Abstract
Males of many animal species perform specialized courtship behaviours to gain copulations with females. Identifying physiological and anatomical specializations underlying performance of these behaviours helps clarify mechanisms through which sexual selection promotes the evolution of elaborate courtship. Our knowledge about neuromuscular specializations that support elaborate displays is limited to a few model species. In this review, we focus on the physiological control of the courtship of a tropical bird, the golden-collared manakin, which has been the focus of our research for nearly 20 years. Male manakins perform physically elaborate courtship displays that are quick, accurate and powerful. Females seem to choose males based on their motor skills suggesting that neuromuscular specializations possessed by these males are driven by female choice. Male courtship is activated by androgens and androgen receptors are expressed in qualitatively and quantitatively unconventional ways in manakin brain, spinal cord and skeletal muscles. We propose that in some species, females select males based on their neuromuscular capabilities and acquired skills and that elaborate steroid-dependent courtship displays evolve to signal these traits.
Keywords: Sexual behaviour, Sexual selection, Courtship, Courtship display, Manakin, Manacus, Androgen, Neuromuscular system
1. Introduction
1.1. Sexual signals and exaggerated displays
Sexual selection was proposed by Charles Darwin (1859, 1871) to explain the origin of traits that seemed to contradict his theory of evolution by natural selection. Within this overarching theory, he found it difficult to explain how, for example, the large, decorative tail of a peacock could evolve when it seemed obvious that the tail had survival costs such as increasing the male's vulnerability to predation and reducing the efficiency of his flight. Typical of Darwin, he envisioned a relatively simple, but powerful solution, referred to as sexual selection. He posited that the peacock's tail would evolve if the gain in reproductive success provided by the tail outweighed its costs. Sexual selection swiftly became a popular branch of biological investigation, now attracting empirical and theoretical biologists alike. The field grew to encompass traits that served to increase reproductive success by both enhancing mate choice (by possessing ornaments) as well as by increasing success in male-male competition (possessing armaments that increase fighting success). The benefits of some traits seem relatively easy to explain, such as armaments like the antlers of deer, that are advantageous in male-male competition and that lead to increased access to females (Andersson, 1994). The origins of other traits are more difficult to comprehend, and likely reflect features that females identify for discrimination of males for purposes of mating. A particularly dramatic category of behavioural ornamentation are the elaborate courtship displays performed by males of many species. Despite their presence in most animal taxa, we know very little about the genomic, physiological and anatomical mechanisms underlying the evolution of elaborate courtship behaviours. Scientists have been seeking answers as to why specific phenotypes, and not others, evolve. A variety of theories have arisen that attempt to capture the great diversity of signals females might use to assess males including the handicap hypothesis (Zahavi, 1975), the parasite hypothesis (Hamilton and Zuk, 1982), Andersson's concept of honest indicator (Andersson, 1994), and the immunocompetence hypothesis (Folstad and Karter, 1992). Yet there remains the particularly challenging question as to what actually drives the evolution of specific behavioural repertoires associated with some courtship displays.
It is widely believed that courtship behaviours carry information that females use in assessing male quality, the basis of their choice for copulation. In some cases, male displays borrow from behaviours that are already employed in other facets of the animals life, such as the ritualized behaviours well-described by Huxley (1938) and Tinbergen (1965). In some cases, courtship behaviours seem designed to show off the male's physical ornaments, likely amplifying the value of these signals (Guilford and Dawkins, 1991; Rowe, 1999). Such seems to be the case for the display of the extraordinary tail of the male peacock (Petrie et al., 1991). It is important to recognize that behavioural features that are used only during periods of reproduction are by their very nature transient and likely provide information to females about the male's current physical condition. In contrast, physical ornaments that are permanent (static) or that require extensive periods of growth likely reflect the male's condition over longer spans of his life (Maynard-Smith and Harper, 2003). Elaborate courtship displays that transiently display long-held physical ornaments may serve to signal to females both the short- and long-term condition of the male (Maynard-Smith and Harper, 2003).
Birds are a taxonomic group with extraordinary variation in male mating displays (Lack, 1968). As noted by Darwin “Secondary sexual characters are more diversified and conspicuous in birds. . .The males sometimes pay their court by dancing, or by fantastic antics performed on the ground or in the air” (Darwin, 1871). Some of the most spectacular of these displays are performed by species that have promiscuous mating systems in which males provide no direct benefits to females and females must rely on courtship and ornaments to gather information on mate choice. In those species in which males gather in leks, the females are able to compare several males displaying simultaneously (Hoglund et al., 1995). Well studied lekking species of the temperate-zone include the male sage grouse, Centrocercus urophasianus (Vehrencamp et al., 1989) and the greater prairie chicken, Tympanuchus cupido (Nooker and Sandercock, 2008). Males of both of species gather in large groups where they perform a dance display that shows off their courtship plumage, which is accompanied by production of unusual drumming sounds produced by airflow through specialized air sacs. Other examples include the well-known birds-of-paradise, a family of tropical Australasian birds that mate promiscuously with males performing truly exceptional courtship displays. The male superb bird-of-paradise, Lophorina superba, has two blue iridescent shields on his breast which create astonishing contrasts when he opens his black wings in his ballerina dance (Frith and Frith, 1988). Male Carola's parotia bird-of-paradise, Parotia carolae, perform a hop and shake dance, spreading their wings into a tutu-like formation to enhance their iridescent colours and the wire-like feathers protruding from their heads (Scholes III and Sodhi, 2006). Some birds enrich their courtship by the construction of specialized display arenas. Males of many bowerbird species, such as the satin bowerbirds Ptilonorhynchus violaceus and the great bowerbird, Chlamydera nuchalis, not only perform exaggerated movements and vocalizations during their display but also build their bowers which they decorate with coloured objects that contrast with their plumage (Endler and Day, 2006; Endler et al., 2005; Patricelli et al., 2003).
Of relevance to the focus of this review, many of the courtship displays highlighted here include movements that are strikingly unique, quite dissimilar from normal body movements used in normal daily life, such as when foraging for food, when interacting in non-reproductive social contexts or when avoiding predation. As we will discuss below, elaborate courtship displays may or may not require an intense expenditure of energy but many displays do appear to require proficient neuromuscular control. The neuro-muscular systems used in courtship may be adapted from existing structures or they may evolve independently for dedicated use in these spectacular behaviours. Several overlapping predictions emerge from observations about male and female neuromuscular systems, given that males are generally the sex that engages in courtship.
Neuromuscular specializations will be found in males who perform the displays, compared to females (or juvenile males) that do not perform courtship.
Sexually dimorphic phenotypes, like those identified in prediction #1, can arise developmentally, by the organizational actions of sex-hormones, or they can arise in sexually mature adults by the activational effects of sex-hormones. Thus, neuromuscular systems will evolve sensitivity to gonadal steroid hormones, most likely to testicular androgens, by their expression of androgen receptors.
Courtship behaviours provide information about the neural and muscular proficiency of the male.
The relevant visual and auditory sensory capabilities of the female will undergo selection for detecting honest indicators of male motor skills. Alternatively, female sensory capabilities will drive the evolution of behaviours that are well-perceived by the female.
As we will see, our studies of the golden-collared manakin, described in detail below, show that some, but not all, of these predictions are met. Moreover, our studies add some new concepts that expand upon these predictions as they might then apply to other species. One central theme that connects these predictions is the pervasive influence of gonadal hormones on the organization and activation of courtship. In what follows, we will introduce basic concepts concerning neuroendocrine control of courtship and then address each of the predictions outlined above. The intensive study of the neurohormonal control of birdsong, the complex learned vocalizations of oscine songbirds that are often used in courtship, provides an important framework for this current discussion, so we will invest some of this introduction to this system.
1.2. The neuromuscular control of song
The song of oscine songbirds is produced by airflow over membranes of the syrinx, an organ lining the trachea, which is composed of 9 intrinsic muscles (for review see Zeigler and Marler, 2008). Song is produced during both the inspiration and expiration, allowing uninterrupted vocalizations over relatively long periods. Thus, oscine song, which can be rich in structure and amplitude, requires accurate control of several effector muscle systems, including those that control respiration, the syringeal muscles themselves, the muscles that control the opening of the mouth and the overall posture of the bird. The neural song system has features specialized for auditory processing, encoding, song learning, and pre-motor control of song structure, and these circuits ultimately drive motor neurons of the pars tracheosyringealis of the nervus hypoglossus to control muscles of the syrinx and song output. Importantly, a parallel circuit exists that controls the ‘calls’ expressed by many birds, with the midbrain nucleus intercollicularis (ICo) serving significant pre-motor control over the syrinx.
It is important to recognize that songbirds are not the only taxa with sex-specific neuromuscular systems controlling vocal production used in courtship, and the study of these other animals has shed considerable light on basic principles in neurobiology. Amongst these include those studies of the underwater courtship vocalizations of species of toadfish (Bass and McKibben, 2003) and some frogs (Moore et al., 2005). As in songbirds, males of these species vocalize mainly to attract females for mating, suggesting that sexual selection drives the evolution of these vocalizations. Indeed, the midbrain circuit described above that controls the calls of many birds appears to be a highly conserved system found throughout the vertebrate lineage that is central for motor control of a variety of systems involved in vocal and visual communication (Bass and Chagnaud, 2012).
1.3. Seasonality and gonadal hormone control of courtship
In birds, as in most vertebrate species, male reproductive behaviours are activated when the testes secrete testosterone (T) during seasonally appropriate periods of reproduction (reviewed by Wingfield and Farner, 1993). These behaviours include vocalizations such as the song of oscine songbirds, aggressive behaviours associated with territorial defence, and the appetitive (courtship) and consummatory (copulation) phases of male sexual behaviour (Balthazart, 1983). In most species studied, male courtship behaviours are under control of testicular hormones (reviewed by Fusani, 2008), indeed, some of these studies were instrumental in advancing the field of behavioural endocrinology (Berthold, 1849; Hutchison, 1967; Silver et al., 1973).
Because it is the substrate for the formation of oestrogens (by local action of the oestrogen synthetic enzyme aromatase), as well as acting as an androgen per se [or after its conversion to 5α-dihydrotestosterone (DHT) by neural 5α-reductase activity], effects of T on brain and behaviour can occur via the activation of oestrogen-receptors (ER), androgen-receptors (AR), or both. There is evidence that courtship behaviours require activation of both steroid signalling pathways. In several well-studied oscine song-birds, for example, activation of both AR and ER is needed to fully activate courtship song (Schlinger and Brenowitz, 2009), with each pathway responsible for promoting the performance of different song features (Fusani and Gahr, 2006). In classic studies of captive doves, oestradiol was found to stimulate the performance of non-aggressive components of courtship (nest solicitation) whereas aggressive components of courtship (chasing and bowing) are stimulated by T or by 5α-DHT (Cheng and Lehrman, 1975; Fusani et al., 2001; Hutchison and Steimer, 1984). In the well-studied Japanese quail, 5α-DHT activates the courtship strutting display and oestradiol and 5α-DHT together activate crowing used in courtship (Ball and Balthazart, 2004, 2006). As may be obvious, much of this foundational work was performed on captive species. We provide additional evidence below demonstrating a role for gonadal hormones in the complex courtship of wild male golden-collared manakins.
A critical feature of the hormonal control of courtship involves an appreciation of the ecological constraints that are imposed on reproduction itself. In temperate and high latitude species, T is elevated for relatively brief periods of time during the seasonally restricted periods of reproduction (Balthazart, 1983; Silver and Ball, 1989; Wikelski et al., 1999). A substantial elevation of T levels does not appear to be required to activate these same behaviours in some lowland tropical species where lengthy breeding seasons and/or year round territoriality are accompanied by low and relatively constant levels of plasma T (Goymann et al., 2004; Wikelski et al., 2003). This latitudinal difference among avian species may be related to the fact that elevated T levels can be costly, i.e. they can reduce immunocompetence (Mougeot et al., 2004). Tropical species with their protracted reproductive seasons may need to find alternative means of regulating reproductive behaviours to avoid having prolonged exposure to high T levels. For example, it is possible that dehydroepiandrosterone (DHEA) may act as a precursor to the neural formation of active androgens and oestrogens that activate courtship in some species, as has been shown for its role in aggressive behaviour of some species (Hau et al., 2004; Soma et al., 2004). Alternatively, brain sensitivity to year-round circulating androgen may be seasonally modulated as shown for example for year-round territorial spotted antbirds (Canoine et al., 2007). Moreover, although T levels may remain generally low across the lengthy reproductive seasons of some tropical species, it is possible that T secretion is rapidly up regulated in response to social situations, such as territorial intrusion (Wikelski et al., 1999), and this ephemeral exposure to T is sufficient to sustain performance of courtship behaviour.
1.4. Sexual selection and the nervous system
Sexually dimorphic phenotypes are an obvious consequence of the forces of sexual selection. In polygamous species, for example, the sexes are often readily distinguished from each other; males are often larger than females and they frequently exhibit ornaments or armaments that females lack. These traits are termed secondary sex characters and they arise developmentally, by the actions of sexspecific gene expression or by the actions of gonadal hormones, or in adults by the activational effects of gonadal steroids.
Although behaviour was known to undergo sexual differentiation by the developmental actions of sex steroids (Phoenix et al., 1959), up until 1976 there was only limited evidence for neural sexual dimorphisms (e.g. Raisman and Field, 1973). Describing their studies of canaries (Serinus canaria), an oscine songbird, Nottebohm and Arnold (1976) reported large differences in the volumes of specific brain areas of the telencephalon known to be responsible for the learning and production of the complex “songs” of this group of birds (Nottebohm and Arnold, 1976). In the case of the canary and some other oscine songbirds, singing is a sexually dimorphic behaviour, produced by males and not females. Thus, this sexual dimorphic song system formed the basis of the sexually dimorphic expression of a behaviour often used in courtship. Sexually dimorphic brain areas have now been described in a number of taxa, especially for control of conserved sex-specific behaviours such as performance of copulatory behaviour (Arnold et al., 2004), but we know less about brain sex differences involved in the control of physical courtship displays.
The mechanisms producing the neural sex differences in oscine songbirds has been the focus of considerable investigation, largely involving zebra finches, a species that is like the canary in that only adult males sing and possess a masculine neural song system. A number of early studies of zebra finches pointed to a significant role for gonadal hormones, particularly oestradiol, in the masculine development of the song system (Schlinger, 1998). However, subsequent work, largely out of the lab of Art Arnold, points to a significant involvement of sex-specific genes (likely present on sexchromosomes) that dictate song system development (McCarthy and Arnold, 2011). In the end, there may well be a combination of hormonal and direct genetic factors that contribute to the development of the zebra finch masculine song system. An important distinction is seen in canaries where adult females can be induced to sing by treatment with male-levels of T, and they also show significant growth of their neural song system (reviewed by Fusani and Gahr, 2006; Nottebohm, 1980). These kinds of effects are considered activational, as they reflect a transient growth of the song system in the presence of T acting on androgen receptors which are present also in the female brain (Fusani et al., 2003b). On the contrary, the neural and behavioural sex difference in the zebra finch develops once early in life and is retained permanently thereafter. For example, treatment of adult female zebra finches with T does not activate song nor does it stimulate growth of a male-like song system (Arnold, 1975).
Studies like these point to a crucial feature of oscine birdsong, its high responsiveness to testicular hormones. First, as pointed out above, there is considerable evidence that T and its androgenic and estrogenic metabolites are crucial for the organization and the activation of neural circuits and some of the musculature to allow for appropriate song learning and production (reviewed by Schlinger et al., 2013; Schlinger and Brenowitz, 2009). Second, the various nuclei of the neural song system, as well as the muscular syrinx itself, express receptors for androgens and/or oestrogens, and respond to these hormones to enable the learning and production of song (Schlinger and Brenowitz, 2009). Thus, the hormonal and neuromuscular control of song serves as a template on which to evaluate neuromotor systems that control other courtship behaviours, including the elaborate physical courtship of many lekking species of birds.
2. The acrobatic kings
2.1. Exaggerated displays in neotropical manakins
Manakins (Pipridae) are a family of Neotropical sub oscine passerines including around 50 species, most of which are polygynous and have a lek mating system. Most species are sexually dimorphic, with the adult male being brightly coloured while the female has a cryptic green plumage. During the breeding season, male manakins perform elaborate courtship displays involving acrobatic manoeuvres, production of mechanical sounds (sonations) using their wings, and coordinated displays involving one or more males (Fig. 1). The existance of morphological specializations in male manakins related to courtship behaviour – and sonation in particular – was already acknowledged by Darwin [1871, p. 2; Vol. II, Cap. XIII]. Display locations vary between species, they can be vertical or horizontal branches high up in the canopy or closer to the ground, fallen logs or cleared display arenas on the forest floor (Prum, 1990). Members of the genera Chiroxiphia produce coordinated, highly elaborated displays. Displays of lance-tailed, Chiroxiphia lanceolata (DuVal and McDonald, 2007; DuVal and Kempenaers, 2008) and long-tailed manakins, Chiroxiphia linearis (Trainer et al., 2002) include coordinated movements between an alpha and a beta male, with the alpha obtaining all of the copulations. Males dance in vertical loops, jumping and using the stabilizing power of fluttering wings while producing sounds, until the alpha male mates with the female or the female leaves. Members of the genera Pipra have complex displays that are performed by individual males. A well-known example is the “moonwalk” dance of the red-capped manakin, Ceratopipra mentalis. Males take off from their horizontal perch and, after performing some loops in the air, they return to the perch to hop back and forth with such a velocity that the human eye only perceives a smooth moonwalk (Prum, 1990). Members of the genera Manacus not only possess modified wings to produce loud mechanical sounds, but modify their dancing grounds similar to what has been described above for bower birds (Chapman, 1935).
Fig. 1.
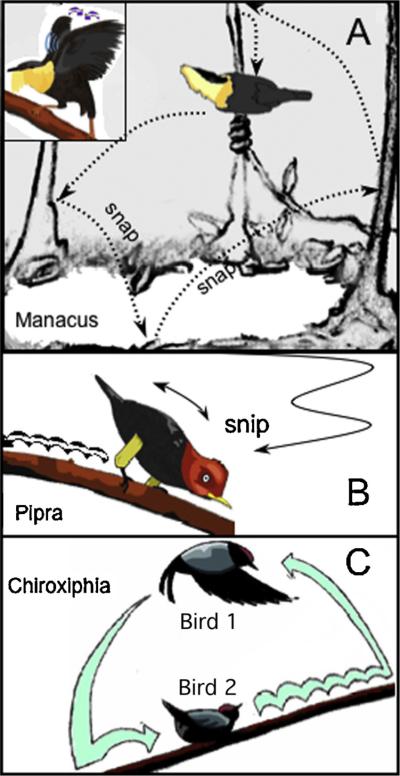
Schematic drawings of male courtship displays of three manakin species (Pipridae). (A) Golden-collared manakins (Manacus vitellinus) hop or flip from twig to twig on a cleared arena loudly snapping their wings. The male is depicted with the bright feathers of the collar erected in the ‘beard-up’ posture, which they resume immediately after landing. Inset: The rollsnap is a series of wingsnaps performed at 50 Hz when perched. (B) Red-capped manakins (Pipra mentalis) take off from perch with quiet rollsnap, swoop in with “S” flight landing with a quiet wingsnip, quiver the tail and take tiny backward hops – ‘moonwalks’. They then hop forward, sometimes pivoting to moonwalk in a new direction. (C) Lance-tailed manakins (Chiroxiphia lanceolata) perform cooperatively. Each male flutters up then backwards over the partner, lands, then hops up to take the others place; only the alpha male mates.
2.2. The dances of bearded manakins
The bearded manakins (Pipridae: Manacus) are a complex of four different, but closely related species: the white-collared manakin (Manacus candei), orange-collared Manakin (Manacus aurantiacus), golden-collared Manakin (Manacus vitellinus) and white-bearded Manakin (Manacus manacus). These species perform very similar courtship displays (Bostwick and Prum, 2003; Brumfield and Braun, 2001; Chapman, 1935; Fusani et al., 2007; Snow, 1962). At the beginning of the breeding season, the male returns to his traditional lek, sometimes to the exact position, to prepare his courtship arena, an elliptical area (~1 m2) on the forest floor, which is surrounded by saplings. Males clear material, mostly fallen leaf matter, from their arena, creating a homogeneous background for their performance (Stein and Uy, 2006; Uy and Endler, 2004). During their courtship dance, males jump between the saplings that delimit the arena using the propulsive power of the legs. During these jumps, in mid-air, males produce a loud snap with their wings (wingsnap). Males dance in the absence of females, likely to attract them to the arena, or for practice. However, they dance especially vigorously when females are present, speeding up their movements (Barske et al., submitted). Females visit leks regularly during the days, with peaks in the first hours of the morning and in the early afternoon (Barske et al., 2014). Before copulating, females join males in their arenas and follow them from one sapling to the next observing their displays. Other behaviours that appear to be relevant for both intraand inter-sexual selection are the roll-snap, a mechanical sound produced by the wings that are clapped repeatedly at a frequency above 50 Hz, and the cheepoo, a two-note vocalization produced when males are socially aroused. Very often the roll-snap is followed immediately by a cheepoo, and the combination of these two signals – a sonation and a vocalization – appears to be mainly used to mark a male's position in the lek and to attract females to the court (Chapman, 1935; Lill, 1974). Bearded manakins possess both modified primary feathers but also modified wing bones for the production of mechanical sounds (Chapman, 1935; our observations).
2.3. The golden-collared manakin
For nearly two decades, we have focused our work on the golden-collared manakin, M. vitellinus. The adult male is coloured in conspicuous black, golden and green feathering whereas juvenile males and all females are feathered green and indistinguishable. Golden-collared manakins live in the lowlands of Panama (Fusani and Schlinger, 2012; Schlinger et al., 2013). Our study site is around Gamboa, a small village along the Panama Canal surrounded by secondary forest, the preferred habitat of golden-collared manakins. We used high speed videography to record and analyze the rapid movements of the golden-collared male courtship dance – the jump-snap display (Fusani et al., 2007). A typical display sequence is as follows: Perched on his favourite branch a few metres above the arenas, a male performs a series of wingsnaps and then descends to his arena, landing on one of the delimiting vertical saplings, with his bright yellow beard feathers extended towards the centre of the arena. Next, he performs a series of rapid jumps keeping the wings closed against the body until the mid-point across the arena, when the wings are suddenly opened and rapidly and powerfully clapped over the back to produce a loud wingsnap. As the male reaches the sapling on the opposite end of the arena, he uses his wings to quickly manoeuvre, turning ~180° to face the centre of the arena. He remains perched for a short interval with his beard exposed before initiating another jump. All these movements combined take ~1 s, which explains the need for high-speed videography for quantitative analysis. Golden-collared manakins perform on average 9 jumps per display lasting ~8.5 s. Males differ significantly between each other for several behavioural elements of the display, providing sufficient variance in the signals to allow for female choice (Fusani et al., 2007), and this variance, we believe, serves as information used by females for selection of males for mating (Barske et al., 2011). The male has one additional feature of his jump-snap display, one often performed just prior to copulating, but also produced in the absence of females. In this case, the male jumps from the ‘mating sapling’ to the ground in the centre of his arena with a mid-air half-twist. The male then performs a strange helicoptering-like flutter-flight producing a “grunt” sound much like the quack of a duck (Chapman, 1935) before landing at a higher position on the mating sapling. If the female accepts his invitation, she moves to the mating sapling, the male then lands above her and slides down the perch onto her back for a quick copulation.
3. Anatomical specializations and sex-differences in manakins
3.1. Musculoskeletal system and feathers
Golden-collared manakins stand out from other similar-sized tropical birds by their compact and stout stature associated with hypertrophied muscles involved in lifting and retracting the wings (supracoracoideus and scapulohumeralis dorsalis) and in extending the legs to execute the powerful jumps of the jump-snap display (gluteal or iliotibialis Chapman, 1935; Lowe, 1942). In addition, Chapman (1935) noted that the sexually dimorphic primaries and secondaries are specialized in males being curved and heavy with stiffened vanes that are asymmetric and especially narrow. He posited that these primaries allow for the whipping together of the wings that produces the resounding snap of displaying males (Chapman, 1935). Building on this earlier work, we found that some wing muscles are significantly larger and have a greater fibre diameter in manakins compared to male zebra finches and to adult female manakins, birds that do not ordinarily wing-snap (Schultz et al., 2001). In addition to this unique musculature, manakins also have a uniquely flattened radius compared to the more typically rounded structure of the radius of other manakin or non-manakin species that do not make wingsnapping sounds (unpublished observations). Together, these data indicate that male manakins do indeed possess musculoskeletal specializations that likely are required for the rapidly and forceful wing motion and collision that produces the loud snapping sounds of the birds various wing-snap behaviours (Table 1).
Table 1.
Neural and muscular specializations found in golden-collared manakins (Manacus vitellinus) likely related to their elaborate courtship displays.
| Tissue/structure | Particularity | Reference |
|---|---|---|
| Brain | ||
| Arcopallium | Sexually dimorphic (M > F); Region-wide expression of androgen receptors | Day et al. (2011) and Fusani et al. (2003a) |
| Hippocampal area | Sexually dimorphic (M > F) | Day et al. (2011) |
| Ventrolateral mesopallium | Sexually dimorphic (F > M) | Day et al. (2011) |
| Spinal cord | ||
| Brachial and lumbosacral enlargements | Widespread expression of androgen- (F> M) and oestrogen receptors | Fuxjager et al. (2012) and Schultz and Schlinger (1999) |
| Motor and sensory neurones innervating limbs | Widespread expression of androgen receptor | Fuxjager et al. (2012) |
| Limb muscles | ||
| Supracoracoideus | Hypertrophied (M > F, M > ZF); Larger fibre size (M > F); Higher proportion of fast-twitch fibres (M > F) | Lowe (1942) and Schultz et al. (2001) |
| Scapulohumeralis dorsalis | Hypertrophied (M > F, M > ZF); Larger fibre size (M > F); Higher proportion of fast-twitch fibres (M > F) | Lowe (1942) and Schultz et al. (2001) |
| Iliotibialis | Hypertrophied (M > F) | Lowe (1942) and Schultz et al. (2001) |
3.2. Brain
As mentioned previously, sex differences in behaviour are associated with sexual dimorphism in brain regions related to those behaviours. In songbirds where only males sing, song nuclei, such as HVC and the robust nucleus of the arcopallium (RA), are larger in males than in females; in species were both sexes sing there is no such dimorphism (Airey et al., 2000; Brenowitz, 1997; Kirn et al., 1989; Tramontin et al., 1998). The size of the hippocampus, a region that plays a prominent role in spatial ability in birds and mammals, can be related to sex-specific demands for locating dispersed resources (Day et al., 2008; Galea et al., 1996; Gaulin, 1992; Jacobs et al., 1990; Reboreda et al., 1996). For example, while sex differences in brood parasitic nesting behaviour favour a larger hippocampus in female cowbirds that search for egg laying sites, differences in singing behaviour favour a larger HVC in male cowbirds (Hauber et al., 1999), indicating sex specific neural phenotypes.
Examination of the brains of golden-collared manakins has also revealed sex differences that may underlie the performance of courtship behaviour. We were especially interested in the possibility that there might be some association of brain areas that control vocal communication with areas that controlled wing-snapping, given that they are both auditory signals produced for purposes of courtship. Examination of the manakin brain revealed no evidence for a neural song system with well-delimited forebrain nuclei as in oscine songbirds (Saldanha et al., 2000) or for widespread expression of the oestrogen synthetic enzyme aromatase, a second characteristic feature of the oscine brain (Saldanha et al., 2000). We did gain some clues regarding the structure of the manakin brain from studies examining the expression of neural expression of AR (described in more detail below). We were struck by prominent expression of AR throughout the arcopallium (Fusani, in press, p. 148), a region that houses the pre-motor androgen-sensitive nucleus RA of the oscine neural song system. In non-oscine song-birds, the non-amygdaloid arcopallium is AR sparse including areas outside of RA in oscine songbirds (Ball and MacDougall-Shackleton, 2001; Bernard et al., 1999; Fusani et al., in press; Metzdorf et al., 1999). Interestingly, when corrected for telencephalon size, we found that the whole manakin arcopallium was larger in males than in females. Previous cytological descriptions of the avian arcopallium revealed five sub regions with distinct cellular morphology: the dorsal (AD), intermediate (AI), and medial arcopallium (AM), the posterior amygdala (PoA) and nucleus teniae of the amygdala (TnA) (Reiner et al., 2004; Saint-Dizier et al., 2009; Zeier and Karten, 1971). Across the manakin arcopallium, neuronal soma size was similar in males and females and no one sub region was sexually dimorphic (Day et al., 2011). However, males had more cells (greater density) in the AD, AI, AM as compared to the PoA and TnA, whereas females demonstrated the opposite pattern, that is cell density was greater in the PoA and TnA as compared to the AD, AI, AM (Day et al., 2011).
While we think it most likely that males larger arcopallium is associated with finer motor control in males than in females, the AP is both sensorimotor and limbic in nature (Sadananda et al., 2007; Saint-Dizier et al., 2009; Zeier and Karten, 1971) and plays roles in ingestive behaviours (Campanella et al., 2009; da Silva et al., 2009), fear conditioning (Saint-Dizier et al., 2009), copulation (Charlier et al., 2005), courtship (Sadananda et al., 2007), and memory functions (Knudsen and Knudsen, 1996) such as imprinting (Lowndes et al., 1994) and passive avoidance (Aoki et al., 2006; Lowndes and Davies, 1995). Thus, in the absence of direct electrophysiological, tract-tracing or evidence for activation by immediate-early gene expression, we cannot be certain that the sex-differences we detect are involved in courtship. Studies are currently underway to assess this possibility.
In addition to the arcopallium, we found that males have a larger hippocampal region. The hippocampus plays a role in complex forms of learning and memory, particularly in spatial learning (Day, 2003; Goodrich-Hunsaker and Hopkins, 2010; Rodriguez et al., 2002; Sherry and Hoshooley, 2010). It is known to be particularly plastic and is both experience and steroid sensitive (Clayton, 1995, 1996; Galea et al., 2006; Garza-Meilandt, 2005; Lathe, 2001; Maguire et al., 2000; Oberlander et al., 2004; Roof and Havens, 1992; van Praag et al., 2002). We suspect the hippocampus is larger in male manakins than females because males maintain both within and between season arena site fidelity and presumably learn spatial features of their arena to optimize the jumps between the saplings during the jump-snap display (Day et al., 2011).
Female manakins appear able to visually discriminate very fast behavioural elements of the male's courtship display (Barske et al., 2011; Fusani et al., 2007). Thus we speculated that females might have enhancements to visual processing regions of the brain. In keeping with this idea, we found that females had a larger ventro-lateral mesopallium (MVL), a tectofugal visual region (Day et al., 2011). The volumetric sexual dimorphism was significant both when controlling for the size of the remaining telencephalon and also by direct comparison. The function of MVL is not well understood but is likely related to modulation of visual guided behaviour via descending striatal-brainstem pathways (Krützfeldt and Wild, 2004). Some portions of the mesopallium may also be involved in sexual imprinting networks (Sadananda and Bischof, 2006). We reason that the size of this visual brain region might be larger in females than in males for reacting to and positioning oneself opposite the male to best view the display and to discriminate male performance. Obviously, many signals used during courtship are directed to neighbour males as well as to courted females, but there might be visual components of the display that target females specifically.
4. An androgen-dependent phenotype
4.1. Widespread sensitivity to androgen
Androgens are known to influence neuromuscular systems underlying some reproductive behaviours, leading us to ask whether androgens acted widely to facilitate complex courtship behaviour. One of the first experiments to address this hypothesis compared the capacity of sex steroids to act throughout the spinal cord (Schultz and Schlinger, 1999). We used autoradiography to assess accumulation of radioactivity in spinal cords removed from wild adult male and female golden-collared manakins that had been captured and injected with radiolabeled T (3H-T). All male birds showed abundant accumulation of radioactive sex steroid throughout their spinal cords, with the vast majority of this accumulation in the ventral horns of the brachial and lumbosacral enlargements. These two areas house the motor neurons that inner-vate the wing and leg muscles, suggesting that sex steroids were acting on these cells to influence motor control of the appendages. In contrast, only one of the five females in this experiment showed significant accumulation of radioactivity. Thus, these results suggested a sex difference in the capacity for sex steroids action on the manakin spinal cord and a possible site where steroids controlled male courtship behaviour.
Additional work has further unravelled the pathways through which sex steroids act in the spinal cord to potentially drive male motor performance. First, we observed that both AR and ER mRNA were expressed in the spinal cords of both male and female manakins, but the AR expression predominated (Fuxjager et al., 2012). This suggests that the abundant 3H-T detected in the spinal column (Schultz and Schlinger, 1999) was largely the result of 3H-T or its more potent metabolite 3H-dihydrotestosterone (3H-DHT) bound to AR. Surprisingly, we found that female golden-collared manakins actually expressed more AR mRNA in their spinal cord than did males. This result seems counter-intuitive, in that autoradiography showed greater accumulation in the spinal cord of males. It is possible that males possess greater amounts of AR protein because they translate AR mRNA to a greater degree than do females or that there are sex differences in AR phosphorylation that affect the receptors ligand binding properties. Alternatively, the AR protein may have a shorter half-life in the spinal cord of females. It is also possible that sex-differences in spinal steroid accumulation may stem from sex differences in T metabolism. Testosterone might be inactivated more in females by the enzyme 5β-reductase (an abundant enzyme in many avian tissues, Hutchison and Steimer, 1984), or that T might be converted into the more potent androgen 5α-DHT by the enzyme 5α–reductase to a greater extent in males than in females (e.g. Jurman et al., 1982).
We obtained additional evidence indicating that androgens can function within the spinal cord to impact motor control of the appendages during courtship (Fuxjager et al., 2012). In this study, male and female birds were captured from the wild, and several wing and leg muscles were injected with fluorescent retrograde tracers to backfill innervating motor and sensory neurons. The spinal cords and dorsal root ganglia of these birds were then dissected and AR mRNA were labelled by in situ hybridization. Results showed that, in both males and females, the motor and sensory neurons that innervated the musculature of the wings and legs transcribed considerable AR, especially when compared to the rest of the spinal cord (Fuxjager et al., 2012). Thus, these data highlight pathways through which androgens could directly influence that neuromuscular circuitry to mediate courtship-related movements. Functionally, however, it is unclear exactly what activation of AR on these tissues does and how it might support male-typical courtship displays. Studies in mammals suggest that such effects likely change the morphology and physiological properties of motor neuron pools (Breedlove and Arnold, 1981; Holmes et al., 2009; Kurz et al., 1986; Matsumoto et al., 1988), which in theory could adjust balance, agility, and speed. Future studies will explore these possibilities in manakins.
In addition to the spinal cord, we also find that skeletal muscles of manakins are important androgen targets. Indeed, AR expression in several muscles controlling the wings, legs, and even the syrinx, were greater in golden-collared manakins compared to other passerine species (Feng et al., 2010). This AR expression appears to yield functional AR protein. Using quantitative PCR (qPCR) on wing musculature of manakins, we asked if androgens could alter expression patterns of putative androgen-dependent genes (Fuxjager et al., 2012). We found that T up-regulated expression of parvalbumin and insulin-like growth factor I, genes whose products enhance Ca(2+) cycling (Celio and Heizmann, 1982; Hou et al., 1991) and growth of skeletal muscle fibres (Musarò et al., 1999, 2001). Further, when we examined wing muscles of manakins and other species, we found that expression of these genes varied in a manner consistent with a function for AR-dependent gene regulation. These results suggest that androgens do indeed impact avian skeletal muscles and may do so in manakins to facilitate their elaborate courtship display (Fuxjager et al., 2012). Female golden-collared manakins transcribe similar amounts of AR in their wing muscles, suggesting that there is no sex difference in musculoskeletal androgenic sensitivity (Feng et al., 2010). Because T circulates at low levels in females, the AR in the muscles of females may naturally have little function.
The results described in these previous sections provide support for the predictions #1 and 2 raised in the introduction that (a) males that perform elaborate courtship displays do so by the use of specialized neural, skeletal and muscular systems and that (b) these neuromuscular systems are targets of androgen action whereby androgens can enhance contraction strength and speed to facilitate courtship displays.
4.2. Circulating testosterone and courtship activity
The courtship season of the population of golden-collared manakins that we study begins at the beginning of the dry season, around mid-January, and lasts until June when the wet season is well underway. Outside of the breeding season, T levels are basal, but they are elevated when males first appear at their arenas in mid-January. By comparison with birds breeding at higher latitudes, these breeding levels of T are relatively low (Day et al., 2006, 2007; Fusani et al., 2007). Moreover, T levels among adult lekking males are quite variable, ranging from 0.38 to 16.0 ng/ml plasma (Day et al., 2007). When six individual males were tracked from the beginning of their arrival at their courtship arenas, we found that T was elevated initially in all birds, but levels were decidedly lower three weeks later (Fusani et al., 2007). We also recorded several of the male's courtship behaviours over the same time interval and found that they were expressed at relatively constant levels. Thus, it appears that the onset of courtship coincides with elevated T, but the maintenance of courtship activity does not require circulating T to remain constantly elevated much above non-breeding levels (Day et al., 2007; Fusani et al., 2007). Nevertheless, outside of the breeding season, when circulating T levels are basal in adult male manakins that are displaying much less if at all, and when T levels are also basal in females and juvenile males, exogenous T treatments potently activates courtship activity both in the lab (wingsnaps) and in the field (full courtship) (Day et al., 2007). These results support the hypothesis that T is required for the activation of courtship displays.
Despite the variation in T levels of reproductively active males, we believe that T still supports courtship because pharmacological blockage of androgen receptors curtails courtship activity (see below). Nevertheless, what explains the individual variation in circulating T levels we observe over the course of the prolonged courtship season of lekking adult males? It is likely that this variability reflects rapid changes in T in response to dynamic social (lek) variables. This could include the arrival of a female at or near a male's arena, unsettled male-male competition for arena space, or changes in the overall social network of a lek by the arrival of other males. Thus, while establishing territories males might have high T levels that drop when territorial contests over arena ownership are settled. Variability may also be linked to changes in arena ownership when intruder males occupy vacant arenas upon death of a resident bird. We have observed at least one episode of ownership change, with a new male challenging the resident across several days until the latter departed and the intruder gained occupancy (L. Fusani, unpublished observation).
4.3. Androgen-dependent motor skills
We have considerable evidence that androgens play a dominant role in the activation of male courtship in male golden-collared manakins. First, we found that treatment of actively displaying males with the AR-blocker flutamide for three weeks decreased courtship behaviour at one-week post treatment in wild and captive birds (Fusani et al., 2007). A rebound of courtship activity under flutamide treatment measured at weeks two and three of treatment suggested some plasticity in the physiological reaction to AR blockage. Flutamide crosses the blood-brain barrier, so it is capable of exerting its antagonist effects on both central and peripheral targets. To address whether the actions of T could be ascribed to peripheral vs. central actions, in a second set of experiments, we treated reproductively active males with a peripherally selective anti-androgen, called bicalutamide (Freeman et al., 1989; Furr, 1989). In effect, this means that treated birds maintained functionally intact AR centrally, but not peripherally, an effect that we confirmed by measures of AR-dependent gene expression patterns in brain and peripheral skeletal muscles of captive golden-collared manakins (Fuxjager et al., 2012). Once implanted, individuals were quickly released back into wild and observed for many days. The results were striking, as treated males rapidly changed their display repertoires. Within one day of implantation, treated males reduced the frequency with which they produced wing-snaps, roll-snap, and jump-snap displays (Fuxjager et al., 2012). Treatment also influenced acoustic elements of the cheepoo, namely its duration and its fundamental frequency (unpublished observations). At the same time, there was no apparent effect on the frequency with which treated males broadcast cheepoo calls, or on the probability that males actively maintained and defended their courtship arena. Activation of AR outside of the central nervous system therefore influenced specific display traits, namely those that require superior motor agility and coordination and possibly vocal filtering.
Additionally, in the work using bicalutamide, we tape-recorded roll-snap sonations of treated and untreated males. These mechanical, firecracker-like sounds are produced when males lift their wings above their head and snap them together multiple times in rapid succession (roughly 50 snaps per second). Roll-snaps therefore require exceptional control of the wings, and examination of the spectrograms and waveform diagrams of the resulting sonations provides a metric through which natural motor coordination can be assessed. The results from this analysis revealed that, on average, treated males produced roll-snaps that not only incorporated fewer independent snaps, but also that had greater time intervals between each snap. Moreover, the data also suggested an interaction between treatment and snap interval, such that males given bicalutamide produced snaps within a roll-train more slowly at first, but then sped this process up as the roll-train progressed (Fig. 2). Together, these findings show that peripheral AR helps modulate the fine motor patterning necessary to completely some of the male's display moves.
Fig. 2.
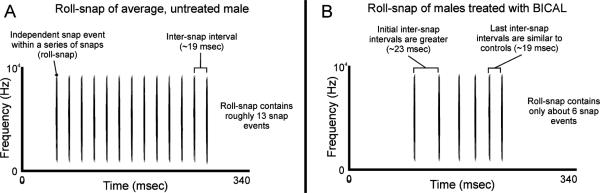
Comparison of roll-snaps produced by untreated male Golden-collared manakins (A) and males treated with the peripherally active androgen receptor blocker, bicalutamide (B). Treated males produced roll-snaps composed of less independent snaps and greater time intervals between each snap. Their roll-snaps were also more irregular, i.e. they were more spaced in time at the beginning and reached the typical inter-snap interval only towards the end of the sonation.
Overall, the effects of peripheral AR are likely manifest through the wing muscles themselves, especially since the functional inhibition of these receptors significantly disrupts behaviour that relies on complex and acrobatic physical movements. Given that male display behaviour is adaptive, the logical assumption is that sexual selection favours androgenic action within the neuromuscular system as a mechanism to fine-tune the physiological control of male display output. This has been observed in other species, whereby androgenic effects on select skeletal muscles presumably change these tissues, so that they are “prepared” for reproductive behaviour (Arnold, 1975; Brantley et al., 1993; Regnier and Herrera, 1993).
Although interference with AR inhibits courtship behaviour, we have some evidence that oestrogens also participate in the full activation of male manakin courtship. First, a combination of oestradiol and DHT activated display elements more potently than either steroid alone, but only when DHT followed treatment with oestradiol (Fusani et al., 2007). The combination of oestradiol + DHT increased courtship display to a degree similar to that of a single T implant (Day et al., 2010). Simultaneous treatment of non-breeding birds with T and fadrozole, an inhibitor of the enzyme aromatase (oestrogen synthase), significantly reduced several display elements compared to males treated with T only (Day et al., 2010). These data suggest that oestrogens participate in the activation of male manakin courtship and combine with androgens to stimulate full male courtship behaviour.
4.4. Displays by female manakins
When testing whether T could activate courtship display elements in non-breeding manakins (discussed above), we implanted many green-plumaged birds. T-treatment induced most of these birds to produce wingsnaps, including birds that we later discovered were females, after they were sexed genetically (Day et al., 2006, 2007, 2010; Fusani et al., 2007). This surprising result was instructive in several ways. First, we had previously expected that the capacity to wingsnap was restricted to males because (a) they possessed uniquely shaped primary and secondary wing feathers thought to be used to create the snaps; (b) the males possessed hypertrophied wing musculature that was thought necessary for wingsnaps and (c) we thought that the neural circuits underlying courtship might be organized in a sexually dimorphic manner in manakins such that only males would produce wingsnaps. These results showed us that we and others were wrong on all accounts. First, it was clear the modified wing feathers of males are not necessary to produce wingsnaps since females could wingsnap, despite lacking these unusual feathers. We now know that the sound is produced by the collision of the radii at the top of the wing stroke (unpublished observations). Second, the enlarged wing muscles of males were likely not a constitutive male trait that enabled wingsnaps. We now assume that wing muscles are enlarged in males due to the actions of T, due to repeated muscle use during courtship, or a combination of these actions. By increasing the motivation to produce wingsnaps and direct actions on muscle, prolonged T exposure in females might produce enlarged musculature.
These results also indicate that the neural circuitry underlying the motivation and capacity to make wing- and roll-snaps is not a permanent sexually dimorphic trait. In other words, and in contrast to one of our predictions, these neural circuits are not differently organized developmentally in male and female manakins. They seem to exist in females but are inactive in the absence of sufficient circulating T. This is similar to what observed in female canaries: in this species, only male sing and the neural song system is sexual dimorphic, but T-treatment of adult females induce a masculinization of some areas of the song system and the development of male-like song (reviewed by Fusani and Gahr, 2006; Nottebohm, 1980). It is important to add here that both male and female golden-collared manakins possess modified radii that appear to create the snapping sounds as the wings collide over the bird's head during wing- and roll-snaps. Thus, neural and skeletal attributes that are necessary for the production of behavioural elements used in the male courtship display are present in females, though we have no evidence that they are used in nature.
The only evidence we have at this point for a pure sexually dimorphic trait concerns plumage characteristics. In experiments that involved exogenous T treatment of green birds (that included both females and juvenile males), we plucked body contour feathers from tracts that become sexually dimorphic in colour as males mature. We also plucked primary wing feathers that are sexually dimorphic in structure in adult males. In all cases, the feathers that grew back during exposure to T were identical to those that were plucked and had grown in the absence of T exposure (Day et al., 2006). Thus, plumage in golden-collared manakins appears to grow in a sexually dimorphic pattern that may be independent of hormonal exposure. In addition, because some of these birds were able to produce wing snaps the study provided indirect evidence that the structure of the wing feathers is not involved in creating the wingsnap sonation. As adult male manakins fly about their leks, they do produce a soft shuffling sound. Perhaps the modified primary feathers are involved in creating these sounds during flight.
We return now to our final prediction which assumes that sensory capabilities may drive enhancements of the male's display. As we discussed previously, at least one visual processing brain region is enlarged in females manakins as compared to males. If this size difference reflects function, then it may be that female visual processing capabilities are indeed superb and their ability to perceive small differences in the timing, and perhaps accuracy, of the male's display may have coevolved with the capacity of males to display more quickly and with exceptional control. In the following chapter, we will discuss why we think that female choice has a primary role in the evolution of male manakin displays.
5. Evolutionary forces behind manakin courtship
5.1. Is mate choice involved?
The unique specializations that underlie the acrobatic capacities of manakins are thought to be the results of an intense process of sexual selection and mate choice in particular (Prum, 1990, 1997). Surprisingly, evidence for a role of female choice in the evolution of elaborate courtship that involves fine motor skills is scarce (Byers et al., 2010). In several of the oscine songbirds, the fast repetition of rapidly modulated syllables appear to be constrained by individual capacities of neuromuscular control (Podos et al., 2009), and this ‘virtuosity’ can be discriminated by females (Byers, 2007; Drǎgǎnoiu et al., 2002). What is the evidence that male motor performance during the golden-collared display is related to mate choice? First, our studies show that males vary with respect to how they perform their display. For example, some males perform quantitatively more of the basic display elements, such as wing-snaps, roll-snaps, and jump-snap displays, compared to others in the population (Fusani et al., 2007; Fuxjager et al., 2013). High-speed video recording of males performing their jump-snap routines at their own display courts shows that individuals also differ in the speed with which they jump among saplings (Fusani et al., 2007). Interestingly, this latter form of variation occurs on the order of hundredth of seconds; that is, some males accomplish jump-snaps fractions of seconds faster than other males. These data suggest that male display performance, and thus the requisite motor coordination, is a substrate on which sexual selection can act.
Furthermore, if male motor skills are sexually selected via mate choice, then females must be able to perceive these differences in male behaviour. Studies in females suggest that this is the case, as faster males are preferred as mates compared to slower males (Barske et al., 2011). This study observed male display performance and noted the putative reproductive success of each male. Indeed, males that produced more display elements in general acquired more copulations from females. This indicates that females distinguish males by their activity levels and choose to mate with those males that have more active displays. The results also showed that males that jumped around their display arena hundredths of a second faster were more likely to acquire a mate. Again, these findings not only provide evidence that females can distinguish among extraordinarily rapid movements, but they also suggest that females use information from these observations to help select mates.
5.2. Female choice for a good heart
We have reason to believe that the manakin cardiovascular system may be specialized to provide for the aerobic demands of courtship. As mentioned above, the manakin courtship displays are very rapid behaviours. For example, the rollsnap is produced by a rapid sequence of wings clapping at a frequency of 50–60 Hz. This requires a fast oxygen supply to the relevant muscles. Using recent telemetry technology, we were able to measure heart rates on free-living birds during displays with transmitters weighing about 1 g, i.e. less than 5% of the body weight (~18 g). We found that during their courtship activities manakin show heart rates that are among the highest recorded in the animal world, reaching up to 1300 beats per minute (Barske et al., 2011). These results suggest that intense sexual selection in manakins has led to cardiovascular specializations required to perform acrobatic courtship display. We are currently examining this possibility.
5.3. Learning to dance?
Undoubtedly, the courtship displays of golden-collared manakins are among the most elaborate of non-human animals. Several observations suggest that cognitive abilities are involved in both the production and the response to the displays. We have shown that females can produce elements of the courtship including wingsnapping when treated with T (Day et al., 2006), and it seems likely that the single motor components of the display are hardwired in the manakin physiology – i.e. fixed action patterns in a classical ethological definition. This does not exclude, however, that manakins might use higher cognitive functions in building up their courtship display. It is useful to remind the reader here that courtship is made by several components besides the behavioural elements: the ‘ornaments’, i.e. the bright plumage of the collar and back, and the courtship arena or court, which is prepared by the males by clearing a patch of ground from dead leaves and other debris. The cleaning of the arena is important for the reproductive success of the male, probably because it improves the contrast between the plumage and the background increasing thus its signal value (Stein and Uy, 2006). The cleaning of the arena, similarly to the building of a bower for a bowerbird, requires the capacity of delineating an ellipsoidal perimeter after having chosen an appropriate group of saplings spatially organized so as to provide adequate room for the male's display performance.
However, the most likely contribution of higher cognitive functions to the courtship display is in the choreography itself. As described previously, manakins mate on a specific sapling of the arena designated as the mating sapling. We were puzzled by the existence of this specific sapling, because it does not stand out, resembling the other saplings that delimit the arena. Therefore, we tested whether males could switch quickly to a different sapling to perform the quack or grunt. Before sunrise and the daily start of courtship activity, we placed a piece of branch on the mating sapling and observed the behaviour of the male upon discovering it. The males were agitated by the branch, reacting to it with calls and efforts to remove it. They were also unable to perform a full display including the final quack or grunt, at least for the first full day after the disruption (Coccon et al., 2012). This suggests that the display follows a rigid choreography that cannot be changed abruptly, and that the choreography involves a learned sequence of jumps. We have observed birds that we believe to be young males in green or transitional plumage visiting the courts of lekking males, perhaps in an effort to learn the choreography of successful adults (unpublished observations). A similar behaviour has been described for bowerbirds, which have a similarly complex and elaborate courtship involving landscaping abilities (reported within Madden, 2001). Future studies in this direction will help us to understand how much cognition is involved in manakin courtship displays. For example, the court might serve to constrain females to observe males from specific points, similarly to the “theatres with forced perspectives” that have been described for bowerbirds (Endler et al., 2010).
5.4. The aesthetic of manakin dances
In this review, we examined the elaborate courtship of manakins mainly from the prospective of the individual that perform the courtship. If the trait has evolved by sexual selection, one cannot ignore however the role of the receiver of the signal. The contribution of intra-sexual selection in shaping the courtship of manakins has been studied with a particular focus on vocal and colour signals. The bright coloured collar of males plays an important function in male-male interactions and seems to be a key factor in the process of speciation within the genus Manacus, for the different species differ mainly because of the colour of the collar (McDonald et al., 2001). Nevertheless, we have evidence that closely related species within the genus Manacus differ significantly in various aspects of the motor components of the courtship display (Barske et al., in preparation), indicating that the behavioural aspects of courtship contribute to the speciation processes.
It is likely that the selection process does not operate independently on single aspects of the display. Manakin courtship displays contain a number of signals that appear to rely on motion, colour, audio, landscape, contrast, etc. Multimodal signals are not uncommon in courtship, although their function is unclear or at least cannot be generalized (Candolin, 2003; Rowe, 1999). We hypothesize that females might choose males not by dissecting and scoring single components of his displays, but relying on its integrate ‘aesthetic’ value, which is not necessarily reflected by the dimensional or chormatic properties of its component signals, i.e. the best display does not necessarily result from the combination of the longest jump, the brightest plumage, and the cleanest arena. We are well aware of the difficulties of testing this hypothesis, but a first step could be that of examining whether specific combination of traits are particularly successful in eliciting female interest. Note that the hypothesis that the courtship has an aesthetic value does not necessarily exclude that some of the sexually selected traits indicate genetic quality; in this respect we agree with other authors that elaborate displays may be the results of a combination of evolutionary mechanisms that may or may not provide genetic benefits to the chooser (e.g. see Prum, 2012).
6. Conclusions
We now return to the predictions described at the outset regarding neuromuscular control of complex courtship. First, beyond the sexually dimorphic plumage of golden-collared manakins, these birds also possess a number of neuromuscular and skeletal specializations involving the brain, spinal cord, skeletal muscles and perhaps also the heart, that underlie the production of the visually and acoustically elaborate courtship display. Second, at least some of these adaptations develop because many man-akin tissues express considerable AR, giving them widespread and enhanced sensitivity to circulating androgens. In some cases, sex-differences are evident in some body tissues, but not in others, suggesting that steroid-dependent or steroid-independent sexual differentiation applies to only a subset of body tissues. Third, androgen-dependent function of the neural and muscular systems involved in courtship affects the features of the courtship display, particularly those that appear to be important to signal male motor skills, neuromuscular coordination, and, perhaps, cardiovascular function. Lastly, our data supports the conclusion that females have the capacity to assess, at a very fine-scale, the performance capabilities of males, perhaps because females themselves possess unique sensory abilities. Thus, mate choice drives the evolution of structural and behavioural elements of male courtship. The novelty and diversity of the behavioural repertoires likely provides information to the female about the physiological competence of individual males. Thus, the systems that evolve androgen-sensitivity are those most important to the female. We hope that this research shows that golden-collared manakins are an excellent example of how non-classical model species can contribute to our understanding of neurobehavioural complexity and variety and inspires additional research on this subject.
Acknowledgments
A large number of people contributed to data collection in the field and laboratory. Funding for our researches on manakins in the last two decades came from NSF (currently IOS-1147288) and the National Geographic Society. The work in Panama was possible only because of the help and support of the Smithsonian Tropical Research Institute and various government agencies of the Republic of Panamà including the Autoridad Nacional del Ambiente (ANAM). We want to thank in particular the staff of STRI for their help with sometimes difficult logistical issues and the Panamanian citizens for their hospitality. We thank John F. Ball for assistance in drawing Fig. 1.
References
- Airey DC, Buchanan KL, Szekely T, Catchpole CK, DeVoogd TJ. Song, sexual selection, and a song control nucleus (HVc) in the brains of European sedge warblers. J. Neurobiol. 2000;44:1–6. [PubMed] [Google Scholar]
- Andersson M. Sexual Selection. Princeton University Press; Princeton: 1994. [Google Scholar]
- Aoki N, Csillag A, Matsushima T. Localized lesions of arcopallium inter-medium of the lateral forebrain caused a handling-cost aversion in the domestic chick performing a binary choice task. Eur. J. Neurosci. 2006;24:2314–2326. doi: 10.1111/j.1460-9568.2006.05090.x. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Effects of castration and androgen replacement on song, courtship, and aggression in zebra finches (Poephila guttata). J. Exp. Zool. 1975;191:309–325. doi: 10.1002/jez.1401910302. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Xu J, Grisham W, Chen X, Kim Y-H, Itoh Y. Minireview: sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol. Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Androgen metabolism and the activation of male sexual behavior: it’s more complicated than you think! Horm. Behav. 2006;49:1–3. doi: 10.1016/j.yhbeh.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Ball GF, MacDougall-Shackleton SA. Sex differences in songbirds 25 years later: what have we learned and where do we go? Microsc. Res. Tech. 2001;54:327–334. doi: 10.1002/jemt.1146. [DOI] [PubMed] [Google Scholar]
- Balthazart J. Hormonal correlates of behaviour. In: Farner DS, King JR, Parker KC, editors. Avian Biology. Academic Press; New York: 1983. pp. 221–365. [Google Scholar]
- Barske J, Fusani L, Wikelski M, Feng NY, Santos M, Schlinger BA. Energetics of the acrobatic courtship in male golden-collared manakins (Manacus vitellinus). Proc. R. Soc. B: Biol. Sci. 2014;281:20132482. doi: 10.1098/rspb.2013.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barske J, Schlinger BA, Wikelski M, Fusani L. Female choice for male motor skills. Proc. R. Soc. Lond. B: Biol. Sci. 2011;278:3523–3528. doi: 10.1098/rspb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal–acoustic and pectoral–gestural signaling. PNAS. 2012;109:10677–10684. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Berthold AA. Transplantation der Hoden. Arch. Anat. Physiol. 1849;16:42–46. [Google Scholar]
- Bostwick KS, Prum RO. High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves). J. Exp. Biol. 2003;206:3693–3706. doi: 10.1242/jeb.00598. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Marchaterre MA, Bass AH. Androgen effects on vocal muscle structure in a teleost fish with intersexual and intrasexual dimorphism. J. Morphol. 1993;216:305–318. doi: 10.1002/jmor.1052160306. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Comparative approaches to the avian song system. J. Neurobiol. 1997;33:517–531. doi: 10.1002/(sici)1097-4695(19971105)33:5<517::aid-neu3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Brumfield RT, Braun MJ. Phylogenetic relationships in bearded manakins (Pipridae: Manacus) indicate that male plumage color is a misleading taxonomic marker. Condor. 2001;103:248–258. [Google Scholar]
- Byers BE. Extrapair paternity in chestnut-sided warblers is correlated with consistent vocal performance. Behav. Ecol. 2007;18:130–136. [Google Scholar]
- Byers J, Hebets E, Podos J. Female mate choice based upon male motor performance. Anim. Behav. 2010;79:771–778. [Google Scholar]
- Campanella LC, da Silva AA, Gellert DS, Parreira C, Ramos MC, Paschoalini MA, Marino-Neto J. Tonic serotonergic control of ingestive behaviours in the pigeon (Columba livia): the role of the arcopallium. Behav. Brain Res. 2009;205:396–405. doi: 10.1016/j.bbr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Canoine V, Fusani L, Schlinger B, Hau M. Low sex steroids, high steroid receptors: increasing the sensitivity of the non-reproductive brain. J. Neurobiol. 2007;67:57–67. doi: 10.1002/dneu.20296. [DOI] [PubMed] [Google Scholar]
- Celio M, Heizmann C. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982;297:504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Chapman FM. The courtship of Gould’s manakin (Manacus vitellinus vitellinus) on Barro Colorado Island, Canal Zone. Am. Mus. Nat. Hist. Bull. 1935;68:472–521. [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Lehrman DS. Gonadal hormone specificity in the sexual behavior of ring doves. Psychoneuroendocrinology. 1975;1:95–102. [Google Scholar]
- Clayton NS. The neuroethological development of food-storing memory: a case of use it, or lose it! Behav. Brain Res. 1995;70:95–102. doi: 10.1016/0166-4328(95)00133-e. [DOI] [PubMed] [Google Scholar]
- Clayton NS. Development of food-storing and the hippocampus in juvenile marsh tits (Parus palustris). Behav. Brain Res. 1996;74:153–159. doi: 10.1016/0166-4328(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Coccon F, Schlinger BA, Fusani L. Male golden-collared manakins Manacus vitellinus do not adapt their courtship display to spatial alteration of their court. Ibis. 2012;154:173–176. [Google Scholar]
- da Silva AA, Campanella LC, Ramos MC, Parreira C, Faria MS, Marino-Neto J, Paschoalini MA. Arcopallium, NMDA antagonists and ingestive behaviors in pigeons. Physiol. Behav. 2009;98:594–601. doi: 10.1016/j.physbeh.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the Origins of Species by Means of Natural Selection. Murray; London: 1859. [Google Scholar]
- Darwin C. The Descent of Man, and Selection in Relation to Sex. Murray; London: 1871. [Google Scholar]
- Day LB. The importance of hippocampal-dependent non-spatial tasks in analyses of homology and homoplasy. Brain Behav. Evol. 2003;62:96–107. doi: 10.1159/000072440. [DOI] [PubMed] [Google Scholar]
- Day LB, Fusani L, Hernandez E, Billo TJ, Sheldon KS, Wise PM, Schlinger BA. Testosterone and its effects on courtship in golden-collared manakins (Manacus vitellinus): seasonal, sex, and age differences. Horm. Behav. 2007;51:69–76. doi: 10.1016/j.yhbeh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Day LB, Fusani L, Kim C, Schlinger BA. Sexually dimorphic neural pheno-types in golden-collared manakins (Manacus vitellinus). Brain Behav. Evol. 2011;77:206–218. doi: 10.1159/000327046. [DOI] [PubMed] [Google Scholar]
- Day LB, Guerra M, Schlinger BA, Rothstein SI. Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater obscurus) Behav. Neurosci. 2008;122:527–534. doi: 10.1037/0735-7044.122.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day LB, McBroom JT, Schlinger BA. Testosterone activates courtship display but does not alter plumage in the tropical golden-collared manakin (Manacus vitellinus). Horm. Behav. 2006;49:223–232. doi: 10.1016/j.yhbeh.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Day LB, Wilkening SR, Brensinger J, Schlinger BA. Estradiol plays a role in activating the courtship display of golden-collared manakins (Manacus vitellinus). Southeast. Biol. 2010;57:133. [Google Scholar]
- Drǎgǎnoiu T, Nagle L, Kreutzer M. Directional female preference for an exaggerated male trait in canary (Serinusanaria) song. Proc. R. Soc. Lond. B: Biol. Sci. 2002;269:2525–2531. doi: 10.1098/rspb.2002.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuVal EH, Kempenaers B. Sexual selection in a lekking bird: the relative opportunity for selection by female choice and male competition. Proc. R. Soc. Lond. B: Biol. Sci. 2008;275:1995–2003. doi: 10.1098/rspb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuVal EH, McDonald DB. Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata) Auk. 2007;124:1168–1185. [Google Scholar]
- Endler JA, Day LB. Ornament colour selection, visual contrast and the shape of colour preference functions in great bowerbirds, Chlamydera nuchalis. Anim. Behav. 2006;72:1405–1416. [Google Scholar]
- Endler JA, Endler LC, Doerr NR. Great bowerbirds create theaters with forced perspective when seen by their audience. Curr. Biol. 2010;20:1679–1684. doi: 10.1016/j.cub.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Endler JA, Westcott DA, Madden JR, Robson T. Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. [DOI] [PubMed] [Google Scholar]
- Feng NY, Katz A, Day LB, Barske J, Schlinger BA. Limb muscles are androgen targets in an acrobatic tropical bird. Endocrinology. 2010;151:1042–1049. doi: 10.1210/en.2009-0901. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. [Google Scholar]
- Freeman SN, Mainwaring WIP, Furr BJA. A possible explanation for the peripheral selectivity of a novel non-steroidal pure antiandrogen, Casodex (ICI 176,334). Br. J. Cancer. 1989;60:664–668. doi: 10.1038/bjc.1989.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith DW, Frith CW. Courtship display and mating of the Superb Bird of Paradise Lophorina superba. Emu. 1988;88:183–188. [Google Scholar]
- Furr BJA. Casodex (ICI – 176,334): a new, pure, peripherally selective anti-androgen – preclinical studies. Horm. Res. 1989;32:69–76. doi: 10.1159/000181315. [DOI] [PubMed] [Google Scholar]
- Fusani L. Testosterone control of male courtship in birds. Horm. Behav. 2008;54:227–233. doi: 10.1016/j.yhbeh.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fusani L, Day LB, Canoine V, Reinemann D, Hernandez E, Schlinger B. Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm. Behav. 2007;51:62–68. doi: 10.1016/j.yhbeh.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fusani L, Donaldson Z, London SE, Fuxjager MJ, Schlinger BA. Expression of androgen receptor in the brain of a sub-oscine bird with an elaborate courtship display. Neurosci. Lett. 2014;578:61–65. doi: 10.1016/j.neulet.2014.06.028. http://dx.doi.org/10.1016/j.neulet.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, Donaldson Z, Schlinger B. The distribution of steroid receptors and aromatase in the brain and spinal cord of a suboscine bird with elaborate courtship display, the golden-collared manakin. Horm. Behav. 2003a;44:50. [Google Scholar]
- Fusani L, Gahr M. Hormonal influence on song development: the role of oestrogen. Neuroscience. 2006;138:939–946. doi: 10.1016/j.neuroscience.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Fusani L, Gahr M, Hutchison JB. Aromatase inhibition reduces specifically one display of the ring dove courtship behavior. Gen. Comp. Endocrinol. 2001;122:23–30. doi: 10.1006/gcen.2001.7608. [DOI] [PubMed] [Google Scholar]
- Fusani L, Metzdorf R, Hutchison JB, Gahr M. Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J. Neurobiol. 2003b;54:370–379. doi: 10.1002/neu.10141. [DOI] [PubMed] [Google Scholar]
- Fusani L, Schlinger B. Proximate and ultimate causes of male courtship behavior in golden-collared manakins. J. Ornithol. 2012;153:119–124. [Google Scholar]
- Fuxjager MJ, Longpre KM, Chew JG, Fusani L, Schlinger BA. Peripheral androgen receptors sustain the acrobatics and fine motor skill of elaborate male courtship. Endocrinology. 2013;154:3168–3177. doi: 10.1210/en.2013-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxjager MJ, Schultz JD, Barske J, Feng NY, Fusani L, Mirzatoni A, Day LB, Hau M, Schlinger BA. Spinal motor and sensory neurons are androgen targets in an acrobatic bird. Endocrinology. 2012;153:3780–3791. doi: 10.1210/en.2012-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Kavaliers M, Ossenkopp K-P. Sexually dimorphic spatial learning in meadow voles Microtus Pennsylvanicus and deer mice Peromyscus maniculatus. J. Exp. Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A. The Effects of Estrogen on Spatial Learning and Neuronal Morphology in the Rat Hippocampus. The University of Texas at San Antonio; United States – Texas: 2005. [Google Scholar]
- Gaulin S. Evolution of sex differences in spatial ability. Yearb. Phys. Anthropol. 1992;35:125–151. [Google Scholar]
- Goodrich-Hunsaker NJ, Hopkins RO. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav. Neurosci. 2010;124:405–413. doi: 10.1037/a0019193. [DOI] [PubMed] [Google Scholar]
- Goymann W, Moore IT, Scheuerlein A, Hirschenhauser K, Grafen A, Wingfield JC. Testosterone in tropical birds: effects of environmental and social factors. Am. Nat. 2004;164:327–334. doi: 10.1086/422856. [DOI] [PubMed] [Google Scholar]
- Guilford T, Dawkins MS. Receiver psychology and the evolution of animal signals. Anim. Behav. 1991;42:1–14. [Google Scholar]
- Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm. Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Clayton NS, Kacelnik A, Reboreda JC, DeVoogd TJ. Sexual dimorphism and species differences in HVC volumes of cowbirds. Behav. Neurosci. 1999;113:1095–1099. doi: 10.1037//0735-7044.113.5.1095. [DOI] [PubMed] [Google Scholar]
- Hoglund J, Alatalo RV, Hoglund J, Alatalo RV. Monographs in Behavior and Ecology: Leks. Monographs in Behavior and Ecology; Leks. 1995:xiii+248. [Google Scholar]
- Holmes MM, Musa M, Lonstein JS, Monks DA. Sexual dimorphism and hormone responsiveness in the spinal cord of the socially monogamous prairie vole (Microtus ochrogaster). J. Comp. Neurol. 2009;516:117–124. doi: 10.1002/cne.22095. [DOI] [PubMed] [Google Scholar]
- Hou T, Johnson JD, Rall JA. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J. Physiol. 1991;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JB. Initiation of courtship by hypothalamic implants of testosterone proprionate in castrated doves (Streptopelia risoria). Nature. 1967;216:591–592. doi: 10.1038/216591a0. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T. Androgen metabolism in the bra. In: Behavioural correlates. In: de Vries GJ, de Bruin JPC, Uylings HBM, Corner MA, editors. Sex Differences in the Brain. Elsevier; Amsterdam: 1984. pp. 23–51. [Google Scholar]
- Huxley JS. Darwin's theory of sexual selection and the data subsumed by it, in the light of recent research. Am. Nat. 1938;72:416–433. [Google Scholar]
- Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman ME, Erulkar SD, Krieger NR. Testosterone 5-[H9251]-reductase in the spinal cord of Xenopus laevis. J. Neurochem. 1982;38:657–661. doi: 10.1111/j.1471-4159.1982.tb08681.x. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Clower RP, Kroodsma DE, Devoogd TJ. Song-related brain-regions in the red-winged blackbird are affected by sex and season but not repertoire size. J. Neurobiol. 1989;20:139–163. doi: 10.1002/neu.480200304. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Disruption of auditory spatial working memory by inactivation of the forebrain archistriatum in barn owls. Nature. 1996;383:428–431. doi: 10.1038/383428a0. [DOI] [PubMed] [Google Scholar]
- Krützfeldt NO, Wild JM. Definition and connections of the entopallium in the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 2004;468:452–465. doi: 10.1002/cne.10972. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lack D. Ecological Adaptations for Breeding in Birds. Methuen; London: 1968. [Google Scholar]
- Lathe R. Hormones and the hippocampus. J. Endocrinol. 2001;169:205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- Lill A. Sexual behaviour of the lek forming white-bearded manakin, M. manacus trinitatis. Z. Tierpsychol. 1974;36:1–36. doi: 10.1111/j.1439-0310.1974.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Lowe PR. The anatomy of Gould’s manakin (Manacus vitellinus) in relation to its display. Ibis. 1942;84:50–83. [Google Scholar]
- Lowndes M, Davies DC. The effect of archistriatal lesions on ‘open field’ and fear/avoidance behaviour in the domestic chick. Behav. Brain Res. 1995;72:25–32. doi: 10.1016/0166-4328(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Lowndes M, Davies DC, Johnson MH. Archistriatal lesions impair the acquisition of filial preferences during imprinting in the domestic chick. Eur. J. Neurosci. 1994;6:1143–1148. doi: 10.1111/j.1460-9568.1994.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Madden J. Sex, bowers and brains. Proc. R. Soc. Lond. B: Biol. Sci. 2001;268:833–838. doi: 10.1098/rspb.2000.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J. Neurosci. 1988;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard-Smith J, Harper D. Animal Signals. Oxford University Press; Oxford: 2003. [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DB, Clay RP, Brumfield RT, Braun MJ. Sexual selection on plumage and behavior in an avian hybrid zone: experimental tests of male–male interactions. Evolution. 2001;55:1443–1451. doi: 10.1111/j.0014-3820.2001.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;405:1–15. [PubMed] [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Historical perspective: hormonal regulation of behaviors in amphibians. Horm. Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mougeot F, Irvine JR, Seivwright L, Redpath SM, Piertney S. Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav. Ecol. 2004;15:930–937. [Google Scholar]
- Musarò A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Nooker JK, Sandercock BK. Phenotypic correlates and survival consequences of male mating success in lek-mating greater prairie-chickens (Tympanuchus cupido). Behav. Ecol. Sociobiol. 2008;62:1377–1388. [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm. Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Patricelli GL, Uy JAC, Borgia G. Multiple male traits interact: attractive bower decorations facilitate attractive behavioural displays in satin bowerbirds. Proc. R. Soc. Lond. B: Biol. Sci. 2003;270:2389–2395. doi: 10.1098/rspb.2003.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie M, Halliday T, Sanders C. Peahens prefer peacocks with elaborate trains. Anim. Behav. 1991;41:323–331. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizational action of prenatally administered testosterone proprionate on the tissues mediating behavior in the female guinea pig. Endocrinology. 1959:65. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Podos J, Lahti DC, Moseley DL. Vocal performance and sensorimotor learning in songbirds. Adv. Study Behav. 2009;40:159–195. [Google Scholar]
- Prum RO. Phylogenetic analysis of the evolution of display behaviour in the neotropical manakins (Aves: Pipridae). Ethology. 1990;84:202–231. [Google Scholar]
- Prum RO. Phylogenetic tests of alternative intersexual selection mechanisms: trait macroevolution in a polygynous clade (Aves: Pipridae). Am. Nat. 1997;149:668–692. [Google Scholar]
- Prum RO. Aesthetic evolution by mate choice: Darwin's really dangerous idea. Philos. Trans. R. Soc. B: Biol. Sci. 2012;367:2253–2265. doi: 10.1098/rstb.2011.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the neurophil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Reboreda JC, Clayton NS, Kacelnik A. Species and sex differences in hippocampus size in parasitic and non-parasitic cowbirds. NeuroReport: Int. J. Rapid Commun. Res. Neurosci. 1996;7:505–508. doi: 10.1097/00001756-199601310-00031. [DOI] [PubMed] [Google Scholar]
- Regnier M, Herrera AA. Changes in the contractile properties by androgen hormones in sexually dimorphic muscles of male frogs (Xenopus laevis). J. Physiol. (Cambridge) 1993;461:565–581. doi: 10.1113/jphysiol.1993.sp019529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Lopez JC, Vargas JP, Broglio C, Gomez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish. Brain Res. Bull. 2002;57:499–503. doi: 10.1016/s0361-9230(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Rowe C. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 1999;58:921. doi: 10.1006/anbe.1999.1242. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Bischof HJ. Afferentation of the lateral nidopallium: a tracing study of a brain area involved in sexual imprinting in the zebra finch (Taeniopygia guttata). Brain Res. 2006;1106:111–122. doi: 10.1016/j.brainres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Korte S, Bischof H-J. Afferentation of a caudal forebrain area activated during courtship behavior: a tracing study in the zebra finch (Taeniopygia guttata). Brain Res. 2007;1184:108–120. doi: 10.1016/j.brainres.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Saint-Dizier H, Constantin P, Davies DC, Leterrier C, Lèvy F, Richard S. Subdivisions of the arcopallium/posterior pallial amygdala complex are differentially involved in the control of fear behaviour in the Japanese quail. Brain Res. Bull. 2009;79:288–295. doi: 10.1016/j.brainresbull.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schultz JD, London SE, Schlinger BA. Telencephalic aromatase but not a song circuit in a sub-oscine passerine, the golden-collared manakin (Manacus vitellinus). Brain Behav. Evol. 2000;56:29–37. doi: 10.1159/000006675. [DOI] [PubMed] [Google Scholar]
- Schlinger BA. Sexual differentiation of avian brain and behavior: current views on gonadal hormone-dependent and independent mechanisms. Annu. Rev. Physiol. 1998;60:407–429. doi: 10.1146/annurev.physiol.60.1.407. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Barske J, Day L, Fusani L, Fuxjager MJ. Hormones and the neuromuscular control of courtship in the golden-collared manakin (Manacus vitellinus). Front. Neuroendocrinol. 2013;34:143–156. doi: 10.1016/j.yfrne.2013.04.001. http://dx.doi.org/10.1016/j.yfrne.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach E, Rubin RT, editors. Hormones, Brain and Behavior. San Diego. Academic Press; 2009. pp. 897–941. [Google Scholar]
- Scholes III E, Sodhi NS. Courtship ethology of Carola's Parotia (Parotia carolae). Auk. 2006;123:967–990. [Google Scholar]
- Schultz JD, Hertel F, Bauch M, Schlinger BA. Adaptations for rapid and forceful contraction in wing muscles of the male golden-collared manakin: sex and species comparisons. J. Comp. Physiol. A. 2001;187:677–684. doi: 10.1007/s00359-001-0239-9. [DOI] [PubMed] [Google Scholar]
- Schultz JD, Schlinger BA. Widespread accumulation of [H-3]testosterone in the spinal cord of a wild bird with an elaborate courtship display. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10428–10432. doi: 10.1073/pnas.96.18.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DF, Hoshooley JS. Seasonal hippocampal plasticity in food-storing birds. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2010;365:933–943. doi: 10.1098/rstb.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Ball GF. Brain, hormone, and behavior interactions in avian reproduction: status and prospectus. Condor. 1989;91:966–978. [Google Scholar]
- Silver R, Feder HH, Lehrman DS. Situational and hormonal determinants of courtship, aggressive and incubation behavior in male ring doves (Streptopelia risoria). Horm. Behav. 1973;4:163–172. [Google Scholar]
- Snow DW. A field study of the black-and-white manakin, Manacus manacus, in Trinidad. Zoologica. 1962;57:65–104. [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J. Neurobiol. 2004;58:413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Stein AC, Uy JAC. Plumage brightness predicts male mating success in the lekking golden-collared manakin, Manacus vitellinus. Behav. Ecol. 2006;17:41–47. [Google Scholar]
- Tinbergen N. Some recent studies of the evolution of sexual behavior. In: Beach FA, editor. Sex and Behavior. John Wiley & Sons; New York: 1965. pp. 1–33. [Google Scholar]
- Trainer JM, McDonald DB, Learn WA. The development of coordinated singing in cooperatively displaying long-tailed manakins. Behav. Ecol. 2002;13:65–69. [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J. Comp. Neurol. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Uy JAC, Endler JA. Modification of the visual background increases the conspicuousness of golden-collared manakin display. Behav. Ecol. 2004;15:1003–1015. [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehrencamp SL, Bradbury JW, Gibson RM. The energetic cost of display in male sage grouse. Anim. Behav. 1989;38:885–896. [Google Scholar]
- Wikelski M, Hau M, Robinson DW, Wingfield JC. Reproductive seasonality of seven neotropical passerine species. Condor. 2003;105:683–695. [Google Scholar]
- Wikelski M, Hau M, Wingfield JC. Social instability increases plasma testosterone in a year-round territorial neotropical bird. Proc. R. Soc. Lond. B: Biol. Sci. 1999;266:551–556. [Google Scholar]
- Wingfield JC, Farner DS. Endocrinology of reproduction in wild species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Academic Press; San Diego: 1993. pp. 163–327. [Google Scholar]
- Zahavi A. Mate selection – a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res. 1971;31:313–326. doi: 10.1016/0006-8993(71)90185-5. [DOI] [PubMed] [Google Scholar]
- Zeigler HP, Marler P. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. [Google Scholar]


