Abstract
Objective
The corticofugal system can alter coding along the ascending sensory pathway. Within the auditory system, electrical stimulation of the auditory cortex (AC) paired with a pure tone can cause egocentric shifts in the tuning of auditory neurons, making them more sensitive to the pure tone frequency. Since tinnitus has been linked with hyperactivity across auditory neurons, we sought to develop a new neuromodulation approach that could suppress a wide range of neurons rather than enhance specific frequency-tuned neurons.
Approach
We performed experiments in the guinea pig to assess the effects of cortical stimulation paired with broadband noise (PN-Stim) on ascending auditory activity within the central nucleus of the inferior colliculus (CNIC), a widely studied region for AC stimulation paradigms.
Main results
All eight stimulated AC regions induced extensive suppression of activity across the CNIC that was not possible with noise stimulation alone. This suppression built up over time and remained after the PN-Stim paradigm.
Significance
We propose that the corticofugal system is designed to decrease the brain’s input gain to irrelevant stimuli and PN-Stim is able to artificially amplify this effect to suppress neural firing across the auditory system. The PN-Stim concept may have potential for treating tinnitus and other neurological disorders.
Keywords: corticofugal, hyperacusis, neuromodulation, plasticity, tinnitus
1. Introduction
There has been tremendous growth in the number of studies using invasive or noninvasive cortical neuromodulation to treat various brain disorders such as epilepsy, depression, pain, tremor, stroke recovery, schizophrenia, addiction, and tinnitus (Gomez Palacio Schjetnan et al., 2013; Johnson et al., 2013; Zhang, 2013; Schulz et al., 2013; Fenoy et al., 2006; Seidman et al., 2008). Unfortunately, the results have varied dramatically across patients. More basic research is needed to further understand the role of cortex in altering descending and ascending networks in order to guide clinicians activating the cortex to provide therapeutic outcomes.
Within normal sensory systems, it has been shown that the cortex can shift or adjust the gain of coding within the ascending pathways (Yan et al., 2005; Zhang et al., 1997; Suga et al., 2011; Massopust and Ordy, 1962; Andersen et al., 1972; Ryugo and Weinberger, 1976; Syka and Popelar, 1984; Xiong et al., 2009; Suga, 2011; Sillito et al., 1994; Malmierca and Nunez, 1998; Ergenzinger et al., 1998; Krupa et al., 1999). A majority of this research has been conducted within the auditory system, though similar trends have been observed within the visual and somatosensory systems (Suga, 2011). For example, repeated presentation of a pure tone paired with electrical stimulation of neurons coding for the same frequency in primary auditory cortex (A1) causes the ventral division of the medial geniculate body (VMGB), central nucleus of the inferior colliculus (CNIC), cochlear nucleus, and A1 itself to become less sensitive to neighboring frequencies and/or more sensitive to the presented frequency, altering the underlying tonotopic map of these regions. This type of plasticity has also been observed for other sound features such as threshold, duration tuning, spatial tuning, and response latency (Suga et al., 2011; Xiong et al., 2009).
Pairing cortical stimulation with an acoustic stimulus to induce controlled plasticity may be relevant for treating various hearing disorders such as tinnitus. Tinnitus is a phantom sound percept that is annoying and/or debilitating for about 5% of the population (statistics provided by Centers for Disease Control and Prevention) and is a major health issue in our society (Moller et al., 2011). Tinnitus has been linked to abnormal tonotopic reorganization within the auditory system (Norena et al., 2003; Muhlnickel et al., 1998; Eggermont and Roberts, 2004). Pairing cortical stimulation with pure tones could potentially restore normal tonotopic coding in tinnitus patients and has recently been investigated in non-tinnitus subjects in a proof-of-concept study using transcranial magnetic stimulation (Schecklmann et al., 2011). However, other studies suggest that tinnitus may be coded as hyperactivity and/or hypersynchrony across neurons (Jastreboff and Sasaki, 1986; Bauer et al., 2008; Moller et al., 2011; Lanting et al., 2009; Chen et al., 2013; Middleton and Tzounopoulos, 2012; Kaltenbach, 2011; Eggermont and Roberts, 2012) and that tonotopic map reorganization may not be a necessary biomarker for tinnitus (Langers et al., 2012). Thus, the ability to suppress neurons tuned to several different frequencies may more effectively treat tinnitus.
Therefore, we were interested if pairing cortical stimulation with other acoustic stimuli could alter ascending neural activity across more widespread neural populations that may be relevant for the treatment of tinnitus. Several animal studies have combined cortical stimulation with acoustic clicks in order to alter subcortical auditory neurons (Torterolo et al., 1998; Amato et al., 1970; Watanabe et al., 1966; Massopust and Ordy, 1962). However, the extent of suppressive versus facilitatory effects varied across these studies, which may reflect differences in stimulation and recording locations across and within studies. It may also be attributed to the use of a click stimulus, which has strong spectral energy only within a limited bandwidth depending on its duration and may not have consistently activated neurons across the tonotopic map. There is one study by He et al. (He et al., 2002) that paired cortical stimulation with broadband noise in guinea pigs. Though the majority of neurons sampled in MGBv were facilitated when using an interstimulus interval of 100 ms, they presented data from three neurons suggesting that pairing cortical stimulation with broadband noise may achieve stronger suppressive than facilitatory effects with shorter cortical-to-acoustic-delays (10–30 ms; Figure 3 from (He et al., 2002)). However, questions remain as to whether this effect is observed consistently across a larger number of neurons throughout the ascending auditory system and whether delays shorter than 10 ms would produce an even stronger suppressive effect.
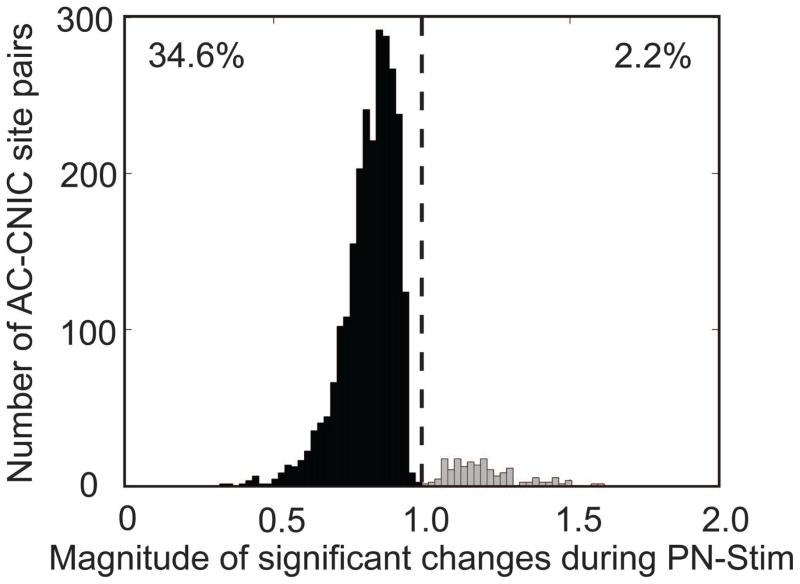
Figure 3. Magnitude of changes in CNIC responses during PN-Stim.
The change in spike count during PN-Stim compared to the baseline response to acoustic stimulation is plotted for the 2,744 AC-CNIC site pairs (out of 7,448 total) that were significantly suppressed (black) or facilitated (gray). A value of 1 (dotted line) signifies equal spiking in both conditions, while data to the left (right) of the line signifies suppression (facilitation) of neural firing. Out of all AC-CNIC site pairs, 34.6% were significantly suppressed, while only 2.2% were significantly facilitated.
To address the questions described above, we investigated a new approach of pairing cortical stimulation approximately simultaneously with broadband noise, which we term PN-Stim, to potentially suppress ascending neural activity. We initially focused on changes in firing in the CNIC because a large proportion of studies investigating corticofugal effects from auditory cortex (AC) have focused on the CNIC (Suga et al., 2011; Xiong et al., 2009) and because the inferior colliculus (IC) has shown neural changes associated with tinnitus (Bauer et al., 2008; Melcher et al., 2009). We stimulated multiple subregions across AC and recorded from neurons fully spanning the CNIC. In our experiments, we observed that PN-Stim elicited substantial suppression of neural activity throughout the CNIC, with the majority of stimulated AC regions inducing much greater suppressive than facilitatory changes in CNIC activity. The brain may be designed to reduce its neural/perceptual gain to noise-like and meaningless inputs and corticofugal activation reinforces this mechanism when precisely paired with that noise stimulus. Clinically, these findings open up the potential for a new neuromodulation paradigm of pairing cortical stimulation with an irrelevant/meaningless stimulus to induce extensive suppression across the auditory system for the treatment of tinnitus.
2. Methods
2.1. Animal surgeries and electrode implantation
Basic surgical procedures and methods for recording and stimulation are similar to those presented in previous studies (Markovitz et al., 2013; Lim and Anderson, 2007; Straka et al., 2013) and are only briefly presented here. Experiments were performed on 16 young Hartley guinea pigs (282–541 g; Elm Hill Breeding Labs, Chelmsford, MA) in accordance with policies of the University of Minnesota Institutional Animal Care and Use Committee. Each animal was anesthetized with an intramuscular mixture of ketamine (40 mg/kg) and xylazine (10 mg/kg) with 0.1 mL supplements every 45–60 minutes to maintain an areflexive state. Atropine sulfate (0.05 mg/kg) was administered periodically to reduce mucous secretions in the airway. Heart rate and blood oxygenation were continuously monitored via a pulse oximeter and body temperature was maintained at 38.0 ± 0.5°C using a heating blanket and rectal thermometer.
With the animals fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), a craniotomy was performed to expose the right auditory and visual cortices and two 32-site electrode arrays (NeuroNexus Technologies, Ann Arbor, MI) were inserted via hydraulic micro-manipulators into the right AC and CNIC. The AC array consists of four 5 mm long shanks separated by 500 μm with eight iridium sites linearly spaced 200 μm (center-to-center) along each shank. Before each experiment, AC electrodes sites were activated from iridium to iridium oxide via cyclic voltammetry for recording and stimulation capabilities (Lim and Anderson, 2007), lowering the site impedances to approximately 0.1–0.3 MΩ at 1 kHz. The array was placed perpendicular to the cortical surface and inserted to a depth of approximately 1.6 mm. In eight experiments, the CNIC array consisted of two 10 mm long shanks separated by 500 μm with 16 iridium sites linearly spaced 100 μm along each shank. In the other eight experiments, the CNIC array had four 10 mm long shanks separated by 500 μm with eight iridium sites linearly spaced 200 μm along each shank. In all experiments, the array was inserted 45° off the sagittal plane through the occipital cortex into the CNIC to align it along the tonotopic gradient of the CNIC (Snyder et al., 2004; Lim and Anderson, 2006; Markovitz et al., 2012). CNIC site impedances ranged between 0.8–3.0 MΩ at 1 kHz. The recording ground wire was positioned in the neck muscles and the stimulation ground needle was implanted into the brain tissue near the intersection of the midline and bregma. After placement of the arrays, the brain was covered with agarose to reduce swelling, pulsations, and drying during the recording sessions.
2.2. Recording and stimulation
Experiments were performed within a sound attenuating, electrically-shielded room using custom software interfaced with TDT hardware (Tucker-Davis Technology, Alachua, FL). All acoustic stimulation was presented to the animal’s left ear canal via a speaker coupled to a custom-made hollow ear bar, which was calibrated using a 0.25 in condenser microphone (ACO Pacific, Belmont, CA). Multi-unit neural data were sampled at a rate of 25 kHz, passed through analog DC-blocking and anti-aliasing filters up to 7.5 kHz, and digitally filtered between 0.3 and 3.0 kHz for analysis of neural spikes. Spikes were determined as voltages exceeding 3.5 times the standard deviation of the noise floor.
2.3. Electrode array placement
Acoustic stimuli were presented to the animal’s left ear canal and acoustic-driven responses were used to guide and verify accurate electrode placement within the CNIC and AC. Pure tones (60 ms duration, 5 ms ramp/decay) of varying frequencies (0.6–40 kHz, 8 steps/octave) and levels (0–70 dB SPL, 10 dB steps) were randomly presented for 4 trials/parameter. The acoustic-driven spike rates were calculated for responses recorded in the CNIC (taken 5–60 ms after tone onset) and AC (5–20 ms after tone onset for most cortical regions; 35–50 ms for late onset response types) to create frequency response maps (FRMs) for each site. Best frequencies (BFs) were calculated from the FRMs as the frequency centroid at 10 dB above the visually determined threshold.
2.3.1. CNIC properties
Array placement within the CNIC was confirmed by observing FRMs that systematically increased in BF with increasing depth (Markovitz et al., 2012; Lim and Anderson, 2007; Straka et al., 2014). FRMs for sites outside of the CNIC in external regions of the IC typically exhibited broad and weak tuning and/or multiple FRM peaks and were excluded from the analysis in this paper.
2.3.2. AC properties and subregions
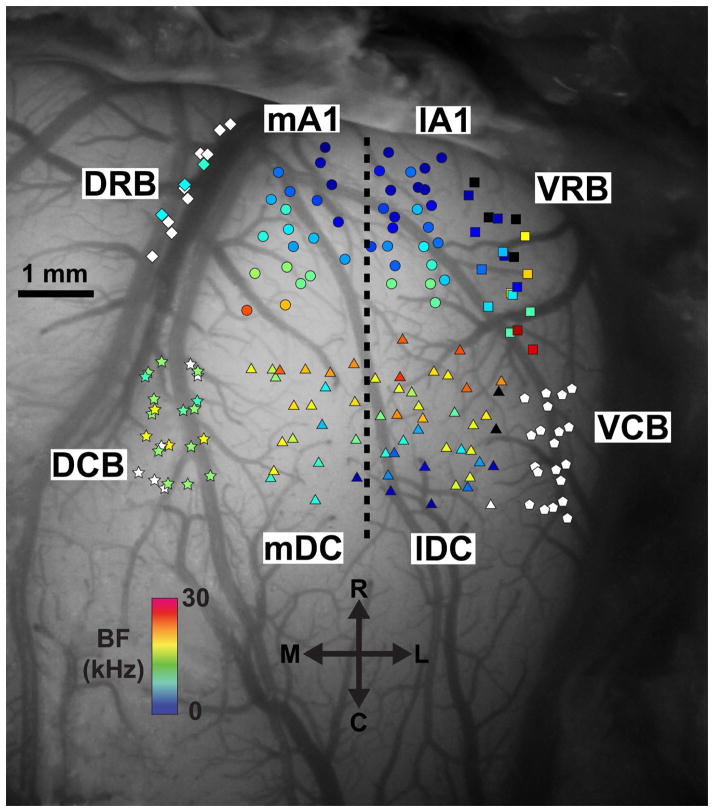
The exposed cortical surface with an inserted electrode array was imaged using a microscope-mounted camera (OPMI 1 FR pro, Zeiss, Dublin, CA). Guinea pig cortical regions, based on response properties characterized in previous studies (Wallace et al., 2000; Redies et al., 1989), are labeled in Figure 1. Primary auditory cortex (A1) exhibited increasing BFs in the rostrolateral-to-caudomedial direction and short first-spike latencies of approximately 12–20 ms. The dorsocaudal area (DC) shares a high frequency border with A1 and continues with decreasing BFs in the rostrolateral-to-caudomedial direction. A1 and DC, the largest cortical areas in the guinea pig, were split approximately in half perpendicular to the tonotopic gradient of these regions for analysis, creating medial A1 (mA1), lateral A1 (lA1), medial DC (mDC), and lateral DC (lDC) regions. The dorsocaudal belt (DCB) lies medial of DC and responds strongest to broadband noise, though weak responses to pure tones with BFs around 15 kHz were often observed. The dorsorostral belt (DRB) lies along the pseudosylvian sulcus, rostral of DCB and medial of A1, and shows variable responses to pure tones and broadband noise. The ventrorostral belt (VRB) is located lateral of A1 and has a similar tonotopic organization as A1 in the rostrolateral-to-caudomedial direction, but it is differentiated from A1 by its longer first-spike latency of approximately 25–35 ms and more sustained firing patterns. The ventrocaudal belt (VCB) lies caudal of VRB and lateral of DC and responds strongest to broadband noise. The small field (S) and transition (T) regions previously described in the literature have been excluded from this study as it was difficult to differentiate these regions from neighboring cortical regions. Each placement of the cortical array was verified to have all four shanks within the same cortical region before proceeding to the stimulation paradigm, which enabled us to easily separate the cortical regions for offline analysis. Multiple AC electrode placements were made per experiment. The order in which the cortical regions were stimulated was randomized within and across experiments to minimize cumulative effects.
Figure 1. Location of cortical stimulation sites across experiments.
A dorsal view of the right auditory cortex is shown approximately perpendicular to the cortical surface. Cortical regions were separated based on anatomical locations and response properties to acoustic stimuli. Primary auditory cortex (A1) and the dorsocaudal area (DC) were separated into their medial and lateral portions (indicated by the dotted line) approximately parallel to their tonotopic gradients. Further details on the identification and separation of these regions are provided in Section 2.3.2. Electrode shanks were inserted approximately perpendicular to the cortical surface and one site along each shank near layer V output neurons was stimulated for the experiment. Each shank location is labeled with a colored marker corresponding to the best frequency (BF) of the layer V site shown in the scale bar. Black symbols represent sites that respond to pure tones but a single BF could not be determined. White symbols represent sites that respond stronger to broadband noise than pure tones. The high frequency region separating A1 and DC was generally avoided to prevent confusion between these regions. Abbreviations: mA1 (circles), medial portion of primary auditory cortex; lA1 (circles), lateral portion of primary auditory cortex; VRB (squares), ventrorostral belt; VCB (pentagons), ventrocaudal belt; lDC (triangles), lateral dorsocaudal area; mDC (triangles), medial dorsocaudal area; DCB (stars), dorsocaudal belt; DRB (diamonds), dorsorostral belt; BF, best frequency; R, rostral; L, lateral; C, caudal; M, medial.
2.4. Stimulation protocol and analysis
The stimulation paradigm consisted of three blocks of trials presented sequentially but separated by 5–10 seconds in time. In Block 1, 50 trials of 70 dB SPL, 60 ms long broadband noise (flat spectrum from 0.625 to 40 kHz; same frozen stimulus for all trials and blocks across experiments) were presented to the animal’s left ear canal at a rate of 2/s and responses in the right CNIC were recorded.
In Block 2, we presented PN-Stim in which broadband noise was paired with AC electrical stimulation. Each trial consisted of the same broadband noise as in Block 1 paired with a single electrical stimulation pulse presented 4 ms after the onset of the noise stimulus. Previous studies in guinea pigs (Markovitz et al., 2013; Lim and Anderson, 2007) have shown that stimulation of A1 activates neurons projecting to CNIC with average first-spike latencies between about 7–10 ms. The average first-spike latency for acoustic-driven responses within the CNIC of guinea pigs is about 13 ms (Syka et al., 2000). We selected 4 ms to attempt to have spikes occur approximately simultaneously into the CNIC from acoustic and cortical stimulation (at least from A1), based on the findings presented in the Introduction for conditions that may cause more suppressive than facilitatory effects. Electrical stimulation consisted of a biphasic, charge-balanced pulse (205 μs/phase, cathodic-leading) at either 12, 16, 20, or 32 μA, with only one site being stimulated per trial. The presented results are generally based on data collected for stimulation of 32 μA, though lower levels were used for specific analyses when stated. We used a maximum of 32 μA because a previous study by our group demonstrated that this current level was low enough to locally activate specific frequency regions within AC in a similar animal model (Markovitz et al., 2013), which enables us to investigate frequency-specific changes in neural activity within the CNIC induced by AC activation. One cortical site located within the main output layer V in the guinea pig cortex was used from each of the four shanks for stimulation. Sites located in layer V were determined using current source density analysis, which calculates the second spatial derivative of the sound-evoked local field potentials recorded across the sites along each shank to obtain the current sources and sinks corresponding to the different cortical layers. Layer V sites were identified as those with a current source located at a depth of approximately 900–1500 μm (Wallace et al., 2000; Lim and Anderson, 2007). Further details on this current source density technique in guinea pig is provided in (Lim and Anderson, 2007). Each stimulus parameter was run for 50 trials and the stimulation was randomized across the four levels and four cortical sites, resulting in a total of 400 trials at a rate of 2/s for Block 2. The CNIC response to PN-Stim in Block 2 for each of the 16 stimulus parameters was then compared to the CNIC response to acoustic stimulation alone in Block 1, which allowed us to assess how PN-Stim directly alters the acoustic-driven activity.
Block 3 consisted of 50 additional trials of broadband noise also at a rate of 2/s. The CNIC response to acoustic stimulation alone from Block 3 was compared to the response to acoustic stimulation alone from Block 1, which allowed us to compare the residual changes caused from PN-Stim.
Spike counts for all conditions were measured over a 60 ms window starting from the beginning of the acoustic response. All statistical comparisons were performed using an unequal variance, two-tailed t-test on ranked data across trials with significance defined as p<0.01 (Ruxton, 2006). After performing all three blocks, the AC electrode was placed into a new location and the procedure was repeated after waiting at least 30 minutes to reduce cumulative effects.
2.5. Histology and electrode site reconstructions for CNIC
A detailed explanation of the computer reconstructions of the midbrain for identifying the locations of CNIC sites was presented in a previous publication (Markovitz et al., 2012) and is only briefly described here. The CNIC array was dipped in a red fluorescent dye (3 mg Di-I per 100 μL acetone; Sigma-Aldrich, St. Louis, MO) prior to its insertion into the brain. Immediately following each experiment, the animal was euthanized with an overdose (0.22 mL/kg) of Beuthanasia-D Special (active ingredients: pentobarbital sodium (390 mg/mL) and phenytoin sodium (50 mg/mL); Merck, Summit, NJ) into the heart and the animal was decapitated. The brain was immersed in 3.7% paraformaldehyde for approximately 10 days. The midbrain was then blocked, cryosectioned into 60 μm thick sagittal slices, and fully reconstructed along with the location of all of the electrode shank tracks (marked with the red Di-I stain) using computer software (Rhinoceros, Seattle, WA). To create computer models of isofrequency laminae, the midbrains were three-dimensionally normalized to each other based on the size and orientation of the IC surface across animals, and the electrode tracks were superimposed within one standard midbrain. A cut perpendicular to the tonotopic axis was made at a depth that corresponded to a specific frequency lamina based on our FRM data and previous studies (Markovitz et al., 2012; Markovitz et al., 2013; Straka et al., 2014). For plotting purposes, the distances in the caudal-to-rostral and medial-to-lateral directions along an isofrequency lamina were normalized based on the most proximal site location in each direction. Though the actual laminae are curved and oriented at different angles between the medial-lateral and dorsal-ventral axes, we use the term “medial-to-lateral” to represent this dimension since this is what is commonly used in other physiological studies that have mapped properties across the isofrequency laminae of the CNIC (Ehret, 1997; Hage and Ehret, 2003; Langner et al., 2002; Schreiner and Langner, 1988).
3. Results
Multi-unit recordings were made from a total of 1,012 CNIC sites after removing sites that did not show a strong response to broadband noise (<2%). The BFs of the recorded CNIC population ranged from 1.0–25.3 kHz and sites were distributed across the different isofrequency laminae. In AC, a total of 176 layer V sites were stimulated throughout the eight cortical regions. In the three tonotopic cortical regions, BFs ranged from 1.0–23.7 kHz for A1, 1.2–23.9 kHz for DC, and 1.5–28.3 kHz for VRB. Since multiple cortical sites were stimulated for each CNIC site, a total of 7,448 AC-CNIC site pairs were sampled to analyze the effects of PN-Stim on acoustic-driven activity (Table 1), and 1,862 AC-CNIC site pairs were sampled to analyze the residual effects of PN-Stim (Table 2).
Table 1.
Effect of PN-Stim on CNIC responses per cortical region.
| Cortical region | Number of AC-CNIC site pairs | Number (percentage) of sites suppressed at p<0.01 | Number (percentage) of sites facilitated at p<0.01 | Average (SD) magnitude of significantly suppressed sites | Average (SD) magnitude of significantly facilitated sites |
|---|---|---|---|---|---|
| mA1 | 1,548 | 792 (51.2%) | 2 (0.1%) | 0.777 (0.092) | 1.286 (0.226) |
| lA1 | 1,664 | 547 (32.9%) | 75 (4.5%) | 0.815 (0.085) | 1.266 (0.209) |
| mDC | 1,136 | 415 (36.5%) | 5 (0.4%) | 0.844 (0.086) | 1.217 (0.186) |
| lDC | 972 | 225 (23.1%) | 41 (4.2%) | 0.885 (0.054) | 1.201 (0.068) |
| DCB | 664 | 230 (34.6%) | 15 (2.3%) | 0.835 (0.085) | 1.261 (0.163) |
| DRB | 344 | 66 (19.2%) | 2 (0.6%) | 0.904 (0.036) | 1.180 (0.009) |
| VRB | 584 | 152 (26.0%) | 6 (1.0%) | 0.869 (0.057) | 1.095 (0.037) |
| VCB | 536 | 150 (28.0%) | 21 (3.9%) | 0.876 (0.049) | 1.100 (0.033) |
| Total | 7,448 | 2,577 (34.6%) | 167 (2.2%) | 0.824 (0.090) | 1.220 (0.167) |
Table 2.
Residual effect of PN-Stim on CNIC responses per cortical region.
| Cortical region | Number of AC-CNIC site pairs | Number (percentage) of sites suppressed at p<0.01 | Number (percentage) of sites facilitated at p<0.01 | Average (SD) magnitude of significantly suppressed sites | Average (SD) magnitude of significantly facilitated sites |
|---|---|---|---|---|---|
| mA1 | 387 | 138 (35.7%) | 7 (1.8%) | 0.805 (0.128) | 1.336 (0.242) |
| lA1 | 416 | 122 (29.3%) | 34 (8.2%) | 0.862 (0.081) | 1.226 (0.161) |
| mDC | 284 | 118 (41.5%) | 4 (1.4%) | 0.844 (0.088) | 1.190 (0.061) |
| lDC | 243 | 80 (32.9%) | 5 (2.1%) | 0.909 (0.057) | 1.102 (0.052) |
| DCB | 166 | 67 (40.4%) | 7 (4.2%) | 0.81 (0.109) | 1.168 (0.071) |
| DRB | 86 | 21 (24.4%) | 4 (4.7%) | 0.880 (0.044) | 1.210 (0.067) |
| VRB | 146 | 25 (17.1%) | 26 (17.8%) | 0.900 (0.060) | 1.228 (0.112) |
| VCB | 134 | 46 (34.3%) | 12 (9.0%) | 0.831 (0.069) | 1.159 (0.166) |
| Total | 1,862 | 617 (33.1%) | 99 (5.3%) | 0.847 (0.099) | 1.214 (0.147) |
3.1. PN-Stim induces extensive suppression of CNIC
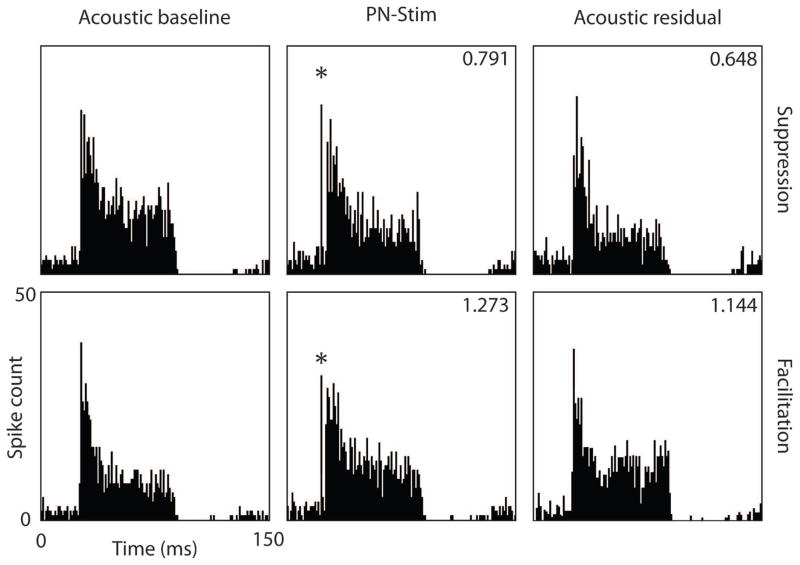
Cortical stimulation caused a variety of effects on acoustic-driven neural activity in the CNIC, including suppressing activity (Figure 2: top panels), facilitating activity (Figure 2: bottom panels), and inducing no significant change in activity. Overall, cortical stimulation had a much stronger suppressive than facilitatory effect on neural firing in the CNIC. Of the 7,448 AC-CNIC site pairs sampled, 34.6% (2,577) were significantly suppressed by PN-Stim, while only 2.2% (167) were significantly facilitated. This analysis was performed by comparing the CNIC response during PN-Stim with the baseline response to acoustic stimulation alone (e.g., columns 2 and 1 of Figure 2). Figure 3 shows all of the magnitudes of significantly modulated CNIC sites in response to PN-Stim relative to their baseline response. Cortical stimulation typically suppressed CNIC neural firing to 0.750–0.900 of its baseline level, with an average magnitude for significantly suppressed sites of 0.824. Few sites were suppressed to less than 0.500 or facilitated to greater than 1.500 of their baseline response.
Figure 2. Examples of CNIC responses to acoustic stimulation or PN-Stim.
Post-stimulus time histograms for two CNIC sites are shown in response to acoustic stimulation (baseline response; left panels), during the PN-Stim paradigm (paired cortical and acoustic stimulation; middle panels), and in response to acoustic stimulation after the PN-Stim paradigm (residual response; right panels). In the top example, the site’s response to PN-Stim was suppressed to 0.791 of the baseline response to acoustic stimulation alone (comparing columns 2 and 1). This suppression continued residually, in which the response to acoustic stimulation after the PN-Stim paradigm was 0.648 of the baseline response (comparing columns 3 and 1). In the bottom example, a different CNIC site’s response to PN-Stim was facilitated to 1.273 of the baseline response, while the residual response remained elevated at 1.144 of the baseline response. The asterisks in the middle panels correspond to the electrical artifact. The acoustic stimulus used was broadband noise. Further details on the stimulation parameters are provided in the Methods: Stimulation protocol and analysis section.
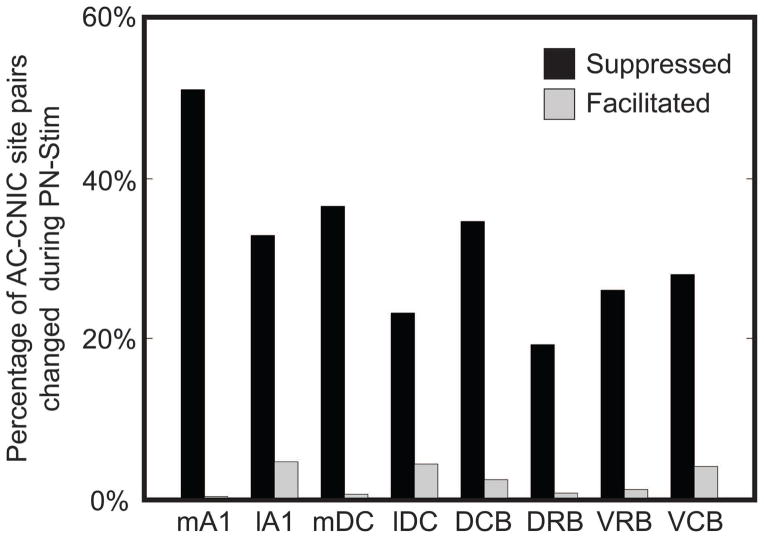
3.2. Predominantly suppressive effects for all stimulated AC regions
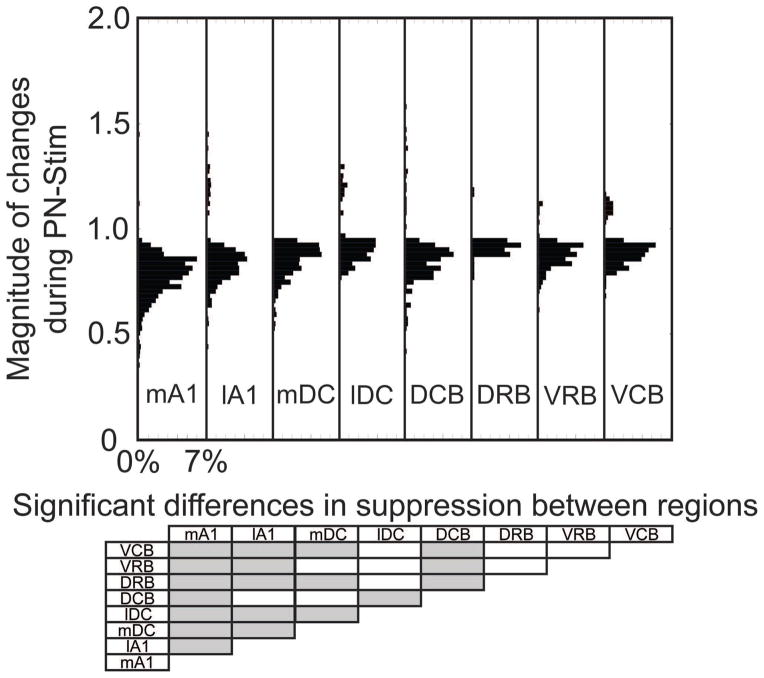
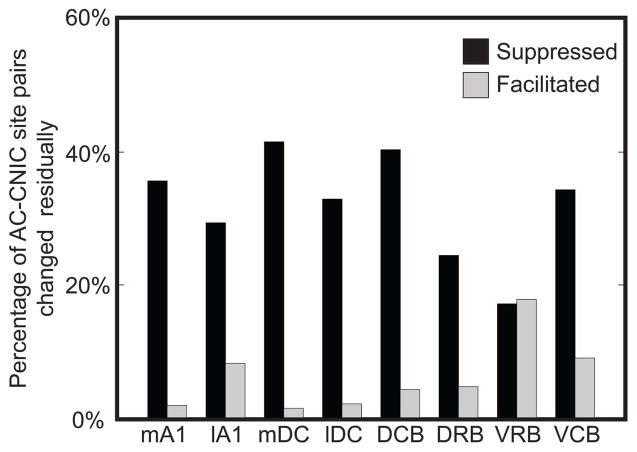
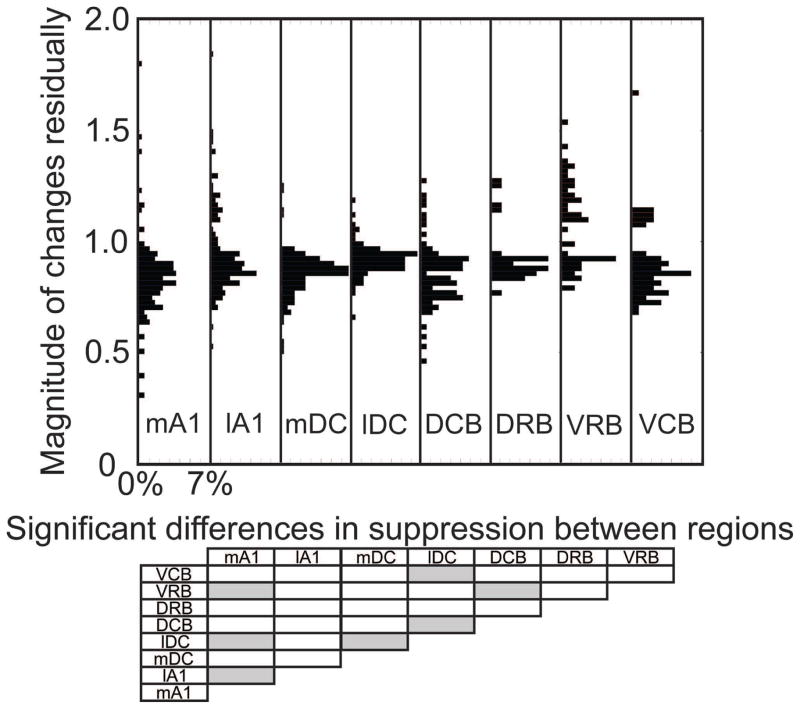
To assess how stimulation of different cortical regions affects CNIC firing, we separated the AC-CNIC site pairs based on the stimulated AC region (Table 1). Electrical stimulation of each of the eight cortical regions caused primarily suppressive effects on CNIC sites (Figure 4). Activation of mA1 suppressed the highest percentage of CNIC sites, with over half of all sites sampled being significantly suppressed, and nearly no sites were significantly facilitated. The magnitudes of suppression and facilitation for significantly changed sites based on the stimulated cortical region are shown in Figure 5. In addition to suppressing the highest percentage of sites, stimulation of mA1 also suppressed these sites to the strongest extent, with sites being suppressed to an average of 0.777 of its baseline response to acoustic stimulation alone (Figure 5). Based on Figures 4 and 5 and Table 1, there are some differences in the extent of suppressed versus facilitated CNIC sites depending on the stimulated AC region. However, all AC regions elicited much greater suppressive than facilitatory effects on CNIC activity.
Figure 4. Percentage of CNIC sites suppressed or facilitated during PN-Stim for different AC regions.
AC-CNIC site pairs were separated based on the stimulated cortical region. The percentages are relative to the total number of AC-CNIC site pairs sampled for the corresponding cortical region. All eight cortical regions had a much stronger suppressive than facilitatory effect on acoustic-driven firing in the CNIC in response to PN-Stim. Abbreviations: mA1, medial portion of primary auditory cortex (n=1,548); lA1, lateral portion of primary auditory cortex (n=1,664); mDC, medial dorsocaudal area (n=1,136); lDC, lateral dorsocaudal area (n=972); DCB, dorsocaudal belt (n=664); DRB, dorsorostral belt (n=344); VRB, ventrorostral belt (n=584); VCB, ventrocaudal belt (n=536).
Figure 5. Magnitude of changes in CNIC responses during PN-Stim for different AC regions.
In the top panel, the change in spike count during PN-Stim compared to the baseline response to acoustic stimulation is plotted for the AC-CNIC site pairs that were significantly suppressed or facilitated based on the cortical region stimulated. Percentages indicated on the abscissa are relative to the total number of AC-CNIC site pairs for each cortical region. The bottom panel shows which cortical regions are statistically different from the others (gray boxes) based on the magnitude of changes in suppressed responses using a Bonferroni-adjusted t statistic multiple comparison test with p<0.01. None of the cortical regions are statistically different when comparing the magnitude of changes in facilitatory responses.
3.3. CNIC distribution of suppression and facilitation
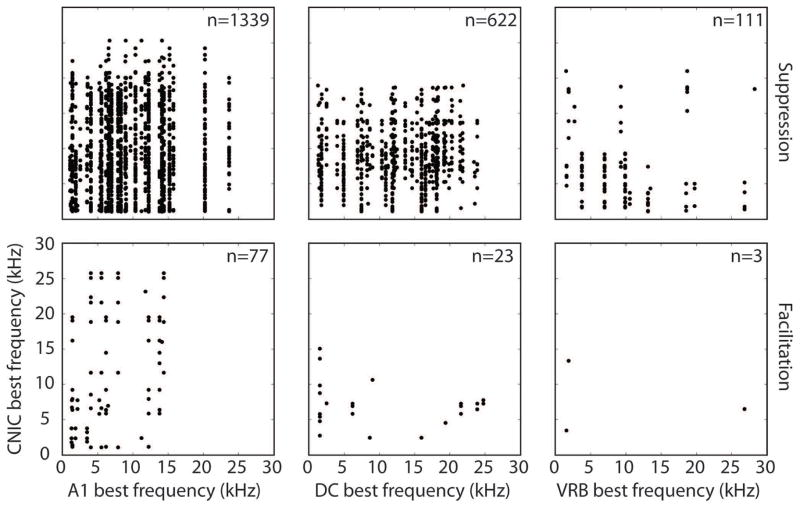
Direct pathways from A1 to the CNIC are glutamatergic and tonotopically arranged (Saldana et al., 1996; Markovitz et al., 2013; Lim and Anderson, 2007; Andersen et al., 1980; Feliciano and Potashner, 1995; Bajo and Moore, 2005; Bajo et al., 2007). Therefore, we wanted to determine whether the facilitation and/or suppression of acoustic-driven activity were organized along the tonotopic gradient. We selected the three tonotopic cortical regions - A1, DC, and VRB - and plotted the BFs of the stimulated sites against the BFs of the CNIC sites that were significantly suppressed or facilitated. The medial and lateral portions of A1 and DC were combined for this analysis. As shown in the top panels of Figure 6, suppression of the CNIC was widespread across the tonotopic axis for all three stimulated AC regions. For instance, stimulation of an A1 site with a BF of 20.2 kHz suppressed CNIC sites with BFs ranging from 1.1 kHz – 24.3 kHz. Similarly, stimulation of the three tonotopic regions produced facilitation in the CNIC with no clear trend across the tonotopic axis, though these plots, especially for VRB, had fewer points for analysis (bottom panels of Figure 6). Similar trends were found using lower stimulation levels (data not shown).
Figure 6. Lack of tonotopic organization of suppression and facilitation in CNIC during PN-Stim.
The best frequency of a stimulated AC site used for PN-Stim is plotted against the best frequency of each CNIC site that was significantly suppressed (top panels) or facilitated (bottom panels) using an electrical stimulation level of 32 μA. Only tonotopically organized cortical regions (A1, DC, and VRB) were analyzed. Electrical stimulation of each of the three cortical regions caused suppression or facilitation across different CNIC sites with no clear tonotopic organization. Note that a single AC site could elicit suppression or facilitation of multiple CNIC sites. Abbreviations: A1, primary auditory cortex; DC, dorsocaudal area; VRB, ventrorostral belt, CNIC: central nucleus of the inferior colliculus.
We do not believe this lack of tonotopic trends is due to current spread since the highest level used, 32 μA, would typically activate a span within a few hundred microns (Lim and Anderson, 2007; Ranck, 1975), which is an order of magnitude smaller than the tonotopic span of A1 and DC. It is also unlikely to be caused by activation of passing fibers since we demonstrated the ability to focally activate frequency-specific corticofugal pathways from layer V of AC to CNIC using a similar stimulation and recording paradigm in a previous study (Markovitz et al., 2013). One possible confounding issue in the methods for this analysis was that four AC sites with different BFs were stimulated in a random order (see details for Block 2 in the Methods: Stimulation protocol and analysis section). For instance, if one of the four AC sites was truly suppressing a BF-aligned CNIC site, this suppression may have accumulated during PN-Stim, making other BF-misaligned AC sites appear to be suppressing that CNIC site as well. However, a reanalysis of the data looking for this trend did not reveal evidence of this confounding effect.
In addition to investigating patterns across the frequency gradient, we were also interested in determining whether suppression could be induced throughout an isofrequency lamina. We did not investigate facilitation effects across a lamina due to the limited number of cases, as evident in Figure 6. In order to create a model of an isofrequency lamina, three-dimensional computer reconstructions of the midbrain were created for each animal, normalized to each other, and superimposed onto a standard midbrain as further described in the Methods: Histology and electrode site reconstructions for CNIC. A planar cut through the standard midbrain was then made perpendicular to the electrode tracks through the center of the CNIC, corresponding to an approximately 8 kHz lamina (Markovitz et al., 2013; Straka et al., 2014). In Figure 7, each plot is normalized in the caudal-to-rostral and lateral-to-medial directions based on the furthest point classified to be within the CNIC based on experiments done for this study and those performed for a previous study which mapped responses across the CNIC (Markovitz et al., 2013). The laminae are not perfect squares, but instead exhibit complex shapes across layers (Malmierca et al., 1995). Laminae responding to frequencies around 8 kHz are nearly elliptical with the long axis going from the caudomedial-to-rostrolateral direction, most clearly shown in the panels with many points (e.g., mA1 and lDC) in Figure 7. Our recordings spanned most of the lamina with a caudal-to-rostral distance of 2.3 mm and a medial-to-lateral distance of 2.7 mm, which is larger than a previous study by Malmierca et al. (Malmierca et al., 1995) that obtained measurements of approximately 1.25 mm and 2 mm, respectively. The discrepancy in dimensions may be attributed to several factors: (1) We defined the CNIC border by functional response properties while Malmierca et al. used anatomical staining; (2) We combined results across several animals; and (3) Malmierca et al. were unsure of the precise CNIC borders due to a lack of distinction in staining density between the CNIC and outer IC regions. Regardless, our extensive mapping of the CNIC across multiple studies (Markovitz et al., 2013; Markovitz et al., 2012; Straka et al., 2014) confirms that we amply sampled the CNIC in all directions. Since no tonotopic trends in suppression were identified based on the analysis in Figure 6, we collapsed data across each electrode shank from different frequency regions onto this lamina. Therefore, if any site along an electrode shank was suppressed by PN-Stim, we counted this as suppression at the given shank location along our modeled lamina, signified by a filled circle in Figure 7.
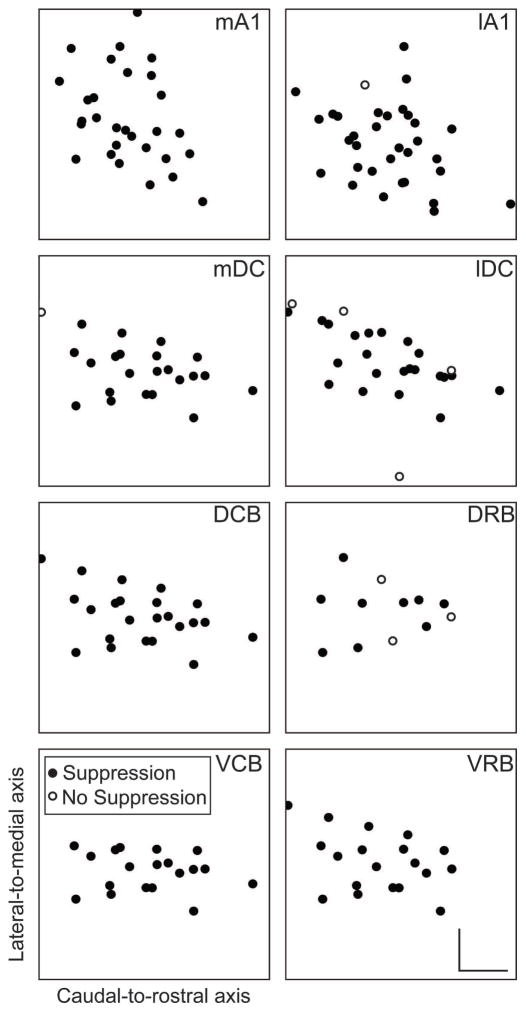
Figure 7. Distribution of suppression across the isofrequency laminae of CNIC during PN-Stim.
Squares representing an isofrequency lamina of the CNIC are plotted for stimulation of eight cortical regions based on three-dimensional computer reconstructions of the midbrain. A planar cut was made through the midbrain reconstruction perpendicular to the tonotopic gradient at a location that represents a middle frequency of approximately 8 kHz. Each panel is normalized similarly in the caudal-to-rostral and lateral-to-medial directions based on the furthest point determined to be inside the CNIC across our mapping studies (Markovitz et al., 2013). Filled circles represent locations in which PN-Stim induced significant suppression on at least one site along the shank that passed through this lamina. Open circles represent locations in which no CNIC suppression was induced by PN-Stim. These plots show that suppression could be induced across an isofrequency lamina by stimulating each cortical region. Scale bars in the bottom-right panel represent 0.5 mm. Abbreviations: mA1, medial portion of primary auditory cortex; lA1, lateral portion of primary auditory cortex; mDC, medial dorsocaudal area; lDC, lateral dorsocaudal area; DCB, dorsocaudal belt; DRB, dorsorostral belt; VCB, ventrocaudal belt; VRB, ventrorostral belt.
As illustrated in Figure 7, CNIC suppression could be elicited throughout the lamina by stimulation of each cortical region. Of the few locations where suppression was not induced, it is possible that we simply did not stimulate the correct cortical location to elicit suppression for those specific sites. However, for the majority of CNIC locations that did exhibit suppressive responses, we did not observe any noticeable trends in their spatial arrangement. In other words, there does not appear to be a specific region of a CNIC lamina that exhibits more or less suppressive versus non-modulatory responses.
3.4. Residual effects of PN-Stim
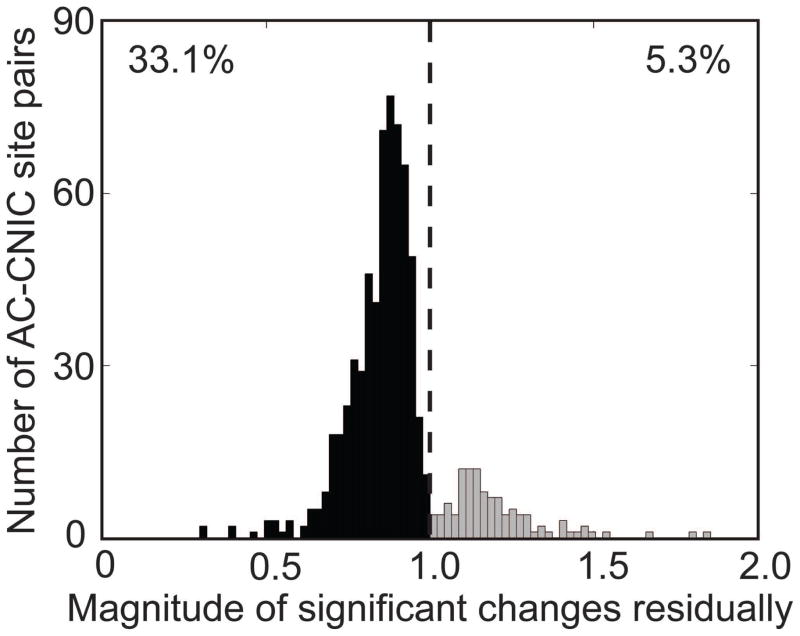
The data presented above indicates that PN-Stim induces a predominantly suppressive effect on acoustic-driven firing in the CNIC. We were further interested in whether this suppression is maintained in the auditory system after PN-Stim has ceased. Immediately after the PN-Stim paradigm was completed, an additional 50 trials of acoustic stimulation was presented and we compared its response to the baseline response (e.g., columns 3 and 1 in Figure 2). Consistent with what was observed during PN-Stim, residual CNIC effects were primarily suppressive, as 33.1% (617) of the 1,862 sampled AC-CNIC site pairs were significantly suppressed while only 5.3% (99) were significantly facilitated. Figure 8 shows all of the magnitudes of AC-CNIC site pairs that were significantly changed residually. Sites significantly suppressed had an average spike count of 0.847 relative to their baseline response.
Figure 8. Magnitude of residual changes in CNIC responses caused by PN-Stim.
The residual change in spike count after PN-Stim compared to the baseline response to acoustic stimulation is plotted for the 716 AC-CNIC site pairs (out of 1,862 total) that were significantly suppressed (black) or facilitated (gray). A value of 1 (dotted line) signifies equal spiking in both conditions, while data to the left (right) of the line signifies suppression (facilitation) of neural firing. Out of all AC-CNIC site pairs, 33.1% were significantly suppressed, while only 5.3% were significantly facilitated.
We separated the residual data based on the stimulated AC region, as shown in Table 2. Electrical stimulation of seven of the eight cortical regions caused primarily suppressive effects on CNIC sites (Figure 9), with only VRB causing a slightly greater percentage of sites being facilitated versus suppressed. While stimulation of mA1 suppressed the highest percentage of CNIC sites during PN-Stim (Figure 4), mDC caused the most sites to remain suppressed residually. Figure 10 shows the magnitudes of suppression and facilitation for significantly changed sites based on the stimulated cortical region. There are a few significant differences in suppressive distributions between different cortical regions (gray boxes in Figure 10). None of the cortical regions are statistically different when comparing the magnitude of residual changes in facilitatory responses. In addition, no location trends were apparent when investigating the residual results across an isofrequency lamina of the CNIC.
Figure 9. Percentage of CNIC sites suppressed or facilitated residually by PN-Stim for different AC regions.
AC-CNIC site pairs were separated based on the stimulated cortical region. The percentages are relative to the total number of AC-CNIC site pairs sampled for the corresponding cortical region. All cortical regions, except for VRB, had a much stronger suppressive than facilitatory residual effect on acoustic-driven firing in the CNIC after the PN-Stim paradigm. Abbreviations: mA1, medial portion of primary auditory cortex (n=387); lA1, lateral portion of primary auditory cortex (n=416); mDC, medial dorsocaudal area (n=284); lDC, lateral dorsocaudal area (n=243); DCB, dorsocaudal belt (n=166); DRB, dorsorostral belt (n=86); VRB, ventrorostral belt (n=146); VCB, ventrocaudal belt (n=134).
Figure 10. Magnitude of residual changes in CNIC responses caused by PN-Stim for different AC regions.
In the top panel, the residual change in spike count after PN-Stim compared to the baseline response to acoustic stimulation is plotted for the AC-CNIC site pairs that were significantly suppressed or facilitated based on the cortical region stimulated. Percentages are relative to the total number of AC-CNIC site pairs for each cortical region. The bottom panel shows which cortical regions are statistically different from the others (gray boxes) based on the magnitude of residual changes in suppressed responses using a Bonferroni-adjusted t statistic multiple comparison test with p<0.01. None of the cortical regions are statistically different when comparing the magnitude of residual changes in facilitatory responses.
3.5. Relationship between the effects during and after PN-Stim
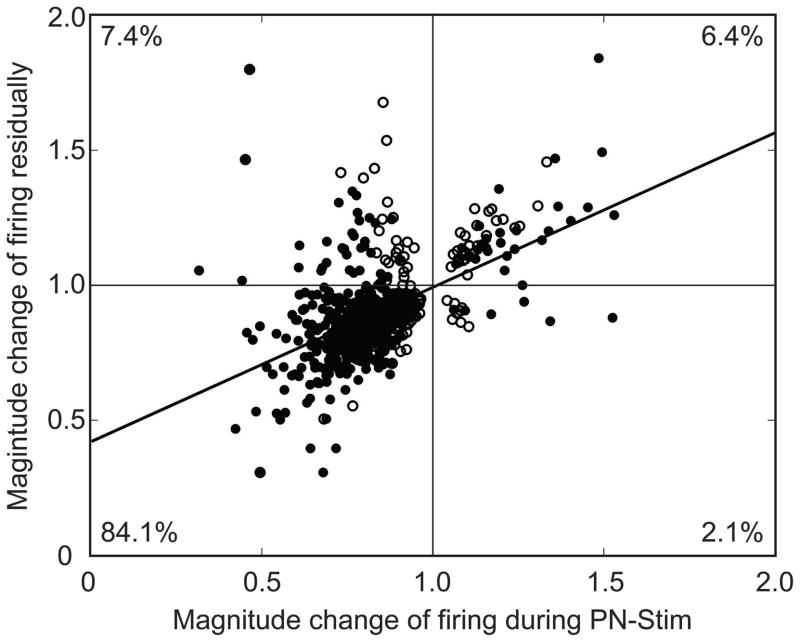
The magnitude changes in acoustic-driven CNIC responses during PN-Stim versus those residually after PN-Stim are shown in Figure 11. For the PN-Stim protocol, we electrically stimulated four cortical sites each at four different current levels (16 total parameters) paired with broadband noise in a randomized order across trials to minimize cumulative effects (see Discussion for further explanation). We also recorded acoustic-driven activity in the CNIC before and after each PN-Stim protocol. As a result, we only had one comparison (change in magnitude value) for the residual effect but 16 comparisons for the changes during PN-Stim. In Figure 11, we only plotted cases in which the residual changes were significant (along the ordinate). For each of those significant residual cases, we plotted the case with the strongest change during PN-Stim (out of the 16 parameters; along the abscissa). The vast majority of points were in the third quadrant, corresponding to suppression during PN-Stim and residual suppression after PN-Stim. The weak linear trend (R2=0.27) reflects the small number of scattered facilitatory points as well as the weak relationship between the magnitude strength of suppression during PN-Stim versus residually. However, Figure 11 clearly shows that AC stimulation causes suppression both during PN-Stim and residually to a much larger extent than facilitation.
Figure 11. Comparison of the magnitude changes for the CNIC responses during PN-Stim versus residually.
Only AC-CNIC site pairs showing significant changes residually are plotted. For each of these AC-CNIC site pairs, we selected the one PN-Stim parameter (out of 16 total; see Methods: Stimulation protocol and analysis section for specific stimulation parameters) that exhibited the largest change in magnitude during PN-Stim. Filled circles correspond to AC-CNIC site pairs that exhibited significant changes both during PN-Stim and residually. Open circles correspond to those that exhibited significant changes only residually, suggesting that some cases did not exhibit significant changes during the PN-Stim paradigm and there may be a build-up effect over time. The black line is a best fit line to the data, which has the equation y=0.57x+0.42 and an R2 value of 0.27. Percentages correspond to the number of points in each corresponding quadrant out of the 716 AC-CNIC site pairs that exhibited significant changes residually. It can be seen that suppression during PN-Stim generally leads to residual suppression, corresponding to quadrant III.
3.6. Strong suppression caused by PN-Stim is due to AC stimulation over time
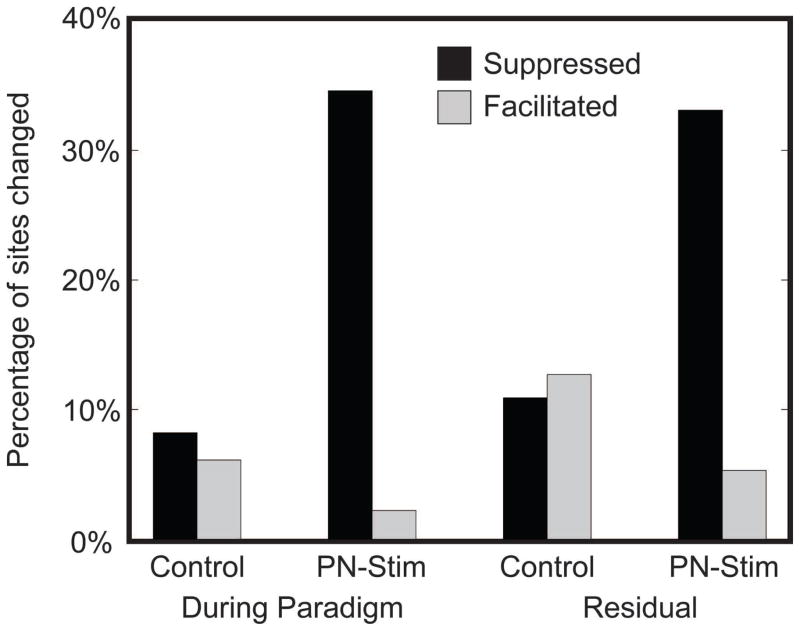
To assess if and how repeated PN-Stim suppresses acoustic-driven CNIC activity which then leads to residual suppression after the PN-Stim paradigm, we further analyzed our data in two different ways. First, we needed to confirm that the suppressive effects were not simply caused by repeated broadband noise stimulation, independent of AC stimulation. In Figure 12, we present the PN-Stim data in comparison to a control condition using acoustic stimulation alone. For the control condition, we performed an identical protocol as with PN-Stim except we removed the cortical electrical stimulation, using only acoustic stimulation. The number of trials, time periods, and stimulation parameters were otherwise similar. As shown in Figure 12, acoustic stimulation alone can induce facilitatory or suppressive changes in acoustic-driven CNIC activity in somewhat equal amounts both during stimulation and residually, which may partially reflect the inherent fluctuations in neuronal firing over time. However, to induce a strong suppressive effect during stimulation and residually, the broadband noise stimulus needed to be paired with AC stimulation.
Figure 12. Changes in CNIC activity from PN-Stim compared to an acoustic stimulation control.
The PN-Stim data for the During Paradigm and Residual conditions from Figures 3 and 8 are replotted here. The Control data had a similar protocol to that of PN-Stim except without cortical electrical stimulation (i.e., broadband noise stimulation alone). The percentages are relative to the total number of CNIC sites sampled for each condition for Control (n=49 for During Paradigm; n=241 for Residual). These data confirm that the strong suppressive changes caused by PN-Stim are due to pairing broadband noise stimulation with AC stimulation and cannot be achieved with acoustic stimulation alone.
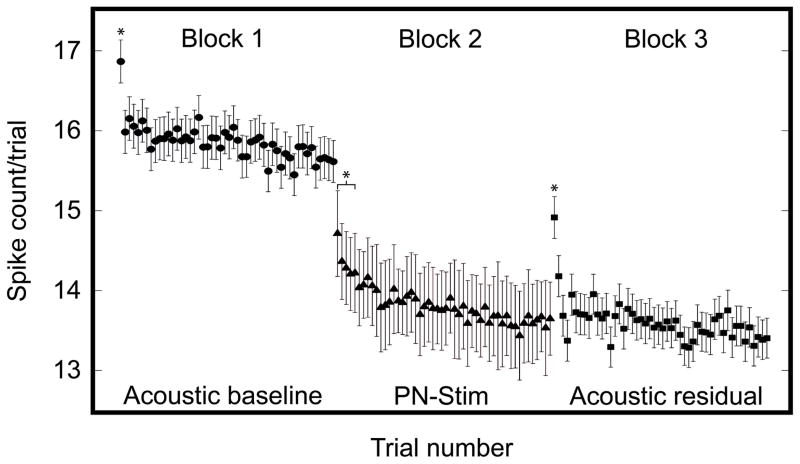
Figure 12 demonstrates that PN-Stim, and not just acoustic stimulation alone, induces strong suppression of CNIC neurons. The question remains as to how this suppressive effect evolves over time. Based on Figure 11, it would be expected that the effects during PN-Stim are somehow driving the residual effects. In Figure 13, we plotted the spike counts trial by trial during the acoustic baseline, PN-Stim, and acoustic residual conditions. For Block 2 (PN-Stim), we used all A1-CNIC site pairs which were significantly suppressed (n=2,577). For Block 1 (Acoustic baseline) and Block 3 (Acoustic residual), we used any CNIC sites (n=960) which were significantly suppressed during PN-Stim by at least one of the four AC stimulation sites. There were smaller n values for the Acoustic baseline and Acoustic residual conditions compared to the PN-Stim condition because there are multiple AC-CNIC site pairs for each CNIC site used in the Acoustic baseline and Acoustic residual conditions. The trial number plotted on the abscissa is generally related to time. Within each stimulation block, the trials occurred at 2/s (500 ms intervals). Between blocks, there was a delay of approximately 5–10 seconds. For the Acoustic baseline and Acoustic residual blocks, trial numbers correspond directly to the inter-trial separation of 500 ms. For the PN-Stim block, trial numbers don’t correspond directly to the inter-trial separation since the 16 stimulation parameters for PN-Stim were presented in a pseudorandom order, in which each parameter was presented on average every 8 seconds (500 ms times 16 parameters; each parameter presented once in a random order before being presented again). The critical point of this figure is not to reflect the exact time of changes but to demonstrate the general trend of how neural changes evolved across trials of stimulation and between blocks. Significant changes across trials within each block were determined by comparing the spike count of the last (50th) trial of a block to the first trial of that same block using Welch’s unequal variance, one-sided t-test with p<0.01. This procedure was then repeated, comparing the 50th trial to the second trial, third trial, and onwards until significance was no longer obtained, which assumes that the spike count stabilizes by the 50th trial (note that we did not find any significant differences among later trials, confirming this assumption).
Figure 13. Suppression of neural firing in CNIC due to PN-Stim over time.
The mean and standard error of spike counts per trial are plotted for the Acoustic baseline (circles; n=960), PN-Stim (triangles; n=2,577), and Acoustic residual (squares; n=960) conditions. Within each block, trials occurred at 500 ms intervals. Between blocks, there was a delay of approximately 5–10 seconds. For Block 1 (Acoustic baseline) and Block 3 (Acoustic residual), we used the CNIC sites which were significantly suppressed during PN-Stim by at least one of the four AC stimulation sites. For these blocks, trial numbers correspond directly to the inter-trial separation of 500 ms. For Block 2 (PN-Stim), we used all A1-CNIC site pairs which were significantly suppressed. For the PN-Stim block, trial numbers do not necessarily correspond directly to the inter-trial separation since the 16 PN-Stim parameters were presented in a pseudorandom order. Instead the trial numbers of the PN-Stim block correspond to an average inter-trial separation of approximately 8 s (equal to 500 ms times 16 PN-Stim parameters). Asterisks correspond to trials with spike counts that are significantly higher than that of the last (50th) trial within the same block using Welch’s unequal variance, one-sided t-test with p<0.01. The significant bracket in Block 2 corresponds to trials 1 through 5.
There were several interesting observations from Figure 13. First, broadband noise stimulation alone (Block 1) caused the CNIC activity to immediately drop after the first trial, which reflects some rapid acoustic-driven adaptive process. Second, pairing broadband noise with AC stimulation (Block 2, after a break of approximately 5–10 seconds) immediately suppressed the acoustic-driven CNIC activity below the level of Block 1, and the spike count continued to drop after the first trial during PN-Stim. However, this decrease in activity occurred to a slower extent than what was observed in Block 1 (note that the inter-trial time is longer for Block 2 compared to Block 1 as explained above). Third, approximately 5–10 seconds after the PN-Stim paradigm for Block 3, the acoustic-driven CNIC activity remained suppressed during broadband noise stimulation in comparison to the Acoustic baseline. It is interesting that the first trial in Block 3 partially recovered in spike count and then immediately dropped back down to the suppressed level observed in Block 2.
Overall, Figures 11–13 suggest that pairing broadband noise stimulation with AC stimulation is suppressing CNIC activity over time to a greater extent than what occurs to broadband noise stimulation alone and that this suppressive effect continues to last beyond the PN-Stim paradigm in concert with some adaptive mechanism(s) inherent within the auditory system for processing broadband noise stimuli.
4. Discussion
The presented results demonstrate that focal activation of all eight different cortical areas using our PN-Stim paradigm causes extensive suppression of acoustic-driven firing throughout the CNIC. This suppression occurs during stimulation and generally continues residually following the PN-Stim paradigm. The greater extent of suppressive versus facilitatory effects caused by PN-Stim is not possible with acoustic stimulation alone, and it appears to evolve over several trials during PN-Stim (tens of seconds; see Figure 13). The ability to induce plasticity and suppress activity within the ascending auditory system may open up a new method for treating tinnitus, which has been linked to hyperactivity and/or hypersynchrony across neurons throughout several central auditory nuclei (Mulders and Robertson, 2013; Jastreboff and Sasaki, 1986; Bauer et al., 2008; Moller et al., 2011; Lanting et al., 2009; Chen et al., 2013; Middleton and Tzounopoulos, 2012; Kaltenbach, 2011; Eggermont and Roberts, 2012).
4.1. Methodological considerations
We cannot rule out the possibility that our use of ketamine-xylazine to anesthetize the animals may have modified the extent or type of plasticity observed within the CNIC associated with corticofugal activation. However, previous studies have generally shown no or minimal changes in spiking activity within the IC caused by ketamine or ketamine-xylazine in different animals, including the guinea pig (Ter-Mikaelian et al., 2007; Suta et al., 2003; Astl et al., 1996). Previous studies have also demonstrated the ability to shift BF tuning of CNIC neurons using cortical stimulation paired with a pure tone in ketamine-xylazine-anesthetized animals that is consistent with the effects observed in awake animals (Yan et al., 2005; Yan and Suga, 1998; Yan and Ehret, 2002; Zhang and Suga, 2000). Therefore, our results showing extensive suppression is likely related to the actual paired paradigm itself, especially since such drastic suppression was not observed in those previous studies regardless of the anesthetic condition or species used. Although ketamine may have limited the ability to induce plasticity within the auditory system since it is a noncompetitive NMDA receptor antagonist (Leong et al., 2004), we still observed significant amounts of plasticity which may occur to a greater extent in an awake preparation.
For the PN-Stim protocol, we randomly presented 16 different parameters (four stimulated sites in a given AC region, each at four different levels). We were limited to four sites due to the design of our electrode array and the desire to only activate sites located in layer V. We used four different levels spanning 12–32 μA because we were not certain which level would be effective in modulating CNIC. Since we were concerned that there may be cumulative effects, we randomized the ordering of sites and levels for stimulation. Due to this protocol, we cannot rule out the possibility that there might have been fewer stimulation locations within a given AC region that cause suppression in the CNIC, and due to their long-lasting effects could make it appear that the other stimulated sites also caused suppressive effects. From our results, we can claim that there are at least some locations across each of the eight AC regions that can induce a greater extent of suppressive versus facilitatory changes in the CNIC.
The main objective of this study was to assess how AC stimulation modulates acoustic-driven activity within the CNIC, in which we were able to show a strong and widespread suppressive effect both during and after the PN-Stim paradigm that did not occur with acoustic stimulation alone. Based on the previous AC stimulation studies described in the Introduction, especially the preliminary data presented by (He et al., 2002), we initially investigated PN-Stim in which AC stimulation and noise stimulation occurred approximately simultaneously since this condition was hypothesized to induce much greater suppressive effects than using other inter-stimulus delays. In future studies, we will need to investigate other inter-stimulus delays and even AC stimulation alone to confirm this hypothesis, which in turn would open up the potential for inducing different types of changes in the brain by varying the inter-stimulus delay for PN-Stim that is not possible by acoustic or AC stimulation alone.
4.2. Potential corticofugal mechanism for CNIC suppression via PN-Stim
One of the most studied pathways in terms of auditory corticofugal function and plasticity is the projection from A1 to the CNIC (Xiong et al., 2009; Suga et al., 2011; Malmierca and Ryugo, 2011). There are both direct and indirect projections between A1 and the CNIC, in which the direct projections have shown to be tonotopic and glutamatergic (presumably excitatory) while some of the indirect projections pass through the dorsal and/or external nuclei of the IC that then provide both suppressive and facilitatory inputs into the CNIC (Saldana et al., 1996; Markovitz et al., 2013; Lim and Anderson, 2007; Andersen et al., 1980; Feliciano and Potashner, 1995; Bajo and Moore, 2005; Jen et al., 2001). Suppressive effects can also occur due to descending activation of GABAergic neurons that have extensive local axons within the IC (Nakamoto et al., 2013).
By activating (via electrical stimulation or drugs) or inactivating (via drugs, lesions, or cooling) A1 or AC in general, studies have shown a wide range of suppressive and facilitatory effects in the CNIC, with some results contradicting each other (Suga et al., 2011; Malmierca and Ryugo, 2011). Through a series of studies using methods for precisely characterizing response properties of the activated and recorded neurons, it was possible to more clearly demonstrate that A1 neurons are designed to residually increase the sensitivity of CNIC neurons that are tuned to similar acoustic features, such as BF (review and a few exceptions presented in (Suga et al., 2011)). This could be achieved, for example, by pairing A1 stimulation with a pure tone at the BF of the stimulated A1 neurons. In contrast, CNIC neurons tuned to different acoustic features (i.e., BF-misaligned or feature-misaligned) tend to be suppressed to their previously tuned feature and/or experience an increase in sensitivity to the features associated with the stimulated A1 neurons (e.g., Figures 4 and 7 in (Yan and Ehret, 2002)). It has been proposed that the A1-to-CNIC projections are part of a larger corticofugal network interacting with cognitive and limbic centers that may assign relevance to certain stimuli and activate corticofugal pathways to enhance ascending coding of those stimuli while suppressing the salience of other less relevant or feature-misaligned sound inputs (Xiong et al., 2009).
In contrast to this feature-specific gain control by the corticofugal system, we observed a widespread shut-down in acoustic-driven activity across the CNIC when pairing cortical stimulation, regardless of AC region, with broadband noise. While a pure tone activates a local region within each auditory nucleus due to the robust tonotopic organization of the auditory system, the broadband noise stimulus used in this study has no precise temporal or spectral structure and therefore activates neurons across the tonotopic gradient. Based on the pure tone studies described above, it could be expected that the non-specificity of the noise stimulus would cause widespread suppressive effects via corticofugal activation due to a greater number of BF-misaligned interactions versus BF-aligned interactions. In other words, we were electrically stimulating in only one AC or BF location but activating CNIC neurons across many BF locations that could result in a greater number of BF- or feature-misaligned neurons.
From Figure 13, it also appears that broadband noise stimulation already induces a rapid adaptive process, after just one trial of presentation, which may relate to an automatic mechanism in which the brain decreases its gain to irrelevant or meaningless inputs. It will be interesting to investigate if this adaptive process occurs to a lesser extent for pure tones or more behaviorally-salient stimuli, such as speech, using a similar paradigm as in our study. It has been shown that stimulus-specific adaptation can occur to some extent in the CNIC for pure tones (Ayala and Malmierca, 2012; Malmierca et al., 2009), but it is unclear if this type of adaptation utilizes a similar mechanism as occurs for our broadband noise adaptation. In addition, AC stimulation of the corticofugal pathway may be reinforcing this proposed gain mechanism to a larger extent that further suppresses ascending activity to the noise input during PN-Stim and residually as observed in Figure 13. Future studies could assess whether paired cortical stimulation with more meaningful stimuli, such as speech, causes greater facilitatory effects due to its relevance to behavior, especially in an awake preparation.
4.3. Clinical implications for a new neuromodulation approach
Currently, cortical stimulation approaches for tinnitus treatment include transcranial magnetic stimulation (TMS), transcranial direct or alternating current stimulation (tDCS or tACS), and invasive cortical stimulation using epidural electrodes. All of these approaches, as is the case for the treatment of many other neurological or psychiatric disorders, have shown quite variable results across tinnitus patients (Friedland et al., 2007; Vanneste and De Ridder, 2012; De Ridder et al., 2011; Song et al., 2012; Hoekstra et al., 2013; Kim et al., 2014; Langguth and De Ridder, 2013; Piccirillo et al., 2013; Johnson et al., 2013; Engelhardt et al., 2014). Only a small proportion of patients obtain full suppression of their tinnitus, and across patients the tinnitus percept typically returns after the termination of the treatment on different time scales. Although there have been many studies performed in animals demonstrating the ability to induce precise and well-controlled plasticity using cortical stimulation paired with acoustic stimuli, there have not yet been any investigations to the best of our knowledge using cortical stimulation paired with an acoustic stimulus in tinnitus patients. There was one proof-of-concept study investigating TMS paired with pure tones in normal hearing, non-tinnitus subjects that demonstrated the ability to alter auditory evoked potentials (Schecklmann et al., 2011), which suggests a paired paradigm may enable therapeutic effects in tinnitus patients.
Assuming that tinnitus can be treated by reducing hyperactive auditory neurons, our findings suggest that pairing auditory cortical stimulation with broadband noise could be a potential treatment option for tinnitus. No previous tinnitus treatments have combined neural stimulation with broadband noise, which may activate the auditory brain with greater suppressive effects compared to pure tones. Furthermore, it appears that PN-Stim of multiple AC regions, particularly within their deeper output layers, may be effective in suppressing tinnitus.
A previous study by our group (Offutt et al., 2014) also combined brain stimulation with broadband noise, except that the stimulation location was the dorsal cortex of the IC (DNIC) in order to evaluate the potential for using an auditory midbrain implant to treat tinnitus. Although there were some differences in the stimulation protocol and analyses between the two studies, (Offutt et al., 2014) also showed a greater percentage of CNIC sites that were residually suppressed (15.5% of DNIC-CNIC site pairs) than were facilitated (9.0%) based on values presented in their Table 1. The average magnitude of residual suppression and facilitation in that study for the CNIC sites were 0.829 and 1.197, respectively, which are quite comparable to the values in this study (suppression: 0.847, facilitation: 1.214). Given these similarities and the lack of tonotopic or isofrequency location trends within the CNIC for both studies, it seems likely that the modulatory effects observed in our study for AC stimulation is largely mediated by a pathway to the CNIC that passes through the DNIC (Offutt et al., 2014; Jen et al., 2001; Nakamoto et al., 2013), which is known to have a weak or nonexistent tonotopic organization (Aitkin et al., 1975; Syka et al., 2000). It is important to note that the study by (Offutt et al., 2014) also observed modulatory effects when stimulating DNIC alone without acoustic stimulation, which suggests that AC stimulation alone may also induce modulatory effects. However, the study by (Offutt et al., 2014) showed greater suppressive than facilitatory effects and stimulation location trends within DNIC when using paired broadband noise and DNIC activation with an acoustic-to-DNIC delay of 18 ms that was not observed for an 8 ms delay or DNIC stimulation alone. We will need to perform additional experiments to further assess if greater suppressive effects and location trends across AC can be observed by using delays other than the acoustic-to-AC delay of 4 ms used in this study (e.g., much longer delays) as well as with presenting AC stimulation alone. Nevertheless, AC stimulation with the parameters in this study still induced a much larger percentage of CNIC sites that exhibited suppression versus facilitation (33.1% versus 5.3%) compared to what was observed for DNIC stimulation. This difference in result may reflect the use of different stimulation protocols between the two studies and/or may reflect a truly stronger suppressive effect induced by AC stimulation which activates pathways and neural interactions not accessed by DNIC stimulation. If future studies can show that PN-Stim with AC stimulation more effectively suppresses tinnitus than DNIC stimulation, and considering that AC is more accessible than the DNIC and could be activated via noninvasive stimulation techniques such as TMS or tDCS/tACS, PN-Stim represents an approach that could be available to a much larger patient population.
In future studies, it will be important to assess how long the suppressive effects manifest and evolve over time after the termination of PN-Stim in order to determine the frequency of treatment needed in patients. These effects will likely vary based on the inter-stimulus delay used for PN-Stim, which needs to be systematically investigated in further experiments. It will also be important to assess the effects of PN-Stim on spontaneous activity and other patterns linked to tinnitus (e.g., hypersychrony or temporal firing patterns; (Bauer et al., 2008; Moller et al., 2011; Eggermont and Roberts, 2004)) across the auditory system, especially within AC which is associated with tinnitus perception. In this study, we investigated the effects of PN-Stim on acoustic-driven activity because it was easier to monitor changes in activity compared to the weaker spontaneous activity and it was less prone to errors associated with multi-unit recordings compared to synchrony measures. It is possible that the suppressive effects of PN-Stim on acoustic-driven activity may be more relevant for treating hyperacusis, which is associated with hypersensitivity to different sound inputs and is also in need of an effective treatment. We are planning to investigate these different questions in further animal studies and eventually translate our approach to tinnitus patients using PN-Stim with different invasive and noninvasive cortical stimulation methods. It will be interesting to also investigate if other neurological or psychiatric disorders may benefit from the PN-Stim concept. For example, it may be possible to pair cortical stimulation of the auditory cortex and/or prefrontal cortex with broadband noise to treat auditory hallucinations or to pair somatosensory cortical stimulation with randomized stimulation across multiple body locations to treat phantom limb pain.
Acknowledgments
This work was supported by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (R03-DC011589); the National Institute on Drug Abuse at the National Institutes of Health (T32-DA022616); the National Science Foundation (IGERT DGE-1069104) the University of Minnesota Institute for Engineering in Medicine Walter Barnes Lange Memorial Award; the University of Minnesota Frieda Martha Kunze Fellowship; and start-up funds from the University of Minnesota (Institute for Translational Neuroscience and the College of Science and Engineering).
Footnotes
Competing financial interests
The authors declare no competing financial interests
Contributor Information
Craig D. Markovitz, Email: cdmarkovitz@gmail.com.
Patrick S. Hogan, Email: hogan216@umn.edu.
Kyle A. Wesen, Email: kyle.wesen@gmail.com.
Hubert H. Lim, Email: hlim@umn.edu.
References
- Aitkin LM, Webster WR, Veale JL, Crosby DC. Inferior colliculus. I. Comparison of response properties of neurons in central, pericentral, and external nuclei of adult cat. Journal of neurophysiology. 1975;38:1196–207. doi: 10.1152/jn.1975.38.5.1196. [DOI] [PubMed] [Google Scholar]
- Amato G, La Grutta V, Enia F. The control of acoustic input in the medial geniculate body and inferior colliculus by auditory cortex. Experientia. 1970;26:55–6. doi: 10.1007/BF01900389. [DOI] [PubMed] [Google Scholar]
- Andersen P, Junge K, Sveen O. Cortico-fugal facilitation of thalamic transmission. Brain, behavior and evolution. 1972;6:170–84. doi: 10.1159/000123705. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder RL, Merzenich MM. The topographic organization of corticocollicular projections from physiologically identified loci in the AI, AII, and anterior auditory cortical fields of the cat. J Comp Neurol. 1980;191:479–94. doi: 10.1002/cne.901910310. [DOI] [PubMed] [Google Scholar]
- Astl J, Popelar J, Kvasnak E, Syka J. Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics. Audiology. 1996;35:335–45. doi: 10.3109/00206099609071954. [DOI] [PubMed] [Google Scholar]
- Ayala YA, Malmierca MS. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front Neural Circuits. 2012;6:89. doi: 10.3389/fncir.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–16. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex. 2007;17:475–91. doi: 10.1093/cercor/bhj164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. Journal of neuroscience research. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-D, Stolzberg D, Lobarinas E, Sun W, Ding D, Salvi R. Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hearing research. 2013;295:100–13. doi: 10.1016/j.heares.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Kovacs S, Sunaert S, Menovsky T, van de Heyning P, Moller A. Transcranial magnetic stimulation and extradural electrodes implanted on secondary auditory cortex for tinnitus suppression. J Neurosurg. 2011;114:903–11. doi: 10.3171/2010.11.JNS10197. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus: understanding abnormal and normal auditory perception. Frontiers in systems neuroscience. 2012:6. doi: 10.3389/fnsys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. In: The Central Auditory System. Ehret G, Romand R, editors. New York: Oxford University Press, Inc; 1997. pp. 259–316. [Google Scholar]
- Engelhardt J, Dauman R, Arne P, Allard M, Dauman N, Branchard O, Perez P, Germain C, Caire F, Bonnard D, Cuny E. Effect of Chronic Cortical Stimulation on Chronic Severe Tinnitus: A Prospective Randomized Double-blind Cross-over Trial and Long-term Follow Up. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nat Neurosci. 1998;1:226–9. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Potashner SJ. Evidence for a glutamatergic pathway from the guinea pig auditory cortex to the inferior colliculus. J Neurochem. 1995;65:1348–57. doi: 10.1046/j.1471-4159.1995.65031348.x. [DOI] [PubMed] [Google Scholar]
- Fenoy AJ, Severson MA, Volkov IO, Brugge JF, Howard MA., 3rd Hearing suppression induced by electrical stimulation of human auditory cortex. Brain Res. 2006;1118:75–83. doi: 10.1016/j.brainres.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland DR, Gaggl W, Runge-Samuelson C, Ulmer JL, Kopell BH. Feasibility of auditory cortical stimulation for the treatment of tinnitus. Otol Neurotol. 2007;28:1005–12. doi: 10.1097/MAO.0b013e318159ebf5. [DOI] [PubMed] [Google Scholar]
- Gomez Palacio Schjetnan A, Faraji J, Metz GA, Tatsuno M, Luczak A. Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke Res Treat. 2013;2013:170256. doi: 10.1155/2013/170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Ehret G. Mapping responses to frequency sweeps and tones in the inferior colliculus of house mice. Eur J Neurosci. 2003;18:2301–12. doi: 10.1046/j.1460-9568.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- He J, Yu YQ, Xiong Y, Hashikawa T, Chan YS. Modulatory effect of cortical activation on the lemniscal auditory thalamus of the Guinea pig. J Neurophysiol. 2002;88:1040–50. doi: 10.1152/jn.2002.88.2.1040. [DOI] [PubMed] [Google Scholar]
- Hoekstra CE, Versnel H, Neggers S, Niesten M, van Zanten G. Bilateral Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Auditory Cortex in Tinnitus Patients Is Not Effective: A Randomised Controlled Trial. Audiology and Neurotology. 2013;18:362–73. doi: 10.1159/000354977. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J Acoust Soc Am. 1986;80:1384–91. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- Jen PH, Sun X, Chen QC. An electrophysiological study of neural pathways for corticofugally inhibited neurons in the central nucleus of the inferior colliculus of the big brown bat, Eptesicus fuscus. Experimental brain research. 2001;137:292–302. doi: 10.1007/s002210000637. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, He B. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60:610–24. doi: 10.1109/TBME.2013.2244890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. Tinnitus: models and mechanisms. Hearing research. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Kim HJ, Kim HI, Oh HS, Sim NS, Moon IS. Long-term Effects of Repetitive Transcranial Magnetic Stimulation in Unilateral Tinnitus. The Laryngoscope. 2014 doi: 10.1002/lary.24722. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Ghazanfar AA, Nicolelis MA. Immediate thalamic sensory plasticity depends on corticothalamic feedback. Proc Natl Acad Sci U S A. 1999;96:8200–5. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers DR, de Kleine E, van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci. 2012;6:2. doi: 10.3389/fnsys.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B, De Ridder D. Tinnitus: therapeutic use of superficial brain stimulation. Brain Stimulation E-Book: Handbook of Clinical Neurology (Series editors: Aminoff, Boller, Swaab) 2013;116:441. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- Langner G, Albert M, Briede T. Temporal and spatial coding of periodicity information in the inferior colliculus of awake chinchilla (Chinchilla laniger) Hear Res. 2002;168:110–30. doi: 10.1016/s0378-5955(02)00367-2. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Leong D, Puil E, Schwarz D. Ketamine blocks non-N-methyl-D-aspartate receptor channels attenuating glutamatergic transmission in the auditory cortex. Acta Otolaryngol. 2004;124:454–8. doi: 10.1080/0001648031000692. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant. J Neurophysiol. 2006;96:975–88. doi: 10.1152/jn.01112.2005. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Antidromic activation reveals tonotopically organized projections from primary auditory cortex to the central nucleus of the inferior colliculus in guinea pig. J Neurophysiol. 2007;97:1413–27. doi: 10.1152/jn.00384.2006. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Nunez A. Corticofugal action on somatosensory response properties of rat nucleus gracilis cells. Brain Res. 1998;810:172–80. doi: 10.1016/s0006-8993(98)00920-2. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Cristaudo S, Perez-Gonzalez D, Covey E. Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat. J Neurosci. 2009;29:5483–93. doi: 10.1523/JNEUROSCI.4153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Rees A, Le Beau FE, Bjaalie JG. Laminar organization of frequency-defined local axons within and between the inferior colliculi of the guinea pig. J Comp Neurol. 1995;357:124–44. doi: 10.1002/cne.903570112. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Ryugo DK. In: The Auditory Cortex. Winer JA, Schreiner CE, editors. New York: Springer Science+Business Media, LLC; 2011. pp. 189–208. [Google Scholar]
- Markovitz CD, Tang TT, Edge DP, Lim HH. Three-dimensional brain reconstruction of in vivo electrode tracks for neuroscience and neural prosthetic applications. Front Neural Circuits. 2012;6:39. doi: 10.3389/fncir.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz CD, Tang TT, Lim HH. Tonotopic and localized pathways from primary auditory cortex to the central nucleus of the inferior colliculus. Front Neural Circuits. 2013;7:77. doi: 10.3389/fncir.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massopust LC, Jr, Ordy JM. Auditory organization of the inferior colliculi in the cat. Exp Neurol. 1962;6:465–77. doi: 10.1016/0014-4886(62)90072-9. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res. 2009;257:63–74. doi: 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Tzounopoulos T. Imaging the neural correlates of tinnitus: a comparison between animal models and human studies. Frontiers in systems neuroscience. 2012;6:35. doi: 10.3389/fnsys.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR, Langguth B, De Ridder D, Kleinjung T, editors. Textbook of Tinnitus. New York: Springer Science+Business Media, LLC; 2011. [Google Scholar]
- Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–3. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hear Res. 2013;298:104–8. doi: 10.1016/j.heares.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Mellott JG, Killius J, Storey-Workley ME, Sowick CS, Schofield BR. Ultrastructural examination of the corticocollicular pathway in the guinea pig: a study using electron microscopy, neural tracers, and GABA immunocytochemistry. Frontiers in neuroanatomy. 2013;7:13. doi: 10.3389/fnana.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Offutt SJ, Ryan KJ, Konop AE, Lim HH. Suppression and facilitation of auditory neurons through coordinated acoustic and midbrain stimulation: investigating a deep brain stimulator for tinnitus. Journal of neural engineering. 2014;11:066001. doi: 10.1088/1741-2560/11/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo JF, Kallogjeri D, Nicklaus J, Wineland A, Spitznagel EL, Vlassenko AG, Benzinger T, Mathews J, Garcia KS. Low-Frequency Repetitive Transcranial Magnetic Stimulation to the Temporoparietal Junction for Tinnitus: Four-Week Stimulation Trial. JAMA Otolaryngology–Head & Neck Surgery. 2013;139:388–95. doi: 10.1001/jamaoto.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Redies H, Sieben U, Creutzfeldt OD. Functional subdivisions in the auditory cortex of the guinea pig. J Comp Neurol. 1989;282:473–88. doi: 10.1002/cne.902820402. [DOI] [PubMed] [Google Scholar]
- Ruxton GD. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behav Ecol. 2006;17:688–90. [Google Scholar]
- Ryugo DK, Weinberger NM. Corticofugal modulation of the medial geniculate body. Experimental neurology. 1976;51:377–91. doi: 10.1016/0014-4886(76)90262-4. [DOI] [PubMed] [Google Scholar]
- Saldana E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Volberg G, Frank G, Hadersdorfer J, Steffens T, Weisz N, Landgrebe M, Hajak G, Greenlee M, Classen J, Langguth B. Paired associative stimulation of the auditory system: a proof-of-principle study. PloS one. 2011;6:e27088. doi: 10.1371/journal.pone.0027088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat. II. Topographical organization. J Neurophysiol. 1988;60:1823–40. doi: 10.1152/jn.1988.60.6.1823. [DOI] [PubMed] [Google Scholar]
- Schulz R, Gerloff C, Hummel FC. Non-invasive brain stimulation in neurological diseases. Neuropharmacology. 2013;64:579–87. doi: 10.1016/j.neuropharm.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Ridder DD, Elisevich K, Bowyer SM, Darrat I, Dria J, Stach B, Jiang Q, Tepley N, Ewing J, Seidman M, Zhang J. Direct electrical stimulation of Heschl’s gyrus for tinnitus treatment. Laryngoscope. 2008;118:491–500. doi: 10.1097/MLG.0b013e31815daf5a. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE, Gerstein GL, West DC. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994;369:479–82. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Vanneste S, Van de Heyning P, De Ridder D. Transcranial direct current stimulation in tinnitus patients: a systemic review and meta-analysis. The Scientific World Journal. 2012;2012:427941. doi: 10.1100/2012/427941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka MM, Schendel D, Lim HH. Neural integration and enhancement from the inferior colliculus up to different layers of auditory cortex. J Neurophysiol. 2013;110:1009–20. doi: 10.1152/jn.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka MM, Schmitz S, Lim HH. Response features across the auditory midbrain reveal an organization consistent with a dual lemniscal pathway. Journal of Neurophysiology. 2014 doi: 10.1152/jn.00008.2014. jn. 00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. In: The Auditory Cortex. Winer JA, Schreiner CE, editors. New York: Springer Science+Business Media; 2011. pp. 513–33. [Google Scholar]
- Suga N, Ji W, Ma X, Tang J, Xiao Z, Yan J. In: Auditory and Vestibular Efferents. Ryugo DK, et al., editors. New York: Springer Sciences+Business Media; 2011. pp. 313–52. [Google Scholar]
- Suta D, Kvasnak E, Popelar J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J Neurophysiol. 2003;90:3794–808. doi: 10.1152/jn.01175.2002. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J. Inferior colliculus in the rat: neuronal responses to stimulation of the auditory cortex. Neurosci Lett. 1984;51:235–40. doi: 10.1016/0304-3940(84)90557-3. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Kvasnak E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Experimental brain research. 2000;133:254–66. doi: 10.1007/s002210000426. [DOI] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci. 2007;27:6091–102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Zurita P, Pedemonte M, Velluti RA. Auditory cortical efferent actions upon inferior colliculus unitary activity in the guinea pig. Neurosci Lett. 1998;249:172–6. doi: 10.1016/s0304-3940(98)00367-x. [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Ridder D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: an overview. Neuromodulation: journal of the International Neuromodulation Society. 2012;15:350–60. doi: 10.1111/j.1525-1403.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res. 2000;132:445–56. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yanagisawa K, Kanzaki J, Katsuki Y. Cortical efferent flow influencing unit responses of medial geniculate body to sound stimulation. Experimental Brain Research. 1966;2:302–17. doi: 10.1007/BF00234776. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang Y, Yan J. The neurobiology of sound-specific auditory plasticity: a core neural circuit. Neuroscience and biobehavioral reviews. 2009;33:1178–84. doi: 10.1016/j.neubiorev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Yan J, Ehret G. Corticofugal modulation of midbrain sound processing in the house mouse. Eur J Neurosci. 2002;16:119–28. doi: 10.1046/j.1460-9568.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Ehret G. Corticofugal shaping of frequency tuning curves in the central nucleus of the inferior colliculus of mice. J Neurophysiol. 2005;93:71–83. doi: 10.1152/jn.00348.2004. [DOI] [PubMed] [Google Scholar]
- Yan W, Suga N. Corticofugal modulation of the midbrain frequency map in the bat auditory system. Nat Neurosci. 1998;1:54–8. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- Zhang J. Auditory cortex stimulation to suppress tinnitus: mechanisms and strategies. Hear Res. 2013;295:38–57. doi: 10.1016/j.heares.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bats. J Neurophysiol. 2000;84:325–33. doi: 10.1152/jn.2000.84.1.325. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature. 1997;387:900–3. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]