Abstract
Protein tyrosine phosphatases (PTPs) regulate T cell receptor (TCR) signalling and thus have a role in T cell differentiation. Here we tested whether the autoimmune predisposing gene PTPN22 encoding for a PTP that inhibits TCR signalling affects the generation of forkhead box protein 3 (FoxP3)+ T regulatory (Treg) cells and T helper type 1 (Th1) cells. Murine CD4+ T cells isolated from Ptpn22 knock-out (Ptpn22KO) mice cultured in Treg cell polarizing conditions showed increased sensitivity to TCR activation compared to wild-type (WT) cells, and subsequently reduced FoxP3 expression at optimal-to-high levels of activation. However, at lower levels of TCR activation, Ptpn22KO CD4+ T cells showed enhanced expression of FoxP3. Similar experiments in humans revealed that at optimal levels of TCR activation PTPN22 knock-down by specific oligonucleotides compromises the differentiation of naive CD4+ T cells into Treg cells. Notably, in vivo Treg cell conversion experiments in mice showed delayed kinetic but overall increased frequency and number of Treg cells in the absence of Ptpn22. In contrast, the in vitro and in vivo generation of Th1 cells was comparable between WT and Ptpn22KO mice, thus suggesting PTPN22 as a FoxP3-specific regulating factor. Together, these results propose PTPN22 as a key factor in setting the proper threshold for FoxP3+ Treg cell differentiation.
Keywords: FoxP3+ Treg cells, PTPN22, Th1 cells
Introduction
Regulation of immune responses towards self- and foreign-antigens depends critically upon the function of T regulatory (Treg) cells. Forkhead box protein 3 (FoxP3)+ peripherally derived Treg cells (pTreg) are pivotal in protecting from autoimmunity and excessive inflammation, and appear to act complementarily with other Treg cells, such as thymus-derived Treg (tTreg) and T regulatory type 1 (Tr1) cells 1–3. FoxP3+ pTreg cells are generated in vivo primarily from naive CD4+ (FoxP3−) T cell precursors exposed to the antigen under tolerogenic conditions, upon homeostatic expansion or under specific inflammatory conditions such as helminth infection 4–7. Transforming growth factor (TGF)-β1 secretion is indispensable for the acquisition of the pTreg cell identity in vivo together with interleukin (IL)-2, co-stimulation signals and activation through the T cell receptor (TCR) 8–10. In vitro, naive CD4+ T cells develop readily into suppressive FoxP3+-induced Treg cells (iTreg) after culture with anti-CD3/CD28 monoclonal antibodies (mAbs) in the presence of TGF-β1 and IL-2. The degree or length of TCR activation affects FoxP3 induction. For example, very weak or extremely strong TCR signals are less potent as compared to intermediate TCR signals at inducing FoxP3 in the presence of equal amounts of TGF-β1 and IL-2 11–16.
PTPN22 encodes for the protein tyrosine phosphatase (PTP), lymphoid phosphatase (LYP) in humans, that is expressed by several cells of the lymphoid and myeloid lineages, and its exact role in T cell development, differentiation and function is unknown 17,18. A genetic association between a single nucleotide polymorphism (SNP) C1858T corresponding to the single amino acid substitution, R620W, and autoimmunity was first described for type 1 diabetes (T1D) and since then for several other autoimmune diseases (e.g. rheumatoid arthritis, systemic lupus erythematosus) 19–21. LYP is involved in TCR signalling, playing important negative regulatory role(s) in T cell activation as supported by data in mice deficient for the homologue gene encoding the Pest-enriched phosphatase (PEP), or mice knock-in (KI), for the equivalent LYP R620W substitution, PEP R619W (i.e. augmented TCR-induced signalling and cellular activation) 22–25. Ptpn22 knock-out (Ptpn22KO) mice develop lymphoproliferative disease with no signs of autoimmunity and accumulate memory-phenotype T cells with age 22. Conversely, PEP R619W KI mice recapitulate several of the features described for Ptpn22KO mice, and can progress to lupus-like autoimmune disease if placed on a mixed genetic background 24. Ptpn22KO mice show increased numbers of Treg cells in the periphery, suggesting that PEP alters Treg cells alongside effector cells establishing an equilibrium that does not advance beyond a certain point of immune dysregulation 26,27. Interestingly, Ptpn22 silencing in non-obese diabetic mice (NOD) by RNA interference also leads to increased Treg cell numbers in the periphery and confers protection from T1D 28. The mechanisms by which reduced levels of PEP contribute to an increase in FoxP3+ pTreg cells remain ill defined, as the source of increased Treg cells in Ptpn22KO mice has been attributed to thymic output 27 or peripheral conversion 26.
Studies with human subjects carrying the predisposing allele of PTPN22, C1858T, have provided controversial data. Lower TCR-induced activation, measured by calcium mobilization and IL-2 cytokine production in carriers compared to donors with the wild-type (WT) PTPN22 allele, was reported initially 19,29,30. Based on this, it was concluded that PTPN22 C1858T is a gain-of-function variant. Later, PTPN22 C1858T was described as a loss-of-function variant, as the expression of LYP in T and B cells from human carriers was lower at steady state 25, a finding that was disproved by two other studies 24,31. The function of the human variant has been examined in T and B cells isolated from carriers in vitro. Several of these reports again appear to be conflicting, and only a small study addressed the role of PTPN22 in Treg cell development and function in humans. In this study, PTPN22 C1858T did not alter peripheral Treg cell numbers but reduced the Treg cell suppressive function 32. Given the fact that most human and murine studies support a role for PTPN22 in TCR signalling, and the importance that TCR signalling has on FoxP3 Treg cell development 33–35, we investigated the role of PTPN22 in Treg cell induction in vitro and in vivo.
During TCR-mediated activation in the presence of a specific cytokine microenvironment, naive CD4+ T cells may differentiate towards a specific T helper (Th) cell lineage, such as Th1, Th2 or Th17 (reviewed in 36). The strength of TCR signalling during in vitro activation can determine Th1/2 polarization 37. Therefore, in the present study we also tested whether Ptpn22 is involved in Th1 cell polarization. We found that at most levels of TCR activation, naive T cells from Ptpn22KO mice differentiated to Th1 cells similarly to those from WT mice. In agreement, in a mouse model of viral infection with lymphocytic choriomeningitis virus (LCMV), LCMV-specific CD4+ T cells from Ptpn22KO mice differentiated in vivo into Th1 cells similarly to those from WT animals. Taken together, in the current study we report that PTPN22 is central for FoxP3+ Treg cell induction, but is dispensable for Th1 cell polarization.
Materials and methods
Mice
Homozygous Ptpn22KO mice were obtained from Dr Souad Rahmouni (University of Liège, Belgium) after Material Transfer Agreement (MTA) with Genentech (San Francisco, CA, USA), and have been previously described 22. Mice were interbred with WT C57BL/6 and the heterozygous offsprings were intercrossed to obtain Ptpn22KO and WT littermates that were used throughout the study. Ptpn22KO and Rag-1-deficient mice (Rag1–/–) (C57BL/6 background) were housed under specific pathogen-free conditions in compliance with the guidelines of the San Raffaele Institutional Animal Care and Use Committee (IACUC number 479).
Human donors
Peripheral blood was obtained from healthy donors and patients with long-lasting T1D at the San Raffaele Hospital. All donors who were recruited into this study were Italian residents. The study was approved by the local ethics committee (protocol HSR-TIGET004/DRI003). All subjects who agreed to participate in the study signed an informed consent form before any procedure.
Mouse cell purification
CD4+CD25− T cells were purified from spleens using the CD4+CD25+ Treg isolation kit and cells were further stained with the fluorescent dye eFluor670 (eBioscience, San Jose, CA, USA) to monitor proliferation. In some experiments, the CD4+CD62L+ naive T cell isolation kit was used to obtain naive CD4+ T cells. All beads and kits were from Miltenyi Biotec (San Diego, CA, USA) and used according to the manufacturer's protocols.
Mouse iTreg and Th1 cell differentiation cultures
For all iTreg and Th1 cell generation, plate-bound anti-CD3 mAb (clone 17A2; BD Biosciences, San Jose, CA, USA) was used at different concentrations together with soluble anti-CD28 mAb (clone 37·51; BD Biosciences) at a 2:1 ratio (e.g. 10 μg/ml anti-CD3 and 5 μg/ml anti-CD28). Cultures were performed in 10% fetal calf serum (FCS) complete RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA). CD4+CD25− or CD4+CD62L+ naive T cells were plated at 5 × 105 cells/ml and cultured in 96-well plates for 2, 3 or 4 days. For iTreg cell polarization, 5 ng/ml of rhTGF-β1 (R&D Systems, Minneapolis, MN, USA) and 50 U/ml rhIL-2 (Proleukin; Chiron, Emeryville, CA, USA) were added to the cultures. For Th1 cell polarization, CD4+CD62L+ naive T cells were cultured in the presence of 10 ng/ml recombinant IL-12 and 200 ng/ml anti-IL-4 mAb. For cytokine detection in iTreg or Th1 cells, LAC [leucocyte activation cocktail containing phorbol myristate acetate (PMA)/ionomycin/GolgiPlug; BD Biosciences] was added at the end of a 4-day culture for 3 h prior to staining, according to the manufacturer's instructions.
Adoptive cell transfer assay
Beads-purified peripheral CD4+CD25− T cells (Miltenyi Biotec) from Ptpn22KO and WT mice were transferred into Rag1–/– recipient mice through tail vein injection. At different time-points after transfer, weight was monitored and blood was withdrawn retro-orbitally. Recipients were sacrificed 45 days after transfer and T cells were analyzed by flow cytometry.
Infection with LCMV and Th1 analysis
Mice were infected with 104 plaque-forming units (PFU) LCMV Armstrong (Arm), as described previously 38. At 8 days post-infection, spleens were harvested and lymphocytes were activated for 3 h with the class II LCMV-specific peptide glycoprotein (GP)61–80 (2 μg/ml) in the presence of brefeldin A, as described previously 39.
Human in vitro Treg cultures and oligonucleotide treatment
Mononuclear cells were prepared from 20 ml of peripheral blood, as described previously 40. Naive human CD4+CD127+CD25− T cells were fluorescence activated cell sorted (FACS) and cultured in 96-well plates at 5 × 105 cells/ml in X-vivo 15 medium and 5% pooled AB human serum (Lonza, Basel, Switzerland). Cells were stimulated with plate-bound anti-CD3 (OKT3, 10 μg/ml; Jansen-Cilag, Raritan, NJ, USA) and soluble anti-CD28 (1 μg/ml; BD Biosciences) mAbs in the presence of 5 ng/ml rTGF-β1 and 100 U/ml rIL-2 for iTreg cell induction and analysed 3, 4 and 5 days after culture. As control, cells were activated similarly in the presence of solely rIL-2 or in the absence of any cytokines. PTPN22-specific or control, scrambled, cell-permeable oligonucleotides were purchased from Gene Tools (Philomath, OR, USA) and used as described previously 41. In some experiments, cells were stained with the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies) to monitor proliferation, as described previously 40.
Quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Relative expression levels of PTPN22 mRNA were measured in human iTreg cells treated with control and specific anti-sense oligonucleotides for PTPN22 2 days after in vitro culture. Cell lysis, cDNA synthesis and quantitative real-time PCR (qPCR) were performed using the TaqMan® Gene Expression Assay (Applied Biosystems, Carlsbad, CA, USA) and the 7900 HT Fast Real Time PCR System (Life Technologies, Carlsbad, CA, USA). Primers were purchased from Applied Biosystems. PTPN22 mRNA expression levels were normalized to those of the housekeeping gene hypoxantine phsophoribosyltransferase (HRPT). For error analysis, the standard deviation (s.d.) was calculated.
Flow cytometry
Cells were stained with anti-CD4, -CD25, -CD69 and -CD127 mAbs (all from BD Biosciences, Biolegend, San Diego, CA, USA and eBioscience) and then intracellularly with anti-FoxP3 and CTLA-4 mAbs (eBioscience). For interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-2, IL-10 and IL-17 cytokine detection from iTreg cultures, the eBioscience FoxP3 Cytofix/Cytoperm kit was used according to the manufacturer's instructions. To detect cytokines in Th1 cell cultures, the Cytofix/Cytoperm kit from BD Biosciences was used. All samples were acquired on a FACSCanto or LSRII flow cytometer (BD Biosciences) and analysed with FlowJo (Tree Star, Ashland, OR, USA) software.
Statistics
Comparisons between groups were performed using the paired or unpaired two-tailed Student's t-test using Prism software (GraphPad, San Diego, CA, USA). For all analyses, a two-tailed P-value ≤ 0·05 was considered significant.
Results
Ptpn22 controls in vitro FoxP3+ Treg cell induction in mice
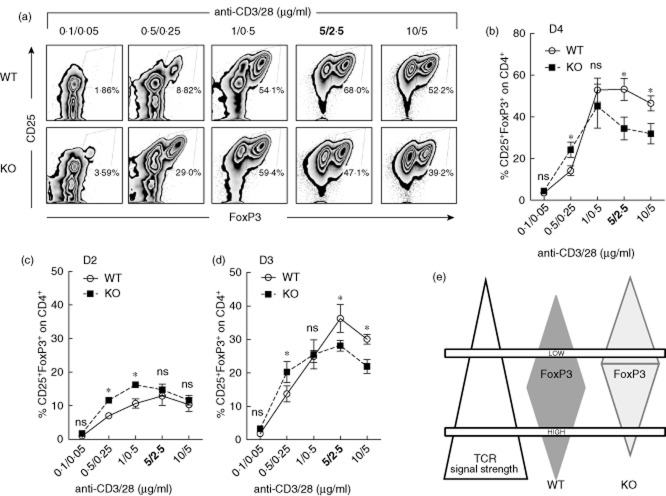
The degree of TCR activation is key for FoxP3+ Treg cell induction in vitro: strong TCR activation correlates inversely with FoxP3 up-regulation, but low TCR activation is inefficient to promote robust FoxP3 expression in iTreg cell polarizing conditions 1,35. Indeed, only intermediate doses of anti-CD3/CD28 mAbs support optimal FoxP3 induction 42,43. Accordingly, CD4+CD25− T cells from WT mice converted efficaciously into FoxP3+ Treg cells in vitro at optimal doses of anti-CD3/28 mAbs (i.e. 5/2·5 μg/ml) in the presence of TGF-β1 and IL-2 4 days after culture (Fig. 1a). To understand whether PEP contributes to FoxP3+ Treg cell induction in vitro, CD4+CD25− T cells from Ptpn22KO mice were cultured as those isolated from WT mice. De-novo FoxP3 expression was reduced in CD4+CD25− T cells lacking Ptpn22 at optimal and high TCR activation (≥5/25 μg/ml, anti-CD3/28 mAbs). Conversely, as already demonstrated 26, CD4+CD25− T cells from Ptpn22KO mice converted more efficiently in FoxP3+ Treg cells at lower doses of TCR activation (Fig. 1a,b).
Fig 1.
Ptpn22 modulates the threshold of T cell activation altering in vitro forkhead box protein 3 (FoxP3)+ T regulatory cell induction in mice. CD4+CD25− T cells from 8–12-week-old wild-type (WT) or PTPN22 knock-out (PTPN22KO) mice were cultured with increasing amounts of anti-CD3/CD28 monoclonal antibodies (mAbs) in the presence of fixed amounts of interleukin (IL)-2 and transforming growth factor (TGF)-β1. (a,b) Expression of CD25 and FoxP3 was evaluated 4 days after culture (gate: CD4+ T cells). In (a), results are representative of five independent experiments with similar results. In (b), cumulative data from all five experiments are shown. (c,d) Frequency of in vitro-induced Treg cells (CD4+ FoxP3+) was evaluated 2 days (c) and 3 days (d) after in vitro culture. Data from two independent experiments are shown. Dots represent data from individual experiments, whereas bars represent standard error. (e) Proposed model for the effect that Ptpn22 has on modulating the threshold of T cell receptor (TCR) activation and consequently iTreg cell induction. Pest-enriched phosphatase (PEP) by inhibiting TCR signalling antagonizes FoxP3 at low TCR-mediated activation while it augments FoxP3-inducing signals at higher TCR activating conditions. n.s. = not statistically significant, *P < 0·05.
The kinetic of FoxP3 expression in Ptpn22KO and WT iTreg cell cultures was then assessed. Whereas CD4+CD25− T cells from Ptpn22KO mice converted into iTreg earlier and more efficiently when activated with low amounts of anti-CD3/CD28 mAbs (Fig. 1c), lack of Ptpn22 reduced iTreg cell induction at high levels of activation (Fig. 1d). This data shows that, in the absence of PEP, T cells are more ‘ready’ to respond to low levels of TCR activation [also confirmed by enhanced up-regulation of CTLA-4 in Ptpn22KO-activated T cells (Supporting information, Fig. S1a,b)], and the optimal TCR strength needed to effectively generate FoxP3+ Treg cells is reduced as compared to that needed for inducing iTreg cells in WT mice. Thus, Ptpn22 sets the threshold for FoxP3 iTreg cell induction in mice by modulating TCR activation (Fig. 1e).
Aged Ptpn22KO mice (>6 months old) develop lymphoproliferative disease and accumulate memory T cells (CD44hiCD62Llo) 22. To define whether memory Ptpn22KO T cells, which might contaminate the starting CD4+CD25− T cells, inhibit Treg cell induction in vitro at optimal FoxP3-inducing culture conditions, the above-mentioned experiments were performed with CD4+CD62Lhi purified naive T cells or with CD4+CD25− T cells isolated from younger mice (5–6 weeks old). Similar results were obtained, showing that the reduced FoxP3 expression in cultures with cells from Ptpn22KO mice is not due to memory T cell contamination (Supporting information, Fig. S1c). Finally, the resulting FoxP3+ Treg cells were tested for IFN-γ and IL-10 production upon polyclonal stimulation. Similar cytokine production was observed in iTreg cells from Ptpn22KO and WT mice, suggesting that the regulation IFN-γ and IL-10 production at increasing TCR activating conditions is not dependent upon the expression of PEP (data not shown).
Ptpn22 does not substantially affect murine Th1 cell polarization in vitro and in vivo
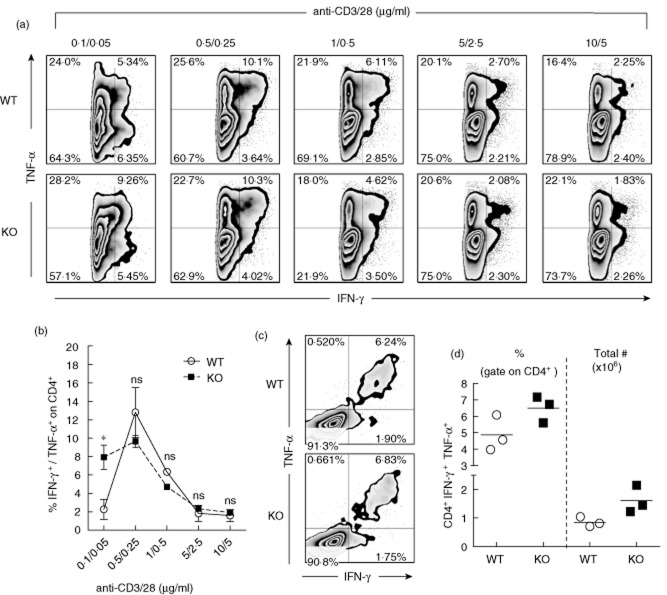
Ptpn22 may dictate not only the capacity of naive T cells to respond and differentiate into iTreg cells, but also their ability to mature effectively into any other cell lineage. To dissect whether Ptpn22 is also a key factor for the differentiation of effector T cells, we tested its function in Th1 cell polarization. CD4+CD62L+ T cells isolated from Ptpn22KO and WT mice were cultured under Th1 cell polarizing conditions at various levels of TCR activation. In contrast to FoxP3+ iTreg cell induction, the absence of PEP did not impinge significantly upon the differentiation of Th1 cells, measured by the production of IFN-γ-and TNF-α 4 days after induction. Interestingly, higher Th1 cell differentiation could be achieved when differentiation was induced with the lowest amount of anti-CD3-mediated activation (Fig. 2a,b). This finding underscores that at very low activating TCR conditions, Ptpn22KO T cells are more prone to differentiate into Th1 cells. To test whether Ptpn22 is implicated in Th1 cell differentiation in vivo, the LCMV model of acute viral infection was used 44. Upon infection with LCMV protective innate and adaptive immune responses emerge, of which the GP61–80-specific Th1 cell effector response predominates among CD4+ T cells 45. The absence of Ptpn22 did not affect the frequency and number of IFN-γ and TNF-α co-producing (Th1) GP61–80-specific CD4+ T cells 8 days after infection with LCMV (Fig. 2c,d). Taken together, these results suggest that Ptpn22 is not involved significantly in Th1 cell lineage commitment in vitro and in vivo.
Fig 2.
Ptpn22 does not significantly alter the T helper type 1 (Th1) cell polarization in mice in vitro or in vivo. CD4+CD62L+ T cells from PTPN22 knock-out (PTPN22KO) and wild-type (WT) mice were cultured under Th1 cell polarizing conditions. (a) Dot-plots showing representative tumour necrosis factor (TNF)-α and interferon (IFN)-γ production (gate: CD4+ T cells) are shown. (b) Results from two experiments are shown. Dots represent data from individual experiments, whereas bars represent standard error. (c,d) Eight to 10-week-old Ptpn22KO and WT mice were infected with lymphocytic choriomeningitis virus (LCMV) and splenocytes were analysed 8 days later for the production of TNF-α and IFN-γ after stimulation with the class II LCMV-specific epitope GP61–80. Representative flow cytometry plots are shown (c). Frequencies (left) and total numbers (right) of Th1 IFN-γ+ TNF-α+ GP61–80-specific cells are shown (one representative experiment out of three with similar results, each symbol represents individual mice) (d). n.s. = not statistically significant; *P < 0·05.
PTPN22 is key for in vitro FoxP3+ Treg cell induction in humans
TGF-β1 also promotes the differentiation of CD4+ T cells into FoxP3-expressing T cells in humans 46,47. To investigate whether PTPN22 is key for FoxP3 induction also in human T cells, CD4+CD127+CD25− T cells were isolated from peripheral blood of healthy donors (HD) and cultured in vitro under FoxP3+ iTreg cell conditions. To knock-down the expression of PTPN22, PTPN22-anti-sense or control (CTRL) non-targeting oligonucleotides 41 were added to the iTreg cell cultures. Considering that the oligo-treated cells survived similarly in culture irrespective of the oligonucleotides used (data not shown), comparisons between CTRL and PTPN22-specific oligo-treated cultures were made. All HD were screened for the PTPN22 polymorphism C1858T, and were homozygous for the WT allele, 1858C/C (Supporting information, Table S1). An average of 50% reduction in CD25+ FoxP3+ T cell induction was observed 5 days after culture when PTPN22 mRNA was down-modulated compared to cultures treated with control oligonucleotides (Fig. 3a,b). Additional analysis at an earlier culture time (i.e. 3 and 4 days) confirmed this reduction (Fig. 3c). Control oligonucleotides had no significant effect on FoxP3+ Treg cell induction compared to untreated cells (data not shown). The same data was generated using cells from patients with established T1D (Fig. 4). All patients were negative for the predisposing allele C1858T (Supporting information, Table S2). The ability to up-regulate FoxP3 in untreated CD4+CD25− T cells from T1D patients was identical to that in HD (data not shown). Considering that PTPN22-anti-sense oligonucleotides reduced PTPN22 mRNA expression by approximately 50% in these primary T cell cultures (Supporting information, Fig. S2a,b), it is likely that a more efficient PTPN22 reduction might lead to further inability in promoting FoxP3 expression in vitro. Overall, these data support that PTPN22 is essential for human FoxP3+ iTreg cell induction in vitro.
Fig 3.
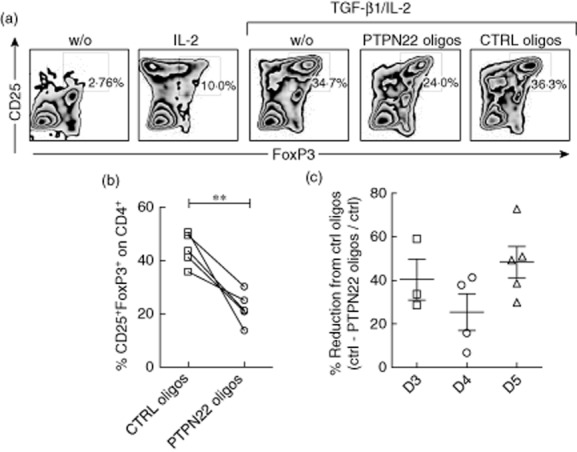
PTPN22 knock-down inhibits in vitro forkhead box protein 3 (FoxP3)+ T regulatory cell induction in human CD4+ T cells from healthy donors. (a) Human CD4+CD127+CD25− T cells were cultured under in vitro-induced (iTreg) [transforming growth factor (TGF)-β1/interleukin (IL)-2] and control (w/o, IL-2) conditions. PTPN22-specific and control (CTRL) oligonucleotides were added to the culture. CD25 and intracellular FoxP3 expression evaluated on gated CD4+ T cells after 5 days of culture are shown (one representative of five experiments is presented). (b) Percentages of CD4+CD25+FoxP3+ induction in all five experiments are presented. Lines connect the sets of experiments done in parallel. (c) Percentage reduction of iTreg cell induction in CD4+ T cells treated with PTPN22-specific anti-sense oligonucleotides compared to CTRL oligos at 3, 4 and 5 days after culture [(%CD4+CD25+FoxP3+ T cells CTRL oligos culture-%CD4+CD25+FoxP3+ T cells PTPN22-oligos culture)/%CD4+CD25+FoxP3+ T cells CTRL oligos culture]. Each symbol represents one donor. Bars represent standard deviation. **P < 0·005, paired t-test.
Fig 4.
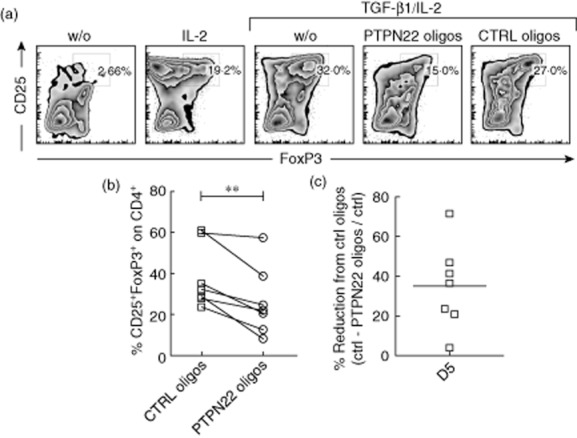
PTPN22 down-modulation reduces in vitro forkhead box protein 3 (FoxP3)+ T regulatory cell induction in CD4+ T cells from patients with type 1 diabetes (T1D). (a) CD4+CD127+CD25− T cells from patients with established T1D were cultured under in vitro-induced (iTreg) and control conditions as described in Materials and methods. PTPN22-specific and control (CTRL) oligonucleotides were added to the culture. CD25 and intracellular FoxP3 were evaluated on gated CD4+ T cells after 5 days of culture. One representative experiment out of five is shown. (b) Percentages of CD4+CD25+FoxP3+ induction in all seven donors with T1D are presented. Lines connect the sets of experiments done in parallel. (c) Percentage reduction of iTreg cell induction in CD4+ T cells treated with PTPN22-specific anti-sense oligonucleotides compared to CTRL oligos 5 days after culture [(%CD4+CD25+FoxP3+ T cells CTRL oligos culture-%CD4+CD25+FoxP3+ T cells PTPN22-oligos culture)/%CD4+CD25+FoxP3+ T cells CTRL oligos culture]. Each open square represents one donor. *P < 0·05.
Ptpn22 determines murine CD4+ T cell expansion and pTreg development in vivo
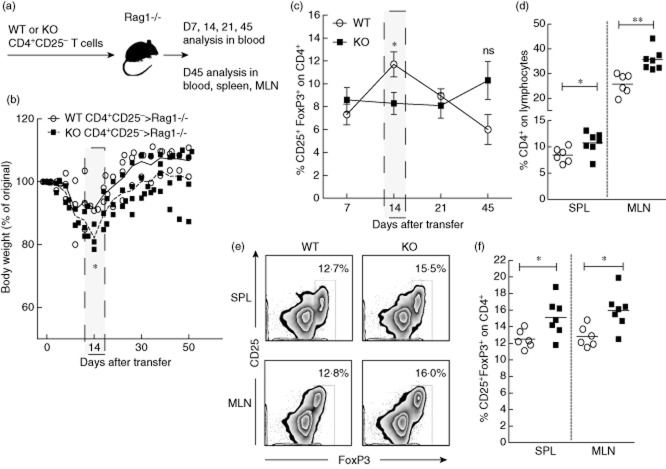
Ptpn22KO mice have increased frequency of FoxP3-expressing Treg cells in secondary lymphoid organs, spleen and lymph nodes (26,27 and our unpublished observations). Enhanced conversion of naive CD4+ T cells into FoxP3+ pTreg cells could have contributed to their accumulation or an increased rate of thymic production and/or turnover in the periphery. To assess whether Ptpn22 also affects FoxP3+ pTreg development in vivo, we compared the ability of CD4+ T cells from Ptpn22KO and WT mice to differentiate into pTreg cells in an in vivo model of lymphopenia-induced differentiation 48. CD4+CD25− T cells purified from 8–12-week-old Ptpn22KO and WT mice were labelled with eFluor670 and injected intravenously into Rag1–/– mice (Fig. 5a). Cells from Ptpn22KO and WT mice proliferated in the peripheral blood to a similar extent in the first 3 and 7 days after transfer (data not shown). As this model of pTreg cell conversion results in mild and transient inflammatory bowel disease (IBD) 48, the weight of recipient mice was recorded. Rag1–/– mice that received T cells from Ptpn22KO mice showed more weight loss 14 days after transfer (Fig. 5b). In line with this, CD4+CD25− T cells from Ptpn22KO mice converted into FoxP3+ pTreg cells less efficiently than those from WT mice 14 days after transfer. However, a trend of increased circulating pTreg cell frequency in mice receiving cells from Ptpn22KO mice was observed 45 days after transfer (Fig. 5c).
Fig 5.
Ptpn22 controls CD4+ T cell expansion and accumulation of forkhead box protein 3 (FoxP3)+ peripherally derived T regulatory cells in mice in vivo. (a) CD4+CD25− purified splenocytes from PTPN22 knock-out (PTPN22KO) and wild-type (WT) 8–12-week-old mice were injected intravenously into Rag1–/– recipient mice (1 × 106 cells per mouse total). The frequency of CD4+CD25+ in transferred cells was comparable before injection (input). The presence of WT and KO cells in the blood of the recipients was monitored on day 1 and was comparable (WT, 0·4936 ± 0·04; KO, 0·5663 ± 0·05). (b) Body weight of Rag1–/– recipient mice given 1 × 106 CD4+CD25− T cells from Ptpn22KO or from WT mice. Average weight loss per group is shown with a line. (c) At 7, 14, 21 and 45 days after transfer, CD4+CD25− cells from Ptpn22KO and WT mice were studied with flow cytometry in the peripheral blood of Rag1–/– recipients for in vivo conversion into FoxP3-expressing peripherally derived (p)Treg cells. (d) Forty-five days after injection, the spleen (SPL) and mesenteric lymph nodes (MLN) were analysed by flow cytometry for the expansion of CD4+ T cells. Graph shows results from multiple mice. The up-regulation of CD25 and FoxP3 was used to determine the conversion into FoxP3-expressing pTregs in the SPL and MLN of recipient mice 45 days after transfer. Representative plots in (e) illustrate the proportions of CD25+FoxP3+ peripherally converted Treg cells. Graph in (f) shows results from multiple mice, with open circles representing individual Rag1–/– mice receiving CD4+CD25− cells from WT mice and closed squares from Ptpn22KO donors. *P < 0·05; **P < 0·005.
The frequencies and total numbers of CD4+ T cells and FoxP3+ pTreg cells were also measured in the spleen (SPL) and mesenteric lymph nodes (MLN) 45 days after cell transfer. pTreg cell frequency and number was higher in the absence of Ptpn22, suggesting increased proliferation and/or survival (Fig. 5d–f and Supporting information, Fig. S3a,b). The majority of transferred CD4+ T cells expressed memory-phenotype due probably to homeostatic expansion, but no differences in the proportion of memory cells between Ptpn22KO and WT cell transfers were seen (data not shown).
Treg cells exert their suppressive functions through various mechanisms, some of which include cell-to-cell contact and cytokine secretion 49. Phenotypical analysis of Ptpn22KO and WT pTreg cells showed similar expression of CD25, CD103 and CTLA-4 (data not shown). Furthermore, Ptpn22KO and WT FoxP3+ (and FoxP3−) CD4+ T cells produced similar amounts of IFN-γ and IL-10 (Supporting information, Fig. S4). Thus, despite differences in their frequency, Ptpn22KO and WT pTreg cells had comparable phenotype and function. In conclusion, 14 days after transfer, CD4+ T cells from Ptpn22KO mice convert into FoxP3+ pTreg cells less efficiently than do those from WT mice. Accordingly, recipient mice develop exacerbated IBD. Later, however, pTreg cell conversion peaks in Ptpn22KO mice, coinciding with weight gain and disease amelioration.
Discussion
In this study we report that PTPN22 is key for the induction of FoxP3+ Treg cells in vitro and in vivo, whereas it has no significant effect on Th1 cell differentiation. Our findings indicate that PTPN22 is critical in setting the threshold of TCR activation and, as a consequence, it modulates the levels of FoxP3 induction, while it does not significantly impact Th1 cell polarization. We found that CD4+ T cells from Ptpn22KO mice develop into iTreg cell more efficiently at low levels of TCR activation at three consecutive time-points, in agreement with previous studies 26. At higher levels of TCR activation, however, the absence of Ptpn22 reduces FoxP3 induction by overactivating the T cells. Under optimal/strong TCR-activating conditions, human CD4+ T cells treated with anti-sense PTPN22-specific oligonucleotides are also less competent in up-regulating FoxP3. PTPN22 also affects the kinetic of FoxP3 expression in vivo; CD4+ T cells from Ptpn22KO mice convert less efficiently into pTreg cells initially but expand and accumulate overall at higher numbers compared to WT. In contrast to iTreg cells, in vitro Th1 cell polarization is not altered significantly in the absence of Ptpn22, except at very low doses of T cell activation.
In vivo pTreg cell formation is mediated by signals derived from the TCR, but also from cytokine receptors 49. Based on the available reported data 23, the absence of Ptpn22 probably altered the kinetic of FoxP3+ pTreg cell formation by modulating the TCR-activation levels, rather than affecting other signalling pathways. However, as the levels of TCR activation can also influence the generation of T effector (Teff) cells it is possible that, in the absence of Ptpn22, an excessive Teff cell expansion affected pTreg cell formation indirectly, i.e. by outcompeting for space and/or cytokines. Whether pTreg formation in Ptpn22KO mice is controlled by such or other mechanism(s) is currently unknown.
Rag1–/– mice receiving CD4+ T cells from Ptpn22KO mice transiently develop worse IBD than CD4+ T cells from WT donors at the peak of expansion. Later, however, the conversion rate and/or survival of pTreg cells increases, tipping the balance towards a sustainable Teff to Treg cell ratio. These results suggest that despite the delayed kinetic, pTreg cells from Ptpn22KO mice are probably functional. The fact that PEP has a double effect on pTreg cell formation points to a ‘yin/yang’ role for the phosphatase in T cell tolerance and corroborates the notion that additional defects in other tolerogenic mechanisms are probably required to trigger clinical autoimmunity in PTPN22 R620W human carriers. Despite this, given that protein similarity between mouse and human PTPN22 is less than 80%, PTPN22 might affect iTreg cell formation in humans differently.
It was described recently that PTPN22 is key for driving type I IFN response in myeloid cells. PEP was required for full induction of type I IFNs and, as a consequence, Ptpn22KO mice produced decreased numbers of LCMV-specific CD8+ cytotoxic lymphocytes (CTLs) at the peak of the LCMV response 50. The type I IFN response is essential to many viruses such as LCMV, but seems to affect predominantly the generation of CTLs rather than the CD4+ virus-specific Teff cells 51,52. We found no differences in the in vivo differentiation of LCMV-specific Th1 cells, but we could confirm the reduction in GP33–41 CTLs (data not shown). Based on these findings, we can presume that the reduction in GP33–41-specific CTLs in this viral infection model is independent of Th1 cells.
Finally, in this study we show that in vitro Th1 cell development is not significantly different in the absence of Ptpn22, except at very low levels of anti-CD3/28 mAb activation. Interestingly, at low-to-intermediate levels of TCR activation, the absence of Ptpn22 favours FoxP3 Treg cell induction. These findings suggest that, in certain situations, Ptpn22KO mice might result as more tolerant, depending on the Th1/Treg cell ratio. Interestingly, Ptpn22KO mice were shown to be resistant to experimental autoimmune encephalomyelitis (EAE) 27, but susceptible to dextran sulphate sodium (DSS)-induced colitis 50,53. While both diseases are mediated mainly by Th17 cells, it is unclear how the lack of Ptpn22 protected from EAE but exacerbated DSS colitis. In this study we also showed that cytokines, and particularly IL-10, is produced at similar levels by Ptpn22KO and WT iTreg cells. These results indicate that Ptpn22KO iTreg cells might possess similar suppression properties to WT iTreg cells.
In summary, our results demonstrate that PTPN22 is key for inducible Treg cell generation and that it acts mainly through modulating the threshold of T cell activation. Lack of Ptpn22 alters Th1 cell differentiation only at low levels of activation, providing a possible explanation for the expanding natural memory T cell pool that characterizes Ptpn22KO mice 22.
Acknowledgments
This work was supported by the following grants: Giovani Ricercatori 2007 (D.lgs 502/92) from the Italian Ministry of Health to M. B., Marie-Curie Reintegration grant FP7 PEOPLE to G. F. (276745) and NIH R01AI070544 to N. B. G. F. and S. M. S. are supported by JDRF postdoctoral fellowships. We would like to thank all members of M. Battaglia's, M. G. Roncarolo's and M. von Herrath's laboratory, especially Andrea Valle, Cristina Morsiani, Grazia Andolfi and Philippe Pagni. We would like to acknowledge the excellent sorting service by the San Raffaele Research Institute's flow cytometry facility. The online version of this paper contains supplementary material.
Disclosures
The authors have no financial conflicts of interest.
Author contributions
G. F. designed, performed experiments, analysed the data and wrote the paper. T. J., I. D. and S. S. performed some of the experiments. A. L. collected human peripheral blood. N. B. provided human PTPN22-specific oligonucleotides, assisted with the interpretation of the results and corrected the paper. M. B. supervised the study and wrote the paper. All authors read the manuscript and contributed to the discussion of the findings.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. PTPN22 affects forkhead box protein 3 (FoxP3) induction by inhibiting T cell activation in mice. CD4+CD25− T cells from 8–12-week-old wild-type (WT) or PTPN22 knock-out (PTPN22KO) mice were cultured under in vitro-induced regulatory T cell (iTreg) polarizing conditions with increasing amounts of anti-CD3/CD28 monoclonal antibodies (mAbs). (a) Histogram overlay depicting the levels of CTLA-4 in WT and PTPN22KO CD4+ T cells 4 days after culture. Blue line indicates WT cells, whereas red indicates KO. (b) The mean fluorescence intensity (MFI) levels of cytotoxic T lymphocyte antigen 4 (CTLA-4) in WT and KO cells depicted in (c) shown in a graph. Results from one representative experiment are shown. (c) Cumulative data showing the % difference in CD25+FoxP3+ induction between WT and KO cells from 10 independent experiments are displayed in a graph. Each coloured line indicates results from independent experiments. In some experiments, naive T cells (CD4+CD62Lhi) or cells from young animals (>6 weeks old) were cultured (indicated in the figure, including the experiment that was selected as representative in Fig. 1). Not all anti-CD3/28 activating conditions were tested in each experiment.
Fig. S2. Anti-sense PTPN22-specific oligonucleotide treatment reduces by 50% the endogenous PTPN22 expression levels in human in vitro-induced regulatory T cell (iTreg) cultures. (a) Human CD4+CD127+CD25− T cells from healthy donors (HD) were fluorescence activated cell sorted (FACS) and cultured under iTreg polarizing conditions as described in Materials and methods. PTPN22-specific and control (CTRL) oligonucleotides were added at the beginning of iTreg cultures with CD4+ T cells derived from HD. Expression levels of PTPN22 were evaluated 2 days later by real-time quantitative polymerase chain reaction (qPCR), P < 0·05. (b) Percentage reduction of PTPN22 expression levels in human CD4+ T cells compared to CTRL oligos. Each open square represents individual donor.
Fig. S3. CD4+ T cell expansion and regulatory T cell (Treg) cell development upon lymphopenic expansion in the absence of PTPN22. (a,b) Forty-five days after transfer into lymphopenic B6.Rag1–/– hosts, CD4+CD25− T cells from PTPN22 WT and PTPN22 knock-out (PTPN22KO) mice were studied for expansion (total number of CD4+ T cells) and peripheral (p)Treg conversion in the spleen (SPL) and mesenteric lymph nodes (MLN). Each symbol represents individual animal and horizontal bar shows the mean of values. *P < 0·05; **P < 0·005.
Fig. S4. Cytokine production by peripherally induced regulatory T cells (Treg) and effector T cells (Teff) in the absence of PTPN22. (a,b) Forty-five days after transfer into lymphopenic B6.Rag1–/– hosts, CD4+CD25− cells from PTPN22.WT and PTPN22 knock-out (PTPN22KO) mice converted into forkhead box protein 3 (FoxP3)-expressing peripherally derived (p)Tregs and memory cells in the spleen (SPL) and mesenteric lymph nodes (MLN). Total lymphocytes were activated with leucocyte activation cocktail (LAC) prior to staining intra-cytoplasmatically for FoxP3 and interferon (IFN)-γ (a,b) or interleukin (IL)-10 (c,d) anti-cytokine antibodies. One representative experiment of two with similar results is shown in (a) and (c) after gating on total CD4+ T cells. Each dot represents individual mice in (b) and (d) and horizontal bar shows the mean of values.
Table S1. Information on healthy donors (HD) used for this study.
Table S2. Information on type 1 diabetic subjects used for study.
References
- Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Edwards B, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri G, Jasinski J, Dave A, et al. Following the fate of one insulin-reactive CD4 T cell: conversion into Teffs and Tregs in the periphery controls diabetes in NOD mice. Diabetes. 2012;61:1169–1179. doi: 10.2337/db11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu XZ. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. 2011;117:3096–3103. doi: 10.1182/blood-2010-08-301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- Soligo M, Camperio C, Caristi S, et al. CD28 costimulation regulates FOXP3 in a RelA/NF-kappaB-dependent mechanism. Eur J Immunol. 2011;41:503–513. doi: 10.1002/eji.201040712. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Ouaked N, Ruckert B, et al. Molecular mechanisms underlying Foxp3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- Fousteri G, Liossis SN, Battaglia M. Roles of the protein tyrosine phosphatase PTPN22 in immunity and autoimmunity. Clin Immunol. 2013;149:556–565. doi: 10.1016/j.clim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Vang T, Congia M, Macis MD, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- Veillette A, Rhee I, Souza CM, Davidson D. PEST family phosphatases in immunity, autoimmunity, and autoinflammatory disorders. Immunol Rev. 2009;228:312–324. doi: 10.1111/j.1600-065X.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett. 2011;585:3689–3698. doi: 10.1016/j.febslet.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- Stanford SM, Rapini N, Bottini N. Regulation of TCR signalling by tyrosine phosphatases: from immune homeostasis to autoimmunity. Immunology. 2012;137:1–19. doi: 10.1111/j.1365-2567.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, James RG, Habib T, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zahir N, Jiang Q, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- Brownlie RJ, Miosge LA, Vassilakos D, Svensson LM, Cope A, Zamoyska R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci Signal. 2012;5 doi: 10.1126/scisignal.2003365. : ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine C, Hamilton-Williams E, Cheung J, et al. PTPN22 alters the development of regulatory T cells in the thymus. J Immunol. 2012;188:5267–5275. doi: 10.4049/jimmunol.1200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Kissler S. PTPN22 Silencing in the NOD model indicates the type 1 diabetes-associated allele is not a loss-of-function variant. Diabetes. 2013;62:896–904. doi: 10.2337/db12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo E, Orru V, Stanford SM, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010;285:26506–26518. doi: 10.1074/jbc.M110.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- Vang T, Liu WH, Delacroix L, et al. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang T, Landskron J, Viken MK, et al. The autoimmune-predisposing variant of lymphoid tyrosine phosphatase favors T helper 1 responses. Hum Immunol. 2013;74:574–585. doi: 10.1016/j.humimm.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Nuclear GS. factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Sauer S, Bruno L, Hertweck A, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- Fousteri G, Dave A, Juedes A, et al. Increased memory conversion of naive CD8 T cells activated during late phases of acute virus infection due to decreased cumulative antigen exposure. PLOS ONE. 2011;6:e14502. doi: 10.1371/journal.pone.0014502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri G, Dave A, Juntti T, von Herrath M. CD103 is dispensable for anti-viral immunity and autoimmunity in a mouse model of virally-induced autoimmune diabetes. J Autoimmun. 2009;32:70–77. doi: 10.1016/j.jaut.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro A, Socci C, Stabilini A, et al. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes. 2011;60:2903–2913. doi: 10.2337/db11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SM, Panchal RG, Walker LM, et al. High-throughput screen using a single-cell tyrosine phosphatase assay reveals biologically active inhibitors of tyrosine phosphatase CD45. Proc Natl Acad Sci USA. 2012;109:13972–13977. doi: 10.1073/pnas.1205028109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- Homann D, Lewicki H, Brooks D, et al. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology. 2007;363:113–123. doi: 10.1016/j.virol.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa G, Zheng L, Medvec A, et al. TCR affinity and specificity requirements for human regulatory T-cell function. Blood. 2012;119:3420–3430. doi: 10.1182/blood-2011-09-377051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SA, Rieck M, Tatum M, et al. Low-dose antigen promotes induction of FOXP3 in human CD4+ T cells. J Immunol. 2011;187:3511–3520. doi: 10.4049/jimmunol.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille M, Lafaille J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shaked I, Stanford SM, et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity. 2013;39:111–122. doi: 10.1016/j.immuni.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel M, Crouse J, Bedenikovic G, Sutherland A, Joller N, Type-I OA. IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur J Immunol. 2012;42:320–329. doi: 10.1002/eji.201142091. [DOI] [PubMed] [Google Scholar]
- Zuniga EI, Hahm B, Oldstone MB. Type I interferon during viral infections: multiple triggers for a multifunctional mediator. Curr Top Microbiol Immunol. 2007;316:337–357. doi: 10.1007/978-3-540-71329-6_16. [DOI] [PubMed] [Google Scholar]
- Chang HH, Miaw SC, Tseng W, et al. PTPN22 modulates macrophage polarization and susceptibility to dextran sulfate sodium-induced colitis. J Immunol. 2013;191:2134–2143. doi: 10.4049/jimmunol.1203363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. PTPN22 affects forkhead box protein 3 (FoxP3) induction by inhibiting T cell activation in mice. CD4+CD25− T cells from 8–12-week-old wild-type (WT) or PTPN22 knock-out (PTPN22KO) mice were cultured under in vitro-induced regulatory T cell (iTreg) polarizing conditions with increasing amounts of anti-CD3/CD28 monoclonal antibodies (mAbs). (a) Histogram overlay depicting the levels of CTLA-4 in WT and PTPN22KO CD4+ T cells 4 days after culture. Blue line indicates WT cells, whereas red indicates KO. (b) The mean fluorescence intensity (MFI) levels of cytotoxic T lymphocyte antigen 4 (CTLA-4) in WT and KO cells depicted in (c) shown in a graph. Results from one representative experiment are shown. (c) Cumulative data showing the % difference in CD25+FoxP3+ induction between WT and KO cells from 10 independent experiments are displayed in a graph. Each coloured line indicates results from independent experiments. In some experiments, naive T cells (CD4+CD62Lhi) or cells from young animals (>6 weeks old) were cultured (indicated in the figure, including the experiment that was selected as representative in Fig. 1). Not all anti-CD3/28 activating conditions were tested in each experiment.
Fig. S2. Anti-sense PTPN22-specific oligonucleotide treatment reduces by 50% the endogenous PTPN22 expression levels in human in vitro-induced regulatory T cell (iTreg) cultures. (a) Human CD4+CD127+CD25− T cells from healthy donors (HD) were fluorescence activated cell sorted (FACS) and cultured under iTreg polarizing conditions as described in Materials and methods. PTPN22-specific and control (CTRL) oligonucleotides were added at the beginning of iTreg cultures with CD4+ T cells derived from HD. Expression levels of PTPN22 were evaluated 2 days later by real-time quantitative polymerase chain reaction (qPCR), P < 0·05. (b) Percentage reduction of PTPN22 expression levels in human CD4+ T cells compared to CTRL oligos. Each open square represents individual donor.
Fig. S3. CD4+ T cell expansion and regulatory T cell (Treg) cell development upon lymphopenic expansion in the absence of PTPN22. (a,b) Forty-five days after transfer into lymphopenic B6.Rag1–/– hosts, CD4+CD25− T cells from PTPN22 WT and PTPN22 knock-out (PTPN22KO) mice were studied for expansion (total number of CD4+ T cells) and peripheral (p)Treg conversion in the spleen (SPL) and mesenteric lymph nodes (MLN). Each symbol represents individual animal and horizontal bar shows the mean of values. *P < 0·05; **P < 0·005.
Fig. S4. Cytokine production by peripherally induced regulatory T cells (Treg) and effector T cells (Teff) in the absence of PTPN22. (a,b) Forty-five days after transfer into lymphopenic B6.Rag1–/– hosts, CD4+CD25− cells from PTPN22.WT and PTPN22 knock-out (PTPN22KO) mice converted into forkhead box protein 3 (FoxP3)-expressing peripherally derived (p)Tregs and memory cells in the spleen (SPL) and mesenteric lymph nodes (MLN). Total lymphocytes were activated with leucocyte activation cocktail (LAC) prior to staining intra-cytoplasmatically for FoxP3 and interferon (IFN)-γ (a,b) or interleukin (IL)-10 (c,d) anti-cytokine antibodies. One representative experiment of two with similar results is shown in (a) and (c) after gating on total CD4+ T cells. Each dot represents individual mice in (b) and (d) and horizontal bar shows the mean of values.
Table S1. Information on healthy donors (HD) used for this study.
Table S2. Information on type 1 diabetic subjects used for study.