Abstract
Background: Fourier transform infrared (FTIR) spectroscopy has shown its unique advantages in distinguishing cancerous tissue from normal one. The aim of this study was to establish a quick and accurate diagnostic method of FTIR spectroscopy to differentiate malignancies from benign breast tissues intraoperatively. Materials and methods: In this study, a total of 100 breast tissue samples obtained from 100 patients were taken on surgery. All tissue samples were scanned for spectra intraoperatively before being processed for histopathological diagnosis. Standard normal variate (SNV) method was adopted to reduce scatter effects. Support vector machine (SVM) classification was used to discriminate spectra between malignant and benign breast tissues. Leave-one-out cross validation (LOOCV) was used to evaluate the discrimination. Results: According to histopathological examination, 50 cases were diagnosed as fibroadenoma and 50 cases as invasive ductal carcinoma. The results of SVM algorithm showed that the sensitivity, specificity and accuracy rate of this method are 90.0%, 98.0% and 94.0%, respectively. Conclusions: FTIR spectroscopy technique in combination with SVM classification could be an accurate, rapid and objective tool to differentiate malignant from benign tumors during operation. Our studies establish the feasibility of FTIR spectroscopy with chemometrics method to guide surgeons during the surgery as an effective supplement for pathological diagnosis on frozen section.
Keywords: FTIR spectroscopy, breast tumor, support vector machine, intraoperative diagnosis
Introduction
Breast cancer is the most common cancer that affects women (excluding skin cancer) worldwide and the second most common cause of women death from cancer in the United States (after lung cancer). Prognosis and survival rates for breast cancer vary greatly depending on the cancer type, stage, treatment, and geographical location of the patient. Therefore, early detection in order to improve breast cancer outcome and survival remains the cornerstone of breast cancer control.
According to the internationally accepted guidelines, mammography screening, regular clinical breast examination, ultrasound, magnetic resonance imaging (MRI) of women can result in down-staging of breast cancer. Nevertheless, these methods do have well-recognized limitations to differentiate the malignant and benign breast lesions [1-4]. Up to now, the histopathological diagnosis according to the changes in microscopic morphology and composition of breast tissues based on surgical operation is still regarded as the gold standard for breast lesions. However, some clear disadvantages of this method are as follows. Firstly, this method is time consuming and involves fixation, dehydration, embedding, slicing and the complex process of histochemical staining technique [5]. Usually, patients have to wait for nearly a week to get the exact nature of tumor. Secondly, the diagnosis depends on the pathologist’s subjective judgment, experience and range of knowledge. As a result, different diagnoses may be made by different pathologists when judging the same section. Finally, histopathological diagnosis is limited to the area being examined and requires extensive human observations to recognize both the constitutive histologic entities and the pathologic states. From the above, great emphasis is placed on finding diagnostic methods that may enhance the ability to differentiate between benign and malignant tissues, to make fast and accurate decisions during operation, and to avoid performing unnecessary extensive procedures or repeated operations.
Fourier transform infrared (FTIR) technology shows potential as a new, promising diagnostic method to detect pathologic alterations in tissues, which are morphological and molecular in nature [6-13]. In the generation and development of cancer, nuclear acids, proteins, carbohydrates, lipids, water and other biomolecules generate significantly changes, which could not be detected by conventional techniques. FTIR spectroscopy could detect all of these chemical changes of biological tissues at the molecular level. Moreover, this method could detect human tissues directly without any reagent and requires minimal sample preparation, so the operation is more simple, time saving and convenient.
The purpose of our study was to explore the possibility of establishing a quick and accurate diagnostic method by intraoperative application of FTIR spectroscopy to differentiate malignancy from benign breast tissues. Support vector machine (SVM) classification were used to discriminate spectra between malignant and benign breast tissues.
Materials and methods
Tissue specimens
A total of 100 women patients with breast nodules underwent surgery in our hospital from October, 2012 to November 2013. The average age of patients was 46 ± 14 y (range, 19-77 y). Preoperative diagnoses were made based on thorough history inquiry of patients, complete physical examination of breast, and auxiliary tests including serology, biomedical tests, and the report of ultrasonography or mammography. Tissue samples for FTIR scanning were obtained from the center of the breast lesions during operation, and were immediately (within 3 min at maximum) prepared to approximately 1.0 cm × 1.0 cm × 0.5 cm in size and mounted on ATR detection plate attached to FTIR spectroscopic equipment without special sample preparation [14]. Each scan took approximately 1-2 min. After scanning, the same tissue samples were processed as paraffin embedded blocks and sent to pathologists for pathologic diagnosis. Fifty of these patients were histopathologically diagnosed as fibroadenoma, and the others as invasive ductal carcinoma. This study was approved by Peking University Biomedical Ethics Committee and Institutional Review Board of Peking University Third Hospital (IRB00001052-11034) and all participants gave their written, informed consents to the study.
Acquisition of FTIR spectra for breast tissues
Breast tissue samples were measured using attenuated total reflection (ATR) equipped with a liquid-nitrogen-cooled mercury cadmium telluride detector (Fourier transform infrared spectrometer, WQF-660, Beijing Rayleign Analytical Instrument Co., Ltd, Beijing, China). Mid-infrared radiation was passed to and from the ATR accessory. Spectra were measured at a resolution of 8 cm-1 and 32 scans were co-added to achieve an acceptable signal-to-noise ratio, with wave number ranging from 900 cm-1 to 2000 cm-1. Background spectrum was recorded before scanning every sample tissue and was subtracted from sample spectrum automatically to eliminate atmospheric effects.
Spectra preprocessing method
A total of 100 spectra were obtained and the dimension of each sample is 286. The pre-processing steps including analysis and model construction were done in MATLAB R2013a (MathWorks, Inc., Natick, Mass., USA) under operating system Windows 8 for China. Standard normal variate (SNV) method was adopted to weaken the effect to the accuracy of modeling from the differences of sample shape, size, density and nonspecific scatter at the surface of the samples [15].
The spectroscopic data of sample i at wavenumber k could be standard normalized as Equation 1.
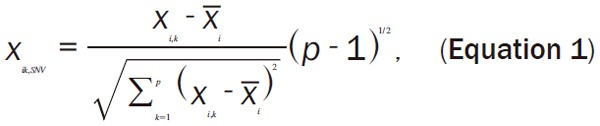 |
Where x̅i represents the average of spectroscopic data of sample i, while p is the number of wavelength, and (p-1) is the freedom degrees.
Discrimination analysis method
The original SVM algorithm was proposed by Vapnik in 1995. This method is based on the idea of hyper-plane classifiers, and it tries to look for the hyper-plane that maximizes the margin between two classes [16]. The SVM employs a non-linear mapping to transform the original training data into higher dimensional data and searches for the linear optimal separating hyperplane within this new dimension. This principle can be explained as follows [17].
For labeled training data of the form (xi, yi), i ∈ {1, ..., I} where xi is an n-dimensional feature vector and the target output y ∈ {-1, 1}, the decision surface of SVM in the form of separating hyperplane is defined as Equation 2.
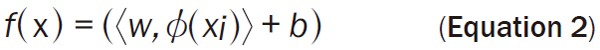 |
Where w is the weight vector, b is the bias and Φ(x) is the kernel function. By projecting the data using a mapping Φ(x), nonlinear decision boundaries in the input data space can be obtained. The separating hyperplane is found by maximizing distances to its closest data points, embedding it in a large margin which is defined by support vectors. Finding the hyperplane while maximizing the margin is formulated as the following optimization problem:
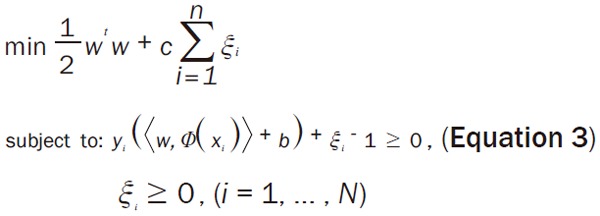 |
Where C denotes the regularization parameter, ξi is parameter for handling non-separable data, and the index i labels the N training cases. Note that y ∈ {-1, 1} is the class labels and xi is the independent variables.
The optimization problem can be efficiently solved using an equivalent formulation to Equation 3 using Lagrangian multipliers. In this formulation, data are represented exclusively by dot products, which can be replaced with a kernel function K(x, x’) = Φ(x)TΦ(x’), allowing large margin separation in the kernel space. The choice of the kernel function determines the mapping Φ(x) of the input data into a higher dimensional feature space, in which the linear separating hyperplane is found (see also Equation 2). In this paper, nonlinear classification using the Gaussian kernel as Equation 4 was investigated.
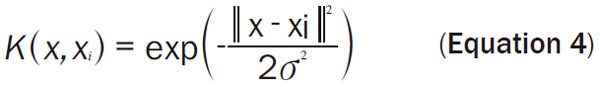 |
It comprises one free parameter, the kernel width σ, which controls the amount of local influence of support vectors on the decision boundary.
In this study, five parameters were chosen to test the efficiency of this algorithm: sensitivity (Se), specificity (Sp), accuracy (Acc), positive predictive value (PPV) and negative predictive value (NPV). Their formulas were as follows (TP: true positive; FP: false positive; TN: true negative; FN: false negative) (Equations 5, 6, 7, 8 and 9):
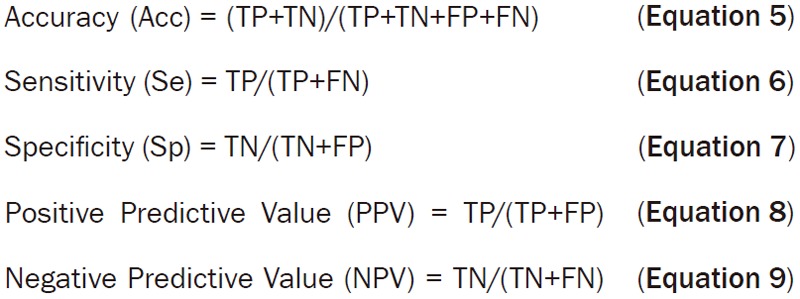 |
Results
Pathologic examination
Surgically removed breast tissues were fixed in formalin and sent to pathologists for diagnosis immediately after FTIR spectroscopy scanning. Fifty invasive ductal carcinomas and fifty fibroadenomas were pathologically diagnosed.
FTIR spectra of breast tissues
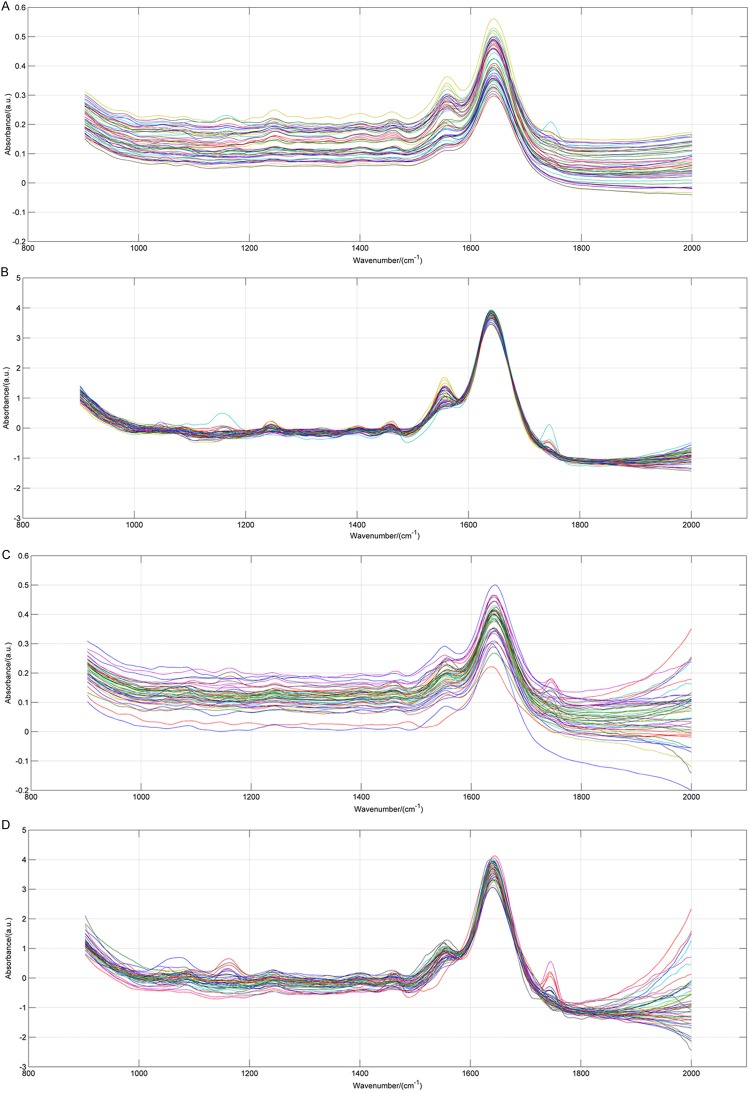
Table 1 is the preliminary assignment of FTIR bands of breast samples. Figure 1 shows the typical FTIR spectra of invasive ductal carcinoma and fibroadenoma. Figure 2 shows the original FTIR spectra of our research and it would be seen that the spectra obtained from two different groups divided into two clusters. After the SNV spectra preprocessing, the differences between FTIR spectra of benign and malignant breast tissues are more pronounced, and the boundary of two clusters is also clearer. However, any single parameter change could not sufficiently distinguish the two groups.
Table 1.
Preliminary assignments of characteristic bands in the FTIR spectra of breast samples
| Peak position (cm-1) | Vibrations | Assignments of substance |
|---|---|---|
| 1743 | v C=O | Lipid |
| 1640 | Amide I band | Protein |
| 1550 | Amide II band | Protein |
| 1460 | δ C-H | Lipid related |
| 1400 | δ C-H, δ C-O-H | Lipid related |
| 1300 | δ C-H, δ C-O-H, Amide III band | Undetermined |
| 1250 | v as, PO2 - | Nucleic acid related |
| 1160 | v C-O, δ C-O-H, δ C-O-C | Carbohydrate related |
| 1080 | v s , PO2 - | Nucleic acid related |
| 1040 | v C-O, δ C-O-H, δ C-H | Carbohydrate related |
v, stretching vibration; δ, bending vibration; vas, asymmetric stretching vibration; v s, symmetric stretching vibration.
Figure 1.
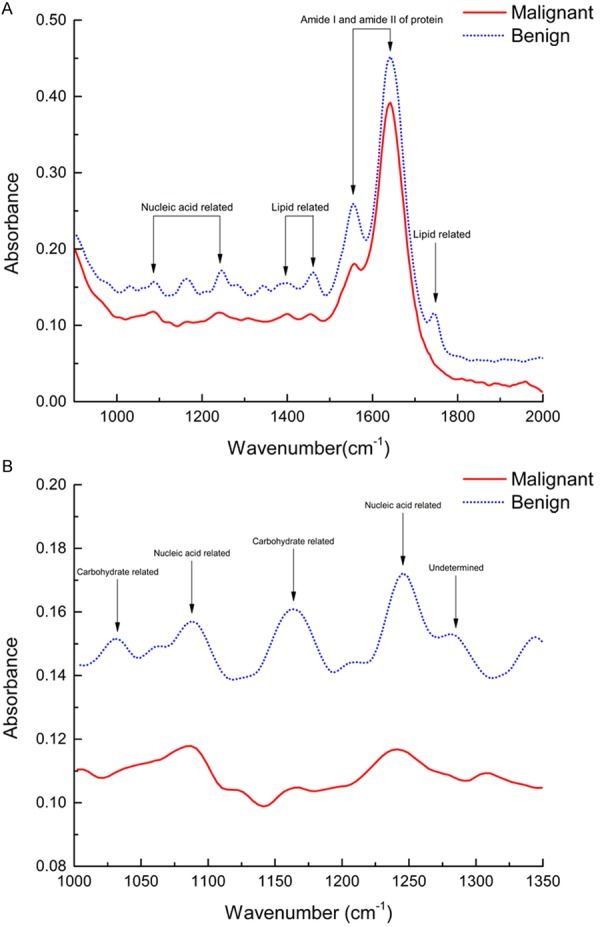
Typical FTIR spectra of invasive ductal carcinoma (malignant) and fibroadenoma (benign). A: Entire spectra of fingerprint region (the most sensitive region) with wavenumber ranging from 900 to 2000 cm-1; B: Wavenumber ranging from 1000 to 1350 cm-1.
Figure 2.
The FTIR spectra of benign and malignant breast tissues. A: The original spectra of 50 benign breast tissues; B: The spectra of benign breast tissues after SNV preprocess; C: The original spectra of 50 malignant breast tissues; D: The spectra of malignant breast tissues after SNV preprocess.
Discrimination analysis
Discrimination model was built for classification of benign and malignant breast tissues and leave-one-out cross validation (LOOCV) was utilized to evaluate the discrimination results of SVM method. The LOOCV method attempts to predict the data of the unknown sample with the data of training sample set. One sample was randomly selected and excluded from the training set. The selected sample regarded is as an unknown one, and then the sample is classified using the model built with the rest training samples. Repeat the above course until all samples had been selected for once and only for once.
Comparison between FTIR spectroscopy technique and the gold standard of histopathologic diagnosis is shown in Table 2. Cross tabulation indicates that the sensitivity (Se) and the specificity (Sp) of SVM method are 90.0% and 98.0%, respectively. The positive predictive value (PPV) and negative predictive value (NPV) were 97.8% and 90.7%, respectively. The accuracy (Acc) of this method is 94.0%.
Table 2.
Discrimination results between FTIR spectroscopy with SVM method and histopathologic examination (gold standard)
| FTIR spectroscopy with SVM method | Histopathologic examination | ||
|---|---|---|---|
|
| |||
| Invasive ductal carcinoma | Fibroadenoma | Sum | |
| Invasive ductal carcinoma | 45 | 1 | 46 |
| Fibroadenoma | 5 | 49 | 54 |
| Total | 50 | 50 | 100 |
Discussion
In this study, FTIR spectroscopy combined with support vector machine method was used to analyze the real-time infrared spectral data of breast tissues obtained in surgery, and the result showed that the accuracy, sensitivity and specificity were all high, indicating that this method helps to distinguish invasive ductal breast cancer from fibroadenoma in surgery and is of great importance for avoiding blind biopsy, providing effective information for resection range and selecting the most reasonable therapy. It is further confirmed that infrared spectroscopy combined with chemometrics could differentiate malignant from benign tumors.
Currently, postoperative pathological diagnosis is still considered the gold standard for diagnosis of breast cancer. Nevertheless, the process of pathological diagnosis is extraordinary complex and it will take at least one week to finally complete. In surgery, the surgeons often need the report of intraoperative frozen section examination to determine whether to undertake the further radical surgery operation. However, as a diagnostic tool, the intraoperative frozen section examination has several limitations. To begin with, the effectiveness of intraoperative frozen section diagnosis is not stable and the delayed diagnosis or misdiagnosis has happen all the time due to the rush time, limited collection, poor quality section, finite experience of pathologists and other reasons. The coincidence rate of frozen and paraffin sections was between 83.8%-98.3%, sensitivity of 65%-100%, and specificity of 87.5%-100% [18-22], and even whether should determine the reasonable resection range according to intraoperative frozen section remains controversial [23]. It was common in clinic that the intraoperative frozen report was benign, causing no radical surgery, which was proved to be a malignant tumor after postoperative pathological examination. Once such a false-negative is there, these patients have to take a second operation. Moreover, each frozen section examination takes about 30 minutes and in some cases this procedure should have to do several times, which further prolong the time of surgery and anesthesia. In the modern hospitals there are often more than one surgery to perform simultaneously and several frozen pathological sections were taken at the same time, which would certainly affect the process of these operations. Last but not least, fundamentally speaking, pathological diagnosis is an invasive examination and tissues removed from surgery are needed to confirm the results. For some essential organs, this method undoubtedly increases the risk of injury. And for the tissues which eventually diagnosed with normal, this step is equivalent to the unnecessary removal. So, currently the most pressing problems for the surgeons are how to make rapid diagnosis during surgery, reduce the erroneous diagnosis or missed diagnosis caused by the subjective factors and lack of experience of the pathologists or the surgeons and avoid unnecessary tissue resection.
Nucleic acids, proteins, carbohydrates, lipids and water are the major constituent components of biological tissues. When these molecules struck by a continuous wavelength infrared light, they absorb some wavelengths and so passes on only part of the spectrum. All that remained is the infrared absorption spectrum of this molecule. So, each molecule has a specific infrared absorption spectrum determined by its composition and structure. Once the patient has a disease, tumor or other abnormalities in the body, the conformation and content of these molecules would changes and the spectrum then change with it [24]. FTIR spectroscopy is based on this principle to detect the infrared absorption spectrum changes of tissues by computer software rapidly and objectively, ultimately differentiate malignant from benign tumors.
The infrared spectroscopy of human tissue is composed of multiple extremely complex overlapping peaks, which contain a great amount of information inside. So how to deal with these information and how to extract efficiently reliable characteristic indexes from the spectrums are the most crucial problems the spectroscopists face. In the long-period activities of spectral classification and pattern recognition, the scientists found two important reasons causing the failure of traditional statistical methods: 1. Due to the performance of most classification algorithms is influenced by potential confounders such as individual differences and interferences of environment, the sample size should be enlarged dramatically; 2. A large number of high-dimensional data generated from the infrared spectrums need for effective management, accurate interpretation and fully utilized, otherwise it will produce so-called “curse of dimensionality” [25,26].
As one algorithm of the machine learning based on statistic theory, SVM has unique advantages in solving small sample, non-linear and high dimension mode pattern recognition. This method constructs a hyperplane or set of hyperplanes in a high dimensional space by the pre-selected nonlinear mapping (kernel), which can be used for classification, regression, or other tasks [16,27]. Intuitively speaking, a good separation is achieved by the hyperplane that has the largest distance to the nearest training data point of any class (so-called functional margin), since in general the larger the margin the lower the generalization error of the classifier. With appropriate non-linear mapping to a sufficiently high dimension, a decision boundary can separate data into two classes. It has been reported that SVM method could compensate the influence of uncertainty and nonlinearity in spectral quantitative analysis due to its high accuracy and good generalization ability [28,29].
According to the experimental results, our method has higher accuracy in diagnosing benign and malignant breast tumors. This means that FTIR spectroscopy technique could be an effective supplement for pathological diagnosis and frozen section in surgery to guide surgeons during the procedure. In addition, the sensitivity of our method is 90.0%. This means that the proposed system can detect a malignant tumor with high probability. Further, the high PPV and NPV indicate that the number of biopsies for benign lesions can be reduced. This may increase diagnostic confidence, enable offering of a second reading to help reduce the misdiagnosis, and dramatically reduce the overall cost of diagnosing breast cancer in practical use. Hence, infrared spectroscopy combined with SVM discrimination method is likely to become an effective diagnostic tool for classifying benign and malignant tumors and can assist physicians in avoiding misdiagnosis.
Unlike conventional techniques, this method can translate the biological information into spectroscopy data based on solid mathematical foundation by computer, which is more objective, scientific and efficient. Furthermore, the method does not require additional processing of specimens and the measurement time is very short (only 2-3 minutes), so it could be used timely to detect and differentiate of malignancy from benign tissues in the operating room. The low costs of this method also establish a good basis for the further research and diagnostic application.
This study still has the following limitations. The study involved only two most common histological types of breast tumor, fibroadenoma and invasive ductal carcinoma, infrared spectroscopy studies for other types remains to be further researched. Furthermore, the number of patients has to be significantly increased for such analysis, which only can be realized in a multi-center study. Lastly, several kernel functions can be used in SVM, including the linear, polynomial, sigmoid and radial basis kernel functions. How to choose the best kernel function for different research directions is still an unsolved problem.
On the basis of the existing research, in the future we will focus on detecting the sentinel nodes of breast cancer patients in situ and in vivo by FTIR spectroscopy to guide the excision extent and determine whether the axillary lymph node dissection is required. Furthermore, combined with endoscopy and puncture biopsy to guide real-time in situ diagnosis and biopsy during surgery is currently under the investigation by the authors. We also believe that expand the sample size and undertake multi-center study can improve the accuracy even further.
The developing trend of the future medicine is combination of basic research and clinical application. With the further development of FTIR technology and deeper improvement of tumor understanding, we have every reason to believe that this technique will soon become an important diagnostic method for malignant tumors and may even as a routine screening tool applied to stage and grade the tumors. All these implementations require good communication and cooperation between spectroscopists and surgeons.
Conclusions
In this paper, FTIR spectroscopy combined with SVM classification was successfully used to distinguish benign and malignant breast tumors in surgery and the method has achieved a high discriminant accuracy with 94.0%. The results indicate that FTIR spectroscopy with chemometrics method is reliable, practical, and could be easily implemented in breast cancer diagnosis. This method can be an effective supplement for pathological diagnosis and frozen section in surgery to guide surgeons during the procedure.
Acknowledgements
This study was supported by grants from Beijing Natural Science Foundation (NO. 2122059), Clinical Research Project of Peking University Third Hospital (B59427-01) and Major Research Project of Peking University Third Hospital (BYSY201207). We also gratefully acknowledge Beijing Rayleign Analytical Instrument Co., Ltd (Beijing, China) for providing excellent assistance.
Disclosure of conflict of interest
None.
References
- 1.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 3.Lord SJ, Lei W, Craft P, Cawson JN, Morris I, Walleser S, Griffiths A, Parker S, Houssami N. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905–1917. doi: 10.1016/j.ejca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Velez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Larsen LH, Barr RG, Farria DM, Marques HS, Boparai K. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rorke LB. Pathologic diagnosis as the gold standard. Cancer. 1997;79:665–667. doi: 10.1002/(sici)1097-0142(19970215)79:4<665::aid-cncr1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Li QB, Sun XJ, Xu YZ, Yang LM, Zhang YF, Weng SF, Shi JS, Wu JG. Diagnosis of gastric inflammation and malignancy in endoscopic biopsies based on Fourier transform infrared spectroscopy. Clin Chem. 2005;51:346–350. doi: 10.1373/clinchem.2004.037986. [DOI] [PubMed] [Google Scholar]
- 7.Liu MJ, Wang Z, Wu RC, Sun SQ, Wu QY. Monitoring all-trans-retinoic acid-induced differentiation of human acute promyelocytic leukemia NB4 cells by Fourier-transform infrared spectroscopy. Leukemia. 2003;17:1670–1674. doi: 10.1038/sj.leu.2403019. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Xu Y, Wu J, Zhang Y, Sun K. Detection of lung cancer tissue by attenuated total reflection-Fourier transform infrared spectroscopy-a pilot study of 60 samples. J Surg Res. 2013;179:33–38. doi: 10.1016/j.jss.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 9.Noreen R, Moenner M, Hwu Y, Petibois C. FTIR spectro-imaging of collagens for characterization and grading of gliomas. Biotechnol Adv. 2012;30:1432–1446. doi: 10.1016/j.biotechadv.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Xu Y, Zhang Y, Wang D, Xiu D, Xu Z, Zhou X, Wu J, Ling X. Detection of cervical metastatic lymph nodes in papillary thyroid carcinoma by Fourier transform infrared spectroscopy. Br J Surg. 2011;98:380–384. doi: 10.1002/bjs.7330. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SE, Cheung KT, Patel II, Trevisan J, Stringfellow HF, Ashton KM, Wood NJ, Keating PJ, Martin-Hirsch PL, Martin FL. Infrared spectroscopy with multivariate analysis to interrogate endometrial tissue: a novel and objective diagnostic approach. Br J Cancer. 2011;104:790–797. doi: 10.1038/sj.bjc.6606094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes C, Brown MD, Clarke NW, Flower KR, Gardner P. Investigating cellular responses to novel chemotherapeutics in renal cell carcinoma using SR-FTIR spectroscopy. Analyst. 2012;137:4720–4726. doi: 10.1039/c2an35632e. [DOI] [PubMed] [Google Scholar]
- 13.Zwielly A, Mordechai S, Brkic G, Bogomolny E, Pelly IZ, Moreh R, Gopas J. Grading of intrinsic and acquired cisplatin-resistant human melanoma cell lines: an infrared ATR study. Eur Biophys J. 2011;40:795–804. doi: 10.1007/s00249-011-0695-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Xu Y, Zhang Y, Wang L, Hou C, Zhou X, Ling X, Xu Z. Intraoperative detection of thyroid carcinoma by fourier transform infrared spectrometry. J Surg Res. 2011;171:650–656. doi: 10.1016/j.jss.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Barnes RJ, Dhanoa MS, Lister SJ. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl Spectrosc. 1989;43:772. [Google Scholar]
- 16.Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20:273–297. [Google Scholar]
- 17.Keerthi SS, Lin CJ. Asymptotic behaviors of support vector machines with Gaussian kernel. Neural Comput. 2003;15:1667–1689. doi: 10.1162/089976603321891855. [DOI] [PubMed] [Google Scholar]
- 18.Cendan JC, Coco D, Copeland EM 3rd. Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg. 2005;201:194–198. doi: 10.1016/j.jamcollsurg.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Weber WP, Engelberger S, Viehl CT, Zanetti-Dallenbach R, Kuster S, Dirnhofer S, Wruk D, Oertli D, Marti WR. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J Surg. 2008;32:2599–2606. doi: 10.1007/s00268-008-9757-8. [DOI] [PubMed] [Google Scholar]
- 20.Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: a systematic review. Ann Surg Oncol. 2012;19:3236–3245. doi: 10.1245/s10434-012-2492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler-Henderson K, Lee AH, Price RI, Waring K. Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast. 2014;23:112–119. doi: 10.1016/j.breast.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Caruso F, Ferrara M, Castiglione G, Cannata I, Marziani A, Polino C, Caruso M, Girlando A, Nuciforo G, Catanuto G. Therapeutic mammaplasties: full local control of breast cancer in one surgical stage with frozen section. Eur J Surg Oncol. 2011;37:871–875. doi: 10.1016/j.ejso.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Manfrin E, Remo A, Falsirollo F, Pollini GP, Parisi A, Nottegar A, Bonetti F. Intra-operative frozen section technique for breast cancer: end of an era. Pathologica. 2011;103:325–330. [PubMed] [Google Scholar]
- 24.Fernandez DC, Bhargava R, Hewitt SM, Levin IW. Infrared spectroscopic imaging for histopathologic recognition. Nat Biotechnol. 2005;23:469–474. doi: 10.1038/nbt1080. [DOI] [PubMed] [Google Scholar]
- 25.Eric PX, Michael IJ, Richard MK. Feature selection for high-dimensional genomic microarray data. 18th International Conference on Machine Learning. 2001;601:39–48. [Google Scholar]
- 26.Trevisan J, Angelov PP, Carmichael PL, Scott AD, Martin FL. Extracting biological information with computational analysis of Fourier-transform infrared (FTIR) biospectroscopy datasets: current practices to future perspectives. Analyst. 2012;137:3202–3215. doi: 10.1039/c2an16300d. [DOI] [PubMed] [Google Scholar]
- 27.Christopher JC. A tutorial on support vector machines for pattern recognition. Data Min Knowl Discov. 1998;2:121–167. [Google Scholar]
- 28.Barman I, Kong CR, Dingari NC, Dasari RR, Feld MS. Development of robust calibration models using support vector machines for spectroscopic monitoring of blood glucose. Anal Chem. 2010;82:9719–9726. doi: 10.1021/ac101754n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mello C, Marangoni A, Poppi R, Noda I. Fast determination of thyroid stimulating hormone in human blood serum without chemical preprocessing by using infrared spectroscopy and least squares support vector machines. Anal Chim Acta. 2011;696:47–52. doi: 10.1016/j.aca.2011.04.015. [DOI] [PubMed] [Google Scholar]