRET sequence.
FIGURE 3.
Peptide-induced α5β1 integrin priming evokes RRET-independent osteogenic differentiation in MSCs. Quantitative PCR analysis showed that cyclized peptides lacking the full RRET sequence (GACRRTAWACGA or GACRETAWACGA, 70 μm) increased the expression of osteoblast marker genes in mMSCs (A and B). Consistently, the peptides increased in vitro matrix mineralization compared with control cells and cells cultured in ascorbic acid (AA) and Pi alone as shown by Alizarin Red staining at 18 days of culture in both mMSCs (C) and hMSCs (D). Data are the mean ± S.D. *, significant difference compared with controls (p < 0.05).
Peptide-induced α5β1 Integrin Priming Promotes Wnt/β-Catenin Signaling in MSCs
We next determined whether peptide-mediated α5β1 integrin priming interacts with Wnt/β-catenin signaling by determining β-catenin transcriptional activity in MSCs. As expected (35), activation of the canonical Wnt/β-catenin signaling by CM increased TCF/LEF transcriptional activity in mMSCs and hMSCs (Fig. 4, A and B). We found that both cyclized CRRETAWAC and peptides lacking the full RRET sequence increased β-catenin transcriptional activity in mMSCs and hMSCs with no significant difference between peptides (Fig. 4, A and B). In hMSCs, the amplitude of the response to the cyclized peptides was similar to that to CM (Fig. 4B), whereas in mMSCs, the response to the peptides was moderate compared with the huge effect of CM (Fig. 4A). Overall, the results indicate that the Wnt/β-catenin transcriptional activity is significantly activated by peptide-mediated α5β1 integrin priming independently of the RGD-like RRET sequence.
FIGURE 4.
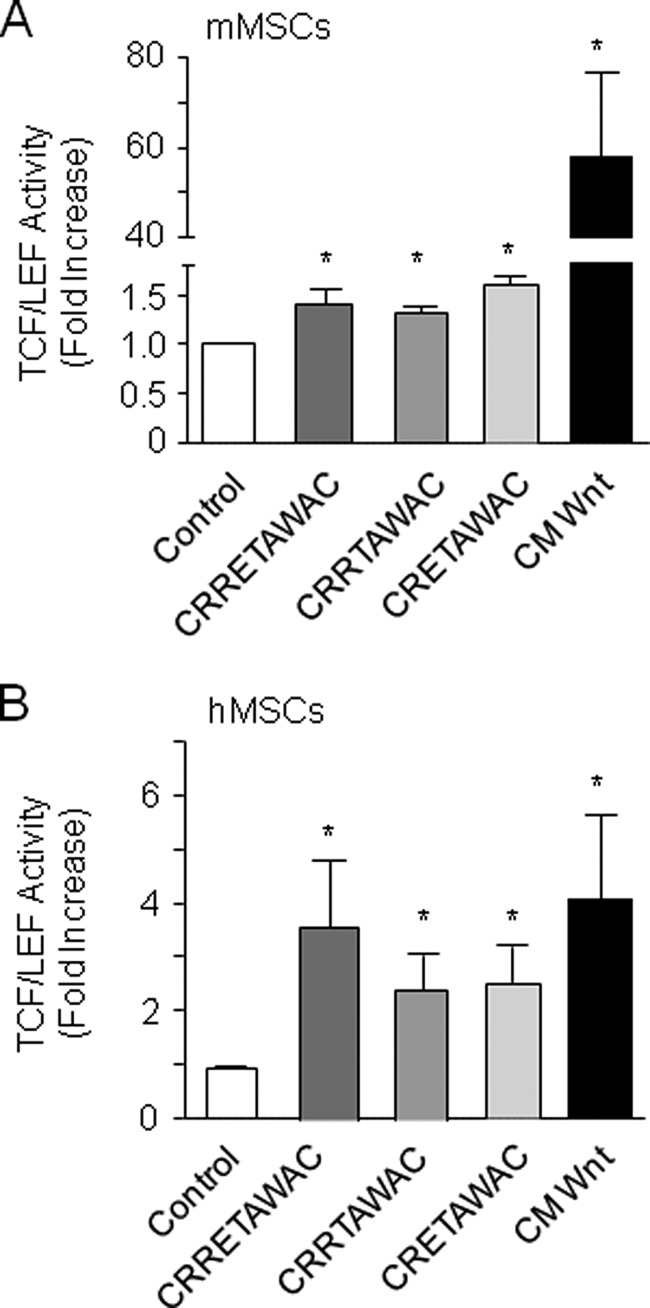
Peptide-induced α5β1 integrin priming enhances β-catenin transcriptional activity in MSCs. mMSCs (A) and hMSCs (B) were treated with cyclized peptides (GACRRETAWACGA, GACRRTAWACGA, or GACRETAWACGA, 70 μm) or CM (used as a positive control), and TCF/LEF transcriptional activity was determined. Data are the mean ± S.D. *, significant difference compared with controls (p < 0.05).
α5β1 Integrin Priming Integrates Wnt Signaling to Promote MSC Osteoblast Differentiation
We then determined the signaling pathway underlying Wnt/β-catenin signaling activation by peptide-mediated α5β1 integrin priming in mMSCs. We focused on PI3K/Akt activity, which negatively regulates GSK-3β activity, leading to inhibition of β-catenin phosphorylation (55). Western blot analysis showed that both cyclized CRRETAWAC and cyclized peptides lacking the full RRET sequence greatly increased phospho-PI3K and phospho-Akt levels in mMSCs compared with controls (Fig. 5, A and B). This effect was transient and was not observed at 24 h. In contrast, CM used as a positive control continuously increased phospho-PI3K and phospho-Akt levels for up to 24 h. These results indicate that α5β1 integrin priming transiently activates PI3K/Akt activity in mMSCs independently of the RRET sequence.
FIGURE 5.
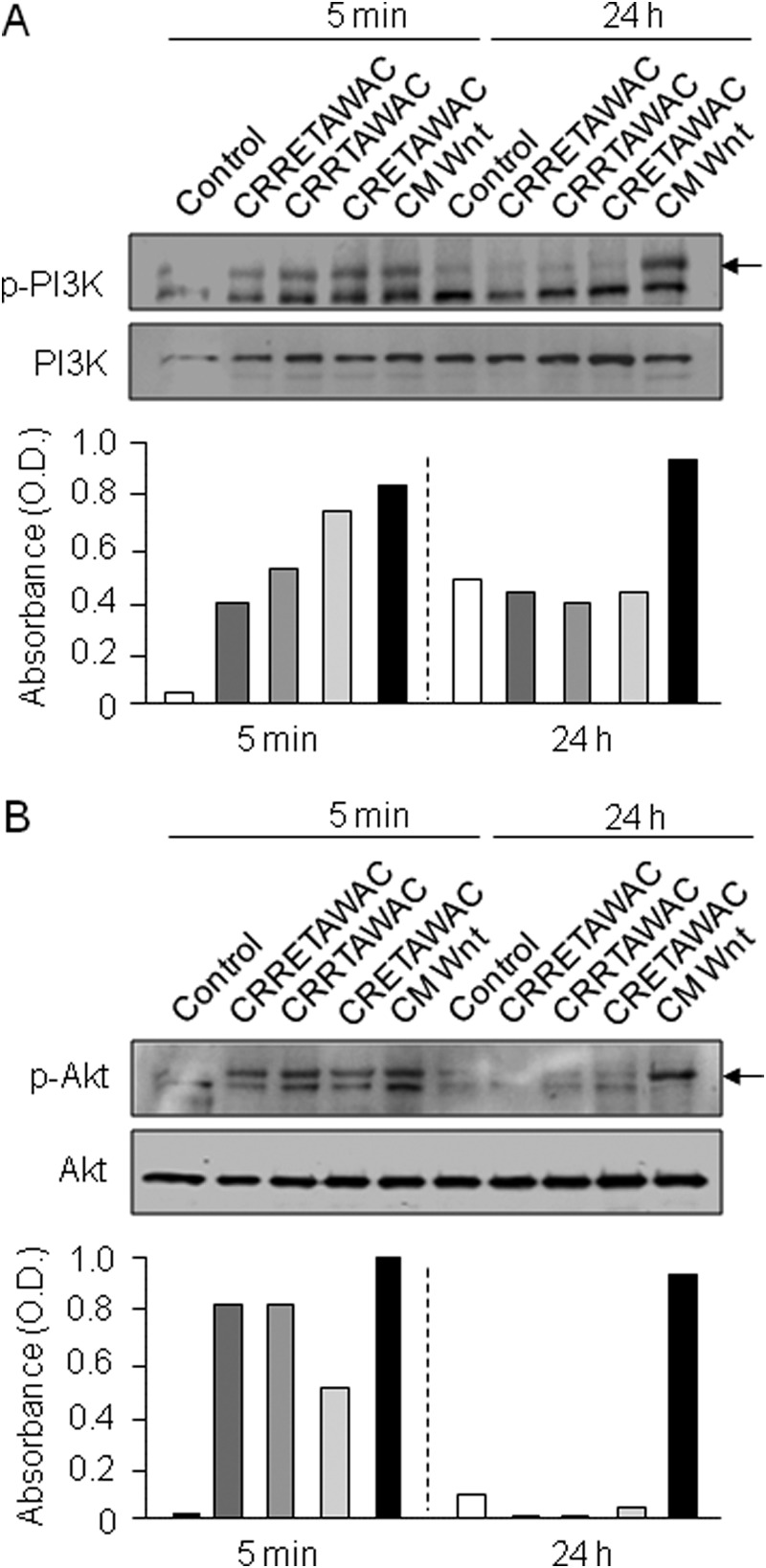
α5β1 integrin-priming peptides transiently activate PI3K and Akt signaling in MSCs. mMSCs were treated with cyclized peptides (GACRRETAWACGA, GACRRTAWACGA, or GACRETAWACGA, 70 μm) or CM (used as a positive control) for 5 min or 24 h. Western blot analysis and quantification of blots showed that all peptides transiently increased phosphorylated (p) PI3K (A) and Akt (B) levels compared with control cells. Relative levels are expressed as a ratio of treated to control after correction to the total levels of PI3K and Akt.
We next determined the functional role of PI3K signaling induced by peptides lacking the full RRET sequence. Toward this goal, mMSCs were treated with α5β1 integrin-priming peptides in the presence of a pharmacological PI3K inhibitor used at a dose that efficiently inhibits PI3K activity in osteoblastic cells (56). As shown in Fig. 6A, pharmacological PI3K inhibition attenuated alkaline phosphatase gene expression induced by the cyclized peptides lacking the full RRET sequence, suggesting that PI3K signaling is involved in MSC differentiation induced by peptide-mediated α5β1 integrin priming. To further determine the functional role of Wnt/β-catenin signaling downstream of PI3K activation, we analyzed the expression of Wnt regulatory genes. We focused on Dkk1 (Dickkopf 1), a direct target of canonical Wnt signaling, and Sfrp1 (secreted Frizzled-related protein 1), which is a Wnt modulator (57–59). Interestingly, α5β1 integrin priming by peptides lacking the RRET sequence increased the expression of both Dkk1 and Sfrp1, and this effect was abolished by PI3K inhibition (Fig. 6, B and C). These data suggest a role for PI3K signaling in activation of these Wnt regulatory genes by peptide-mediated α5β1 integrin priming in MSCs.
FIGURE 6.
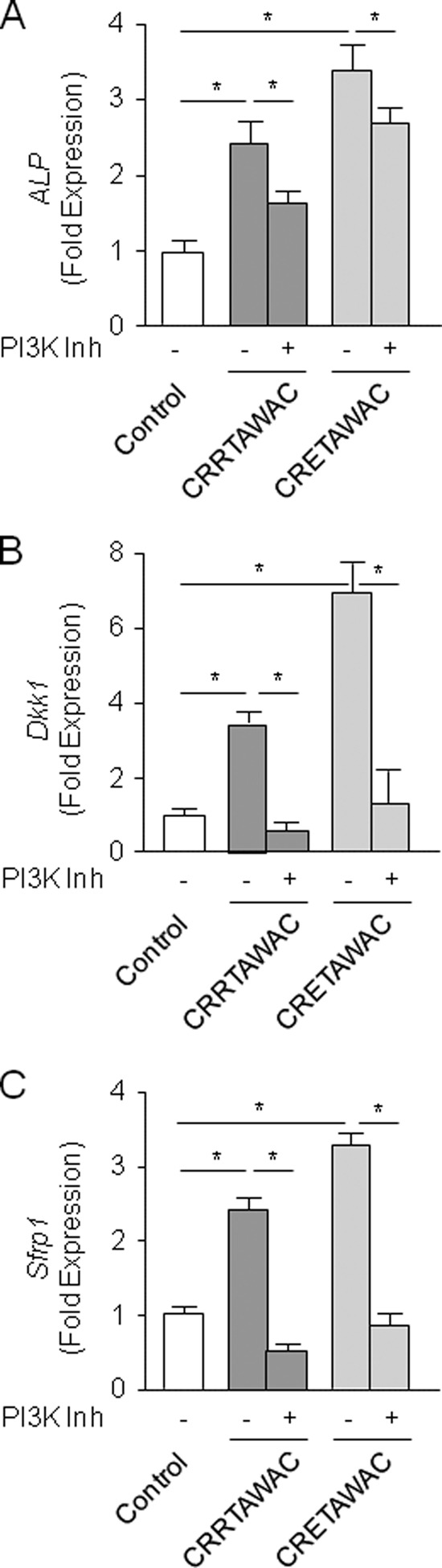
PI3K signaling mediates Wnt signaling activation and osteoblast differentiation induced by α5β1 integrin priming in MSCs. Treatment of mMSCs with cyclized peptides lacking the full RRET sequence (GACRRTAWACGA or GACRETAWACGA, 70 μm) for 5 days increased the expression of the phenotypic osteoblast marker alkaline phosphatase (ALP; A) and Wnt regulatory genes (B and C), an effect that was attenuated by the PI3K inhibitor (Inh) wortmannin (10 μm). Data are the mean ± S.D. *, significant difference (p < 0.05).
Peptide-mediated α5β1 Integrin Priming Improves Bone Microstructure in Senescent Osteopenic Mice
We next performed a pilot study in vivo to test whether the effects of α5β1 integrin-priming peptides observed in vitro translate into beneficial effects in SAMP-6 senescent osteopenic mice, a model of skeletal aging characterized by decreased bone mass and altered microstructure (50, 51). Toward this goal, we selected the cyclized CRETAWAC peptide, the most effective peptide in promoting osteogenesis in mMSCs in vitro (Fig. 3C), and linked the peptide to (DSS)6 to ensure in vivo delivery at active bone-forming sites (46). We chose (DSS)6 as a ligand for the cyclized peptides because it has the potential to selectively bind to bone-forming surfaces rather than to bone-resorbing surfaces in vivo (46). We found that systemic delivery of (DSS)6-GACRETAWACGA tended to increase serum P1NP levels and significantly increased osteocalcin levels compared with the inactive peptide (DSS)6-GREGSP(Fig. 7, A and B), suggesting increased osteoblast differentiation rather than function. Interestingly, systemic delivery of (DSS)6-GACRETAWACGA resulted in a 20% increase in trabecular bone volume in femurs compared with the control peptide (Fig. 7C). This effect was associated with increased trabecular number and decreased trabecular separation with no change in trabecular thickness (Fig. 7, D–F). The administration of the compound had no effect on body weight or soft tissue organs (data not shown), suggesting that there was no deleterious effect of the peptides in these mice. These results suggest that systemic delivery of an α5β1 integrin priming-peptide in osteoblasts can lead to improved bone mass and microarchitecture in senescent osteopenic mice.
FIGURE 7.
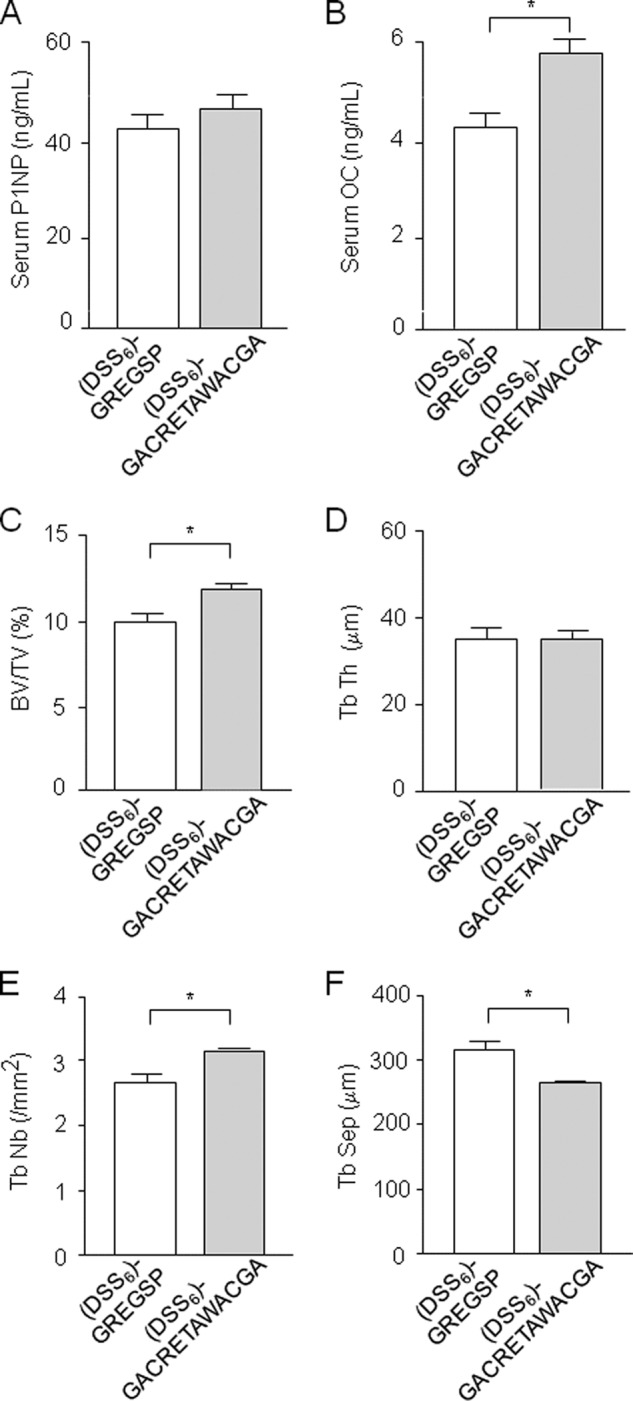
Peptide-mediated α5β1 integrin priming improves bone mass and microarchitecture in senescent osteopenic mice. Systemic delivery of (DSS)6-GACRETAWACGA for 6 weeks to SAMP-6 osteopenic mice tended to increase P1NP levels (A) and significantly increased serum osteocalcin (OC) levels (B) compared with the inactive peptide (DSS)6-GREGSP. This effect was associated with increased trabecular bone volume (BV/TV; C) and trabecular number (Tb Nb; E) and decreased trabecular separation (Tb Sep; F) with no change in trabecular thickness (Tb Th) in femurs (D). Data are the mean ± S.D. (n = 7 mice per group). *, significant difference (p < 0.05).
Taken together, the data indicate that CRRETAWAC-induced α5β1 integrin priming promotes mesenchymal cell osteogenic differentiation independently of cell adhesion mediated by the RGD-like RRET sequence through activation of PI3K/Akt/Wnt/β-catenin signaling and that treatment with an α5β1 integrin-priming peptide can improve bone microarchitecture and bone mass in senescent osteopenic mice (Fig. 8).
FIGURE 8.
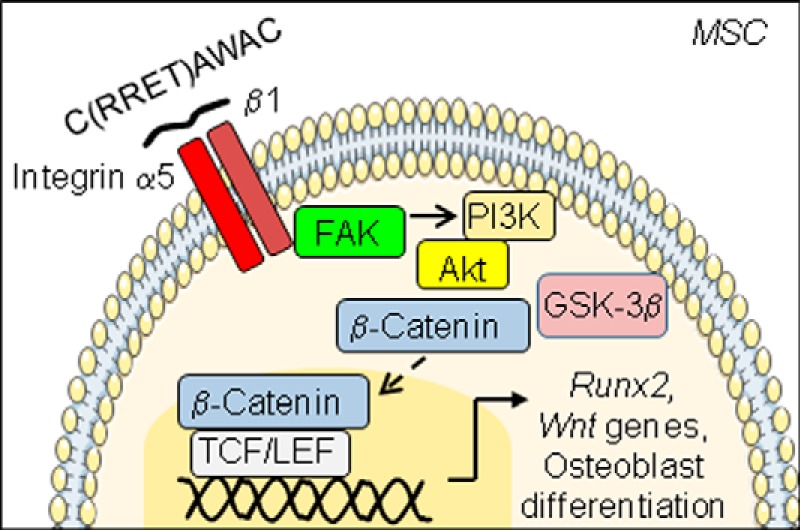
Proposed mechanism whereby peptide-mediated α5β1 integrin priming integrates Wnt/β-catenin signaling to promote osteoblast differentiation in mMSCs. Priming α5β1 integrin with cyclized CRRETAWAC and related peptides lacking the full RGD-like RRET sequence leads to transiently activated PI3K/Akt and to increased Wnt/β-catenin signaling, resulting in increased Runx2 expression, OSE2 transcriptional activity, and osteoblast differentiation independently of the RGD-like RRET sequence, which may translate into improved bone mass and microarchitecture in senescent osteopenic mice.
DISCUSSION
The molecular mechanisms by which integrins control osteogenic differentiation are not fully understood. Here, we report a novel signaling mechanism whereby α5β1 integrin priming promotes osteogenic differentiation in MSCs. We used CRRETAWAC as a tool to prime α5β1 integrin because this high-affinity ligand interacts specifically with α5β1 integrin (29, 30). CRRETAWAC and RGD were found to compete with each other for binding to α5β1 integrin in fibroblasts, but the respective binding sites for RGD and RRETAWA peptides are distinct, although seemingly overlapping (29, 60, 61). Phage display studies revealed no degeneracy in the RRETAWA sequence for integrin binding, suggesting that all amino acids are important for binding (29). To determine the equivalence of RGD and RRET sequences, we investigated the functional roles of the RRET sequence in MSC adhesion and osteogenic differentiation. We showed that cyclized peptides lacking the RRET sequence partially displaced MSC adhesion on FN, indicating that the RRET sequence is required for CRRETAWAC to fully antagonize FN-mediated MSC adhesion. In contrast, peptides without the full RRET sequence increased FAK phosphorylation to the same extent as cyclized CRRETAWAC, indicating that the full RRET sequence is not essential for peptide-induced α5β1 integrin signaling in MSCs. We thus investigated whether the RRET sequence is required for osteogenic differentiation induced by α5β1 integrin priming in MSCs. Our findings that all cyclized peptides increased RUNX2 transcriptional activity, RUNX2-dependent gene expression, and matrix mineralization in mMSCs indicate that the full RGD-like RRET sequence is not required for the osteogenic signals generated by CRRETAWAC/α5β1 integrin interaction in these cells.
Several intracellular signaling pathways may be involved in osteogenic differentiation induced by CRRETAWAC/α5β1 integrin interaction in MSCs. Notably, we previously reported that α5β1 integrin priming by CRRETAWAC promotes mMSC osteogenic differentiation through ERK1/2 activation (24) and IGF2/IGFBP2 signaling (32). Here, we showed that peptide-mediated α5β1 integrin priming increased Wnt/β-catenin transcriptional activity in MSCs independently of the RGD-like RRET sequence. The reason why the FAK and TCF/LEF signaling responses were better in hMSCs compared with mMSCs is unknown but may be related to a higher availability of free ITGA5 in hMSCs. In non-skeletal cells, integrin activation induces integrin-linked kinase phosphorylation, which in turn up-regulates PI3K/Akt signaling, resulting in inhibition of GSK-3β (62, 63). Consistent with this signaling cascade, we found that peptide-mediated α5β1 integrin priming increased PI3K/Akt phosphorylation in mMSCs. This effect was transient, however, compared with the expected long-lasting activation of Wnt3a in PI3K/Akt signaling in osteoblastic cells (56). Moreover, we found that peptide-mediated α5β1 integrin priming increased the levels of Dkk1 and Sfrp1, which are negative regulators of canonical Wnt signaling (57–59), consistent with a negative feedback response to activation of Wnt signaling (64). In mMSCs, the increased expression of Dkk1 and Sfrp1 induced by the peptides may have contributed to limit Wnt signaling activation. Our finding that pharmacological inhibition of PI3K attenuated the stimulatory effect of the peptides on osteoblast gene expression in mMSCs suggests a role for PI3K/Akt signaling in the activation of osteogenic differentiation induced by α5β1 integrin priming in MSCs. Thus, in addition to ERK1/2 and IGF2/IGFBP2 signaling (24, 32), Wnt signaling activation may contribute in part to osteoblast differentiation induced by α5β1 integrin priming in MSCs.
On the basis of the stimulatory effect of the α5β1 integrin-priming peptides on in vitro osteogenesis in mMSCs, we tested whether this effect may translate into improved bone mass and microstructure in SAMP-6 senescent osteopenic mice, a model of skeletal aging characterized by decreased bone mass and altered bone microarchitecture (50, 51). We selected the cyclized GACRETAWACGA peptide, which was the most efficient in matrix mineralization by mMSCs in vitro, and linked this peptide to (DSS)6 to ensure delivery of the peptide to active bone-forming sites, as demonstrated previously in vivo (46). Our finding that systemic delivery of (DSS)6-GACRETAWACGA (which primed α5β1 integrin) increased the levels of serum osteocalcin (which is expressed by mature osteoblasts) suggests that the peptide increased osteoblast differentiation independently of RRET-mediated cell adhesion. Moreover, we found that this effect was associated with increased femoral bone mass and microarchitecture in senescent osteopenic mice, suggesting an anti-osteopenic effect. Although this pilot study suggests a beneficial effect of this peptide on bone mass in senescent osteopenic mice, more studies are needed to establish whether the delivery of α5β1 integrin-priming peptides may fully prevent the altered bone mass and microstructure that occur during aging. Given the lack of substantial side effects observed, it may be possible to increase injection frequency and/or peptide dosage to optimize treatment efficacy. Further studies are also needed to determine the time course changes in osteoblast and osteoclast parameters, as well as dynamic histomorphometric parameters of bone formation during treatment.
In summary, our data support a model wherein α5β1 integrin priming by high-affinity ligands integrates Wnt/β-catenin signaling to promote mesenchymal cell osteogenic differentiation independently of cell adhesion mediated by the RGD-like RRET sequence, a model that could be used to improve bone mass and microarchitecture in the aging skeleton.
This work was supported by grants from the Agence Nationale de la Recherche (Integros, ANR-2010-BLAN-1505-01) and the Association Prévention et Traitement des Décalcifications (Paris, France) (to P. J. M.).
- MSC
- mesenchymal skeletal cell
- hMSC
- human MSC
- mMSC
- mouse MSC
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- FN
- fibronectin
- GSK-3β
- glycogen synthase kinase-3β
- TCF/LEF
- T-cell factor/lymphoid enhancer factor
- CM
- Wnt3a-conditioned medium
- P1NP
- procollagen type 1 N-terminal propeptide.
REFERENCES
- 1. Khosla S., Bellido T. M., Drezner M. K., Gordon C. M., Harris T. B., Kiel D. P., Kream B. E., LeBoff M. S., Lian J. B., Peterson C. A., Rosen C. J., Williams J. P., Winer K. K., Sherman S. S. (2011) Forum on aging and skeletal health: summary of the proceedings of an ASBMR workshop. J. Bone Miner. Res. 26, 2565–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manolagas S. C., Parfitt A. M. (2010) What old means to bone. Trends Endocrinol. Metab. 21, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marie P. J. (2014) Bone cell senescence: mechanisms and perspectives. J. Bone Miner. Res. 29, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 4. Marie P. J., Kassem M. (2011) Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur. J. Endocrinol. 165, 1–10 [DOI] [PubMed] [Google Scholar]
- 5. Baron R., Hesse E. (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J. Clin. Endocrinol. Metab. 97, 311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long F. (2012) Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 [DOI] [PubMed] [Google Scholar]
- 7. Marie P. J. (2008) Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 473, 98–105 [DOI] [PubMed] [Google Scholar]
- 8. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 9. Humphries J. D., Byron A., Humphries M. J. (2006) Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giancotti F. G., Ruoslahti E. (1999) Integrin signaling. Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 11. Luo B. H., Springer T. A. (2006) Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 18, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juliano R. L., Haskill S. (1993) Signal transduction from the extracellular matrix. J. Cell Biol. 120, 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damsky C. H., Ilić D. (2002) Integrin signaling: it's where the action is. Curr. Opin. Cell Biol. 14, 594–602 [DOI] [PubMed] [Google Scholar]
- 14. Larsen M., Artym V. V., Green J. A., Yamada K. M. (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 18, 463–471 [DOI] [PubMed] [Google Scholar]
- 15. Damsky C. H. (1999) Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 25, 95–96 [DOI] [PubMed] [Google Scholar]
- 16. Franceschi R. T. (1999) The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral Biol. Med. 10, 40–57 [DOI] [PubMed] [Google Scholar]
- 17. Brunner M., Jurdic P., Tuckerman J. P., Block M. R., Bouvard D. (2013) New insights into adhesion signaling in bone formation. Int. Rev. Cell Mol. Biol. 305, 1–68 [DOI] [PubMed] [Google Scholar]
- 18. Ge C., Xiao G., Jiang D., Franceschi R. T. (2007) Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 176, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao G., Jiang D., Thomas P., Benson M. D., Guan K., Karsenty G., Franceschi R. T. (2000) MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J. Biol. Chem. 275, 4453–4459 [DOI] [PubMed] [Google Scholar]
- 20. Marie P. J. (2013) Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 9, 288–295 [DOI] [PubMed] [Google Scholar]
- 21. Moursi A. M., Globus R. K., Damsky C. H. (1997) Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J. Cell Sci. 110, 2187–2196 [DOI] [PubMed] [Google Scholar]
- 22. Kaabeche K., Guénou H., Bouvard D., Didelot N., Listrat A., Marie P. J. (2005) Cbl-mediated ubiquitination of α5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J. Cell Sci. 118, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 23. Dufour C., Holy X., Marie P. J. (2007) Skeletal unloading induces osteoblast apoptosis and targets α5β1-PI3K-Bcl-2 signaling in rat bone. Exp. Cell Res. 313, 394–403 [DOI] [PubMed] [Google Scholar]
- 24. Hamidouche Z., Fromigué O., Ringe J., Haupl T., Vaudin P., Pagès J. C., Srouji S., Livne E., Marie P. J. (2009) Priming integrin α5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 18587–18591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srouji S., Ben-David D., Fromigué O., Vaudin P., Kuhn G., Müller R., Livne E., Marie P. J. (2012) Lentiviral-mediated integrin α5 expression in human adult mesenchymal stromal cells promotes bone repair in mouse cranial and long-bone defects. Hum. Gene. Ther. 23, 167–172 [DOI] [PubMed] [Google Scholar]
- 26. Fromigué O., Brun J., Marty C., Da Nascimento S., Sonnet P., Marie P. J. (2012) Peptide-based activation of α5 integrin for promoting osteogenesis. J. Cell Biochem. 113, 3029–3038 [DOI] [PubMed] [Google Scholar]
- 27. Ruoslahti E., Pierschbacher M. D. (1987) New perspectives in cell adhesion: RGD and integrins. Science 238, 491–497 [DOI] [PubMed] [Google Scholar]
- 28. Koivunen E., Gay D. A., Ruoslahti E. (1993) Selection of peptides binding to the α5β1 integrin from phage display library. J. Biol. Chem. 268, 20205–20210 [PubMed] [Google Scholar]
- 29. Koivunen E., Wang B., Ruoslahti E. (1994) Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J. Cell Biol. 124, 373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mould A. P., Burrows L., Humphries M. J. (1998) Identification of amino acid residues that form part of the ligand-binding pocket of integrin α5β1. J. Biol. Chem. 273, 25664–25672 [DOI] [PubMed] [Google Scholar]
- 31. Mould A. P., Askari J. A., Humphries M. J. (2000) Molecular basis of ligand recognition by integrin α5β1. I. Specificity of ligand binding is determined by amino acid sequences in the second and third NH2-terminal repeats of the α subunit. J. Biol. Chem. 275, 20324–20336 [DOI] [PubMed] [Google Scholar]
- 32. Hamidouche Z., Fromigué O., Ringe J., Häupl T., Marie P. J. (2010) Crosstalks between integrin α5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westendorf J. J., Kahler R. A., Schroeder T. M. (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341, 19–39 [DOI] [PubMed] [Google Scholar]
- 34. Baron R., Kneissel M. (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 [DOI] [PubMed] [Google Scholar]
- 35. Gordon M. D., Nusse R. (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 281, 22429–22433 [DOI] [PubMed] [Google Scholar]
- 36. Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
- 37. Dedhar S., Williams B., Hannigan G. (1999) Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 9, 319–323 [DOI] [PubMed] [Google Scholar]
- 38. Oloumi A., Syam S., Dedhar S. (2006) Modulation of Wnt3a-mediated nuclear β-catenin accumulation and activation by integrin-linked kinase in mammalian cells. Oncogene 25, 7747–7757 [DOI] [PubMed] [Google Scholar]
- 39. Novak A., Hsu S. C., Leung-Hagesteijn C., Radeva G., Papkoff J., Montesano R., Roskelley C., Grosschedl R., Dedhar S. (1998) Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 95, 4374–4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burkhalter R. J., Symowicz J., Hudson L. G., Gottardi C. J., Stack M. S. (2011) Integrin regulation of β-catenin signaling in ovarian carcinoma. J. Biol. Chem. 286, 23467–23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan C., Costello P., Sanghera J., Dominguez D., Baulida J., de Herreros A. G., Dedhar S. (2001) Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− colon carcinoma cells. Oncogene 20, 133–140 [DOI] [PubMed] [Google Scholar]
- 42. El-Hoss J., Arabian A., Dedhar S., St-Arnaud R. (2014) Inactivation of the integrin-linked kinase (ILK) in osteoblasts increases mineralization. Gene 533, 246–252 [DOI] [PubMed] [Google Scholar]
- 43. Novak A., Dedhar S. (1999) Signaling through β-catenin and Lef/Tcf. Cell. Mol. Life Sci. 56, 523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fonar Y., Frank D. (2011) FAK and WNT signaling: the meeting of two pathways in cancer and development. Anticancer Agents Med. Chem. 11, 600–606 [DOI] [PubMed] [Google Scholar]
- 45. Haÿ E., Faucheu C., Suc-Royer I., Touitou R., Stiot V., Vayssière B., Baron R., Roman-Roman S., Rawadi G. (2005) Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J. Biol. Chem. 280, 13616–13623 [DOI] [PubMed] [Google Scholar]
- 46. Zhang G., Guo B., Wu H., Tang T., Zhang B. T., Zheng L., He Y., Yang Z., Pan X., Chow H., To K., Li Y., Li D., Wang X., Wang Y., Lee K., Hou Z., Dong N., Li G., Leung K., Hung L., He F., Zhang L., Qin L. (2012) A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat. Med. 18, 307–314 [DOI] [PubMed] [Google Scholar]
- 47. Haÿ E., Laplantine E., Geoffroy V., Frain M., Kohler T., Müller R., Marie P. J. (2009) N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/β-catenin signaling, osteoblast function, and bone formation. Mol. Cell. Biol. 29, 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miraoui H., Oudina K., Petite H., Tanimoto Y., Moriyama K., Marie P. J. (2009) Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J. Biol. Chem. 284, 4897–4904 [DOI] [PubMed] [Google Scholar]
- 49. Haÿ E., Dieudonné F. X., Saidak Z., Marty C., Brun J., Da Nascimento S., Sonnet P., Marie P. J. (2014) N-cadherin/Wnt interaction controls bone marrow mesenchymal cell fate and bone mass during aging. J. Cell Physiol. 229, 1765–1775 [DOI] [PubMed] [Google Scholar]
- 50. Jilka R. L., Weinstein R. S., Takahashi K., Parfitt A. M., Manolagas S. C. (1996) Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J. Clin. Invest. 97, 1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saidak Z., Haÿ E., Marty C., Barbara A., Marie P. J. (2012) Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell 11, 467–474 [DOI] [PubMed] [Google Scholar]
- 52. Liao X., Lu S., Zhuo Y., Winter C., Xu W., Wang Y. (2012) Visualization of Src and FAK activity during the differentiation process from HMSCs to osteoblasts. PLoS ONE 7, e42709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu J. K., Chen H. J., Li X. D., Huang Z. L., Xu H., Yang H. L., Hu J. (2012) Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase. J. Biol. Chem. 287, 26200–26212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geoffroy V., Ducy P., Karsenty G. (1995) A PEBP2α/AML-1-related factor increases osteocalcin promoter activity through its binding to an osteoblast-specific cis-acting element. J. Biol. Chem. 270, 30973–30979 [DOI] [PubMed] [Google Scholar]
- 55. Nusse R. (2005) Wnt signaling in disease and in development. Cell Res. 15, 28–32 [DOI] [PubMed] [Google Scholar]
- 56. Haÿ E., Nouraud A., Marie P. J. (2009) N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling. PLoS ONE 4, e8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kawano Y., Kypta R. (2003) Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- 58. Bodine P. V., Zhao W., Kharode Y. P., Bex F. J., Lambert A. J., Goad M. B., Gaur T., Stein G. S., Lian J. B., Komm B. S. (2004) The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18, 1222–1237 [DOI] [PubMed] [Google Scholar]
- 59. Yao G. Q., Wu J. J., Troiano N., Insogna K. (2011) Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J. Bone Miner. Metab. 29, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruoslahti E. (1996) RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715 [DOI] [PubMed] [Google Scholar]
- 61. Mould A. P., Koper E. J., Byron A., Zahn G., Humphries M. J. (2009) Mapping the ligand-binding pocket of integrin α5β1 using a gain-of-function approach. Biochem. J. 424, 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U.S.A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maydan M., McDonald P. C., Sanghera J., Yan J., Rallis C., Pinchin S., Hannigan G. E., Foster L. J., Ish-Horowicz D., Walsh M. P., Dedhar S. (2010) Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3β) phosphorylation. PLoS ONE 5, e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chamorro M. N., Schwartz D. R., Vonica A., Brivanlou A. H., Cho K. R., Varmus H. E. (2005) FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J. 24, 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]