Abstract
The skin protects mammals from insults, infection and dehydration and enables thermoregulation and sensory perception. Various skin-resident cells carry out these diverse functions. Constant turnover of cells and healing upon injury necessitate multiple reservoirs of stem cells. Thus, the skin provides a model for studying interactions between stem cells and their microenvironments, or niches. Advances in genetic and imaging tools have brought new findings about the lineage relationships between skin stem cells and their progeny and about the mutual influences between skin stem cells and their niches. Such knowledge may offer novel avenues for therapeutics and regenerative medicine.
Adult stem cells reside in niches that provide spatially distinct microenvironments for stem cell maintenance and function. The conceptual framework for stem cell niches, their compositions and their operating logistics is constantly being updated. Initially, niches were thought to be composed solely of heterologous cell populations that originate from a lineage different from the stem cells they regulate1. Recent studies have added several important modifications: differentiated progeny and stem cells can coexist within a niche, suggesting that niche signals alone are not sufficient to dictate ‘stemness’2,3; downstream progeny of stem cells can regulate their stem cell parents and thus become a component of the niche4,5; and communications between stem cells and their niches are reciprocal, as stem cells may also regulate the assembly and maintenance of their niches6.
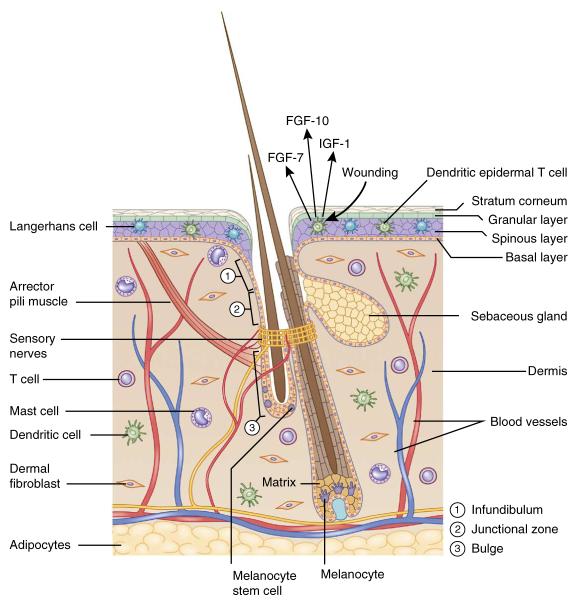
The skin is a complex organ harboring several distinct populations of stem cells and a rich array of cell types (Fig. 1), making it an ideal model for studying the interplay between stem cells and their niches. The outermost layer is the epidermis, a stratified structure that is maintained by stem cells located at the most basal layer and acts as a protective barrier. Underneath the epidermis is the dermis, enriched for dermal fibroblasts that produce collagens and elastic fibers of extracellular matrix (ECM) and give the skin its elasticity. Below the dermis lies the subcutaneous fat, which acts as protective padding, insulation and an energy reservoir.
Figure 1.
The skin: an organ with a diverse array of cell types. The hair follicle is a complex appendage of the epidermis. It is composed of an infundibulum that opens to the skin surface, sebaceous glands, and the junctional zone between the glands and the bulge. Hair follicle and melanocyte stem cells reside in the bulge and the hair germ. In full anagen, hair follicle stem cells regenerate the lower two-thirds of the follicle, including the matrix, which produces the hair and its channel. Melanocyte stem cells generate mature melanocytes, which transfer their pigment to differentiating hair cells. The hair follicle also serves as a hub attracting peripheral nerves, blood vessels and arrector pili muscles. The dermis is populated with dermal fibroblasts and various immune cells such as mast cells, dendritic cells and T cells. Deeper in the dermis is a layer of subcutaneous adipocytes.
Hair follicles are notable appendages of the epidermis. In addition to generating hairs that facilitate thermal regulation, hair follicles also serve as anchors for sensory neurons, arrector pili muscles (APMs) and blood vessels. Hair follicles undergo cycles of regeneration and rest driven by stem cells located in a region known as the bulge, and in a cluster of cells below the bulge known as the hair germ. Melanocyte stem cells (MSCs) are intermingled with hair follicle stem cells (HFSCs) in the bulge and the hair germ. The MSCs generate mature melanocytes that produce melanin, which absorbs ultraviolet (UV) light to prevent DNA damage and gives skin and hairs their distinctive colors.
In this Review, we focus on various stem cell populations in the skin, summarizing and comparing recent advances in research on skin stem cell niches that have contributed to the emergence of new concepts. We summarize the niche components and signals that regulate the behavior of epidermal stem cells, HFSCs and MSCs. In addition, we discuss how the dynamics of stem cell–niche interactions change during aging, wounding, skin cancer initiation and malignant progression. Lastly, we discuss the clinical implications of recent findings and how studying the stem cell niche might shape the future of regenerative medicine.
Stem cells in the interfollicular epidermis
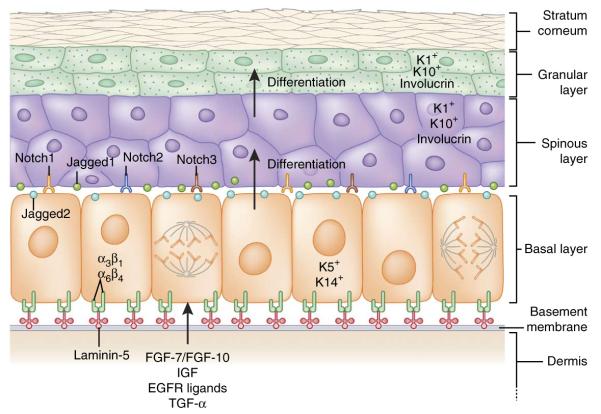
In mammals, the skin’s protective barrier is composed of a stratified epidermis (Fig. 2). The interfollicular epidermis (IFE) between hair follicles is exposed to many external insults, such as UV light, chemicals, allergens and traumatic injuries. To withstand these physical stresses, the epidermal cells, called keratinocytes, form a dense cytoskeletal infrastructure of 10-nm intermediate filaments composed of the keratin subfamily of proteins. Keratin filaments are highly enriched in the vertebrate epidermis and its appendages, but not in the surface epithelium of organisms such as insects, which instead secrete a protective outer shell.
Figure 2.
Interfollicular epidermis: architecture, signaling and lineages. The epidermis is a stratified structure. Self-renewing stem cells reside within the basal layer, which adheres through α3β1 and α6β4 integrins to an underlying basement membrane of laminin-5–rich extracellular matrix that separates the epidermis from the underlying dermis. Secreted factors such as FGF-7, FGF-10, IGF, EGF ligands and TGF-α from dermal fibroblasts promote the proliferation of basal epidermal cells. Proliferative basal progenitors generate columnar units of Notch-activated terminally differentiating cells that go through three stages: spinous layers, granular layers and finally dead stratum corneum layers that eventually are shed from the skin surface. Each cell type expresses a different gamut of keratin (K) proteins.
The innermost (basal) epidermal layer consists of undifferentiated proliferative progenitors that express keratins K5 and K14. These progenitors not only replenish the basal layer, but also give rise to nonproliferative, transcriptionally active spinous and granular layers expressing K1, K10 and involucrin, and finally the outer layers of terminally differentiated, dead stratum corneum cells7 (Fig. 2). As demonstrated by retrovirus- and mutation-based lineage tracing data8–12, these columnar tissue units are in constant flux, as outer layer cells are continually shed and replaced by differentiating cells from inner layers.
Lineage choices of interfollicular epidermal stem cells: hierarchical versus stochastic
Two distinct models have been proposed to explain the behavior of stem cells within the basal layer of the IFE (Fig. 3). The hierarchical model suggests that the IFE is composed of discrete epidermal proliferative units consisting of a slow-cycling stem cell that gives rise to short-lived transit-amplifying cells (TACs), which then depart the basal layer after several divisions to generate upward columnar units of differentiating cells. The stochastic model suggests that basal epidermis is composed of a single type of proliferative progenitor whose daughter cells choose randomly to differentiate or remain as progenitors.
Figure 3.
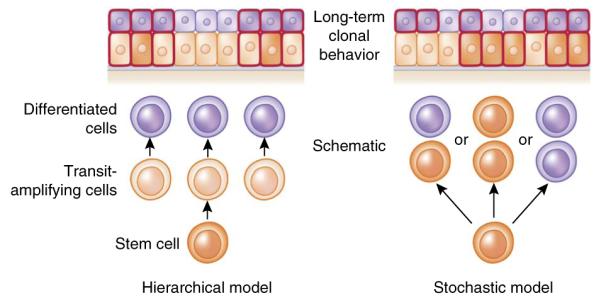
Hierarchical versus stochastic models of epidermal differentiation. In a hierarchical model, rare divisions by stem cells generate rapidly dividing transit amplifying cells, which then give rise to differentiated cells. During lineage tracing, only clones marking the stem cells are long lived, and thus clone sizes become invariant after a period of time. By contrast, in a stochastic model, all basal cells are the same and each division can yield three different outcomes: (i) one differentiated daughter that withdraws from cell cycle and departs from the basal layer, and one progenitor that remains in the basal layer and continues to divide; (ii) two basal progenitors; and (iii) two differentiated daughters. Although the fate choices are random, the probabilities of different outcomes are similar, so that the generation of differentiated cells and the maintenance of committed progenitor pools are balanced at the population level and long-term homeostasis is ensured. In this model, each individual clone will vary in size. Predictions of lineage-tracing results from each model are shown at the top of the diagram; cells outlined in red are the ones retaining lineage-traced marks.
When individually labeled basal cells in tail, ear or hindpaw epidermis were marked by lineage tracing and their progeny were then monitored over the long term, the clonal fate data were compatible with the stochastic model13–15. However, in these various labeling strategies, a crucial issue left unresolved was whether basal cells were marked randomly or selectively. By contrast, a recent study on tail skin employed two inducible Cre-lineage tracers in which Cre recombinases are fused with mutated estrogen receptors (ERs), rendering their inducibilty by tamoxifen. One Cre driver was driven by the K14 promoter, active in all basal cells, and the other by the involucrin promoter, active only in a discrete subset of K14+ basal cells that precociously express this typically differentiation-specific gene. In this study, purportedly slow-cycling basal cells marked by K14-CreER but not involucrin-CreER behaved like long-lived stem cells and gave rise to the subset marked by involucrin-CreER, which displays features of more committed basal progenitors16. These findings support the hierarchical model, yet show that progenitors within the basal layer exist and can behave in a stochastic manner. Although it is tempting to speculate that the differences among studies arise from the tools used to mark the basal cells, it is still possible that variation in the epidermis at different body sites accounts for the differences.
Epidermal stem cell proliferation
Epidermal keratinocytes proliferate before moving upward and differentiating. Dermal fibroblasts facilitate colony formation of human and mouse keratinocytes in vitro and are a rich source for mitogens such as insulin-like growth factors (IGFs), fibroblast growth factor-7 (FGF-7), FGF-10 and epidermal growth factor receptor (EGFR) ligands17–19 (Fig. 2). The physiological importance of these factors in regulating epidermal proliferation has been verified in mouse models: epidermis lacking insulin-like growth factor receptor (IGFR) is impaired in basal epidermal proliferation20. Ectopic expression of mesenchymal factor FGF-7 in epidermal cells causes epidermal hyperproliferation21. EGF signaling, as its name suggests, is a particularly potent pathway for epidermal growth22. In mice, activation of transforming growth factor-α (TGF-α), a positive autocrine regulator of EGFR signaling in the epidermis, or deletion of Mig6, a negative regulator of EGFR signaling in the epidermis, leads to epidermal hyperproliferation23,24. In humans, reduction of expression of the EGFR antagonist, LRIG1, promotes human keratinocyte proliferation in culture.
ECM proteins deposited by basal epidermal keratinocytes and by underlying dermal fibroblasts form a sheath of basement membrane separating epidermis from dermis. Basal epidermal cells adhere to the basement membrane through receptors known as integrins. α3β1 and α6β4 are the major epidermal integrins that bind the ligand laminin-5, the major ECM component of the basement membrane (Fig. 2). In human basal epidermis, greater expression of β1 integrin marks a relatively slow-cycling population in vivo that has a higher colony-forming efficiency when plated in culture, suggesting that this population has greater stem cell potential26–28.
The functions of integrins have also been explored in animal models. Ablation of β1 integrin in mice compromises basement membrane assembly and impairs proliferation29. Deletion of α6 or β4 integrin, or their ECM ligand laminin-5, leads to epidermolysis bullosa, a skin condition that results in severe blistering of the skin30–33. α6β4 integrin signals through RAC1, a small GTPase in basal cells, to mediate their adhesion to the basement membrane; depletion of Rac1 results in epidermal hyperproliferation34. Although the precise mechanisms mediating these phenotypic changes may be complex, these studies demonstrate that ECM is a critical niche component for the stem cells within the basal layer.
Genetic studies in mice suggest that epigenetic factors are also regulators for epidermal proliferation and differentiation35–40. Among these, the histone H3 Lys27 (H3K27) methyltransferases EZH1 and EZH2 are essential for epidermal homeostasis and wound repair38,39, whereas the histone H3K27 demethylase JMJD3 promotes differentiation41. Intriguingly, several epigenetic factors including EZH2 have been implicated in regulating the transcription of genes encoding α6 and β1 integrins in cultured human keratinocytes42, raising the possibility that some epigenetic modifiers might balance epidermal proliferation and differentiation in part by altering the attachment of basal cells to their basement membrane. Most, if not all, adult stem cells rely upon integrins and the ECM for adhesion to their niche, and understanding the interactions between skin stem cells and ECMs will provide a paradigm for understanding similar processes in other adult stem cells.
In addition to spatial cues from their niches, epidermal stem cell behavior may also be modulated by temporal cues such as circadian rhythms. In mice, proliferation of basal epidermal cells peaks at night, when the accumulation of reactive oxygen species is the lowest. Keratinocyte-specific deletion of Arntl (Bmal1), which encodes a transcription factor in the core clock machinery, abrogates this temporal difference by increasing epidermal proliferation during daytime43. Expression of core clock genes also oscillates in cultured human keratinocytes, and perturbation of this oscillation reduces colony-forming efficiency and promotes differentiation44. Although it remains unclear how these successive peaks of expression are established and how circadian rhythms regulate epidermal stem cells, it is tempting to speculate that temporal regulation has evolved to suppress proliferation during daytime, when there are higher risks of DNA damage owing to UV radiation and elevated concentrations of reactive oxygen species.
Epidermal stem cell differentiation
Epidermal stratification is governed by two mechanisms: delamination, in which basal cells lose their attachment to the basement membrane and move upwards45, and asymmetrical cell division, in which the plane of division is perpendicular to the basement membrane, generating a committed suprabasal daughter and a proliferative basal cell46. Transition from the basal to the spinous layer requires Notch signaling, a highly conserved pathway involved in a wide variety of developmental processes. In mouse epidermis, Notch1, Notch2 and Notch3 receptors and one of their ligands, Jagged1, are expressed suprabasally, whereas Jagged2 is expressed basally47,48 (Fig. 2). Upon ligand-receptor interaction, Notch proteins are cleaved by γ-secretase, releasing their intracellular domains (NICD1, NICD2 and NICD3), which bind to the transcriptional repressor RBP-J, enabling them to activate the Hes/Hey family of transcription factors and other target genes.
The presence of nuclear NICD and the transcription factor Hes1, as well as the expression of a Notch reporter gene regulated by RBP-J and NICD, have all been used as indicators that Notch signaling is active in spinous cells49. Indeed, constitutive activation of Notch signaling in the basal epidermis results in massive expansion of spinous layers, reduced integrin expression and detachment of epidermis from underlying dermis. Conversely, deletion of Rbpj in embryonic epidermis suppresses formation of spinous layers and reduces basal cell proliferation49. Postnatal loss of Notch signaling paradoxically results in epidermal hyperproliferation, but this turns out to be an indirect consequence of the impaired skin barrier and ensuing inflammation49,50. Notch signaling also acts genetically downstream of the asymmetric cell division machinery that basal progenitors use to generate spinous cells51. When the core components in asymmetric division are compromised, basal cells fail to activate Notch signaling and spinous cell numbers decline51. In humans, the Notch ligand DELTA1 is expressed by basal cells, and DELTA-NOTCH interactions in cultured human keratinocytes promote differentiation52. Together, these data suggest that Notch activation determines spinous cell fate and promotes delamination, and asymmetric cell division balances epidermal proliferation and differentiation through Notch signaling.
The exact ligand that activates Notch in the mouse epidermis, and the cellular source of that ligand, remains unclear, although evidence thus far implicates cross-talk between stem cells (basal) and their differentiated progeny (suprabasal cells). One possible cell mediator is the primary cilium, a microtubule-based sensory organelle that typically functions in hedgehog signaling but also enhances Notch signaling53. Failure in ciliogenesis leads to compromised Notch signaling and defective epidermal differentiation53.
Hair follicle regeneration
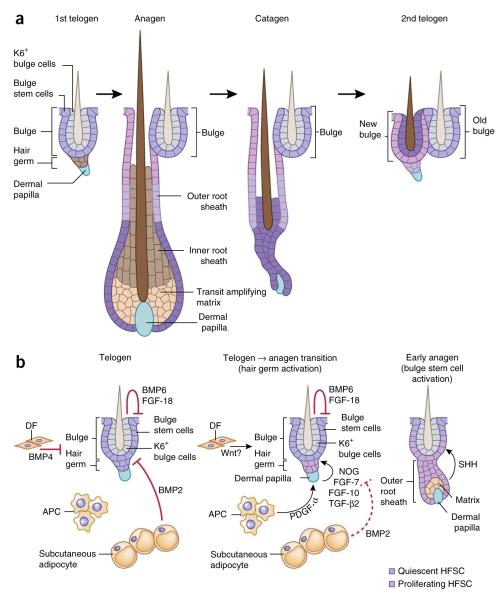
Unlike the epidermis, which regenerates continually, hair follicles undergo cycles of growth (anagen), degeneration (catagen) and rest (telogen) (Fig. 4a). In mice, the first two cycles are synchronized, making hair follicles an ideal system for understanding how stem cells interact with progeny and heterologous cell types in the niche to transition between quiescence and regeneration.
Figure 4.
Hair follicle lineage and niche signals regulate hair follicle stem cells. (a) HFSCs can exist in two states. Quiescent bulge stem cells (Bu-SCs) are located in the outer layer of this niche and contribute to the generation of the outer root sheath. Primed stem cells reside in the hair germ, sandwiched between the bulge and a specialized dermal cluster known as the dermal papilla. They are responsible for generating the transit amplifying cell (TAC) matrix, which then gives rise to the hair shaft and its inner root sheath (IRS) channel. Although matrix and IRS are destroyed during catagen, many of the outer root sheath (ORS) cells are spared and generate a new bulge right next to the original one at the end of catagen. The upper ORS contributes to the outer layer of the new bulge, and the middle ORS contributes to the hair germ. Some of the lower ORS cells become the differentiated inner keratin 6+ (K6+) bulge cells, which provide inhibitory signals to Bu-SCs, raising their activation threshold for the next hair cycle. (b) During telogen, K6+ bulge cells produce BMP6 and FGF-18, dermal fibroblasts (DFs) produce BMP4 and subcutaneous adipocytes express BMP2. Together, these factors maintain Bu-SCs and hair germ in quiescence. At the transition to anagen, BMP2 and BMP4 are downregulated, whereas the expression of activation factors including noggin (NOG), FGF-7, FGF-10 and TGF-β2 from dermal papillae and PDGF-α from adipocyte precursor cells (APCs) is elevated. This, in turn, stimulates hair germ proliferation, and a new hair cycle is launched. Bu-SCs maintain their quiescent state until TAC matrix is generated and starts producing SHH.
HFSCs can be subdivided into two populations that share similar molecular signatures: a quiescent one located in the bulge (Bu-SCs) and a primed population within the hair germ just below the bulge, which is more prone to proliferation54 (Fig. 4). Previous lineage-tracing studies have demonstrated that these two populations are responsible for initiating hair growth2,55,56, and recently live imaging has provided a more precise means of delineating their relative contributions to these early steps57.
Neither Bu-SCs nor hair germ give rise to differentiated cells directly. At anagen onset, the hair germ is always the first to proliferate54,58. Hair germ develops into matrix, a pool of TACs that proliferate rapidly before terminally differentiating to form the hair shaft and its surrounding channel, the inner root sheath (IRS). By contrast, Bu-SCs primarily give rise to the outer root sheath (ORS), a population of cells that retains many stem cell characteristics and envelops the differentiating core of each hair follicle as well as the bulb of matrix TACs at the base of the mature follicle2,57 (Fig. 4a).
During catagen, matrix and most lower ORS cells apoptose, middle ORS cells form a new hair germ and upper ORS cells form a new bulge adjacent to the original one2. The newly formed bulge and hair germ house the HFSCs for the next hair cycle. By contrast, the previous bulge becomes an HFSC reservoir for injury repair. Interestingly, some lower ORS cells escape apoptosis, follow a short-circuited differentiation program and become the inner layer of the new bulge. Marked by K6, these inner bulge cells anchor the hair shaft but have lost stemness, despite returning to the bulge2 (Fig. 4a).
Regulation of hair follicle stem cell quiescence and activation
HFSCs are maintained in a quiescent state during most of the hair cycle and only proliferate early in anagen. Several secreted factors from stem cell progeny and dermal cells are important in regulating the proliferative status of Bu-SCs and hair germs. HFSC quiescence is largely maintained by bone morphogenetic proteins (BMPs). Originally named for their functions in bone and cartilage formation, BMPs have now been implicated in many developmental processes and stem cell systems. During telogen, dermal fibroblasts express BMP4, whereas subcutaneous fat expresses BMP2 (ref. 59). The K6+ inner bulge layer secretes high levels of BMP6 and another quiescence factor, FGF-18 (ref. 2; Fig. 4b). Together, these factors maintain quiescence of both Bu-SCs and hair germ.
The dermal papilla located beneath the hair germ is an essential niche component that initiates hair regeneration. Upon dermal papilla ablation, telogen-phase hair follicles never reenter the hair cycle60,61. Several dermal papilla–specific factors have been implicated in hair follicle activation. During progression from early to late telogen, levels of hair germ–activating factors, including FGF-7, FGF-10, TGF-β2 and the BMP inhibitor noggin, become elevated in dermal papillae54,62, whereas levels of BMP4 in dermal fibroblasts and BMP2 in mature adipocytes are downregulated, lowering the overall threshold for HFSC activation59. In addition, adipocyte precursor cells secrete platelet-derived growth factor-α (PDGF-α) to activate PDGF signaling in dermal papillae, which then relays a yet-to-be-identified signal to activate hair germ63.
WNT signaling, a prominent pathway in development and cancer, is also critical for hair germ activation: the downstream WNT effector, nuclear β-catenin, accumulates in the activated hair germ and leads to target gene activation54. Without β-catenin, hair follicles arrest in telogen58,64. WNT signaling is also required in dermal papillae, as hair follicles regenerate more slowly when β-catenin is conditionally targeted there65. Although the exact WNT ligand(s) and source(s) mediating these effects remain to be identified, potential sources include the hair germ itself54 and dermal fibroblasts59,66. Together, these activation cues overpower the inhibitory signals and launch regeneration (Fig. 4b).
Several lines of evidence suggest that Bu-SC activation may rely upon signals distinct from those used in hair germ activation. In contrast to the hair germ, which proliferates to form the matrix TACs upon anagen initiation, Bu-SCs remain quiescent until TACs emerge, a time when dermal papilla–activating cues are further distanced from the bulge. A recent study shows that Bu-SC activation depends on sonic hedgehog (SHH), a potent mitogen secreted by the newly formed TAC matrix. Moreover, by mid-anagen, as hair follicles grow downward and matrix-derived SHH moves away from the bulge, Bu-SCs resume quiescence. Matrix-derived SHH also signals dermal papillae to intensify expression of noggin and FGF-7, which, together with SHH, maintain the matrix and lower ORS in a highly proliferative state throughout anagen5. Thus, although cross-talk between the dermal papilla and the hair germ initiates anagen, Bu-SCs depend upon signals from the emerging TAC pool for their activation (Fig. 4b).
Circadian rhythms also seem to have an impact on hair follicles, as they do on the epidermis. Expression of core circadian clock genes oscillates throughout the day in Bu-SCs, matrix and dermal papillae67–69. Furthermore, during the resting phase, the Bu-SC population displays an intriguing heterogeneity in core clock gene expression67, and depletion of circadian clock proteins in mice delays anagen entry68. That said, epithelial lineage–specific knockouts of these genes have only mild effects on hair cycle progression43,67,69, suggesting that circadian rhythms may not act on HFSCs directly to control their activities. Future studies should help to delineate the actions of the circadian clock on specific niche components and reveal how these mechanisms might regulate hair cycles.
Interactions of melanocyte stem cells and hair follicle stem cells
In mice, HFSCs and MSCs are intermingled in the bulge and hair germ70, providing a unique opportunity to explore how two different types of stem cells coordinate their actions within a shared niche (Fig. 1). As a new hair cycle initiates, MSCs become activated to generate proliferative committed progenitor melanocytes. In mature hair follicles, these melanocytes reside within the inner core of the matrix, where they produce and transfer melanin pigment to differentiating hair cells. As catagen ensues, melanocytes degenerate along with the rest of the matrix.
Signals from both HFSCs and dermal papillae are essential to synchronize MSC activation and differentiation with those of HFSCs71–74. In the hair bulb during anagen, KIT ligand (secreted by the dermal papilla) and endothelins (secreted by the matrix) work in concert to prompt melanocyte differentiation. Interestingly, when telogen-phase HFSCs are conditionally targeted for loss of transcription factor NFIB, they aberrantly upregulate endothelin-2 (Edn2), which promotes precocious differentiation of MSCs near the KIT ligand–expressing dermal papilla. Adjacent hair germ cells take up melanin, prompting their apoptosis. Once the hair cycle is launched, these defects are resolved as the dermal papilla moves away from the bulge, thereby sparing the remaining HFSCs and MSCs74. Notably, Edn2 expression in mouse skin can be induced upon UV irradiation75, suggesting that the synchrony between HFSCs and MSCs might also be uncoupled in stress conditions.
Endothelin-1 is naturally induced by WNT signaling in early-anagen hair follicles72. Injection of endothelin receptor B antagonists can rescue the melanocyte expansion that results from either HFSC-specific Nfib deletion or elevated WNT signaling72,74. HFSCs might also produce WNTs and TGF-βs, offering other potential routes for coordinating MSC and HFSC behaviors71,72,74. Whether MSCs release instructive signals to HFSCs is an intriguing question that awaits future studies.
Diverse cell types interacting with skin stem cells
Immune cells in the skin
As our body’s first line of defense, the skin is equipped with an impressive arsenal of immune cells. Immune cells and epithelial cells can influence each other’s functionality and behavior. Mouse epidermis is populated with dendritic epidermal γδ T cells (DETCs) and Langerhans cells76,77, whereas dermis is enriched for dendritic cells, mast cells, macrophages, γδ T cells and αβ T cells77 (Fig. 1).
During injuries, compromising the epidermal barrier triggers inflammatory responses, which cause hyperproliferation of epidermal cells. In mice, DETCs in wounded skin produce FGF-7, FGF-10 and IGF-1, which are important for survival, proliferation and migration of epidermal cells78,79. Recently, FGF-9 secreted from dermal γδ T cells has been reported to promote dermal WNT activation and induce hair follicle neogenesis in wounded epidermis80.
Interestingly, there are some similarities and differences between the injury-induced immune responses in mice and humans. Human epidermal resident T cells also upregulate IGF-1 upon wounding81. Nevertheless, a robust population of dermal γδ T cells is lacking in humans, which might account for poor hair follicle neogenesis in humans following injury80. Notably, when p120-catenin, a component of adherens junctions, is conditionally ablated in the epidermis, barrier functions remain seemingly intact. However, nuclear factor-κB signaling is induced, triggering inflammation and epidermal hyperplasia82. These findings suggest that pathogen-independent, intrinsic mechanisms coexist with pathogen-dependent ones to balance immune responses in skin.
The distribution and function of immune cells change during hair morphogenesis and cycling77, suggesting that hair follicles might also influence the immune cell composition in skin. Indeed, hair follicles can act as entry points for immune cells to move into the epidermis: upon temporary ablation of epidermal Langerhans cells and inflammation, the junctional zone and infundibulum of hair follicles produce chemokines CCL2 and CCL20, respectively, to recruit new Langerhans cell precursors to the epidermis, whereas bulge cells express high levels of CCL8 that repel them83.
Intriguingly, both bulge and matrix appear to express immunosuppressants, leading to reduced signaling to immune cells; this has prompted the hypothesis that these sites may be ‘immune privileged’84,85. If so, such immune privilege must have limits, as both hair follicles and epidermis are targeted by immune cells upon allotransplantation. Moreover, in autoimmune disorders such as alopecia areata, immune cells target the hair bulb and the matrix, sparing only Bu-SCs, which leads to reversible hair loss86. In contrast, Bu-SCs are destroyed in discoid lupus erythematosus and lichen planopilaris, resulting in irreversible hair loss87,88. As the molecules mediating cross-talk between epithelial stem cells and immune cells continue to be discovered, more specific and effective therapies should emerge to maintain the skin’s ability to defeat harmful stimuli caused by injuries and infections, but eliminate excess responses during inflammation and autoimmune responses.
Peripheral nerves in the skin
The skin is the largest sensory organ, innervated by numerous fibers of primary sensory neurons whose cell bodies are located in trigeminal and dorsal root ganglia. These neurons are a heterogeneous population, including nociceptors, mechanoreceptors and proprioceptors (Fig. 1).
Anatomically, sensory nerves are in close contact with cells in the epidermis and hair follicles. Free nerve endings terminate at different layers of the epidermis89. Mechanoreceptive nerve endings encase a region of the hair follicle immediately above the bulge90,91. In neonatal animals, the temporal sequence of innervation correlates with hair follicle morphogenesis, suggesting that the two processes are interdependent92. Skin-derived cues have been shown to have an impact on sensory innervation and dendritic arborization93,94. Conversely, signals from peripheral nerves may influence hair follicles. For example, premature hair follicle regression is elicited by the neuropeptides substance P and CGRP, which trigger neurogenic inflammation95. In addition, peripheral nerves that innervate the cells above the bulge secrete SHH and may govern the behavior of these cells in wounding90. As the complex communication circuits between skin and peripheral nerves become better understood at the molecular level, novel strategies may emerge to restore proper wiring and sensory functions after injuries such as severe burns.
Cutaneous blood vessels
The skin vasculature supplies the skin with nutrients, hormones and immune cells and plays a role in thermal control. Although arteries and veins are located in the lower (reticular) dermis, arborizing capillary networks can be found in the region above the bulge96 (Fig. 1). These capillary networks might influence hair cycling: when angiogenesis is inhibited, anagen induction is delayed, indicating that angiocrine factors may regulate HFSC activity97. Intriguingly, the cells above the bulge express angiogenic factor EGFL6, an ECM protein96. Whether EGFL6 recruits blood vessels to the hair follicle remains to be explored. Intriguingly, minoxidil, an active ingredient used to treat androgenic alopecia (male-pattern baldness), is a vasodilator that has been proposed to work by increasing blood flow to the skin and thus potentially stimulating hair follicle growth. Future studies should help to reveal molecular cross-talk between skin vasculature and HFSCs.
Arrector pili muscle
HFSCs also control the formation and attachment of the arrector pili muscle (APM) responsible for piloerection (‘goosebumps’6; Fig. 1). Bu-SCs express nephronectin, an ECM protein in the same family as EGFL6. Both nephronectin and EGFL6 are ligands for α8β1 integrin. Nephronectin is enriched in the bulge basement membrane, where it recruits α8β1+ dermal cells. In turn, nephronectin-integrin activation in these dermal cells induces the expression of smooth muscle actin, an APM marker. In mice lacking nephronectin, fewer APMs are formed and their anchorage is shifted to the EGFL6-expressing cells above the bulge, suggesting that EGFL6 may compensate for nephronectin6. Taken together, these studies suggest that different hair follicle compartments recruit and assemble different hair follicle–associated structures, including peripheral nerves, blood vessels and APMs, in part through expression of different ECM proteins.
Aging and pathological conditions
Aging and the niche
The skin shows profound structural and functional changes with age, including dermal and epidermal thinning, reduction in epidermal proliferation and injury repair, loss of dermal elasticity and wrinkling, and graying, thinning and loss of hair. Aged HFSCs maintain their numbers and gene signatures. However, telogen lengthens with age, suggesting that quiescent HFSCs become increasingly resistant to activation98,99.
In culture, HFSCs from aged mice proliferate more slowly and generate fewer large colonies than their younger counterparts, indicating that intrinsic changes with age affect HFSC proliferation. In vivo, prolonged telogen in aged mice might be due to more BMP inhibitory cues and/or fewer WNT-activating signals, the balance of which determines HFSC activity. Indeed, elevated Bmp2, Bmp4 and Bmp6 expression and protein secretion from aged adipose tissue, and to a lesser extent aged dermal fibroblasts, may further raise the threshold for activating aged HFSCs. Correspondingly, aged HFSCs display upregulation of BMP targets Nfatc1 and Id2, reinforcing HFSC quiescence98. The composition of immune cells in skin also changes with age100, and age-related increases in proinflammatory cytokine signaling have been described101. Because immune-related changes are heavily influenced by pathogen exposure and skin barrier integrity, they add a variable component to the age-related decline in HFSC activity.
Lastly, although age-related systemic changes affect various organs, including those of the nervous system, muscle and heart102–104, only modest increases in HFSC proliferation result from joining the circulatory systems of older and younger mice (parabiosis)98. Therefore, much of the age-related decline in HFSC function appears to arise from more local signals or the HFSCs themselves98. Moreover, these findings suggest that even though some common mechanisms of the aging process are shared, each stem cell niche and the macroenvironment surrounding it have unique regulators, complicating the search for a ‘fountain of youth’.
Wound repair and the niche
When skin is wounded, its stem cells must respond rapidly to restore the compromised barrier and repair tissue damage. Wound healing involves three overlapping phases: inflammation, tissue formation and tissue remodeling105. Inflammation occurs immediately after injury. Following platelet aggregation, various leukocyte lineages, including neutrophils, macrophages, mast cells and T cells, are recruited to the wound site. In addition to clearing dead cells and fighting against infections, these leukocytes secrete cytokines and growth factors such as TGF-βs, IGFs and FGFs that promote angiogenesis, migration and proliferation of keratinocytes and dermal fibroblasts, synthesis of ECMs and sometimes generation of new hair follicles in the process of epidermal regeneration78–80. During tissue formation, granulation tissue, consisting of newly formed blood vessels, macrophages and fibroblasts, begins to cover the wound. Epidermal cells then migrate over the granulation tissue to reepithelialize the wound. Adipocyte precursor cells are also activated at this stage to generate mature adipocytes important for fibroblast recruitment106. During tissue remodeling, the epidermis and dermal fibroblasts deposit new ECM proteins to strengthen the repaired tissue105.
Following full-thickness wounds, cells from both the hair follicles and the IFE migrate into the site of damage16,56,90,107–109. Mice lacking either hair follicles or HFSCs show delayed wound healing110. That said, very few HFSC-derived cells persist within the reepithelialized epidermis of a full-thickness wound. Lineage tracing of Gli1+ cells above the bulge and Lrig1+ cells in the junctional zone show that their progeny can persist longer90,109, but even these hair follicle progeny are largely replaced by epidermal progeny following repair16. Overall, the data on full-thickness wounds have led to the now widely held view that input from hair follicles plays relatively minor roles, whereas IFE plays a major role in wound reepithelialization.
Cancer and the niche
In humans, exposure to UV damage from the sun increases the risk of oncogenic transformation of long-lived skin stem cells. Basal cell carcinoma (BCC), the most common cancer worldwide, is rooted in deregulated, sustained SHH signaling111. By contrast, squamous cell carcinoma (SCC), an aggressive skin cancer with significant risk of metastasis, can arise from oncogenic RAS transformation and accompanying loss of tumor suppressors such as p53, BRCA1 or TGF-β receptors, and/or their downstream effectors112. The roots of both BCCs and SCCs have been traced to multiple stem cell populations in the hair follicles and epidermis, whereas matrix seems to lack tumor-initiating capacity113–116. In mice, cutaneous injuries have been reported to exacerbate BCC malignant progression117,118, a finding consistent with the higher risk of cancer in patients with chronic wounds.
Tumor-initiating cells, frequently referred to as cancer stem cells (CSCs), are also influenced by their niches, which are greatly altered by infiltration of blood vessels and immune cells as well as perturbations in the cross-talk of niches with their malignant stem cell residents (Fig. 5a). CSCs have been purified and characterized from mouse skin papillomas and SCCs119–121. At a near single-cell level, SCC-CSCs can induce the formation of a new SCC when transplanted into a host recipient mouse120. Additionally, the expansion of CSCs and their SCC progeny has been monitored by in vivo lineage tracing122. CSCs exist at the tumor-stroma interface and express high levels of integrins120,121. CSC cycling activities are influenced by cues from their niche, where signals from TGF-β and signals from integrin and focal adhesion kinase counteract each other to inhibit or promote CSC proliferation, respectively120. SCC-CSCs express vascular endothelial growth factor (VEGF), which stimulates elaborate tumor vascularization. Interestingly, VEGF may also act on CSCs in an autocrine fashion to increase their proliferation and thereby sustain tumor growth123 (Fig. 5b). Therefore, CSCs are analogous to their normal counterparts in that they rely upon autocrine and paracrine niche signals for their self-renewal and differentiation.
Figure 5.
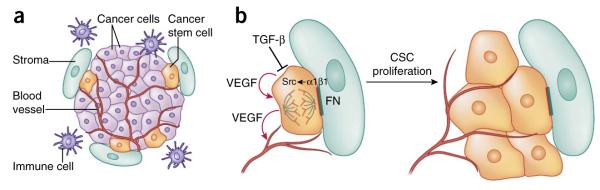
Signaling pathways in skin cancers. (a) The squamous cell carcinoma–cancer stem cell niche. CSCs are often found at the tumor-stroma interface, together with an elaborate vasculature, immune cells and aberrant fibroblasts. (b) Upon activation of αβ1 integrins by extracellular matrix ligands such as fibronectin (FN), focal adhesion kinase (FAK) and its associate tyrosine kinase Src become hyperactivated and promote proliferation of CSCs. By contrast, TGF-β signaling counteracts integrin activity and enhances CSC quiescence. In addition, CSCs secrete VEGF, which acts in an autocrine fashion to enhance CSC proliferation and in a paracrine fashion to promote formation of new blood vessels.
With the identification of CSCs in situ, it is now possible to tackle the question of intrinsic versus extrinsic regulation of CSCs during tumor progression. Gene expression signatures have been reported for purified SCC-CSCs120. Large-scale screens, either genome-wide or with preselected gene sets, have allowed researchers to identify which of the myriad changes in gene expression are causative for SCCs124,125. In the future, it will be interesting to discover whether altering some of these drivers in CSCs also affects CSC niches, as seen, for instance, with VEGF123.
The niche in regenerative medicine
The use of cultured human keratinocytes to treat burn patients has been a success in regenerative medicine17,126,127. However, although these autologous grafts fulfill the need for epidermal barrier protection, they lack hair follicles, sweat glands and peripheral nerves. In addition, although the dermis underneath the engrafted epidermis recovers eventually, the entire process takes months, and the dermis is never restored fully.
The considerable progress in understanding stem cell–niche interactions in hair follicles and sweat glands opens new avenues for therapeutic advances, offering possible improvements for skin grafting and alopecia treatments. During embryonic development, WNT signaling within the epidermis and BMP inhibition in underlying mesenchyme triggers hair follicle formation128,129. Analogously, similar cross-talk between adult hair germs and dermal papillae stimulates each new round of hair cycling54. Intriguingly, fibroblasts from the upper dermis of neonatal skin also possess robust capacity for hair induction, whereas those from the lower dermis are not effective130. Collectively, these studies suggest that proper niche cell types, signaling and spatial organization are all important considerations for regenerating hair follicles.
In their native niche, adult HFSCs become active only upon hair cycle initiation, when they function in making new hair follicles. When transplanted onto immunocompromised mice, however, mixtures of dissociated HFSCs and mesenchymal cells regenerate not only hair follicles but also sebaceous glands and epidermis131. A three-dimensional culture method has recently been developed that involves seeding a mixture of dissociated hair follicle cells and dermal cells from adult mice or humans in collagen gels. Upon transplantation some of these mini-organoids can grow into functional hair follicles that receive nerve innervation, form APMs and undergo hair cycles and piloerection132.
Another major advance is the identification of progenitors that give rise to sweat glands in mice. In homeostasis and mild injury, most adult sweat gland progenitors behave unipotently, only giving rise to myoepithelial or luminal epithelial cells. However, upon engraftment, purified myoepithelial stem cells can generate an entire gland, containing both myoepithelial and luminal epithelial layers; this is reminiscent of their multipotent behavior during development133. Together, these studies further reinforce the idea that stem cell potential and behavior are not fixed, and can be altered upon exposure to different environments. With ever-increasing knowledge of stem cell populations, regulatory signals within the niche, and the ecosystem of the skin, the ability to regenerate a fully functional skin for tissue replacement in regenerative medicine should continue to improve.
Conclusions
Recent findings have brought forth the complexity of cellular and molecular regulators within the skin stem cell niche during development, homeostasis, injury, aging and cancer. Several open questions remain. First, although many putative niche factors have been identified, their functional importance can be firmly established only by knocking out specific factors spatially and temporally from specific niche components that express them. To date, this has rarely been achieved for any given mammalian stem cell system. Second, although several cell types, including blood vessels and sensory neurons, make stereotypic and spatially distinct contacts with epithelial cells, specific signals governing these connections remain to be identified. Third, given the heterogeneity and complexity of tumor development, our understanding of the cancer stem cell niche is still in its infancy. Fourth, biological processes such as pregnancy, lactation, hypoxia and circadian rhythms can also impact skin stem cells44,67,134–136. Whether these influences are mediated through effects on any niche components remains to be explored.
These hurdles will probably be overcome with continued development of stem cell–specific genetic tools, identification of new markers to characterize specific stem cell populations more precisely, and improvements in imaging strategies. With its rich cellular composition, the skin will continue to serve as an important paradigm in the quest to understand stem cell niches. In the era of tissue engineering—driven by the hope that in the future we will be able to manipulate stem cell behavior in situ, suppress tumor formation and progression and grow functional tissues for regenerative medicine—it is even more important and timely to tackle the complexity of niche components within the skin.
ACKNOWLEDGMENTS
We are grateful to members of E.F.’s lab, in particular S. Naik, for comments on the manuscript. Y.-C.H. was a New York Stem Cell Foundation Druckenmiller Postdoctoral Fellow and is now supported by US National Institutes of Health Pathway to Independence Award (K99-R00). L.L. is a Helen Hay Whitney Postdoctoral Fellow. E.F. is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the US National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR031737 to E.F., R01-AR050452 to E.F. and K99-AR063127 to Y.-C.H.), and by a grant from the Ellison Foundation (E.F.).
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 2.Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu Y-C, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:103–114. doi: 10.1038/nrm3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu Y-C, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara H, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie IC. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 1997;109:377–383. doi: 10.1111/1523-1747.ep12336255. [DOI] [PubMed] [Google Scholar]
- 9.Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl. Acad. Sci. USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghazizadeh S, Taichman LB. Organization of stem cells and their progeny in human epidermis. J. Invest. Dermatol. 2005;124:367–372. doi: 10.1111/j.0022-202X.2004.23599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ro S, Rannala B. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep. 2004;5:914–920. doi: 10.1038/sj.embor.7400218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 14.Doupé DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Lim X, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascré G, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 17.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 18.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DA, Travers JB, Somani AK, Spandau DF. The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29:1475–1485. doi: 10.1038/onc.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadagurski M, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol. Cell. Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L, Yu QC, Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993;12:973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 23.Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 24.Ferby I, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat. Med. 2006;12:568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 25.Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl. Acad. Sci. USA. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 27.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 28.Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- 29.Raghavan S, Bauer C, Mundschau G, Li Q. Conditional ablation of β1 integrin in skin severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georges-Labouesse E, et al. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 31.Dowling J, Yu QC, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 33.McGrath JA, et al. Altered laminin 5 expression due to mutations in the gene encoding the beta 3 chain (LAMB3) in generalized atrophic benign epidermolysis bullosa. J. Invest. Dermatol. 1995;104:467–474. doi: 10.1111/1523-1747.ep12605904. [DOI] [PubMed] [Google Scholar]
- 34.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 35.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luis NM, et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–246. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Frye M, Benitah SA. Chromatin regulators in mammalian epidermis. Semin. Cell Dev. Biol. 2012;23:897–905. doi: 10.1016/j.semcdb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Ezhkova E, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezhkova E, et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mejetta S, et al. Jarid2 regulates mouse epidermal stem cell activation and differentiation. EMBO J. 2011;30:3635–3646. doi: 10.1038/emboj.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulder KW, et al. Diverse epigenetic strategies interact to control epidermal differentiation. Nat. Cell Biol. 2012;14:753–763. doi: 10.1038/ncb2520. [DOI] [PubMed] [Google Scholar]
- 43.Geyfman M, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA. 2012;109:11758–11763. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janich P, et al. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13:745–753. doi: 10.1016/j.stem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Watt FM, Green H. Stratification and terminal differentiation of cultured epidermal cells. Nature. 1982;295:434–436. doi: 10.1038/295434a0. [DOI] [PubMed] [Google Scholar]
- 46.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell BC, Passmore EA, Nesci A, Dunn SM. The Notch signalling pathway in hair growth. Mech. Dev. 1998;78:189–192. doi: 10.1016/s0925-4773(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, et al. γ-secretase functions through notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev. Cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowell S, JONES P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 53.Ezratty EJ, et al. A role for the primary cilium in notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 56.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lien W-H, et al. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol. 2014;16:179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–1683. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rompolas P, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances bmp-mediated repressionin hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi YS, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plikus MV, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janich P, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- 68.Lin KK, et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plikus MV, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc. Natl. Acad. Sci. USA. 2013;110:E2106–E2115. doi: 10.1073/pnas.1215935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 71.Nishimura EK, et al. Key roles for transforming growth factor β in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabbani P, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanimura S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Chang C-Y, et al. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adur J, Takizawa S, Uchide T, Casco V, Saida K. High doses of ultraviolet-C irradiation increases vasoactive intestinal contractor/endothelin-2 expression in keratinocytes of the newborn mouse epidermis. Peptides. 2007;28:1083–1094. doi: 10.1016/j.peptides.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Paus R, Hofmann U, Eichmüller S. Distribution and changing density of γ-δ T cells in murine skin during the induced hair cycle. Br. J. Dermatol. 1994;130:281–289. doi: 10.1111/j.1365-2133.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 77.Paus R, et al. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Invest. Dermatol. 1998;111:7–18. doi: 10.1046/j.1523-1747.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- 78.Jameson J, et al. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 79.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 80.Gay D, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toulon A, et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Moreno M, et al. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagao K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer KC, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br. J. Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 85.Christoph T, et al. The human hair follicle immune system: cellular composition and immune privilege. Br. J. Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 86.Kang H, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J. Invest. Dermatol. 2010;130:2677–2680. doi: 10.1038/jid.2010.180. [DOI] [PubMed] [Google Scholar]
- 87.Al-Refu K, Edward S, Ingham E, Goodfield M. Expression of hair follicle stem cells detected by cytokeratin 15 stain: implications for pathogenesis of the scarring process in cutaneous lupus erythematosus. Br. J. Dermatol. 2009;160:1188–1196. doi: 10.1111/j.1365-2133.2009.09074.x. [DOI] [PubMed] [Google Scholar]
- 88.Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J. Cutan. Pathol. 2005;32:675–679. doi: 10.1111/j.0303-6987.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 89.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 90.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peters EMJ, et al. Developmental timing of hair follicle and dorsal skin innervation in mice. J. Comp. Neurol. 2002;448:28–52. doi: 10.1002/cne.10212. [DOI] [PubMed] [Google Scholar]
- 93.Salzberg Y, et al. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell. 2013;155:308–320. doi: 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honig MG, Camilli SJ, Surineni KM, Knight BK, Hardin HM. The contributions of BMP4, positive guidance cues, and repulsive molecules to cutaneous nerve formation in the chick hindlimb. Dev. Biol. 2005;282:257–273. doi: 10.1016/j.ydbio.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Peters EMJ, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp. Dermatol. 2006;15:1–13. doi: 10.1111/j.0906-6705.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 96.Xiao Y, et al. Perivascular hair follicle stem cells associate with a venule annulus. J. Invest. Dermatol. 2013;133:2324–2331. doi: 10.1038/jid.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mecklenburg L, et al. Active hair growth (anagen) is associated with angiogenesis. J. Invest. Dermatol. 2000;114:909–916. doi: 10.1046/j.1523-1747.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- 98.Keyes BE, et al. Nfatc1 orchestrates aging in hair follicle stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:E4950–E4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C-C, et al. Regenerative hair waves in aging mice and extra-follicular modulators follistatin, Dkk1, and Sfrp4. J. Invest. Dermatol. 2014 Apr 17; doi: 10.1038/jid.2014.139. published online, doi:10.1038/jid.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–259. doi: 10.1111/j.1474-9726.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–2153. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 104.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ito M, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 108.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 109.Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J. Invest. Dermatol. 2008;128:1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 111.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J. Clin. Invest. 2012;122:464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Youssef KK, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 114.Wang GY, Wang J, Mancianti M-L, Epstein EH., Jr. Basal cell carcinomas arise from hair follicle stem cells in Ptch1+/− mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lapouge G, et al. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. USA. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.White AC, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kasper M, et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc. Natl. Acad. Sci. USA. 2011;108:4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on ∣[bgr]∣-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 120.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lapouge G, et al. Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness. EMBO J. 2012;31:4563–4575. doi: 10.1038/emboj.2012.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beck B, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 124.Beronja S, et al. RNAi screens in mice identify physiological regulators of oncogenic growth. Nature. 2013;501:185–190. doi: 10.1038/nature12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schramek D, et al. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science. 2014;343:309–313. doi: 10.1126/science.1248627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Connor NE, Mulliken JB, Banks-Schlegel S. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;317:75–78. [PubMed] [Google Scholar]
- 128.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 129.Noramly S, Freeman A, Morgan BA. β-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- 130.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 132.Toyoshima K-E, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012;3:784. doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu CP, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldstein J, et al. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:983–994. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McGee HM, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J. Invest. Dermatol. 2013;133:1321–1329. doi: 10.1038/jid.2012.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]