Abstract
Objectives: To investigate the value of contrast-enhanced ultrasound (CEUS) in the differential diagnosis between gallbladder adenomas and gallbladder adenomas canceration. Methods: CEUS data from 34 patients (25 patients with gallbladder adenomas and 9 patients with gallbladder adenomas canceration) were retrospectively analyzed, including the characteristics of contrast arrival time, time to peak enhancement, enhancement extend, enhancement morphology and the intactness of gallbladder wall below the lesions. Results: On CEIS, the contrast arrival time and the time to peak enhancement were significantly shorter in patients with gallbladder adenomas than in patients with gallbladder adenomas canceration (12.63 ± 3.37 s vs. 18.11 ± 3.26 s, P < 0.001; 17.42 ± 3.69 s vs. 24.56 ± 4.36 s, P < 0.001). The time to iso-enhancement showed no significant difference between the two groups; while the time to hypo-enhancement was significantly shorter in patients with gallbladder adenomas canceration than in patients with gallbladder adenomas (55.56 ± 15.48 s vs. 84.71 ± 36.07 s, P = 0.027), and the enhancement time of the liver was significantly slower in patients with gallbladder adenomas canceration than in patients with gallbladder adenomas (22.78 ± 5.28 s vs. 16.63 ± 4.66 s, P = 0.004). Using receiver operating characteristic (ROC) analysis, the time to peak enhancement greater than 20 s had 89% sensitivity and 84% specificity for detecting patients with gallbladder adenomas canceration. The enhancement level showed no difference between the two groups. Inhomogeneous enhancement was found in 33% (3/9) gallbladder adenoma canceration and none (0/25) of gallbladder adenoma (P < 0.01). Destruction of gallbladder wall intactness was found in 66.7% (6/9) gallbladder adenoma canceration and none (0/25) of gallbladder adenoma (P < 0.01). Conclusion: CEUS is useful in differentiation between gallbladder adenoma and gallbladder adenoma canceration. The time to peak enhancement, the enhancement morphology and the intactness of gallbladder wall below the lesions are the diagnostic clues in differentiating diagnosis between gallbladder adenoma and gallbladder adenoma canceration.
Keywords: Contrast-enhanced ultrasound, gallbladder adenoma, gallbladder adenoma canceration
Introduction
Gallbladder adenoma is a rare benign gland-forming neoplasm arising from mucosal epithelium with malignant potential. Adenomas have been reported in 0.2% to 0.5% of surgically resected gallbladders [1,2]. It has been proven that patients with gallbladder adenoma are at higher risk of adenoma canceration and the rate is about 30% [3-5]. Therefore, it is of great importance to make an early and accurate diagnosis of gallbladder adenoma and adenoma canceration.
Conventional ultrasound is the preferred initial screening method for gallbladder disease because it is usually less expensive than computed tomography (CT) or magnetic resonance imaging (MRI), without ionizing radiation, and with high accuracy [6]. However, the diagnostic accuracy of conventional ultrasound is subjected to various influencing factors such as the inappropriate position of the gallbladder, the artifacts associated with ultrasound imaging and insufficiency for obtaining low speed blood flow information in the gallbladder lesions [7]. Recently, real-time contrast-enhanced ultrasound (CEUS) has been established as a reliable tool in the detection and characterization of gallbladder lesions [7-9], which has the advantages such as real-time scanning, repeatability, scanning from arbitrary direction, no radiation, and high sensitivity in depicting macro- and micro-circulation [10].
The present study was aimed to evaluate the value of CEUS for differential diagnosis between gallbladder adenoma and adenoma canceration. To the best of our knowledge, differentiation between gallbladder adenoma and adenoma canceration with CEUS has not been reported yet.
Materials and methods
Study population
The subjects were 34 patients with gallbladder diseases underwent CEUS examination between May 2009 and February 2012. The subjects were retrospectively collected from a CEUS data bank when they met the following criteria: 1) aged 18-80 years; 2) gallbladder polypoid lesions found at conventional ultrasound examination; 3) patients who were referred for exclusion of gallbladder malignancy by clinicians; 4) agreed to underwent CEUS examination; 5) absence of severe cardiopulmonary diseases; 6) gallbladder adenoma or adenoma canceration confirmed by pathological examination after gallbladder surgery; 7) the data of conventional ultrasound and CEUS were complete. The patients consisted of 16 males and 18 females and were aged 21 to 80 years with an average age of 54.7 ± 18.8 years old. All the patients underwent surgery and the diagnoses were confirmed by histopathological evaluation. Finally, 25 patients were diagnosed as gallbladder adenoma and 9 patients as adenoma canceration (Table 1).
Table 1.
Clinical and conventional ultrasound data of two groups of patients
| Gallbladder adenoma (n = 25) | Adenoma canceration (n = 9) | P | |
|---|---|---|---|
| Age (year) | 49 ± 18 | 67 ± 10 | 0.008* |
| Gender | |||
| Female | 12 | 6 | 0.225 |
| Male | 13 | 3 | |
| Gallbladder wall thickness (cm) | 0.31 ± 0.06 | 0.32 ± 0.04 | 0.556 |
| Tumor size (cm) | 2.14 ± 0.91 | 2.80 ± 1.03 | 0.081 |
| Tumor stalk | |||
| Wide | 14 | 9 | |
| Narrow | 11 | 0 | 0.002* |
Indicates statistically significant difference.
Informed consent was obtained from all patients before the study and the study protocol was approved by the local ethics committee.
Contrast-enhanced ultrasound examination
The following ultrasound machines were used: LOGIQ E9 (GE Healthcare, Milwaukee, WI, USA), LOGIQ 9 (GE Healthcare, Milwaukee, WI, USA), Aplio XV (Toshiba Medical System, Tokyo, Japan), Acuson Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA), and IU 22 (Philips Medical Systems, Bothell, Wash, USA). The imaging settings were used consistently for the same type of ultrasound machine. Abdominal convex transducers were used in all the cases and the transducer frequency ranged from 1.0 to 6.0 MHz. Contrast specific imaging (CSI) modes were used for CEUS in all the US systems at a low mechanical index (< 0.2) in real time, which enables effective tissue cancellation and avoids destruction of microbubbles in the circulation. The contrast agent used in this study was SonoVue (BR1; Bracco SpA, Milan, Italy), consisting of phospholipid-stabilized shell microbubbles filled with sulfur hexafluoride gas.
Conventional ultrasound and CEUS examinations were performed by one author who had more than 5-years’ experience in CEUS and was not involved in the data and images analysis. Each patient was fasted at least 8 h before ultrasound examination. First, the entire gallbladder and adjacent liver parenchyma were thoroughly scanned using conventional gray-scale ultrasound and the target lesions were identified; the position, size, shape, echogenicity and lesion number were evaluated. Subsequently, contrast-specific imaging mode was initiated and 1.5 to 2.4 ml of contrast agent was injected into the antecubital vein with a bolus fashion through a 20-gauge intravenous cannula, followed by a flush of 5 ml 0.9% sodium chloride solution [8,9]. The timer was activated promptly from the beginning of contrast agent administration and the lesion was observed continuously for at least 3 minutes.
The enhancement process of gallbladder lesion was classified as early phase (10-30 s after contrast injection) and late phase (31-180 s after the injection) since the blood supply of the gallbladder is entirely arterial [8,11].
Image reading
The images of conventional ultrasound and CEUS were recorded and analyzed in consensus by two experienced investigators who were not involved in the ultrasound examination and were unknown of the clinical histories, histopathological results and other data of the patients. On CEUS, the enhancement extent of the lesion was referred to adjacent liver parenchyma and was divided into non-, hypo-, iso- and hyper-enhancement. The highest enhancement of the lesion was considered if different enhancement levels were present. The enhancement pattern was divided into homogeneous and inhomogeneous enhancement. The following parameters were measured: contrast arrival time, time to peak enhancement, time to iso-enhancement, time to hypo-enhancement and enhancement time of the liver. The intactness of gallbladder wall beneath the lesion was defined as distinct and indistinct. The continuity of the gallbladder wall was also classified as intact or destroyed.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation. Student’s two-tailed t-test and chi-squared test were used when appropriate. The diagnostic performance of contrast arrival time, time to peak enhancement, time to iso-enhancement, and time to hypo-enhancement, was assessed with receiver operating characteristic (ROC) curve and the optimal cut-off values were estimated. Youden’s index was calculated as sensitivity + specificity -1. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, Illinois, USA) and the differences were considered significant at P < 0.05.
Results
Conventional ultrasound
The mean diameter of gallbladder adenoma was 2.14 ± 0.91 cm (range, 1.2-4.4 cm). Among 25 cases with gallbladder adenoma, 17 cases had only one adenoma in each, 8 cases with multiple lesions in each and 4 cases had coexisted stones. In patients with multiple lesions, only the largest one was included in the analysis. In 19 cases, the lesions were located in gallbladder body, 3 in the bottom, 1 in the neck, 1 in the bottom and body, and 1 in the neck and body. A total of 11 lesions had narrow stalks (3 cases with pedicles) and the remaining 14 cases had wide stalks.
The mean diameter of gallbladder adenoma canceration was 2.80 ± 1.03 cm (rage 1.8-5.8 cm). All cases showed single lesion in each and one case had coexisting stone. In 4 cases, the lesions were located in the gallbladder body, 3 in the bottom, and 2 in the neck. All 9 cases had wide stalks (Table 1).
Enhancement morphology and extent of contrast-enhanced ultrasound
Homogeneous enhancement was found in all patients with gallbladder adenoma, while in patients with gallbladder adenoma canceration, homogeneous enhancement was found in 3 patients and heterogeneous enhancement was found in the remaining 6 cases (P < 0.001).
In the early phase, hyper-enhancement was found in 24 patients and iso-enhancement was found in 1 case in patients with gallbladder adenoma; hyper-enhancement was found in all 9 patients with gallbladder adenoma canceration (P > 0.05). In the late phase, hypo-enhancement was found in 24 patients and iso-enhancement was found in 1 case in patients with gallbladder adenoma; hypo-enhancement was also found in all 9 patients with gallbladder adenoma canceration (P > 0.05).
Enhancement time
As shown in Table 2 and Figures 1, 2, the contrast arrival time and the time to peak enhancement were significantly shorter in patients with gallbladder adenoma than in patients with gallbladder adenoma canceration (12.63 ± 3.37 s vs. 18.11 ± 3.26 s; 17.42 ± 3.69 s vs. 24.56 ± 4.36 s; both P < 0.001). The time to iso-enhancement, however, showed no significant difference between the two groups. The time to hypo-enhancement was significantly shorter in patients with gallbladder adenomas canceration than in patients with gallbladder adenomas (55.56 ± 15.48 s vs. 84.71 ± 36.07 s, P = 0.027), and the enhancement time of the liver was significantly slower in patients with gallbladder adenomas canceration than in patients with gallbladder adenomas (22.78 ± 5.28 s vs. 16.63 ± 4.66 s, P = 0.004). Using ROC curve analysis, the time to peak enhancement greater than 20 s had a sensitivity of 89% and a specificity of 84% for discriminating gallbladder adenoma canceration from gallbladder adenoma. The Youden’s index was 0.73, which is the highest among all the parameters (Table 3).
Table 2.
CEUS enhancement time in gallbladder adenoma and adenoma canceration group
| Gallbladder adenoma (n = 25) | Adenoma canceration (n = 9) | P | |
|---|---|---|---|
| Contrast arrival time (s) | 13.11 ± 3.63 | 18.11 ± 3.26 | 0.000* |
| Time to peak enhancement (s) | 17.42 ± 3.69 | 24.56 ± 4.36 | 0.000* |
| Time to isoenhancement (s) | 27.54 ± 10.79 | 32.33 ± 5.74 | 0.218 |
| Time to hypoenhancement (s) | 84.71 ± 36.07 | 55.56 ± 15.48 | 0.027* |
| Liver enhancement time (s) | 16.63 ± 4.66 | 22.78 ± 5.28 | 0.004* |
Indicates statistically significant difference.
Figure 1.
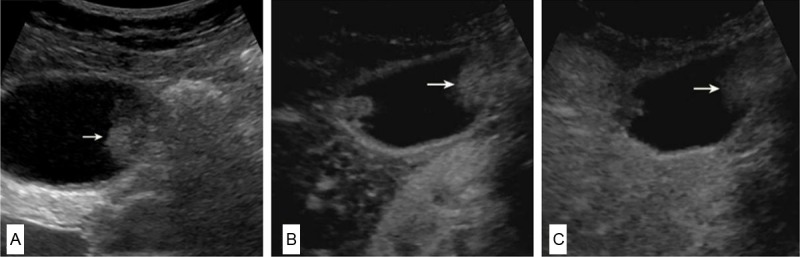
Conventional ultrasound and CEUS images of gallbladder adenoma canceration. A: An isoechoic nodule (arrow) is seen in the body of the gallbladder wall on conventional ultrasound. The diameter of the lesion is about 1.8 cm; B, C: CEUS images: 18 s after injection of contrast agent, the lesion (arrow) shows hyperenhancement and the gallbladder wall structure beneath the lesion is not clear (B); 34 s after injection of contrast agent, the lesion (arrow) becomes hypoenhancement (C).
Figure 2.
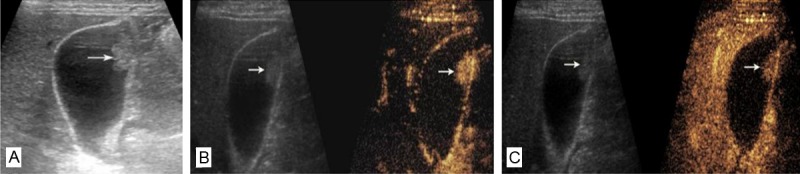
Conventional ultrasound and CEUS images of gallbladder adenoma. A: A hyperechoic nodule is present in the body of the gallbladder wall on conventional ultrasound. The diameter of the lesion (arrow) is about 1.7 cm; B, C: CEUS images: 13 s after injection of contrast agent, lesion appears hyperenhancement and the gallbladder wall structure beneath the lesion is intact (B); 45 s after injection of contrast agent, the lesion becomes hypoenhancement (C).
Table 3.
The area under the ROC curve for each CEUS enhancement parameter
| Enhancement time | AUROC | P | Cut-off value | Sen | Spe | Youden’s index |
|---|---|---|---|---|---|---|
| Contrast arrival time (s) | 0.848 (0.705-0.991) | 0.003* | 14 | 89% | 63% | 0.52 |
| Time to peak enhancement (s) | 0.901 (0.787-1.000) | 0.001* | 20 | 89% | 84% | 0.73 |
| Time to isoenhancement (s) | 0.746 (0.565-0.926) | 0.039* | 28 | 78% | 68% | 0.46 |
| Time to hypoenhancement (s) | 0.161 (0.120-0.310) | 0.004* | 28 | 7% | 67% | -0.26 |
| Liver enhancement time (s) | 0.819 (0.660-0.978) | 0.007* | 17 | 89% | 63% | 0.52 |
Indicates statistically significant difference.
AUROC: the area under the ROC curve. rocC-Hong Xu difference between them only the largest one was included in the analysis.
Intactness of gallbladder wall
The intactness of gallbladder wall beneath the lesions was identified as distinct and the continuity of gallbladder wall was intact in all 25 patients with gallbladder adenoma. In patients with gallbladder adenoma canceration, the intactness of the gallbladder wall was depicted as indistinct in 6 cases and the continuity of gallbladder wall was defined as destroyed in 5 patients (P = 0.000).
Discussion
Gallbladder adenoma is an uncommon benign epithelial neoplasm that often seen in association with cholelithiasis, chronic cholecystitis, and pyloric gland metaplasia [12]. Gallbladder adenomas can be classified histologically as tubular, papillary and tubulopapillary and they are more common in women, with a female-to-male ratio of 2.4:1 [2,13]. It has been reported that the prevalence of gallbladder adenoma was 0.3-0.5% in gallbladder after cholecystectomy due to chronic cholecystitis and calculosis [1,2]. With the increasing use of transabdominal ultrasound in daily clinical practice, more and more gallbladder adenomas are detected [14]. Gallbladder adenoma has been suggested as precancerous lesions [3-5,15], and it is well established that the prognosis of gallbladder carcinoma is poor with less than a 50% 5-year survival rate [15].
Conventional ultrasound is usually the first imaging test used to evaluate gallbladder disease. Ultrasound images often fail to distinguish between gallbladder carcinoma and chronic cholecystitis, especially in the early stage of gallbladder carcinoma [16,17], as these lesions are mucosal and do not necessarily rise to appreciable wall thickening [17]. Besides that, the destruction of the gallbladder wall beneath the lesion, and the infiltration to the adjacent liver tissue, is hard to be visible by conventional ultrasound [8]. CEUS has been introduced in the diagnosis of gallbladder disease in recent years since CEUS allows depiction of microvasculization and the microvasculization between the lesion and the surrounding tissue and the difference between them might be different [8,9,18]. In a previous study, using CEUS, it was found that CEUS was more reliable in differential diagnosis between benign and malignant gallbladder disease when compared with conventional ultrasound [8,9,18]. Adamietz et al. [19] found that patients with acute cholecystitis showed a hyper-enhancement, while patients with chronic cholecystitis showed hypo-enhancement during CEUS examination. As aforementioned, gallbladder adenoma is a precancerous lesion, conventional ultrasound plays a pivotal role in identifying the size of the lesion and follow-up for those patients, but it cannot distinguish benign from malignant gallbladder diseases accurately.
In the present study, we included 34 patients with gallbladder adenoma or gallbladder adenoma canceration who had undergone CEUS examination and gallbladder surgery and the results of CEUS were compared with the histological results after the surgery. We found that all patients with gallbladder adenoma showed homogeneous enhancement, while most patients with gallbladder adenoma canceration showed heterogeneous enhancement. Although the enhancement morphology and extent showed significant difference between two groups, those results might be subjected to the observer variability. In order to assessment the value of CEUS in differential diagnosis of gallbladder adenoma and adenoma canceration more accurately, we compared the enhancement time between the two groups. It was found that the contrast arrival time and the time to peak enhancement were significantly shorter in patients with gallbladder adenoma than in patients with gallbladder adenoma canceration, whereas the time to hypo-enhancement and enhancement time of the liver were significantly longer in patients with gallbladder adenoma canceration than in patients with gallbladder adenoma. In the current study, patients with adenoma canceration showed a slow-enhancing and fast-weakening model. Inconsistent with the results of our study, Hattori et al [20] found that patients with gallbladder carcinoma showed a fast-enhancing and fast-weakening model. This discrepancy between the studies may be explained by the differences in patient population such as clinical stage, age, etc. In the present study, we only included patients with adenoma canceration, which could be classified as early stage gallbladder carcinoma. Despite some overlap, the time to peak enhancement value greater than 20 s had a sensitivity of 89% and a specificity of 85% for discriminating gallbladder adenoma canceration from gallbladder adenoma, indicating that the time to peak enhancement maybe a useful index to identify patients with adenoma canceration in clinical practice.
The intactness of gallbladder wall beneath the lesion and the continuity of the gallbladder wall can be detected by CEUS distinctly. In our study, the intactness and the continuity of gallbladder were identified as distinct and intact in all 25 patients with gallbladder adenoma, whereas in 66.7% patients with gallbladder adenoma canceration the intactness and the continuity of gallbladder wall were destroyed.
Despite of the intriguing findings of this study, some limitations should be stressed. First, the number of the enrolled patients was small, thus the diagnostic value of CEUS should be validated in a subsequent study with more patients. Second, the observed enhancement pattern on CEUS was a subjective judgment based on visual assessment so that quantitative CEUS might be a solution in the future.
In conclusion, CEUS might be useful in making a distinction between patients with gallbladder adenoma and those with gallbladder adenoma canceration. The time to peak enhancement, the enhancement morphology and the integrity of gallbladder wall beneath the lesion might be diagnostic clues for differentiation between gallbladder adenoma and gallbladder adenoma canceration.
Acknowledgements
This work was supported in part by Grant 20114003 and 2013SY066 from Shanghai Municipal Commission of Health and Family Planning, Grant 81371570 from the National Natural Scientific Foundation of China, and Grant 2012045 from Shanghai Municipal Human Resources and Social Security Bureau.
Disclosure of conflict of interest
None.
References
- 1.Chafe BW, Chung JP, Park YN, Yoon DS, Yu JS, Lee SJ, Lee KS, Chung JB, Lee SI, Moon YM, Kang JK. Villous adenoma of the bile ducts: a case report and a review of the reported cases in Korea. Yonsei Med J. 1999;40:84–89. doi: 10.3349/ymj.1999.40.1.84. [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. Washington: DC: Armed Forces Institute of Pathology; 2000. Fasc 27, Ser 3. [Google Scholar]
- 3.Nakano S, Yamamoto M, Tahara E. Morphometric analysis of gallbladder adenocarcinoma: discrimination between carcinoma and dysplasia. Virchows Arch A Pathol Anat Histopathol. 1989;416:133–40. doi: 10.1007/BF01606318. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZX, Yin WH, Zhu ZY. Adenoma of the gallbladder and its canceration: an analysis of 88 cases. Zhonghua Wai Ke Za Zhi. 1994;32:400–402. [PubMed] [Google Scholar]
- 5.Kozuka S, Tsubone N, Yasui A, Hachisuka K. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50:2226–2234. doi: 10.1002/1097-0142(19821115)50:10<2226::aid-cncr2820501043>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Balfe DM, Ralls PW, Bree RL, DiSantis DJ, Glick SN, Levine MS, Megibow AJ, Saini S, Shuman WP, Greene FL, Laine LA, Lillemoe K, Kidd R. Imaging strategies in the initial evaluation of the jaundiced patient. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(Suppl):125–133. [PubMed] [Google Scholar]
- 7.Numata K, Oka H, Morimoto M, Sugimori K, Kunisaki R, Nihonmatsu H, Matsuo K, Nagano Y, Nozawa A, Tanaka K. Differential diagnosis of gallbladder diseases with contrast-enhanced harmonic gray scale ultrasonography. J Ultrasound Med. 2007;26:763–774. doi: 10.7863/jum.2007.26.6.763. [DOI] [PubMed] [Google Scholar]
- 8.Liu LN, Xu HX, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS, Qian LX, Luo BM, Wen YL. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7:e48371. doi: 10.1371/journal.pone.0048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie XH, Xu HX, Xie XY, Lu MD, Kuang M, Xu ZF, Liu GJ, Wang Z, Liang JY, Chen LD, Lin MX. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010;20:239–248. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 10.Meacock LM, Sellars ME, Sidhu PS. Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. Br J Radiol. 2010;83:615–627. doi: 10.1259/bjr/60619911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M, Evans DH, Filice C, Greiner L, Jäger K, Jong Nd, Leen E, Lencioni R, Lindsell D, Martegani A, Meairs S, Nolsøe C, Piscaglia F, Ricci P, Seidel G, Skjoldbye B, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 12.Albores-Saavedra J, Chablé-Montero F, González-Romo MA, Ramírez Jaramillo M, Henson DE. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum Pathol. 2012;43:1506–1513. doi: 10.1016/j.humpath.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Albores-Saavedra J, Vardaman CJ, Vuitch F. Non-neoplastic polypoid lesions and adenomas of the gallbladder. Pathol Annu. 1993;28:145–177. [PubMed] [Google Scholar]
- 14.Spaziani E, Di Filippo A, Picchio M, Lucarelli P, Pattaro G, De Angelis F, Francioni P, Vestri A, Petrozza V, Narilli F, Drudi FM, Stagnitti F. Prevalence of adenoma of gallbladder, ultrasonographic and histological assessment in a retrospective series of 450 cholecystectomy. Ann Ital Chir. 2013;84:159–164. [PubMed] [Google Scholar]
- 15.Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218–229. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 16.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671–681. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 17.Pandey ML, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28:227–232. doi: 10.1002/(sici)1097-0096(200006)28:5<227::aid-jcu4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SG, Xu HX, Liu LN, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS, Qian LX, Luo BM. Contrast-enhanced ultrasound versus conventional ultrasound in the diagnosis of polypoid lesion of gallbladder: a multi-center study of dynamic microvascularization. Clin Hemorheol Microcirc. 2013;55:359–374. doi: 10.3233/CH-121651. [DOI] [PubMed] [Google Scholar]
- 19.Adamietz B, Wenkel E, Uder M, Meyer T, Schneider I, Dimmler A, Bautz W, Janka R. Contrast enhanced sonography of the gallbladder: a tool in the diagnosis of cholecystitis? Eur J Radiol. 2007;61:262–266. doi: 10.1016/j.ejrad.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Hattori M, Inui K, Yoshino J, Miyoshi H, Okushima K, Nakamura Y, Naito T, Imaeda Y, Horibe Y, Hattori T, Nakazawa S. Usefulness of contrast-enhanced ultrasonography in the differential diagnosis of polypoid gallbladder lesions. Nihon Shokakibyo Gakkai Zasshi. 2007;104:790–798. [PubMed] [Google Scholar]


