Abstract
Multiple organ systems, including the gastrointestinal tract, pancreas, and hepatobiliary systems, are affected by cystic fibrosis (CF). Many of these changes begin early in life and are difficult to study in young CF patients. Recent development of novel CF animal models has expanded opportunities in the field to better understand CF pathogenesis and evaluate traditional and innovative therapeutics. In this review, we discuss manifestations of CF disease in gastrointestinal, pancreatic, and hepatobiliary systems of humans and animal models. We also compare the similarities and limitations of animal models and discuss future directions for modeling CF.
Keywords: cystic fibrosis, gastrointestinal tract, liver, pancreas, cystic fibrosis transmembrane conductance regulator
cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CF transmembrane conductance regulator (CFTR) (97, 101, 129). CFTR is an anion channel that transports Cl− and HCO3− and, thereby, regulates fluid secretion (22, 48, 108). While there are many theories about mechanisms of CF disease pathogenesis (18, 29, 92, 122), the fundamental lack of CFTR function produces luminal secretions with reduced pH and fluid volume (91). These changes in luminal environment are thought to biophysically and functionally alter mucus and proteinaceous secretions that obstruct and alter function in various organs (47, 60, 114, 122).
CF research has benefited from animal models; historically, this has been limited to mice with multiple CFTR mutations, which are summarized elsewhere (54, 130). CFTR-deficient mice have intestinal disease but lack characteristic pancreatic and lung phenotypes commonly seen in humans (54, 94, 111). Even so, mouse models have been useful. Recently, researchers developed mouse models with conditional, cell-specific CFTR expression, and such novel approaches may be useful to further dissect CF pathogenesis (16, 58, 59). Because CF mice lacked a lung phenotype similar to that of humans, new model species were recently developed to study CF (Table 1). Targeted mutations in CFTR have been described in at least four mammalian species, including mouse (111), pig (100), ferret (121), and, most recently, rat (124), with other species being considered.
Table 1.
CFTR mutations recently developed in novel CF animal models
| Species |
|||
|---|---|---|---|
| Mutation | Pig | Ferret | Rat |
| CFTR−/− | 2008 (100) | 2010 (121) | 2014 (124) |
| CFTRΔF508/ΔF508 | 2011 (87) | ||
| CFTR−/−;iFABP-CFTR (“gut-corrected”) | 2013 (115) | 2010 (121) | |
Data represent year of publication, with reference number in parentheses.
CFTR-deficient animal models offer several advantages for study of CF pathogenesis and disease. 1) Study of early CF pathogenesis is limited in young CF infants and children because of appropriate ethical concerns. Animal models can be studied early in life, prior to significant remodeling and secondary features (e.g., inflammation and destruction) that can confound the interpretation. 2) Animal models allow for controlled comparison of organ systems in the study of CF pathophysiology and in response to treatments. CF patients often receive multiple prophylactic and therapeutic treatments, all of which could confound organ-specific interpretations. 3) Comparison of the spectrum of CF disease between species can lead to a better understanding of CF disease pathogenesis and/or identify candidate modifier genes.
We review CF pathophysiology and disease of gastrointestinal, pancreatic, and hepatobiliary systems. For context, CF disease in humans is presented first, followed by contemporary CF animal models.
Gastrointestinal System
CFTR expression.
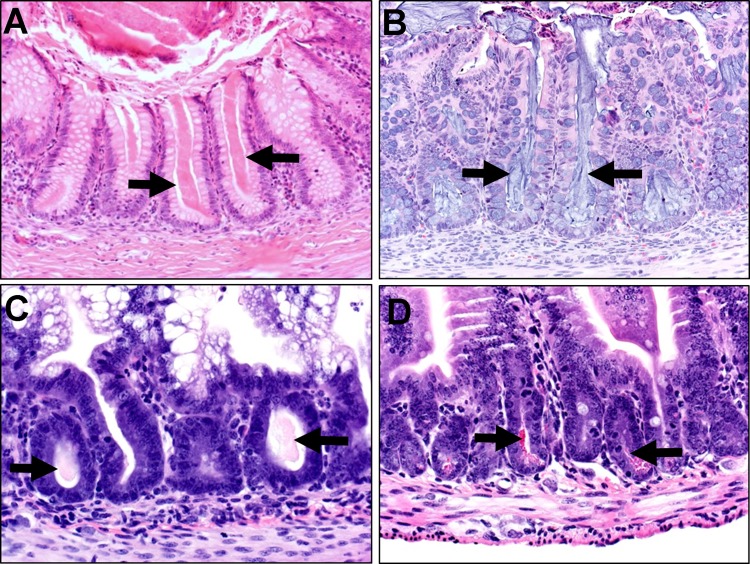
CFTR expression in the human gastrointestinal tract is defined by anatomic location and, often, relevant function (117). In the stomach, mRNA expression is low throughout the mucosa. At the entrance to the small intestine, robust CFTR expression is found in Brunner's glands, consistent with the immediate requirement for bicarbonate-rich secretions to buffer acidic ingesta. In the small intestine, the expression gradient decreases from duodenum to ileum; in the colon, levels of expression are moderate. In the small intestine and colon, CFTR mRNA expression levels are highest in the crypts, and the expression gradient decreases toward cells extending into the lumen. In the small intestine, scattered high-expressing cells have been described in the duodenum and jejunum, but the relevance of these is not clear. The high expression of CFTR in the crypts corresponds with the anatomic location of the mucoproteinaceous secretions obstructing and distending the crypts, a diagnostic feature of CF intestinal disease in humans and CF animal models (Fig. 1) (15, 54, 79, 120).
Fig. 1.
Hematoxylin-eosin-stained sections of cystic fibrosis (CF) colon (A and B) and small intestine (C and D). Absence of CF transmembrane conductance regulator (CFTR) in the crypts can result in multifocal plugging/obstruction (arrows). A: CF infant, 4 mo old. B: CF pig, newborn. C: CF ferret, 2.5 wk old. D: CF mouse, 1 mo old (courtesy of Lynda Ostedgaard, University of Iowa).
Intestinal obstruction.
Meconium ileus, an intestinal obstruction in ∼15–20% of CF births, is often detected in the first 48 h following birth (Fig. 2) (41, 86, 127). Typically, the obstruction is located in the distal ileum to proximal colon, on either side of the ileocecal junction. Immediately proximal to the obstruction, the bowel is distended by abnormally sticky and tenacious meconium, and distal to the obstruction the bowel is of small caliber (“microcolon”), with multifocal luminal casts (“cords” or “pellets”) consisting of mucous, proteinaceous, and cellular debris (5). Abnormal intestinal secretions are believed to play an important role in pathogenesis of the intestinal obstruction (20, 96), but the exact mechanism is not well defined. Interestingly, recent studies in the CF pig lung suggest that abnormal mucus may not be able to detach properly from its site of origin (60). It can be speculated that a similar mechanism in the intestine could possibly contribute to the development of intestinal obstruction.
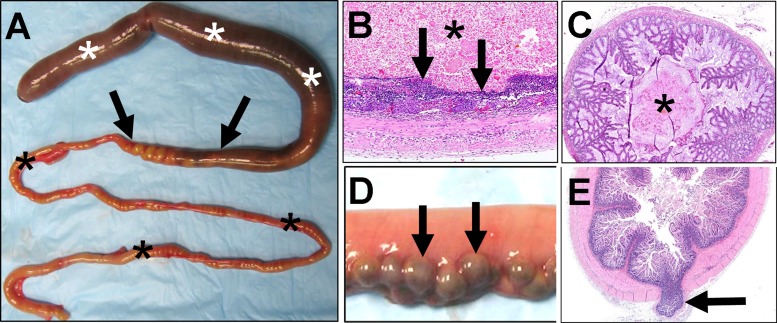
Fig. 2.
Meconium ileus in newborn CF pig model. A: meconium ileus obstruction in distal small intestine. Proximally, intestine is distended by sticky meconium (white asterisks). Site of obstruction (arrows) represents an abrupt transition from dilated meconium-filled bowel to small meconium-devoid bowel. Distal to obstruction (black asterisks), intestine is of small caliber (“microcolon”). B: meconium (asterisk) proximal to obstruction (white asterisks in A) can cause distension and thinning (arrows) of intestinal mucosa and, in severe cases, hemorrhage and necrosis, leading to perforation. C: microcolon distal to the obstruction can have multifocal cords (“casts,” “pellets”; asterisk) composed of mucus, protein, and cellular debris. D: proximal to obstruction, diverticula can be detected (arrows). E: diverticula are mucosal herniations through the tunica muscularis (arrow). Sections in B, C, and E were stained with hematoxylin-eosin.
In meconium ileus, approximately half of the cases are “complicated” by atresia, perforations, adhesions, pseudocyst formation, or generalized peritonitis, requiring surgical intervention (5, 96, 110), whereas the remainder are considered “uncomplicated” (81, 86). A recent review of 59 cases of meconium ileus reported that Gastrografin (Bracco Diagnostics, Anjou, PQ, Canada) enemas were successful in only ∼9% (5 of 19) of cases and laparotomy was required for 91% (54 of 59) of cases (41). In 3 of these 54 cases, major surgical approaches, including stoma formation (72%), enterotomy with washout (13%), or resection and anastomosis (15%), were employed. All cases received N-acetyl cysteine until enteral feeding was resumed. With improved medical and surgical therapies, most patients with meconium ileus survive the neonatal obstruction.
Distal intestinal obstruction syndrome (DIOS), also called “meconium ileus-equivalent,” is an intestinal obstruction that occurs at a rate of ∼6.2 events per 1,000 patient years and can be seen at any age after the newborn period. The obstruction is located on either side of the ileocecal junction and can generally appear similar to meconium ileus, but without meconium filling the bowel (61). Coincidence rates of meconium ileus and later DIOS presentations have been reported to be 44% (61), and it has been suggested that a history of meconium ileus is a significant risk factor for the necessity of surgical intervention for DIOS cases (118). Genetic analysis indicates that DIOS development is influenced by environmental factors, and not by modifier genes like meconium ileus (13, 61). Consensus diagnosis of DIOS in CF patients has recently been categorized as either impending or complete. Impending DIOS is characterized by the presence of a viscid fecal mass in the ileocolon, along with abdominal pain/distension. Complete DIOS has, in addition to impending DIOS, evidence of complete obstruction, such as bilious vomiting and/or fluid levels in abdominal radiographs (61, 127). In a retrospective study, DIOS medical therapy with Gastrografin (oral or enema administration) was reported to be successful in 71% of cases, with the remainder of the cases requiring laparotomy (41).
Constipation is clinically less severe than DIOS and is reported to occur in 47% of patients, but this condition may be underrecognized in CF patients (126, 127). Diagnostic definitions of constipation include 1) abdominal pain and/or distension or 2) reduced frequency and/or increased consistency of bowel movements and 3) improvement of these symptoms with the use of laxatives. In CF animal models, diagnostic parameters and techniques to distinguish DIOS from constipation are lacking. Accordingly, discussion of intestinal obstructions that occur after the 1st wk of life will be referred to as “DIOS/constipation,” unless otherwise specified. Gastrointestinal manifestations in CF are summarized in Table 2.
Table 2.
Gastrointestinal manifestation of CF disease in humans and animal models
| Species |
|||||
|---|---|---|---|---|---|
| Manifestation | Human | Mouse | Pig | Ferret | Rat |
| Meconium ileus obstruction | ∼15% incidence (41, 86, 127) | Up to 3-fold increased mortality compared with controls in first few days of life (54, 94, 111) | 100% incidence (87, 100) | 75% incidence (121) | 0%* (124) |
| DIOS obstruction/constipation | DIOS incidence: 6.2 events per 1,000 patient yr (61) Constipation: ≤47% of CF patients (126) | ∼0–100% mortality weeks after weaning, dependent on background (54, 94, 111) | Prophylaxis treatment with some “episodes” (113) | Prophylaxis treatment with some “episodes” (119, 121) | ∼70% mortality due to intestinal obstruction by 6 wk of age (124) |
| Diverticula | 7- to 14-fold increased risk (50) | NR | Incidence may be proportional to meconium ileus severity (79) | NR | NR |
| Atresia | CF infants have >200-fold increased risk (99) | NR | Incidence may be proportional to meconium ileus severity (79) | NR | NR |
| GERD | ∼35–80% incidence (106, 132) | NR | NR, but treated as prophylaxis (87, 113) | NR, but treated as prophylaxis (121) | NR |
| Motility | Delayed transit with prominent intestinal smooth muscle (15, 49, 55, 134) | Increased peak isometric force and increased relaxation in CF intestines with increased smooth muscle (98) | Increased size of tunica muscularis from duodenum to colon (79) | NR | NR |
| Dysbiosis | Genotype and disease severity influence dysbiosis; SIBO also reported (36, 71, 72, 104) | SIBO/dysbiosis reported, may alter motility and inflammation; defective antimicrobial peptides from crypt may accentuate (25, 32, 33, 75) | NR | Dysbiosis/SIBO influenced by environment, and not genotype (119) | NR |
| Rectal prolapse | Historically ≤20%, especially in young children (5, 15) | NR | NR | Up to 30% at ∼1 mo of age (119) | NR |
DIOS, distal intestinal obstruction syndrome; GERD, gastroesophageal reflux disease; NR, not reported; SIBO, small intestinal bacterial overgrowth.
Based on preliminary phenotypic study.
MOUSE MODEL.
In 1992, the first CF mouse model was described, and intestinal disease was the major defect in the CF mouse phenotype (111). Incidence of intestinal obstruction in CF mice is highly variable and is dependent on genetic background, CFTR mutation, and also dietary factors (54). In CF mice, the rate of mortality can be increased up to threefold compared with control mice in the first few days of life, and this increase in mortality rate is attributed to intestinal obstruction (i.e., meconium ileus) (54, 94, 111). Thereafter, disease is generally subclinical until weaning, when a significant wave of mortality occurs; this is again attributed to intestinal obstruction (i.e., DIOS/constipation). Dietary supplementation of a 6% polyethylene glycol-and-electrolyte solution significantly increased the survival rates postweaning (53). A common pathological finding in small intestinal crypts of laxative-treated and untreated CF mice is crypt filling and distension by Paneth cell cryptins, an α-defensin antimicrobial peptide (25).
One of the historic advantages of mouse models has been the ability to control genomic and tissue-specific expression of target genes. Ectopic CFTR expression in intestinal epithelium of CF mice leads to mitigation of intestinal obstruction (88, 133). Furthermore, a recent conditional CFTR expression model demonstrated that intestinal epithelium was a significant regulator of intestinal obstruction, but not of weight loss, even though CF mice lack pancreatic disease (59). As another example, genetic analysis has shown that methionine sulfoxide reductase A gene (MSRA) is a modifier gene of meconium ileus in human infants. This premise was validated using CF mice with wild-type, heterozygous, or mutated MSRA. In mice deficient in both CFTR and MSRA, severity of intestinal obstruction was reduced, suggesting that therapeutic modifications to reduce MSRA expression may be protective in CF (57). The variation of phenotypes seen in humans or in mice with the same CFTR mutation has raised questions about alternative anion channels that may modulate the CF mouse phenotype (54). A recent evaluation of candidate gene expression profiles in two strains of CF mice did not identify genes that might rescue CFTR mutation but did identify one candidate gene product (mTTYH3) that was consistently downregulated in CF and was speculated to possibly increase severity of CF disease (19).
PIG MODEL.
Genetically engineered CF pigs were first reported in 2008 (100). At birth, 100% of CF pigs with homozygous null or ΔF508 CFTR mutations had meconium ileus (87, 100). The obstruction was characterized proximally by dilated meconium-filled bowel and distally by small-caliber to atretic bowel containing mucus changes and multifocal mucocellular cords (“casts,” pellets) (79). The obstruction was often located in the distal small intestine or proximal spiral colon (79) and was unresponsive to medical therapy (laxatives, enemas, supportive care) but often complicated by small-caliber bowel, atresia, perforation, and/or peritonitis (79, 87, 113). Interestingly, the site of obstruction was dynamic and could sometimes transition distally after oral feeding, as demonstrated by the accumulation of mucocellular cords in the colon, similar to that reported in humans (79, 110). Since CF pigs required surgery for meconium ileus and were similar in size to a small infant, the CF pig has become a useful model for pediatric surgeons to optimize surgical techniques (ileostomies, cecostomies) in the management and postoperative care of complicated meconium ileus cases (87, 113). Furthermore, improved knowledge and surgical skills gained from this spontaneous obstruction model can be applied as a learning tool for other types of intestinal obstruction in which animal models are sparse and often artificially induced (90). Recently, the generation of a CFTR−/− pig model with intestinal-specific CFTR expression under the intestinal fatty acid-binding protein promoter alleviated the meconium ileus. Interestingly, correction of meconium ileus obstruction requires only ∼20% of wild-type CFTR expression levels, which gives a target threshold for future gene therapy interventions (115).
In CF pigs, DIOS/constipation was prophylactically treated with daily oral administration of polyethylene glycol (113). In some cases, CF pigs had reduced stools and became anorexic, suggestive of postnatal intestinal obstruction. Supportive treatments with enemas and increased oral laxatives with fluids have been used to correct the situation. Surgical treatment was not pursued in refractory cases because of the requirement to evaluate respiratory disease in this model and to avoid potentially confounding lung disease with surgical complications.
FERRET MODEL.
A CFTR−/− ferret model (CF ferret), first reported in 2010, had a 75% incidence of meconium ileus at birth (121). While CF ferrets lack a macroscopically distinct landmark for the ileocecal junction, the site of the meconium ileus in CF kits was usually located within the distal third of the length of the small and large intestine, resulting in a 50% perforation rate. Meconium dilation and distal microcolon were similar to human infants. Interestingly, evaluation of breeding animals showed that the genetic background of the hob (paternal male) had a significant influence on the incidence of meconium ileus, suggesting the presence of genetic modifiers. Intestinal expression of CFTR using a fatty acid-binding protein promoter has also been reported to alleviate intestinal obstruction in the CF ferret (121). DIOS/constipation is reportedly treated prophylactically in ferrets, but occasional DIOS/constipation episodes still occurred and were successfully managed medically (119).
RAT MODEL.
In 2014, a CFTR−/− rat (CF rat) model was initially characterized (124). At birth, CF rats lack meconium ileus, but by 2 wk of age they start to have reduced weight gain and begin to die of intestinal obstruction (DIOS/constipation) after weaning. By 6 wk of age only ∼30% of the CF rats survive. Use of GoLYTELY (Braintree Laboratories) significantly improves the survival to ∼64%. Intestinal histology showed cell sloughing and crypts dilated with a mucoid substance. While the CF rat has a DIOS/constipation phenotype, the pancreas and liver appear normal.
Diverticulosis.
CF patients have a 7- to 14-fold increased risk of diverticulosis, which has been observed in the vermiform appendix and in the colon (11, 50). In the general population, diverticulosis/diverticulitis does not become apparent until approximately the fourth decade of life; however, in one study of CF patients, diverticula were detected in 8 of 57 (14%) 6- to 22-yr-old (median 14.5 yr) patients. Diverticula are proposed to form from chronic/repeated exposure to luminal distension and pressure, causing herniation of the mucosa/submucosa through the tunica muscularis at the weakest muscular sites, i.e., sites of vascular entry along the mesenteric border (50, 77). The total incidence in CF patients from autopsy studies is likely underestimated, as diverticula can be difficult to discern during pathological examination.
ANIMAL MODELS.
Diverticula, as a consequence of CF disease, have only been reported in CF pig models. This may be a reflection of the severity and/or chronicity of these intestinal obstructions. At birth, CF pigs frequently have diverticula along the mesenteric border, suggesting that they formed in utero as a result of increased luminal pressures from the meconium ileus obstruction (79). Development of diverticula seems to be related to increased severity of meconium ileus in CF pigs, and when meconium ileus was not present, such as in the CF pig model with intestinal correction of CFTR expression, intestinal diverticula were absent (115).
Atresia.
CF infants have increased (>200 times) risk for intestinal atresia. The mechanisms of intestinal atresia are poorly understood. Theories include intestinal ischemia from meconium ileus or malformations during fetal development (99). Volvulus, reported in up to 50% of meconium ileus cases, is suggested as a possible contributing cause of intestinal ischemia during development (2).
ANIMAL MODELS.
Atresias have only been reported in the CF pig model. Atretic bowel is associated with meconium ileus and, when present, is consistently found distal to the obstruction (79, 100). Atresia in CF pigs seems to be related to the severity of meconium ileus obstruction, and, in pigs with intestinal CFTR correction, atresias are lacking (115).
Gastroesophageal reflux disease.
CF patients, including children, are prone to acidic gastroesophageal reflux disease (GERD), with incidence rates ranging as high as 35–80% (106, 132). A comparison of GERD patients suggested that those with CF did not have an increased frequency of reflux but, rather, had decreased ability to buffer and restore esophageal pH following reflux events. This may be a result of hyperacidity of the gastric fluids (132) or inadequate bicarbonate-rich secretions in saliva and/or esophageal glands (22, 56, 80). Additionally, the proximal small intestine has a diminished ability to buffer acidified gastric contents (49), likely a result of decreased bicarbonate-rich secretions from Brunner's glands, pancreas, and, to a lesser extent, intestinal epithelium. CF patients are often placed on proton pump inhibitors or H2 antagonists, in part, to mitigate the GERD symptoms (21). The use of proton pump inhibitors to mitigate gastric pH has been recently questioned because of potential for adverse changes in CF lung disease (21, 37).
ANIMAL MODELS.
Clinical signs of GERD have not been defined in animal models of CF. Even so, CF pigs and ferrets typically follow a postnatal protocol that includes proton pump inhibitors or H2 blocker principally to mitigate their susceptibility to gastric ulceration (87, 113, 121), but also to minimize potential for GERD-related disease. In contrast, these therapies are not routinely used in studies involving CF mice and rats.
Motility.
Alterations in intestinal motility can contribute to intestinal disease phenotype. Delays in transit time in the CF intestine, especially the small intestine, have been reported when traditional H2 breath tests (8) and, more recently, techniques such as a magnet-based motility-tracking system (55) and wireless motility capsule (49) were used. During end-stage lung disease, the esophagus and stomach of CF patients are also reported to have similar delayed transit (44). Histopathological examination of the intestine from CF patients at autopsy reported prominent smooth muscle (i.e., tunica muscularis) in the intestinal wall (15, 134), which could be a marker of an adaptive change or even a primary smooth muscle phenotype.
ANIMAL MODELS.
Among the CF animal models, only in mice and pigs have alterations in intestinal motility or smooth muscle phenotypes been reported. Ex vivo ileal smooth muscle from CF mice exhibited increased peak isometric forces following stimulation, as well as increased relaxation following isoproterenol administration, compared with non-CF controls. Smooth muscle mass in the wall of the ileum was also variably increased, and many of these changes were specific to background strain, suggesting a role for modifier genes (98). CF pigs also have an intestinal smooth muscle phenotype with increased thickness of the tunica muscularis from the duodenum to the colon (79). Since these changes are found at birth, bacterial regulation is not likely the cause but could possibly contribute to the phenotype postnatally.
Dysbiosis.
Intestinal dysbiosis is defined as a change in the microbiome and/or bacterial populations of the intestine. A recent study of CF patients found that changes in fecal bacterial populations were associated with both genotype and clinical disease severity. Homozygous ΔF508 mutations or CF patients with severe disease exhibited an increase in the growth of pathogenic bacterial populations (e.g., Escherichia coli) and a decrease in the presence of beneficial bacterial populations (e.g., Bifidobacterium spp) (104). Another recent study suggested that probiotic supplementation can mitigate clinical gastrointestinal disease and significantly reduce pathogenic proteobacterial populations (36). Small intestinal bacterial overgrowth (a form of dysbiosis) is also recognized in CF, but any link with intestinal inflammation is not yet clear (71, 72).
ANIMAL MODELS.
Much of the modeling of CF dysbiosis has been performed in the mouse and, more recently, the ferret model. CF mice have reduced diversity of beneficial bacteria (e.g., Lactobacilliales members) and increased pathogenic bacteria (e.g., Bacteroides fragilis), which are associated with intestinal inflammation and immunomodulation. The increases in Enterobacteriaceae and Clostridiaceae in CF mice are speculated to have a negative effect on regulation of growth of beneficial bacteria (75). Another study found that cryptins (α-defensins from Paneth cells) and bacterial numbers were increased in CF mice, but even with increased activated cryptin protein levels, the capacity to kill cryptin-sensitive bacteria was significantly reduced in intestines from CF mice (25). This may be due to the poor dissolution and entrapment of cryptins in the villus crypts (25). Additionally, deficient bicarbonate transport in CF mice (48) could produce an abnormally acidic microenvironment that could potentially dampen the efficacy of endogenous antimicrobials (1). In CF mice, intestinal microbiota can modulate intestinal motility, as evidenced by treatment with antibiotics or laxatives to flush out enteric bacteria; both can normalize intestinal motility (33). Additionally, the postnatal onset of the smooth muscle phenotype in mice directly correlated with the development of bacterial overgrowth. The influence of bacteria appears to be related to inflammation regulated through phospholipase A genes, which suggests a prostaglandin-mediated effect (32).
In the ferret, diversity of fecal bacteria appears to be genotype-independent between CF and non-CF pairs, suggesting that the environment plays an important role in the intestinal microbiome (119). Bacterial overgrowth is common in CF ferrets and characterized by a dilated, ingesta-filled small intestine. Microscopically, blunted villi lined by abundant bacteria were common, along with lymphoplasmacytic inflammation. Aerobic and anaerobic titers from the small intestine of CF ferrets were significantly higher; several types of bacteria distinguished CF from non-CF ferrets (119).
Rectal prolapse.
Rectal prolapse was historically seen in up to 20% of CF patients, with a preferential incidence in the first few years of life (5, 15). Predisposing factors may include weakened muscular tone of the pelvic floor, numerous bulky stools, and respiratory disease (e.g., increased intra-abdominal pressure from persistent/recurrent cough). Conservative medical therapy aimed at mitigating the inciting cause is often successful, but surgical therapies may be required in refractory cases (109).
ANIMAL MODELS.
Rectal prolapse is a common finding in CF ferrets, with up to 30% affected (119), but is not described in other CF animal models. Rectal prolapse in CF animals occurs within a very narrow window at ∼1 mo of age. Proactive laxative and pancreatic enzyme therapy has been found to be effective in treating the majority of the affected CF ferrets.
Cancer.
CF patients have an overall low cancer burden compared with the general population (82). However, long-term studies have suggested that CF may predispose patients to specific cancers. For instance, CF patients have increased incidence of digestive tract cancers, and this risk is further elevated following lung transplantation (76, 82). Increased incidence of testicular cancer and lymphoid leukemias has also been reported in CF patients (76).
Studies that validate a predisposition to cancer in mouse models and the more recent CF animal models (e.g., pig, ferret, rat) are lacking. The CF pig, ferret, and rat models are relatively novel; thus, aging studies to assess cancer incidence have yet to be reported. CF mice have intestinal disease characterized by a variety of histological changes, including increased cell proliferation (47a), but whether these changes contribute to eventual cancer development in the model remains unclear.
Future directions.
All the species with targeted CFTR disruption demonstrate gastrointestinal phenotypes, most notably intestinal obstruction. Better treatments and prophylaxis for intestinal obstructions are an obvious target goal for the animal models and require better understanding of the molecular mechanisms initiating the obstruction. Furthermore, CF animal models will be useful toward understanding the intestinal microbiome and its effects on gastrointestinal function, as well as overall health status (e.g., lung disease) of CF patients. Lastly, emerging technologies such as organoids may be useful for study of intestinal CF disease (66, 73, 78). These cultured cell systems are a hybrid of in vivo and in vitro models that produce small cellular aggregates with cellular differentiation, three-dimensional structure, and physiological properties that resemble specific organs or tissues. While organoid research is in its early stages, it has potential for use in study of CF pathogenesis as well as testing the efficacy and safety of novel therapeutics.
Liver/Gallbladder
The incidence of CF liver disease (CFLD) is 5–10%, and it results in 2.5% of the overall mortality in CF patients (28). The mean age of diagnosis of CFLD is 10 yr (65, 105). A spectrum of hepatobiliary diseases, including focal biliary cirrhosis, multilobular biliary cirrhosis, and portal hypertension, are defined as CFLD; the most common is focal biliary cirrhosis. For the diagnosis of CFLD, at least two of the following criteria are required: hepatomegaly, abnormalities in liver function tests [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] over a 12-mo period, ultrasonographic evidence of liver involvement, or portal hypertension (34). Elevations in AST, ALT, and γ-glutamyltransferase are not predictive of liver disease and/or fibrosis, and up to 41% of children with CF will have abnormalities in liver transaminases by the time they reach 12 yr of age (65). Hepatic steatosis, likely associated with nutritional deficiencies, is common in CF patients and has not proven to be associated with cirrhosis in CF patients (28).
The pathogenesis of CFLD is complex and not well defined. CFTR is expressed in cholangiocytes (bile duct cells), and not in other cells of the liver (27). Dysfunction of CFTR results in abnormal biliary sections and decreased bile flow, which leads to thickening of the bile and formation of plugs within the bile ducts. Bile plugging/obstruction is present focally to multifocally, and not in every duct. Duct obstruction results in upregulation of chemokines (93), activation of hepatic stellate cells (68), and peribiliary fibrogenesis, which is characteristic of CFLD. In a subset of patients, focal biliary cirrhosis progresses to multilobular biliary cirrhosis and portal hypertension.
Early diagnosis of CFLD is confounded by the focal nature of the disease. Ultrasound can detect changes associated with cirrhosis but does not reliably detect early stages of fibrosis. A recent study demonstrated that fibrosis staging on liver biopsy predicted the development of clinically significant CFLD and advised that detection was improved when two biopsy core samples were obtained for histopathological evaluation (69). Because ∼40% of CF patients exhibit liver function test abnormalities but only a very small percentage develop cirrhosis and hypertension, other factors must be involved in the pathogenesis of CFLD. No specific CFTR mutation has been associated with the occurrence or severity of liver disease; however, CF patients with the α1-antitrypsin (SERPINA1) Z allele are at increased risk of CFLD, indicating that modifier genes likely play a role in pathogenesis (9).
There is no effective treatment for CFLD. The use of ursodeoxycholic acid (UDCA) is recommended in the best-practice guidelines for the management of CFLD, but the effectiveness of the treatment has been controversial (23, 34, 83). Liver transplantation may be considered for CFLD patients, but there are no guidelines for the optimal timing for transplantation.
Neonatal cholestasis is an uncommon early manifestation of liver disease. Leeuwen et al. (67) reported cholestasis in 5.7% (23 of 401) of infants and a higher incidence of cholestasis in infants with meconium ileus than in those without meconium ileus. They found that cholestasis resolved in all the children and was not associated with the later development of CFLD (67). Even without clinical liver disease or evidence of cholestasis at biopsy, liver function tests can be elevated in the majority of pediatric cases during the first 2–3 yr of life (70).
CFTR is also expressed in gallbladder epithelial cells. Gallbladder abnormalities, including microgallbladder and gallbladder dysfunction, are found in 24–50% of CF patients (45, 131). No treatment is required for microgallbladder.
Mouse models.
The majority of CF mouse models do not have liver changes, but when such changes are present, they are often mild. As previously discussed, the severity of the intestinal manifestations often limits the survival of CF mice. In a long-term study, CF mice demonstrated liver changes that progressed with aging. At the culmination of the study at 12–24 mo, CF mice developed focal biliary cirrhosis, with some showing lobular cirrhosis very similar to CF patients (39). CFTRΔF508/ΔF508 mice exhibit mild histopathological liver disease, characterized by mild and patchy bile ductular proliferation and very mild fibrosis (46).
Although CF mouse models have minimal liver disease, they have been utilized to investigate bile acid flow and bile acid metabolism. CF mice are reported to have enlarged gallbladders with emptying defects that can contribute to altered bile flow and shunting. Expression of fibroblastic growth factor 15, a key hormone regulating gallbladder filling, was decreased in the ileum (a normal site of expression) but overexpressed in the gallbladder, suggesting a possible mechanism contributing to CFLD (35). Additionally, increased hydrophobicity of the bile salt is thought to be more toxic to the hepatobiliary system, thereby causing injury (10). CFTRΔF508/ΔF508 mice have more hydrophobic bile acid and reduced bile salt-dependent bile flow, possibly contributing to biliary epithelial injury (46). In a dextran sulfate sodium-induced colitis model, CFTR−/− (C57BL/6J-Cftrtm1Unc) mice had increased biliary epithelial cell damage in response to endotoxin administration compared with controls and had an exaggerated innate immune response mediated by Toll-like receptor 4 and NF-κB (42). This could suggest that targeting inflammatory processes may be a potential therapy for CFLD.
Given the controversial nature of UDCA treatment in CF patients, several CF mouse studies have investigated mechanisms of UDCA therapy. Chronic UDCA treatment in CF mice reduced the hydrophobicity of the bile salt pool and stimulated bile flow independent of CFTR (14). These results support a potential beneficial effect of UDCA on bile flow and bile salt metabolism in CF conditions; however, beneficial effects in CF patients remain controversial.
Pig.
Neonatal CF pigs have focal biliary cirrhosis with portal inflammation, biliary hyperplasia, and mild fibrosis (79). With age, multilobular cirrhosis can develop (115). Microgallbladder was present in all CF pigs, with diffuse epithelial mucinous change and cystic epithelial proliferation in some cases (79). In microgallbladders, the lumen was plugged with thick bile/mucus material. Liver and gallbladder lesions in newborn CFTRΔF508/ΔF508 pigs were similar to those in CFTR−/− pigs (87). The pH of gallbladder bile was significantly lower in CF neonatal pigs (125), consistent with loss of bicarbonate transport.
Ferret.
Neonatal ferrets demonstrate early abnormalities in liver enzymes without histological evidence of cholestasis, similar to CF infants (11, 67). Treatment with UDCA resulted in normalization of these values. CF ferrets and controls had lymphocytic portal inflammation but no evidence of biliary proliferation or fibrosis (15). Gallbladder changes were not evident at birth, but as CF ferrets aged, dark gallbladders were observed on gross examination in several animals, and thick bile was present within the lumen. Histologically, in the majority of older CF ferrets, mucinous change and cystic proliferation were observed in gallbladder epithelium (15). However, unlike the CF pig, gallbladder bile pH was not significantly lower in newborn CF ferret than non-CF control (43).
Future directions.
Future goals utilizing animal models will be directed at the prevention or reversal of liver changes to mitigate the potential development of multilobular cirrhosis. Development of diagnostics for early detection of liver disease and understanding factors that result in increased susceptibility of CF patients would also be valuable.
Exocrine Pancreas
The pancreas is one of the organs most commonly affected in CF. CFTR is highly expressed in ductal epithelial cells and functions to transport fluid and anions into the ductal lumen. The pancreas secretes large quantities of digestive enzymes, along with alkaline fluid. Altered fluid and anion transport in CF results in reduced luminal fluid volume, decreased pH, and hyperconcentration of macromolecules, which obstruct and damage exocrine acini (Fig. 3) (38, 103).
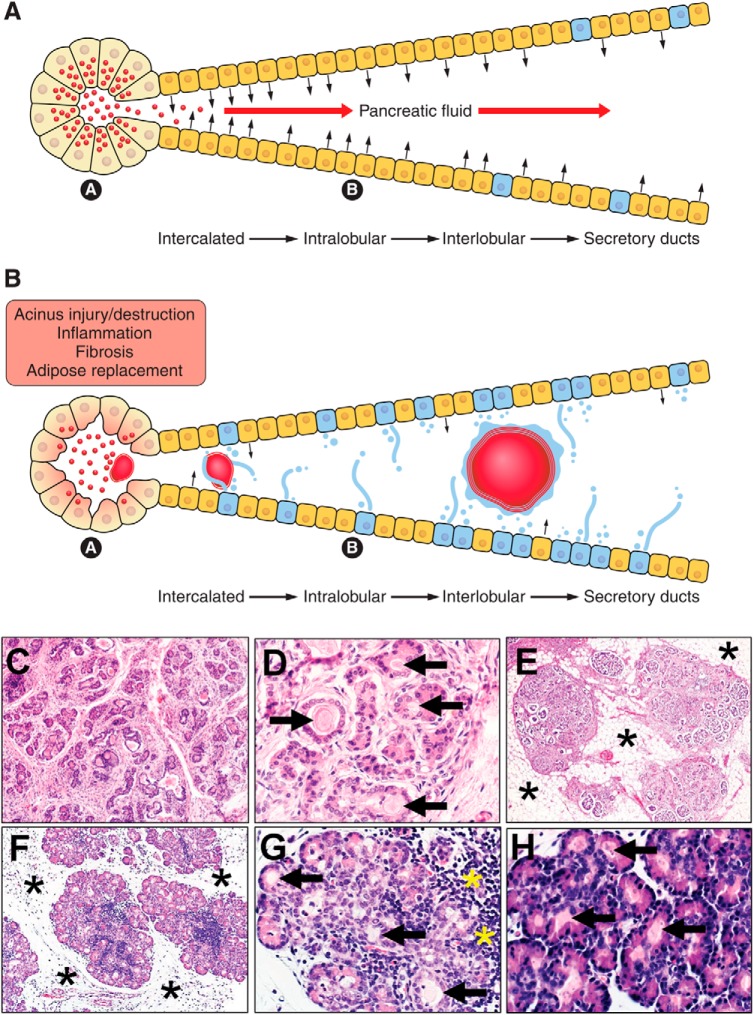
Fig. 3.
Pancreas in health and CF disease. A: schematic of healthy pancreas. Acini have numerous zymogen granules (red circles) in the apical cytoplasm that, upon stimulation, are secreted into the duct system (a). Duct cells secrete bicarbonate-rich fluids into the lumen (black arrows) to mix with the acinar secretions to form pancreatic fluid (red arrows; b). Note that mucous cells (blue) are normally restricted to the larger ducts. B: pathophysiology of CF disease in the pancreas. Acini secrete altered zymogen material and ducts secrete reduced liquid (small black arrows) into a microenvironment that promotes obstructions (red masses; a). Obstructions injure nearby acini and ducts, leading to destruction, inflammation, fibrosis, and adipose replacement of the organ (b). Note that, during CF disease, mucous cells increase in number and secretions (blue color in lumen), contributing to obstructions. C and D: CF pancreas from 2-mo-old infant. In C, many pancreatic acini have been destroyed and replaced by connective tissue. In D, remnant acini and ducts are often distended and obstructed by altered zymogen secretions. E: CF pancreas from 14-yr-old child. With progressive destruction, adipose tissue (asterisks) replaces the pancreas. Remaining lobules are composed of scattered ducts, a few acini, and multiple islets. Continued disease progression can result in nearly complete destruction of exocrine tissue, with replacement by adipose tissue, leaving scattered islets as the only evidence of pancreas. F and G: newborn CF pig pancreas. In F, CF pig pancreas has small degenerate lobules separated by loose connective tissue (asterisks). In G, acini and ducts are distended by altered zymogen secretions (arrows) and show evidence of inflammation (yellow asterisks). H: newborn CF ferret pancreas. Altered zymogen secretions result in obstructions (arrows) in pancreatic acini and ducts, with little evidence of inflammation or remodeling.
Pancreatic disease severity is highly dependent on genotype (85, 102), and these CFTR mutations can be organized into classes on the basis of their functional biology (102). Pancreatic-insufficient (PI) patients tend to have mutations that result in absent or nonfunctional CFTR at the cell surface (class I–III mutations), while pancreatic-sufficient (PS) individuals have more mild CFTR mutation with partially functional CFTR on the cell surface (class IV and V mutations) (6). In contrast, CFTR mutations can produce disproportional decreases in anion transport, leading to altered HCO3−-to-Cl− ratios, which can influence phenotype. For example, patients with CFTR mutations producing a low HCO3−-to-Cl− transport ratio were more likely PI patients, whereas higher HCO3−-to-Cl− transport ratios were often associated with PS patients and milder disease (24).
Pancreatic lesions begin in utero and progress from early plugging (obstruction) in acini and small ducts, with eventual acini injury and destruction, resulting in inflammation, fibrosis, and fatty replacement (62). As acini are destroyed, duct proliferations (“tubular complexes”) are sometimes observed and have been suggested to represent a reparative response (79, 116). Altered zymogen secretions form much of the early luminal obstruction material. In more severe or advanced disease, secondary changes such as mucous cell metaplasia with its mucus secretions may further complicate luminal obstructions (123).
Serum immunoreactive trypsinogen (IRT) (64), a pancreatic enzyme precursor, is elevated in the blood of CF infants and utilized as a newborn screening tool (112). Evaluation of feces for pancreatic enzymes such as elastase-1 is also useful in diagnosis of pancreatic insufficiency (74). It is important to note that these immunological tests use antibodies that do not cross react with elastase in porcine-derived pancreatic replacement supplements. Additionally, fecal fat levels are used to classify CF patients as PS or PI. IRT levels and fecal fat levels are inversely correlated in CF patients (112). Transition from PS to PI increases in frequency throughout the 1st yr of life and has a significant impact on growth and nutrition (62, 128). Approximately 85% of CF patients are classified as PI and require lifelong pancreatic enzyme supplementation. PS patients have evidence of pancreatic damage, as demonstrated by high IRT during neonatal screening, with reduced, but sufficient, exocrine pancreatic function for normal digestion (7, 40, 85). The estimated occurrence of pancreatitis among CF patients is 1.24%, but PS patients are at increased risk (10.3%) (30). Interestingly, patients with idiopathic pancreatitis and no history of CF have a higher frequency of CFTR gene mutations (12, 107).
Mouse model.
Of the CF mouse models, the major phenotype has been intestinal obstruction, but only subtle pancreatic disease is detectable. The lack of pancreatic disease may be due to several factors: 1) developmental/structural differences of the mouse pancreas, 2) low CFTR expression in the rodent pancreas, with higher expression of alternative anion channels, and 3) modifier genes and/or short life-span of CF mice (26, 39, 52, 63, 89).
Many CF mouse strains (see Ref. 130 for a recent overview of CF mouse models) are available for pancreas study, but examples of those that demonstrate pancreatic changes are discussed here. It is important to note that, in some studies, CF mice may have altered physiology (e.g., CCK secretion, fluid homeostasis) as a result of being maintained on a long-term liquid diet, and the extent to which this contributes to any observed pancreatic changes is not clear (17, 31). In one study, CF mice maintained on a liquid diet developed mild intraluminal obstruction that progressed with age, with a decrease in acinar cell volume by 15–24 mo of age (39). CFTRtm1CAM mice (15–50 days of age) demonstrated mild pancreatic pathology, characterized by focal pancreatic duct plugging in 50% of the mice (94). In another study, Muc6 expression was increased in CF mice maintained with polyethylene glycol 4000 in water (51). This mucin is similarly localized to acini and luminal plugs in human CF pancreas (95). Additional studies may be useful to determine if Muc6 has a role in the pathogenesis of CF pancreas disease.
Pig model.
Pancreatic pathology in CF newborn pigs is similar to that in CF patients, with plugging/obstruction and acinus destruction with mild inflammation. The newborn CF pig pancreas is characterized by small lobules separated by increased loose connective tissue, scattered inflammation, dilation of centroacinar spaces, duct proliferation, and mucinous metaplasia (79, 100). Interestingly, duct proliferation and mucinous metaplasia were not present in fetal pancreas at 90 days gestation (full term ∼115 days), indicating that remodeling occurred in the weeks prior to birth (3). While inflammation is present in the CF pig pancreas at birth, there is a lack of systemic inflammation, indicating a pancreas-specific response (4). Pancreatic enzyme replacement therapy is started shortly after birth in CF pigs (113). As animals age, acini are destroyed and are replaced by fibrosis and adipose tissue (79). The volume and pH of pancreatic fluid are lower in newborn CF pigs with higher protein concentration. Similarly, CF pigs have diminished response following the administration of secretin (125). Reduced volume and pH of pancreatic fluid are also features of human CF disease (36).
Ferret model.
The CF ferret presents with mild pancreatic disease at birth, characterized by multifocal acinar lumen dilation by zymogen material with few dilated ducts (11). Within the 1st mo of life, there is rapid progression of disease characterized by acinar loss, duct dilation, inflammation, and fibrosis (126). This pancreatic destruction coincides with glycemic instability in CF ferrets and progressive glucose intolerance (84), which may highlight the CF ferret as a suitable model to study CF-related diabetes. The majority of CF ferrets have progressive pancreatic destruction that results in exocrine PI and necessitates enzyme replacement therapy. Interestingly, a small percentage (∼15%) of CF ferrets have a predominantly normal pancreas with normal fecal elastase levels (15), indicating that other genetic modifiers may play a role in pancreatic disease.
Future directions.
The current treatment for PI is pancreatic enzyme replacement therapy, which can help prevent the most severe complications. Enzymes are not fully effective, given the acidity of the duodenal fluid; therefore, acid-resistant lipases are being developed. PS patients may benefit from therapies that target the basic defect, inflammation, or other factors that play a role in progression of the disease. Animal models will allow us to better evaluate the spectrum and time course of pancreatic disease, which would facilitate therapeutic development and testing.
Summary Observations
CF research is fortunate to have a rich diversity of animal models for the study of CF disease. Interorgan and interspecies comparison of gastrointestinal, pancreatic, and hepatobiliary pathogenesis allows some fundamental observations. First, altered secretions are highly relevant features of disease in these organs, and mucus is often the targeted culprit. However, altered proteinaceous secretions (and not just mucus) are clearly evident histopathologically in intestinal crypts and as common components of pancreatic obstructions. These underreported observations suggest that the CF luminal microenvironment could have a profound influence on many secreted materials (proteins and mucus), both in structure and function. Second, comparison between CF species may allow for better targeting of genetic or environmental modifiers of CF disease severity. For instance, why is meconium ileus obstruction absent in early reports of the rat model, yet meconium ileus has variable penetrance in the other three models? Could outbred models such as the CF ferret or CF pig offer wider genetic diversity to help identify candidate modifier genes that could then be further tested in inbred models such as the CF rat and mouse? In conclusion, CF researchers can take advantage of the similarities and differences in CF models to optimize CF disease modeling, advance the understanding of CF pathogenesis, and validate therapeutic targets.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-51670 and HL-091842, National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK-54759 and R24 DK-096518, and the Cystic Fibrosis Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.O. and D.K.M. developed the concept and designed the research; A.K.O., K.N.G.-C., and D.K.M. prepared the figures; A.K.O. and D.K.M. drafted the manuscript; A.K.O., K.N.G.-C., and D.K.M. edited and revised the manuscript; A.K.O., K.N.G.-C., and D.K.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Marcus Nashelsky and Morris Dailey for assistance in retrieval of archival autopsy material. We also thank the University of Iowa Comparative Pathology Laboratory for technical services.
REFERENCES
- 1.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J, Welsh MJ. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA 111: 18703–18708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson SJ, Baker DH, Amodio JB, Berdon WE. Gastrointestinal manifestations of cystic fibrosis. Semin Roentgenol 22: 97–113, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Abu-El-Haija M, Ramachandran S, Meyerholz DK, Abu-El-Haija M, Griffin M, Giriyappa RL, Stoltz DA, Welsh MJ, McCray PB Jr, Uc A. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol 181: 499–507, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB Jr, Butler J, Uc A. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology 11: 506–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrons GA, Corse WR, Markowitz RI, Suarez ES, Perry DR. Gastrointestinal manifestations of cystic fibrosis: radiologic-pathologic correlation. Radiographics 16: 871–893, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N, Corey M, Forstner G, Zielenski J, Tsui LC, Ellis L, Tullis E, Durie P. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut 52: 1159–1164, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augarten A, Ben Tov A, Madgar I, Barak A, Akons H, Laufer J, Efrati O, Aviram M, Bentur L, Blau H, Paret G, Wilschanski M, Kerem BS, Yahav Y. The changing face of the exocrine pancreas in cystic fibrosis: the correlation between pancreatic status, pancreatitis and cystic fibrosis genotype. Eur J Gastroenterol Hepatol 20: 164–168, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bali A, Stableforth DE, Asquith P. Prolonged small-intestinal transit time in cystic fibrosis. Br Med J 287: 1011–1013, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, Castaldo G, Castellani C, Cipolli M, Colombo C, Colombo JL, Debray D, Fernandez A, Lacaille F, Macek M Jr, Rowland M, Salvatore F, Taylor CJ, Wainwright C, Wilschanski M, Zemkova D, Hannah WB, Phillips MJ, Corey M, Zielenski J, Dorfman R, Wang Y, Zou F, Silverman LM, Drumm ML, Wright FA, Lange EM, Durie PR, Knowles MR, Gene Modifier Study Group. Genetic modifiers of liver disease in cystic fibrosis. JAMA 302: 1076–1083, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La Rosa T, Di Sario A, Baiocchi L, Jezequel AM. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology 26: 9–21, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Benya EC, Nussbaum-Blask AR, Selby DM. Colonic diverticulitis causing partial bowel obstruction in a child with cystic fibrosis. Pediatr Radiol 27: 918–919, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Bishop MD, Freedman SD, Zielenski J, Ahmed N, Dupuis A, Martin S, Ellis L, Shea J, Hopper I, Corey M, Kortan P, Haber G, Ross C, Tzountzouris J, Steele L, Ray PN, Tsui LC, Durie PR. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet 118: 372–381, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Blackman SM, Deering-Brose R, McWilliams R, Naughton K, Coleman B, Lai T, Algire M, Beck S, Hoover-Fong J, Hamosh A, Fallin MD, West K, Arking DE, Chakravarti A, Cutler DJ, Cutting GR. Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology 131: 1030–1039, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodewes FA, Wouthuyzen-Bakker M, Bijvelds MJ, Havinga R, de Jonge HR, Verkade HJ. Ursodeoxycholate modulates bile flow and bile salt pool independently from the cystic fibrosis transmembrane regulator (Cftr) in mice. Am J Physiol Gastrointest Liver Physiol 302: G1035–G1042, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Bodian M. Fibrocystic Disease of the Pancreas: A Congenital Disorder of Mucus Production-Mucosis. New York: Grune & Stratton, 1953. [Google Scholar]
- 16.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 92: 1111–1122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, De Lisle RC, Lewindon P, Lichtman SM, Sinaasappel M, Baker RD, Baker SS, Verkade HJ, Lowe ME, Stallings VA, Janghorbani M, Butler R, Heubi J. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr 41: 273–285, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 58: 157–170, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Braun J, Mundhenk L, Range F, Gruber AD. Quantitative expression analyses of candidates for alternative anion conductance in cystic fibrosis mouse models. J Cyst Fibros 9: 351–364, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan DJ, Rapoport S. Chemical comparison of normal meconium and meconium from a patient with meconium ileus. Pediatrics 9: 304–310, 1952. [PubMed] [Google Scholar]
- 21.Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, Passariello A, Manguso F, Morelli L, Guarino AM, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB Jr, Butler J, Uc A, Working Group on Intestinal Infections of the Italian Society of Pediatric Gastroenterology and Nutrition. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics 117: e817–e820, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev 10: CD000222, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3− transport in mutations associated with cystic fibrosis. Nature 410: 94–97, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE, Shull GE, Tanabe H, Ouellette AJ, Franklin CL, Walker NM. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol 286: G1050–G1058, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr−/− mice. Proc Natl Acad Sci USA 91: 479–483, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology 105: 1857–1864, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, Giunta A. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcome. Hepatology 36: 1374–1382, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 173: 475–482, 2006. [DOI] [PubMed] [Google Scholar]
- 30.De Boeck K, Weren M, Proesmans M, Kerem E. Pancreatitis among patients with cystic fibrosis: correlation with pancreatic status and genotype. Pediatrics 115: e463–e469, 2005. [DOI] [PubMed] [Google Scholar]
- 31.De Lisle RC. Increased expression of sulfated gp300 and acinar tissue pathology in pancreas of CFTR−/− mice. Am J Physiol Gastrointest Liver Physiol 268: G717–G723, 1995. [DOI] [PubMed] [Google Scholar]
- 32.De Lisle RC, Meldi L, Mueller R. Intestinal smooth muscle dysfunction develops postnatally in cystic fibrosis mice. J Pediatr Gastroenterol Nutr 55: 689–694, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lisle RC, Sewell R, Meldi L. Enteric circular muscle dysfunction in the cystic fibrosis mouse small intestine. Neurogastroenterol Motil 22: e341–e387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 10Suppl 2: S29–S36, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Debray D, Rainteau D, Barbu V, Rouahi M, El Mourabit H, Lerondel S, Rey C, Humbert L, Wendum D, Cottart CH, Dawson P, Chignard N, Housset C. Defects in gallbladder emptying and bile acid homeostasis in mice with cystic fibrosis transmembrane conductance regulator deficiencies. Gastroenterology 142: 1581–1591, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Campo R, Garriga M, Perez-Aragon A, Guallarte P, Lamas A, Maiz L, Bayon C, Roy G, Canton R, Zamora J, Baquero F, Suarez L. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: a double blind prospective study. J Cyst Fibros 13: 716–722, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Dimango E, Walker P, Keating C, Berdella M, Robinson N, Langfelder-Schwind E, Levy D, Liu X. Effect of esomeprazole versus placebo on pulmonary exacerbations in cystic fibrosis. BMC Pulm Med 14: 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durie PR, Forstner GG. Pathophysiology of the exocrine pancreas in cystic fibrosis. J R Soc Med 82Suppl 16: 2–10, 1989. [PMC free article] [PubMed] [Google Scholar]
- 39.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol 164: 1481–1493, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durno C, Corey M, Zielenski J, Tullis E, Tsui LC, Durie P. Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology 123: 1857–1864, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Farrelly PJ, Charlesworth C, Lee S, Southern KW, Baillie CT. Gastrointestinal surgery in cystic fibrosis: a 20-year review. J Pediatr Surg 49: 280–283, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology 141: 1498–1508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher JT, Tyler SR, Zhang Y, Lee BJ, Liu X, Sun X, Sui H, Liang B, Luo M, Xie W, Yi Y, Zhou W, Song Y, Keiser N, Wang K, de Jonge HR, Engelhardt JF. Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets. Am J Respir Cell Mol Biol 49: 837–844, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisichella PM, Jalilvand A. The role of impaired esophageal and gastric motility in end-stage lung diseases and after lung transplantation. J Surg Res 186: 201–206, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. J Cyst Fibros 12: 116–124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freudenberg F, Broderick AL, Yu BB, Leonard MR, Glickman JN, Carey MC. Pathophysiological basis of liver disease in cystic fibrosis employing a ΔF508 mouse model. Am J Physiol Gastrointest Liver Physiol 294: G1411–G1420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harbor Perspect Med 2: a009563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Gallagher AM, Gottlieb RA. Proliferation, not apoptosis, alters epithelial cell migration in small intestine of CFTR null mice. Am J Physiol Gastrointest Liver Physiol 281: G681–G687, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 58: 2275–2281, 2013. [DOI] [PubMed] [Google Scholar]
- 50.George DH. Diverticulosis of the vermiform appendix in patients with cystic fibrosis. Hum Pathol 18: 75–79, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Gouyer V, Leir SH, Tetaert D, Liu Y, Gottrand F, Harris A, Desseyn JL. The characterization of the first anti-mouse Muc6 antibody shows an increased expression of the mucin in pancreatic tissue of Cftr-knockout mice. Histochem Cell Biol 133: 517–525, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol Cell Physiol 266: C213–C221, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Grubb BR. Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 268: G505–G513, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 273: G258–G266, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Hedsund C, Gregersen T, Joensson IM, Olesen HV, Krogh K. Gastrointestinal transit times and motility in patients with cystic fibrosis. Scand J Gastroenterol 47: 920–926, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Helm JF, Dodds WJ, Riedel DR, Teeter BC, Hogan WJ, Arndorfer RC. Determinants of esophageal acid clearance in normal subjects. Gastroenterology 85: 607–612, 1983. [PubMed] [Google Scholar]
- 57.Henderson LB, Doshi VK, Blackman SM, Naughton KM, Pace RG, Moskovitz J, Knowles MR, Durie PR, Drumm ML, Cutting GR. Variation in MSRA modifies risk of neonatal intestinal obstruction in cystic fibrosis. PLos Genet 8: e1002580, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodges CA, Cotton CU, Palmert MR, Drumm ML. Generation of a conditional null allele for Cftr in mice. Genesis 46: 546–552, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodges CA, Grady BR, Mishra K, Cotton CU, Drumm ML. Cystic fibrosis growth retardation is not correlated with loss of Cftr in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 301: G528–G536, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345: 818–822, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houwen RH, van der Doef HP, Sermet I, Munck A, Hauser B, Walkowiak J, Robberecht E, Colombo C, Sinaasappel M, Wilschanski M, ESPGHAN Cystic Fibrosis Working Group. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J Pediatr Gastroenterol Nutr 50: 38–42, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Imrie JR, Fagan DG, Sturgess JM. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and control infants. Am J Pathol 95: 697–708, 1979. [PMC free article] [PubMed] [Google Scholar]
- 63.Ip WF, Bronsveld I, Kent G, Corey M, Durie PR. Exocrine pancreatic alterations in long-lived surviving cystic fibrosis mice. Pediatr Res 40: 242–249, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Karlas T, Neuschulz M, Oltmanns A, Wirtz H, Keim V, Wiegand J. ARFI and transient elastography for characterization of cystic fibrosis related liver disease: first longitudinal follow-up data in adult patients. J Cyst Fibros 12: 826–827, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 41: 920–925, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345: 1247125, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Leeuwen L, Magoffin AK, Fitzgerald DA, Cipolli M, Gaskin KJ. Cholestasis and meconium ileus in infants with cystic fibrosis and their clinical outcomes. Arch Dis Child 99: 443–447, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Lewindon PJ, Pereira TN, Hoskins AC, Bridle KR, Williamson RM, Shepherd RW, Ramm GA. The role of hepatic stellate cells and transforming growth factor-β1 in cystic fibrosis liver disease. Am J Pathol 160: 1705–1715, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewindon PJ, Shepherd RW, Walsh MJ, Greer RM, Williamson R, Pereira TN, Frawley K, Bell SC, Smith JL, Ramm GA. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology 53: 193–201, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology 30: 1151–1158, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Lisowska A, Madry E, Pogorzelski A, Szydlowski J, Radzikowski A, Walkowiak J. Small intestine bacterial overgrowth does not correspond to intestinal inflammation in cystic fibrosis. Scand J Clin Lab Invest 70: 322–326, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Lisowska A, Wojtowicz J, Walkowiak J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: combined hydrogen and methane measurements are required for its detection. Acta Biochim Pol 56: 631–634, 2009. [PubMed] [Google Scholar]
- 73.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. Functional Cftr in crypt epithelium of organotypic enteroid cultures from murine small intestine. Am J Physiol Cell Physiol 302: C1492–C1503, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loser C, Mollgaard A, Folsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut 39: 580–586, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lynch SV, Goldfarb KC, Wild YK, Kong W, De Lisle RC, Brodie EL. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes 4: 41–47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 105: 122–129, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Makris K, Tsiotos GG, Stafyla V, Sakorafas GH. Small intestinal nonmeckelian diverticulosis. J Clin Gastroenterol 43: 201–207, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Marx V. Models: stretching the skills of cell lines and mice. Nat Methods 11: 617–620, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol 176: 1377–1389, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyers RL, Orlando RC. In vivo bicarbonate secretion by human esophagus. Gastroenterology 103: 1174–1178, 1992. [DOI] [PubMed] [Google Scholar]
- 81.Murshed R, Spitz L, Kiely E, Drake D. Meconium ileus: a ten-year review of thirty-six patients. Eur J Pediatr Surg 7: 275–277, 1997. [DOI] [PubMed] [Google Scholar]
- 82.Neglia JP, FitzSimmons SC, Maisonneuve P, Schoni MH, Schoni-Affolter F, Corey M, Lowenfels AB. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med 332: 494–499, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Nousia-Arvanitakis S, Fotoulaki M, Economou H, Xefteri M, Galli-Tsinopoulou A. Long-term prospective study of the effect of ursodeoxycholic acid on cystic fibrosis-related liver disease. J Clin Gastroenterol 32: 324–328, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 122: 3755–3768, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, Freedman SD, Zielenski J, Berthiaume Y, Corey M, Schibli S, Tullis E, Durie PR. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 140: 153–161, 2011. [DOI] [PubMed] [Google Scholar]
- 86.Oppenheimer EH, Esterly JR. Observations in cystic fibrosis of the pancreas. II. Neonatal intestinal obstruction. Bull Johns Hopkins Hosp 111: 1–13, 1962. [PubMed] [Google Scholar]
- 87.Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 3: 74ra24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ostedgaard LS, Meyerholz DK, Vermeer DW, Karp PH, Schneider L, Sigmund CD, Welsh MJ. Cystic fibrosis transmembrane conductance regulator with a shortened R domain rescues the intestinal phenotype of CFTR−/− mice. Proc Natl Acad Sci USA 108: 2921–2926, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pascua P, Garcia M, Fernandez-Salazar MP, Hernandez-Lorenzo MP, Calvo JJ, Colledge WH, Case RM, Steward MC, San Roman JI. Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflügers Arch 459: 203–214, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Patricolo M, Noia G, Rossi L, Zangari A, Pomini F, Catesini C, Filippetti R, Galli T, Iacobelli BD, Capuano LG, Romano D, Mancuso S, Rivosecchi M. An experimental animal model of intestinal obstruction to simulate in utero therapy for jejunoileal atresia. Fetal Diagn Ther 13: 298–301, 1998. [DOI] [PubMed] [Google Scholar]
- 91.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 79: S3–S22, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Ramm GA, Shepherd RW, Hoskins AC, Greco SA, Ney AD, Pereira TN, Bridle KR, Doecke JD, Meikle PJ, Turlin B, Lewindon PJ. Fibrogenesis in pediatric cholestatic liver disease: role of taurocholate and hepatocyte-derived monocyte chemotaxis protein-1 in hepatic stellate cell recruitment. Hepatology 49: 533–544, 2009. [DOI] [PubMed] [Google Scholar]
- 94.Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet 4: 35–41, 1993. [DOI] [PubMed] [Google Scholar]
- 95.Reid CJ, Hyde K, Ho SB, Harris A. Cystic fibrosis of the pancreas: involvement of MUC6 mucin in obstruction of pancreatic ducts. Mol Med 3: 403–411, 1997. [PMC free article] [PubMed] [Google Scholar]
- 96.Rescorla FJ, Grosfeld JL, West KJ, Vane DW. Changing patterns of treatment and survival in neonates with meconium ileus. Arch Surg 124: 837–840, 1989. [DOI] [PubMed] [Google Scholar]
- 97.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 98.Risse PA, Kachmar L, Matusovsky OS, Novali M, Gil FR, Javeshghani S, Keary R, Haston CK, Michoud MC, Martin JG, Lauzon AM. Ileal smooth muscle dysfunction and remodeling in cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 303: G1–G8, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Roberts HE, Cragan JD, Cono J, Khoury MJ, Weatherly MR, Moore CA. Increased frequency of cystic fibrosis among infants with jejunoileal atresia. Am J Med Genet 78: 446–449, 1998. [DOI] [PubMed] [Google Scholar]
- 100.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet 67: 471–485, 2003. [DOI] [PubMed] [Google Scholar]
- 103.Scheele GA, Fukuoka SI, Kern HF, Freedman SD. Pancreatic dysfunction in cystic fibrosis occurs as a result of impairments in luminal pH, apical trafficking of zymogen granule membranes, and solubilization of secretory enzymes. Pancreas 12: 1–9, 1996. [DOI] [PubMed] [Google Scholar]
- 104.Schippa S, Iebba V, Santangelo F, Gagliardi A, De Biase RV, Stamato A, Bertasi S, Lucarelli M, Conte MP, Quattrucci S. Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLos One 8: e61176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scott-Jupp R, Lama M, Tanner MS. Prevalence of liver disease in cystic fibrosis. Arch Dis Child 66: 698–701, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott RB, O'Loughlin EV, Gall DG. Gastroesophageal reflux in patients with cystic fibrosis. J Pediatr 106: 223–227, 1985. [DOI] [PubMed] [Google Scholar]
- 107.Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 339: 645–652, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45, 1999. [DOI] [PubMed] [Google Scholar]
- 109.Siafakas C, Vottler TP, Andersen JM. Rectal prolapse in pediatrics. Clin Pediatr 38: 63–72, 1999. [DOI] [PubMed] [Google Scholar]
- 110.Siddiqui MM, Burge DM. Neonatal spontaneous colonic perforation due to cystic fibrosis. Pediatr Surg Int 24: 863–864, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. [DOI] [PubMed] [Google Scholar]
- 112.Sontag MK, Corey M, Hokanson JE, Marshall JA, Sommer SS, Zerbe GO, Accurso FJ. Genetic and physiologic correlates of longitudinal immunoreactive trypsinogen decline in infants with cystic fibrosis identified through newborn screening. J Pediatr 149: 650–657, 2006. [DOI] [PubMed] [Google Scholar]
- 113.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stoltz DA, Rokhlina T, Ernst SE, Pezzulo AA, Ostedgaard LS, Karp PH, Samuel MS, Reznikov LR, Rector MV, Gansemer ND, Bouzek DC, Alaiwa MH, Hoegger MJ, Ludwig PS, Taft PJ, Wallen TJ, Wohlford-Lenane C, McMenimen JD, Chen JH, Bogan KL, Adam RJ, Hornick EE, Nelson GA, Hoffman EA, Chang EH, Zabner J, McCray PB Jr, Prather RS, Meyerholz DK, Welsh MJ. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest 123: 2685–2693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133: 1999–2009, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subhi R, Ooi R, Finlayson F, Kotsimbos T, Wilson J, Lee WR, Wale R, Warrier S. Distal intestinal obstruction syndrome in cystic fibrosis: presentation, outcome and management in a tertiary hospital (2007–2012). ANZ J Surg 84: 740–744, 2014. [DOI] [PubMed] [Google Scholar]
- 119.Sun X, Olivier AK, Liang B, Yi Y, Sui H, Evans TI, Zhang Y, Zhou W, Tyler SR, Fisher JT, Keiser NW, Liu X, Yan Z, Song Y, Goeken JA, Kinyon JM, Fligg D, Wang X, Xie W, Lynch TJ, Kaminsky PM, Stewart ZA, Pope RM, Frana T, Meyerholz DK, Parekh K, Engelhardt JF. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol 50: 502–512, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun X, Olivier AK, Yi Y, Pope CE, Hayden HS, Liang B, Sui H, Zhou W, Hager KR, Zhang Y, Liu X, Yan Z, Fisher JT, Keiser NW, Song Y, Tyler SR, Goeken JA, Kinyon JM, Radey MC, Fligg D, Wang X, Xie W, Lynch TJ, Kaminsky PM, Brittnacher MJ, Miller SI, Parekh K, Meyerholz DK, Hoffman LR, Frana T, Stewart ZA, Engelhardt JF. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol 184: 1309–1322, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 120: 3149–3160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thiagarajah JR, Verkman AS. CFTR pharmacology and its role in intestinal fluid secretion. Curr Opin Pharmacol 3: 594–599, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Tucker JA, Spock A, Spicer SS, Shelburne JD, Bradford W. Inspissation of pancreatic zymogen material in cystic fibrosis. Ultrastruct Pathol 27: 323–335, 2003. [PubMed] [Google Scholar]
- 124.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLos One 9: e91253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uc A, Giriyappa R, Meyerholz DK, Griffin M, Ostedgaard LS, Tang XX, Abu-El-Haija M, Stoltz DA, Ludwig P, Pezzulo A, Abu-El-Haija M, Taft P, Welsh MJ. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. Am J Physiol Gastrointest Liver Physiol 303: G961–G968, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van der Doef HP, Kokke FT, Beek FJ, Woestenenk JW, Froeling SP, Houwen RH. Constipation in pediatric cystic fibrosis patients: an underestimated medical condition. J Cystic Fibrosis 9: 59–63, 2010. [DOI] [PubMed] [Google Scholar]
- 127.van der Doef HP, Kokke FT, van der Ent CK, Houwen RH. Intestinal obstruction syndromes in cystic fibrosis: meconium ileus, distal intestinal obstruction syndrome, and constipation. Curr Gastroenterol Rep 13: 265–270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walkowiak J, Sands D, Nowakowska A, Piotrowski R, Zybert K, Herzig KH, Milanowski A. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. J Pediatr Gastroenterol Nutr 40: 199–201, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: The Metabolic and Molecular Basis of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D. New York: McGraw-Hill, 2001, p. 5121–5189. [Google Scholar]
- 130.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cystic Fibrosis 10Suppl 2: S152–S171, 2011. [DOI] [PubMed] [Google Scholar]
- 131.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 56: 1153–1163, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woodley FW, Machado RS, Hayes D Jr, Di Lorenzo C, Kaul A, Skaggs B, McCoy K, Patel A, Mousa H. Children with cystic fibrosis have prolonged chemical clearance of acid reflux compared with symptomatic children without cystic fibrosis. Dig Dis Sci 59: 623–630, 2014. [DOI] [PubMed] [Google Scholar]
- 133.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266: 1705–1708, 1994. [DOI] [PubMed] [Google Scholar]
- 134.Zuelzer WW, Newton WA Jr. The pathogenesis of fibrocystic disease of the pancreas: a study of 36 cases with special reference to the pulmonary lesions. Pediatrics 4: 53–69, 1949. [PubMed] [Google Scholar]