Abstract
In patients with haemophilia A, factor VIII (FVIII) prophylaxis reduces bleeding frequency and joint damage compared with on-demand therapy. To assess the effect of prophylaxis initiation age, magnetic resonance imaging (MRI) was used to evaluate bone and cartilage damage in patients with severe haemophilia A. In this cross-sectional, multinational investigation, patients aged 12–35 years were assigned to 1 of 5 groups: primary prophylaxis started at age <2 years (group 1); secondary prophylaxis started at age 2 to <6 years (group 2), 6 to <12 years (group 3), or 12−18 years (group 4); or on-demand treatment (group 5). Joint status at ankles and knees was assessed using Compatible Additive MRI scoring (maximum and mean ankle; maximum and mean of all 4 joints) and Gilbert scores in the per-protocol population (n = 118). All prophylaxis groups had better MRI joint scores than the on-demand group. MRI scores generally increased with current patient age and later start of prophylaxis. Ankles were the most affected joints. In group 1 patients currently aged 27−35 years, the median of maximum ankle scores was 0.0; corresponding values in groups 4 and 5 were 17.0 and 18.0, respectively [medians of mean index joint scores: 0.0 (group 1), 8.1 (group 2) and 13.8 (group 4)]. Gilbert scores revealed outcomes less pronounced than MRI scores. MRI scores identified pathologic joint status with high sensitivity. Prophylaxis groups had lower annualized joint bleeds and MRI scores vs. the on-demand group. Primary prophylaxis demonstrated protective effects against joint deterioration compared with secondary prophylaxis.
Keywords: cross-sectional study, haemophilia, joint status, magnetic resonance imaging, on-demand treatment, prophylaxis
Introduction
Haemophilia A is a congenital, X-chromosome-linked bleeding disorder resulting from a deficiency in coagulation factor VIII (FVIII). In patients with severe haemophilia, joint destruction following recurrent haemarthroses is a major disease-associated morbidity [1]. Prevention of joint damage is a primary goal of FVIII replacement therapy that can be administered on demand to stop bleedings or prophylactically to prevent them. Compared with patients treated on demand, those receiving FVIII prophylaxis have reduced bleeding frequency, rarely develop chronic arthropathy and have improved quality of life (QoL) [2–7]. Thus, prophylaxis is generally recommended as the ideal treatment for severe haemophilia A [8–11]. Preservation of joint integrity is most evident in patients receiving primary prophylaxis [5], generally defined as continuous prophylactic treatment initiated before the onset of joint damage (before age 2 years or after the first joint bleed) [12]. However, patients receiving secondary prophylaxis (initiated after several joint bleeds have occurred) also have less joint deterioration than those treated on demand [13].
Magnetic resonance imaging (MRI) is a sensitive technology for early detection of joint damage in patients with haemophilia; unlike methods such as radiography, MRI can detect early joint degeneration and changes in cartilage and synovium [14–16]. Several studies have shown the value of MRI in assessing haemophilic arthropathy [2,14–21]; one randomized controlled trial used MRI to evaluate outcomes of haemophilia prophylaxis [2]. Although previous data showed that MRI scores are significantly correlated with joint bleeds and clinical joint scores [18], a more recent study found only a weak correlation of MRI scores with clinical function and no correlation with bleeding incidence [22].
The primary objective of this investigation, using a cross-sectional design with an MRI diagnostic component, was to evaluate bone and cartilage damage in ankles and knees in patients with severe haemophilia A relative to their previous treatment schedule (primary or secondary prophylaxis starting at different ages vs. on-demand therapy).
Methods
Study design
This cross-sectional, multinational, epidemiologic, interventional investigation (ClinicalTrials.gov ID: NCT00927667) was conducted in 15 centres in Europe from June 2009 to December 2010.
Data were collected in patient files. Patients were assigned to 1 of 5 groups according to age at FVIII therapy initiation: group 1 used primary prophylaxis (initiated at <2 years after ≤1 joint bleed); group 2 initiated secondary prophylaxis between ages 2 and <6 years; group 3 initiated secondary prophylaxis between ages 6 and <12 years; group 4 initiated secondary prophylaxis between ages 12 and 18 years; group 5 used only on-demand treatment). The study occurred before publication of the updated World Federation of Hemophilia definitions for primary and secondary prophylaxis [11]. Following an enrolment visit, each patient underwent MRI evaluation of four index joints (two ankles, two knees).
Patients
Male patients aged 12–35 years with severe haemophilia A (FVIII:C < 1%), complete documented history of bleeds and treatments (for ≥5 years for on-demand patients), and no history of FVIII inhibitors were eligible. Prophylaxis patients (groups 1 through 4) must have received ≥2 prophylactic infusions/week (for 45 weeks per year for ≥10 years) without relevant interruption (e.g. gaps in documentation of infusions). Patients who had a history of only on-demand treatment and no prophylaxis for >8 consecutive months were eligible for inclusion in the on-demand group. Patients in the on-demand group must have had >12 bleeds/year during the previous 5 years to ensure that a low bleeding frequency was not the primary reason why these patients did not start prophylaxis.
Exclusion criteria included any other bleeding disorder; contraindication to high magnetic exposure; synovectomy within the past 6 months or planned during the study; and previous replacement of knee or ankle joints. Patients for whom the most clinically severe joint was not one of the four index joints (ankles, knees) were also excluded.
The protocol was approved by each investigational site's independent ethics committee or institutional review board and written informed consent was obtained from all patients (or parents/legal representatives for patients aged <18 years).
Imaging and image evaluation
At all centres, MRI of knees and ankles was conducted using a 1.5T magnet and a uniform protocol comprising coronal and sagittal T2* gradient echo sequences as well as a sagittal turbo spin-echo proton-density fat-suppressed sequence. Maximum slice thickness was 4 mm. Other main parameter settings were TR 300–700 ms, TE 18–20 ms, flip angle 20–40° for the gradient echo images and TR >1000 ms with minimum TE for the spin-echo images. Joint MR images were read by three independent experienced radiologists blinded to patient identity and clinical data. Each radiologist used a comprehensive scoring sheet that included all items of the Compatible Additive (Table1) and Compatible Progressive MRI scales [23], and the International Prophylaxis Study Group (IPSG) MRI scale [24], but only results from the Compatible Additive MRI score were used for the primary analysis. Final scores were derived from median values reported by the radiologists.
Table 1.
Compatible Additive MRI scale.
| Joint characteristic | Points* |
|---|---|
| Synovial hypertrophy | |
| Small | 1 |
| Moderate | 2 |
| Large | 3 |
| Haemosiderin | 1 |
| Changes of subchondral bone or joint margins | |
| Any surface erosion | 1 |
| Any surface erosion in at least 2 bones | 1 |
| Half or more of the articular surface eroded in at least 1 bone | 1 |
| Half or more of the articular surface eroded in at least 2 bones | 1 |
| At least 1 subchondral cyst | 1 |
| More than 1 subchondral cyst | 1 |
| Subchondral cysts in at least 2 bones | 1 |
| Multiple subchondral cysts in each of at least 2 bones | 1 |
| Cartilage loss | |
| Any loss of joint cartilage height | 1 |
| Any loss of joint cartilage height in at least 2 bones | 1 |
| Any loss of joint cartilage height involving more than one-third of the joint surface in at least 1 bone | 1 |
| Any loss of joint cartilage height involving more than one-third of the joint surface in at least 2 bones | 1 |
| Full-thickness loss of joint cartilage in at least some area in at least 1 bone | 1 |
| Full-thickness loss of joint cartilage in at least some area in at least 2 bones | 1 |
| Full-thickness loss of joint cartilage involves at least one-third of the joint surface in at least 1 bone | 1 |
| Full-thickness loss of joint cartilage involves at least one-third of the joint surface in at least 2 bones | 1 |
Maximum value: 20 points.
Adapted from Lundin et al. Compatible scales for progressive and additive MRI assessments of haemophilic arthropathy. Haemophilia 2005;11:109–115. Reprinted with permission.
Efficacy assessments
The primary efficacy variable was the maximum ankle Compatible Additive MRI score (maximum value, 20 points; Table1) [23]. The primary endpoint was assessed in ankles because they are the most frequently affected joints in patients with haemophilia A in previous studies [2,25]. Patients were grouped by current age (12−16, 17−21, 22−26 and 27−35 years). The primary group comparison comprised patients currently aged 27–35 years who were receiving secondary prophylaxis initiated at age 6–18 years (groups 3 and 4) vs. those using on-demand therapy (group 5). Secondary efficacy variables included clinical joint evaluation using Gilbert score (physical examination joint score [with and without pain and bleeding scores] for the mean of index joints), annualized number of joint bleeds (all joints and index joints) for the last 5 years, and maximum index joint Compatible Additive MRI scores.
Other assessments
The McGill pain questionnaire, QoL using the Haemo-QoL and Haemo-QoL-A, and correlation between Compatible Additive MRI and Gilbert scores were also assessed.
Safety assessments
Adverse events (AEs) during the study and those related to MRI procedures were assessed. AEs were coded according to the Medical Dictionary for Regulatory Activities, version 13.1.
Data analysis
Continuous data were summarized using descriptive statistics. Categorical data were summarized using the frequency and percentage of patients in each of the age/analysis groups. Correlations were assessed with Spearman rank correlation coefficients. The primary efficacy evaluation was in the per-protocol (PP) population (all patients without major protocol deviations); the intent-to-treat (ITT) population included all patients entered into the study. Between-group differences and P values were not planned or calculated for these studies, and no post hoc statistical analyses were performed.
Results
Patient characteristics
In total, 129 patients (ITT population) underwent MRI examinations; the PP population comprised 118 patients (Table2). Eleven patients were excluded from the PP population due to the following major protocol violations: most clinically severe joint was not 1 of the 4 index joints analysed (n = 6 patients), history of only 4 years of prophylaxis (n = 2), previous joint replacement (n = 1) and high Gilbert scores with no pathologic findings by MRI (n = 2). All results presented are in the PP population.
Table 2.
Patient enrolment* (per-protocol population).
| Age at prophylaxis start, years | Current Age, years |
Total patients enrolled, n | |||
|---|---|---|---|---|---|
| 12−16 (n = 8) | 17−21 (n = 28) | 22−26 (n = 43) | 27–35 (n = 39) | ||
| <2 (primary) | 4/5 | 8/10 | 9/10 | 4/10 | 25 |
| 2 – <6 (secondary) | 4/5 | 7/10 | 5/10 | 6/10 | 22 |
| 6 – <12 (secondary) | − | 12/10 | 12/25 | 3/30 | 27 |
| 12 – 18 (secondary) | − | − | 7/35 | 14/35 | 21 |
| Never (on demand) | 0/5 | 1/10 | 10/15 | 12/15 | 23 |
Unless indicated otherwise, actual/planned enrolment per group is shown.
Efficacy
Compatible additive MRI score
In the primary group comparison [patients currently aged 27−35 years: secondary prophylaxis started at age 6−18 years (n = 17) vs. on-demand treatment (n = 12), the median maximum ankle MRI score was 16.5 (range, 0−20) with secondary prophylaxis and 18.0 (range, 2−19) with on-demand treatment, indicating a slight nominal trend towards worse affected ankle condition among patients treated on demand. Differences were more pronounced when medians of the mean index joint scores were compared [secondary prophylaxis, 7.5 (range, 0−10.5); on demand, 13.8 (range, 0.5−19.5)].
MRI scores for maximum ankle as well as the mean of all 4 index joints for each group and age subgroup are listed in Table3. Patients in the prophylaxis groups generally had lower median scores than patients treated on demand. The magnitude of differences was largest in groups 1 (primary prophylaxis) and 2 (secondary prophylaxis started at age 2−6 years) vs. group 5 (on-demand treatment) (Table3). Most patients on primary prophylaxis (group 1) and ≤50% of patients in group 2 had an MRI score of zero (indicating a lack of pathologic joint status) for both ankles and for all 4 index joints; markedly fewer patients had no joint damage when secondary prophylaxis was started at age 6–18 years (groups 3 and 4; Table4). Minimal differences were seen between patients who started secondary prophylaxis at ≥12 years of age (group 4) and patients treated on demand (group 5; Table4). Only 1 patient (in group 1, current age 17–21 years) had an abnormal knee MRI score when both ankles were normal.
Table 3.
Compatible Additive MRI scores.
| Age at prophylaxis start, years | Current age, years |
|||
|---|---|---|---|---|
| 12−16 | 17−21 | 22−26 | 27−35 | |
| Maximum ankle | ||||
| <2 (primary), n | 4 | 8 | 8 | 4 |
| Median (range) | 0.0 (0−2) | 0.0 (0−0) | 0.0 (0−9) | 0.0 (0−2) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 1.0 (0−18) | 2.0 (0−19) | 16 (0−19) | 7.0 (0−19) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 14.0 (0−17) | 14.0 (0−18) | 16.0 (15−16) |
| 12 to 18 (secondary), n | − | − | 7 | 13 |
| Median (range) | − | − | 16.0 (7−19) | 17.0 (0−20) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 18.0 (18−18) | 17.0 (12−18) | 18.0 (2−19) |
| Mean index joint | ||||
| <2 (primary), n | 4 | 8 | 9 | 4 |
| Median (range) | 0.0 (0.0−0.5) | 0.0 (0.0−0.8) | 0.0 (0.0−2.8) | 0.0 (0.0−0.5) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 0.3 (0.0−8.5) | 0.5 (0.0−9.3) | 4.0 (0.0−4.8) | 3.5 (0.0−13.3) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 4.0 (0.0−9.0) | 5.1 (0.0−13.0) | 4.0 (3.8−7.5) |
| 12 to 18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 8.0 (1.8−12.0) | 8.1 (0.0−10.5) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 7.7 (7.7−7.7) | 11.6 (5.3−14.8) | 13.8 (0.5−19.5) |
MRI, magnetic resonance imaging.
Table 4.
Proportion of patients without pathologic findings in the Additive MRI score
| Age at prophylaxis start, years | Current age, years |
|||
|---|---|---|---|---|
| 12−16 | 17−21 | 22−26 | 27−35 | |
| All index joints, n/N (%) patients | ||||
| <2 (primary) | 3/4 (75) | 7/8 (88) | 5/9 (56) | 3/4 (75) |
| 2 to 6 (secondary) | 2/4 (50) | 3/7 (43) | 1/5 (20) | 3/6 (50) |
| 6 to 12 (secondary) | − | 1/12 (8) | 2/12 (17) | 0/3 (0) |
| 12 to 18 (secondary) | − | − | 0/7 (0) | 1/14 (7) |
| Never (on demand) | − | 0/1 (0) | 0/10 (0) | 0/12 (0) |
| Both ankles, n/N (%) patients | ||||
| <2 (primary) | 3/4 (75) | 8/8 (100) | 5/9 (56) | 3/4 (75) |
| 2 to <6 (secondary) | 2/4 (50) | 3/7 (43) | 1/5 (20) | 3/6 (50) |
| 6 to <12 (secondary) | − | 1/12 (8) | 2/12 (17) | 0/3 (0) |
| 12 to 18 (secondary) | − | − | 0/7 (0) | 1/14 (7) |
| Never (on demand) | − | 0/1 (0) | 0/10 (0) | 0/12 (0) |
MRI, magnetic resonance imaging.
The results were comparable using the Compatible Progressive and IPSG MRI scores (data not shown).
Annualized joint bleeds
The median annualized number of index joint bleeds was ≤2.5 in all prophylaxis groups; most patients receiving primary prophylaxis experienced <1 index joint bleed in the previous 5 years (Fig.1). The proportion of patients with no index joint bleeds during the previous 5 years decreased with the age at initiation of secondary prophylaxis (group 1, 11/25 patients [44.0%]; group 2, 8/22 [36.4%]; group 3, 6/27 [22.2%]; group 4, 4/21 [19.0%]). The frequencies of bleeds into index joints and into all joints were similar in all prophylaxis and current age groups, although rates were slightly higher in the groups with later secondary prophylaxis initiation (groups 3 and 4; Table5).
Figure 1.
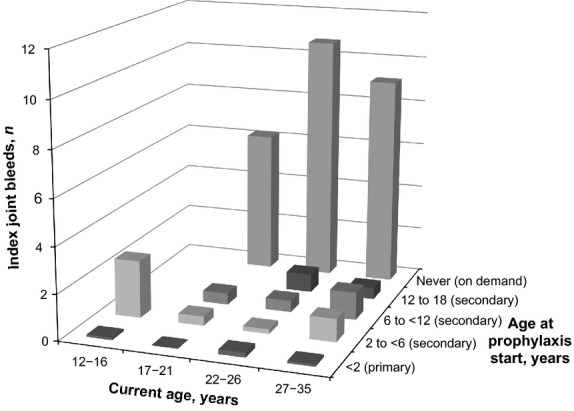
Median annualized number of index joint bleeds in the previous 5 years.
Table 5.
Annualized number of joint bleeds in the previous 5 years
| Parameter and age at prophylaxis start, years | Current age, years |
|||
|---|---|---|---|---|
| 12−16 | 17−21 | 22−26 | 27−35 | |
| All joint bleeds | ||||
| <2 (primary), n | 4 | 8 | 9 | 4 |
| Median (range) | 0.30 (0.0−1.4) | 0.10 (0.0−0.6) | 0.40 (0.0−1.4) | 0.20 (0.0−0.4) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 4.10 (0.2−8.4) | 0.60 (0.0−14.6) | 0.20 (0.0−0.4) | 0.20 (0.0−4.0) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 0.60 (0.0−4.0) | 0.80 (0.0−7.8) | 1.60 (0.4−2.0) |
| 12 to 18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 1.00 (0.2−4.6) | 0.90 (0.0−9.2) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 10.4 (10.4−10.4) | 14.3 (10.8−54.6) | 13.9 (5.2−37.8) |
| Index joint bleeds | ||||
| <2 (primary), n | 4 | 8 | 9 | 4 |
| Median (range) | 0.10 (0.0−1.2) | 0.00 (0.0−0.6) | 0.20 (0.0−0.8) | 0.10 (0.0−0.2) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 2.50 (0.0−4.6) | 0.40 (0.0−10.6) | 0.20 (0.0−0.2) | 1.00 (0.0−2.0) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 0.50 (0.0−3.6) | 0.50 (0.0−4.0) | 1.20 (0.4−1.6) |
| 12 to 18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 0.80 (0.2−3.8) | 0.50 (0.0−8.6) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 6.2 (6.2−6.2) | 10.7 (5.8−39.4) | 9.10 (5.2−28.4) |
Consistent with the inclusion criterion of approximately 12 bleeds per year in the previous 5 years, no patients in the on-demand group (group 5) had 0 index joint bleeds during this time period (0/23 patients [0%]). Overall, the median number of bleeds into all joints during the previous 5 years was ≤4.1 per year in all prophylaxis groups and ≤14.3 per year in patients treated on demand (Table5).
Gilbert score
Similar to the Compatible Additive MRI scores, Gilbert scores of index joints were more severe with increasing age and time of prophylaxis start (Table6). The only exception was in group 3 patients currently aged 27–35 years (n = 3) for whom the physical examination joint score was comparable to the corresponding subgroup of group 5 (n = 12). There was no correlation between the MRI and Gilbert scores in groups 1 and 4 (Spearman correlation coefficients, 0.2 > r > −0.2); but correlations between MRI and Gilbert scores were observed in groups 2, 3 and 5 (r = 0.547, 0.438 and 0.618, respectively).
Table 6.
Gilbert score: physical examination joint score for the mean of index joints.*
| Age at prophylaxis start, years | Current age, years |
|||
|---|---|---|---|---|
| 12−16 | 17−21 | 22−26 | 27−35 | |
| <2 (primary), n | 4 | 8 | 9 | 4 |
| Median (range) | 0.5 (0.0−6.0) | 0.0 (0.0−0.0) | 0.0 (0.0−4.0) | 0.0 (0.0−0.0) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 2.5 (0.0−5.0) | 0.0 (0.0−5.0) | 0.0 (0.0−7.0) | 1.0 (0.0−12.0) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 2.0 (0.0−11.0) | 3.5 (0.0−10.0) | 7.0 (2.0−12.0) |
| 12 to 18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 4.0 (0.0−11.0) | 5.0 (0.0−17.0) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 0.0 (0.0−0.0) | 7.0 (2.0−26.0) | 6.5 (0.0−22.0) |
Total score range, 0–12 points for ankles or knees.
Quality of life
Although there was large variability in pain scores for each group, pain scores for patients currently aged 22–35 years (groups 4 and 5) were higher (indicating more severe pain) than for those in other age groups; patients currently aged ≥22 years had increased pain scores with on-demand therapy and secondary prophylaxis compared with primary prophylaxis (Table7).
Table 7.
Quality-of-life parameters
| Parameter and age at prophylaxis start, years | Current age, years |
|||
|---|---|---|---|---|
| 12−16 | 17−21 | 22−26 | 27−35 | |
| Pain total score | ||||
| <2 (primary), n | 2 | 7 | 9 | 4 |
| Median (range) | 1.0 (0−2) | 0.0 (0−3) | 0.0 (0−37) | 0.0 (0−2) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 0.0 (0−10) | 1.0 (0−12) | 5.0 (0−21) | 0.5 (0−10) |
| 6 to <12 (secondary), n | − | 12 | 12 | 3 |
| Median (range) | − | 2.0 (0−17) | 4.5 (0−27) | 2.0 (0−8) |
| 12 to18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 7.0 (0−22) | 5.5 (0−16) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 0.0 (0−0) | 6.0 (0−16) | 5.0 (0−12) |
| Haemo-QoL-A total score | ||||
| <2 (primary), n | 3 | 7 | 9 | 4 |
| Median (range) | 87.0 (62.3−95.6) | 89.2 (61.1−99.2) | 90.8 (66.4−95.4) | 92.7 (86.5−98.6) |
| 2 to <6 (secondary), n | 4 | 7 | 5 | 6 |
| Median (range) | 82.6 (70.4−93.3) | 92.9 (81.1−99.7) | 89.2 (72.7−89.7) | 93.7 (78.8−99.5) |
| 6 to <12 (secondary), n | − | 11 | 12 | 3 |
| Median (range) | − | 94.4 (48.9−98.5) | 79.5 (44.3−89.7) | 93.6 (84.1−99.1) |
| 12 to 18 (secondary), n | − | − | 7 | 14 |
| Median (range) | − | − | 74.3 (57.4−98.5) | 83.9 (61.7−97.0) |
| Never (on demand), n | − | 1 | 10 | 12 |
| Median (range) | − | 89.2 (89.2−89.2) | 62.1 (42.4−80.9) | 75.2 (37.3−84.8) |
Impairment in haemophilia-related QoL was relatively low in all groups (Table7). In patients aged ≥22 years, those treated with late secondary prophylaxis (group 4) or on demand (group 5) had more impairment than patients in the other prophylaxis groups; this difference mirrored the results for the physical functioning subscore, which is assessed using the physical functioning domain of the Haemo-QoL-A questionnaire (Fig.2).
Figure 2.
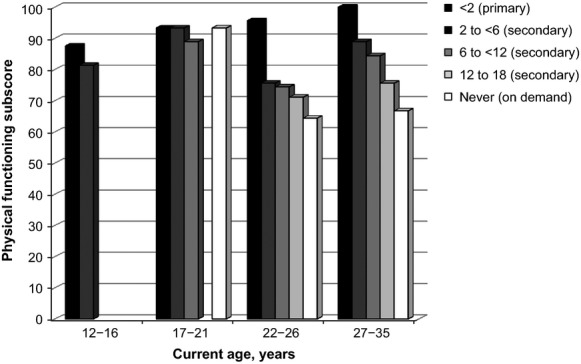
Median Haemo-QoL and Haemo-QoL-A transformed physical functioning subscores. Legend indicates age at prophylaxis start (in years).
Safety
One AE (nasopharyngitis), unrelated to study procedures, was reported in this investigation.
Discussion
This epidemiologic investigation using MRI scoring demonstrates the benefit of FVIII prophylaxis, even secondary prophylaxis initiated at age >6 years, over on-demand therapy for prevention of joint damage in patients with severe haemophilia A. The results are consistent with the clinical studies showing improved QoL and decreased joint damage and bleeding incidence with prophylaxis in general [2,26–28], as well as with reports on benefits over on-demand treatment for secondary prophylaxis initiated later in childhood, adolescence or adulthood [4,7,29,30].
Several MRI scales are described in the literature [23], but there is no international consensus on a single scale. When our study was designed, the IPSG MRI scale [24] was not yet validated. Therefore, we used a comprehensive score sheet and scores according to the Compatible Additive MRI scale as the principal predefined MRI assessment. The primary group comparison in this investigation, Compatible Additive MRI scores for the maximally affected ankle in patients currently aged 27 – 35 years receiving secondary prophylaxis initiated at age 6–18 years vs. on-demand therapy, revealed a trend in favour of prophylaxis. Comparisons of all prophylaxis groups with the on-demand group showed that maximum ankle Compatible Additive MRI scores were lower in patients on prophylaxis and were particularly low in those who started prophylaxis at a younger age. Analyses based on other MRI scales revealed similar results (data not shown).
Performing MRIs of multiple joints is time consuming, cumbersome (strenuous to patients because they must remain strictly stationary in the magnet during the lengthy image acquisition), and therefore difficult to conduct with preserved high image quality, which presently is a hindrance to widespread and systematic use of this diagnostic modality in haemophilia care. To make the procedure more practical, we restricted the number of investigated index joints per patient to 4 (knees and ankles) rather than the 6 (knees, ankles and elbows) used conventionally for radiographic joint scoring in patients with haemophilia. In our study, higher MRI scores for the maximally affected ankle compared with the mean index joint in all patient subgroups and the scarcity of pathologic findings in knees of patients with normal ankles indicate that in most patients, the ankle is the lead target joint of haemophilic arthropathy. These results are consistent with previous reports showing more haemarthroses and worse joint scores for ankles compared with knees or elbows in patients with haemophilia treated with prophylaxis or on demand [2,22,25,31,32]. Based on our and other authors’ results, we suggest that ankles should be the joints of primary interest for monitoring treatment using MRI. Investigation of fewer joints is simpler, and if future experiences show that investigation of only 4 joints, or even only the ankles, can provide information relevant for clinical decision making, it may have implications for haemophilia care.
Assessment of joint bleeds also reflected the protective effect of prophylaxis. Regardless of when prophylaxis was initiated, annual bleeding rates were lower compared with on-demand treatment. A small number of index joint bleeds (<1 over 5 years or ≤0.2 bleeds per year over 5 years) occurred during primary prophylaxis, suggesting that joint bleeding is not completely avoidable. These data are consistent with results from a randomized prospective trial which showed a median of 0.2 annual joint haemorrhages in boys with haemophilia A treated with primary prophylaxis [2]. All prophylaxis groups in our study had fewer joint bleeds and lower MRI scores than the on-demand groups, suggesting that progression of joint damage may be decelerated by secondary as well as primary prophylaxis.
Among the PP population, clinical joint evaluation using the Gilbert score showed trends similar to MRI scoring: decreased joint status was associated with increasing age and later start of prophylaxis, whereas improved joint status occurred with prophylaxis vs. on-demand treatment. However, although the Gilbert subscores for pain and bleedings were excluded because of possibly confounding effects, no clear correlations were observed between Gilbert and Compatible Additive MRI scores, consistent with the fact that clinical scores are less sensitive than MRI scores in detecting early joint damage.
Magnetic resonance imaging is established as the technology of choice for joint status assessment in patients with haemophilia. In previous studies, MRI was a useful diagnostic tool for detecting early joint damage; it showed efficacy superior to physical examination, computed tomography and x-ray scanning and was particularly efficient at detecting early joint arthropathy [2,14–16,18,21]. However, although data indicate that MRI can detect early damage in clinically asymptomatic joints [17], a report assessing baseline joint status in patients enrolled in a prospective study questioned the value of MRI in predicting meaningful joint deterioration in patients with haemophilia and called for further research [22]. Our study suggests that MRI scores do show relevant joint changes because MRI scoring trends with respect to patient age and treatment regimen were similar to trends for bleeding rates, clinical joint data and QoL.
The benefit of later prophylaxis (i.e. secondary prophylaxis initiated at age 6 to <12 and 12−18 years) could possibly be extrapolated to outcomes of prophylaxis initiation in older adults. Indeed, several retrospective and prospective clinical trials have reported benefits of secondary prophylaxis started in older teens or adults compared with on-demand therapy [27,28,33–36].
This investigation used a cross-sectional rather than a longitudinal design with multiple patient arms combined with an MRI diagnostic component, which to our knowledge is an approach not previously applied in haemophilia research. We recognize the benefits of longitudinal studies and their value for confirming cause-and-effect relationships. However, changes in joint status require long-term monitoring (over several years or decades), which makes longitudinal studies more time consuming and costly than cross-sectional studies. Our results demonstrate that a cross-sectional study can be an alternative tool for collecting valuable information about prophylaxis in patients with haemophilia.
A study limitation was that target sample sizes were not met for several patient subgroups; therefore, some subgroup sizes were small (n < 5), and only descriptive statistical tests were performed. Only frequent bleeders (>12 bleeds per year) were included in the on-demand group to avoid clinical variability within this group and also to ensure that a low bleeding frequency was not the primary reason why these patients did not start prophylaxis. The difficulty encountered with enrolling patients who initiated prophylaxis at age ≥6 years and those in the on-demand subgroups currently aged 12–21 years suggests that prophylaxis is the current standard of care for young patients with haemophilia in Europe.
Conclusion
Pathologic joint status can be assessed using MRI scoring in patients with severe haemophilia A, with the ankle suggested to be the leading joint. Observed MRI scores depended on patient age (age at initiation of FVIII prophylaxis and current age) and therapy regimen (prophylaxis or on-demand therapy). Prophylaxis, in general, is beneficial vs. on-demand treatment in maintaining joint health over time, with primary prophylaxis being more beneficial than secondary prophylaxis.
Acknowledgments
This study was funded by Bayer HealthCare AG (Leverkusen, Germany). We would like to thank Jane A. Phillips, PhD, from Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA) for medical writing assistance, which was fully funded by Bayer HealthCare.
APPENDIX
Appendix
Cross-sectional MRI study investigators: Germany: D. Franke (Magdeburg), J. Oldenburg (Bonn), H. Pollmann (Münster), R. Zimmermann (Heidelberg); Greece: O. Katsarou (Athens), G. Theodossiades (Athens); Italy: G. Mazzucconi (Rome), E. Santagostino (Milan), A. Tagliaferri (Parma), E. Zanon (Padova); Spain: F. Lopez (La Coruna), F. Querol (Valencia); Sweden: E. Berntorp (Malmö), M. Homström (Stockholm); United Kingdom: P. Chowdary (London).
M. L. Manco-Johnson (Denver, CO, USA) and M. Werk (Berlin, Germany) served as blinded MRI readers for the study. R. Walsch (Leverkusen, Germany) served as the study manager and L. Schwartz (Whippany, NJ, USA) served as the statistician for the study.
Author contributions
J. Oldenburg was the principal investigator of the study, contributed to the clinical study protocol, design, and study report and reviewed all manuscript drafts. B. Lundin was the lead radiologist of the study, supervised the design and implementation of the MRI protocol as well as served as a blinded reader, and reviewed all manuscript drafts. E. Kellermann contributed to the design and conduct of the study and the clinical study protocol and report, and reviewed all manuscript drafts. R. Zimmermann, O. Katsarou, G. Theodossiades, E. Zanon and B. Niemann were co-investigators for the study and reviewed all manuscript drafts.
Disclosures
J. Oldenburg received reimbursement for attending symposia/congresses and/or honoraria for speaking or consulting, and/or funds for research from Bayer HealthCare, Baxter, Biogen Idec, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Swedish Biovitrum and Pfizer. O. Katsarou is a member of the European Haemophilia Standardisation Board sponsored by Baxter. G. Theodossiades is a member of the ADVANCE working group sponsored by Bayer. E. Zanon has acted as a paid consultant for Bayer, Baxter, Novo Nordisk, CSL Behring and Grifols. E. Kellermann is an employee of Bayer Vital GmbH. B. Lundin received funds for conference participation from Bayer. B. Niemann and R. Zimmermann have no conflicts of interest to declare.
References
- 1.National Hemophilia Foundation. Medical and Scientific Advisory Council (MASAC) recommendation regarding the need for collaboration. Document #102. Available at: http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57%26contentid=307. Accessed January 14, 2014.
- 2.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 4.Liesner RJ, Khair K, Hann IM. The impact of prophylactic treatment on children with severe haemophilia. Br J Haematol. 1996;92:973–8. doi: 10.1046/j.1365-2141.1996.420960.x. [DOI] [PubMed] [Google Scholar]
- 5.Lofqvist T, Nilsson IM, Berntorp E, Pettersson H. Haemophilia prophylaxis in young patients–a long-term follow-up. J Intern Med. 1997;241:395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- 6.Brackmann HH, Eickhoff HJ, Oldenburg J, Hammerstein U. Long-term therapy and on-demand treatment of children and adolescents with severe haemophilia A: 12 years of experience. Haemostasis. 1992;22:251–8. doi: 10.1159/000216332. [DOI] [PubMed] [Google Scholar]
- 7.Collins P, Faradji A, Morfini M, Enriquez MM, Schwartz L. Efficacy and safety of secondary prophylactic vs. on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-month crossover study. J Thromb Haemost. 2010;8:83–9. doi: 10.1111/j.1538-7836.2009.03650.x. [DOI] [PubMed] [Google Scholar]
- 8.Berntorp E, Astermark J, Bjorkman S, et al. Consensus perspectives on prophylactic therapy for haemophilia: summary statement. Haemophilia. 2003;9:1–4. doi: 10.1046/j.1365-2516.9.s1.17.x. [DOI] [PubMed] [Google Scholar]
- 9.Colvin BT, Astermark J, Fischer K, et al. European principles of haemophilia care. Haemophilia. 2008;14:361–74. doi: 10.1111/j.1365-2516.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 10.National Hemophilia Foundation. Medical and Scientific Advisory Council (MASAC) recommendations concerning prophylaxis (regular administration of clotting factor concentrate to prevent bleeding). Document #179. Available at: http://www.hemophilia.org/NHFWeb/Resource/StaticPages/menu0/menu5/menu57/masac179.pdf. Accessed January 14, 2014.
- 11.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 12.Ljung R. Second Workshop of the European Paediatric Network for Haemophilia Management, 17-19 September 1998 in Vitznau/Switzerland. Haemophilia. 1999;5:286–91. doi: 10.1046/j.1365-2516.1999.00328.x. [DOI] [PubMed] [Google Scholar]
- 13.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 14.Pergantou H, Platokouki H, Matsinos G, et al. Assessment of the progression of haemophilic arthropathy in children. Haemophilia. 2009;16:124–9. doi: 10.1111/j.1365-2516.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu W, Lin Q, Guermazi A, et al. Comparison of radiography, CT and MR imaging in detection of arthropathies in patients with haemophilia. Haemophilia. 2009;15:1090–6. doi: 10.1111/j.1365-2516.2009.02044.x. [DOI] [PubMed] [Google Scholar]
- 16.Dobon M, Lucia JF, Aguilar C, et al. Value of magnetic resonance imaging for the diagnosis and follow-up of haemophilic arthropathy. Haemophilia. 2003;9:76–85. doi: 10.1046/j.1365-2516.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 17.Olivieri M, Kurnik K, Pfluger T, Bidlingmaier C. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia. 2012;18:369–74. doi: 10.1111/j.1365-2516.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 18.Lundin B, Ljung R, Pettersson H. MRI scores of ankle joints in children with haemophilia–comparison with clinical data. Haemophilia. 2005;11:116–22. doi: 10.1111/j.1365-2516.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- 19.Lundin B, Pettersson H, Ljung R. A new magnetic resonance imaging scoring method for assessment of haemophilic arthropathy. Haemophilia. 2004;10:383–9. doi: 10.1111/j.1365-2516.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 20.Feldman BM, Funk S, Lundin B, et al. Musculoskeletal measurement tools from the International Prophylaxis Study Group (IPSG) Haemophilia. 2008;14(Suppl 3):162–9. doi: 10.1111/j.1365-2516.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 21.Hassan TH, Badr MA, El-Gerby KM. Correlation between musculoskeletal function and radiological joint scores in haemophilia A adolescents. Haemophilia. 2011;17:920–5. doi: 10.1111/j.1365-2516.2011.02496.x. [DOI] [PubMed] [Google Scholar]
- 22.Den Uijl IE, De Schepper AMA, Camerlinck M, Grobbee DE, Fischer K. Magnetic resonance imaging in teenagers and young adults with limited haemophilic arthropathy: baseline results from a prospective study. Haemophilia. 2011;17:926–30. doi: 10.1111/j.1365-2516.2011.02513.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundin B, Babyn P, Doria AS, et al. Compatible scales for progressive and additive MRI assessments of haemophilic arthropathy. Haemophilia. 2005;11:109–15. doi: 10.1111/j.1365-2516.2005.01049.x. [DOI] [PubMed] [Google Scholar]
- 24.Lundin B, Manco-Johnson ML, Ignas DM, et al. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia. 2012;18:962–70. doi: 10.1111/j.1365-2516.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 25.Su Y, Wong WY, Lail A, Donfield SM, Konzal S, Gomperts E. Long-term major joint outcomes in young adults with haemophilia: interim data from the HGDS. Haemophilia. 2007;13:387–90. doi: 10.1111/j.1365-2516.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 26.Royal S, Schramm W, Berntorp E, et al. Quality-of-life differences between prophylactic and on-demand factor replacement therapy in European haemophilia patients. Haemophilia. 2002;8:44–50. doi: 10.1046/j.1365-2516.2002.00581.x. [DOI] [PubMed] [Google Scholar]
- 27.Manco-Johnson MJ, Kempton CL, Reding MT, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART) [published correction appears in J Thromb Haemost. 2014;12: 119–122.] J Thromb Haemost. 2013;11:1119–27. doi: 10.1111/jth.12202. [DOI] [PubMed] [Google Scholar]
- 28.Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10:359–67. doi: 10.1111/j.1538-7836.2011.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manco-Johnson MJ, Nuss R, Geraghty S, Funk S, Kilcoyne R. Results of secondary prophylaxis in children with severe hemophilia. Am J Hematol. 1994;47:113–7. doi: 10.1002/ajh.2830470209. [DOI] [PubMed] [Google Scholar]
- 30.Cohen A, White E, Bernstein C, et al. Effects of secondary prophylaxis in hemophilia children with frequent hemarthrosis. Transfusion (Paris) 1997;37:534–5. [Google Scholar]
- 31.Kraft J, Blanchette V, Babyn P, et al. Magnetic resonance imaging and joint outcomes in boys with severe hemophilia a treated with tailored primary prophylaxis in Canada. J Thromb Haemost. 2012;10:2494–502. doi: 10.1111/jth.12025. [DOI] [PubMed] [Google Scholar]
- 32.Groen W, van der Net J, Bos K, et al. Joint health and functional ability in children with haemophilia who receive intensive replacement therapy. Haemophilia. 2011;17:783–90. doi: 10.1111/j.1365-2516.2011.02606.x. [DOI] [PubMed] [Google Scholar]
- 33.Siegmund B, Richter H, Pollmann H. Need for prophylactic treatment in adult haemophilia A patients. Transfus Med Hemother. 2009;36:283–8. doi: 10.1159/000225965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh CE, Valentino LA. Factor VIII prophylaxis for adult patients with severe haemophilia A: results of a US survey of attitudes and practices. Haemophilia. 2009;15:1014–21. doi: 10.1111/j.1365-2516.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 35.Miners AH, Sabin CA, Tolley KH, Lee CA. Assessing the effectiveness and cost-effectiveness of prophylaxis against bleeding in patients with severe haemophilia and severe von Willebrand's disease. J Intern Med. 1998;244:515–22. [PubMed] [Google Scholar]
- 36.Tagliaferri A, Franchini M, Coppola A, et al. Effects of secondary prophylaxis started in adolescent and adult haemophiliacs. Haemophilia. 2008;14:945–51. doi: 10.1111/j.1365-2516.2008.01791.x. [DOI] [PubMed] [Google Scholar]


