Somatic mutations (for example, CALR, ASXL1) and karyotype have been shown to independently influence survival in patients with myelofibrosis (MF).1, 2 The objectives of the current study were to determine if such genetic markers also influence treatment response in MF patients receiving single agent momelotinib and whether such therapy overcomes the detrimental effect of prognostically relevant mutations in MF. Momelotinib is a Janus kinase (JAK)-1/2 inhibitor currently being evaluated in phase-3MF clinical trials (NCT01969838, NCT02101268). In an earlier phase-1/2 study (n=166), the drug was shown to improve anemia (53% response rate), reduce spleen size (39% response rate) and alleviate constitutional symptoms (>50% response rate) in MF patients.3 The current study considers 100 consecutive patients, who were part of the aforementioned phase-1/2 study and received momelotinib therapy at the Mayo Clinic.
The protocol was approved by the Mayo Clinic institutional review board. Diagnosis of MF was per the 2008 World Health Organization criteria.4 Subjects for the current study were recruited at our center from those enrolled in the open-label, non-randomized study CCL09101 (NCT00935987). All subjects provided written informed consent for blood sample collection for research use. The archived samples were interrogated for specific MPN-relevant mutations and molecular data examined for correlation with treatment response and clinical outcome as per an unplanned sponsor-independent study analysis conducted in July 2014.
Study CCL09101 was conducted in two phases from November 2009 to April 2012: a single-center (Mayo Clinic) dose-escalation phase with supernumerary patient addition (Part 1), to determine the safety and tolerability of momelotinib, and to identify a therapeutic dose for the second phase, and a multicenter dose-confirmation phase (Part 2), with cohort expansion at or below the maximum tolerated dose. Momelotinib capsules were administered orally once daily with a treatment plan for continuous therapy for 36 weeks (nine × 28-day cycles). Intra-patient dose escalation was permitted after completion of at least three cycles at the starting dose. Treatment beyond nine cycles was permitted on an extension study (CCL09101E; NCT01236638) if deemed beneficial to the patient and if well tolerated.
Results from Part 1 of the study (CCL09101) have been previously published.5 The published report includes details regarding study eligibility criteria and assessment of toxicity and treatment response. Specifically, responses were measured every 4 weeks per the 2006 IWG-MRT criteria;6 clinical data including palpable spleen and liver size and details regarding packed red blood cell (RBC) transfusions were recorded at every study visit. Interim data for the overall study (Parts 1 and 2) have been previously reported in abstract form.3 Previously published methods were used for mutation analyses.7
All statistical analyses considered clinical and laboratory parameters obtained at the time of entry to study CCL09101. Differences in the distribution of continuous variables between categories were analyzed by either Mann–Whitney or Kruskal–Wallis test. Patient groups with nominal variables were compared by chi-square test. Survival analysis was considered from the date of study entry to the date of death (uncensored) or last contact (censored). Leukemia-free survival calculations considered leukemic transformation as the uncensored variable. Survival curves were prepared by the Kaplan–Meier method and compared by the log-rank test. Cox proportional hazard regression model was used for multivariate analysis of survival. Logistic regression methods were used for multivariable analysis of response predictors. P values <0.05 were considered significant. The Stat View (SAS Institute, Cary, NC, USA) statistical package was used for all calculations.
One hundred patients with intermediate- or high-risk MF were enrolled in study CCL09101 at our site (60 and 40 patients in Parts 1 and 2, respectively); 64 patients had PMF, 22 post-polycythemia vera MF and 14 post-essential thrombocythemia MF. Detailed demographic, clinical and laboratory characteristics of this cohort are presented in Table 1. Twenty-one patients (21%) had been previously treated with an alternative JAK inhibitor; other treatments included thalidomide in 11 patients, pomalidomide in 18 patients and lenalidomide in 11 patients. The momelotinib starting dose was 100 mg/day (n=3), 150 mg/day (n=21), 150 mg twice daily (n=20), 200 mg/day (n=3), 300 mg/day (n=47) and 400 mg/day (n=6). The maximum momelotinib dose achieved was 150 mg/day (n=6), 150 mg twice daily (n=20), 300 mg/day (n=68) and 400 mg/day (n=6). Seventy-one patients (71%) required dose reduction at some point during treatment. After a median (range) follow-up of 36 months (1–52), 83 patients (83%) had discontinued momelotinib treatment. During the follow-up period, 57 (57%) deaths and 12 (12%) leukemic transformations were recorded.
Table 1. Clinical and laboratory characteristics of momelotinib-treated patients stratified by JAK2V617F, CALR exon 9, MPL exon 10 and ASXL1 mutation status.
| Variables | All patients (n=100) | JAK2+ (n=73; 73%) | CALR+ (n=16; 16%) | MPL+ (n=7; 7%) | Triple negative (n=4; 4%) | P-value | CALR+ (n=16; 19%) | CALR-/ASXL1- (n=41; 49%) | CALR-/ASXL1+ (n=27; 32%) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age in years; median (range) | 66 (34–89) | 65 (34–89) | 68 (47–78) | 72 (66–77) | 71 (49–75) | 0.2 | 68 (47–78) | 65 (34–86) | 68 (47–85) | 0.5 |
| Age >65 years; n (%) | 54 (54%) | 35 (48%) | 9 (56%) | 7 (100%) | 3 (75%) | 0.051 | 9 (56%) | 20 (49%) | 16 (59%) | 0.7 |
| Males (%) | 58 (58%) | 44 (60%) | 8 (50%) | 3 (43%) | 3 (75%) | 0.6 | 8 (50%) | 27 (66%) | 16 (59%) | 0.5 |
| Hemoglobin, g/dl; median (range); non-transfusion dependent at baseline=51 | 10.5 (8.0–14.4) | 10.8 (8.0–14.4), n=40 | 10.0 (8.4–13.6), n=9 | 9.0, n=1 | 9.9, n=1 | 0.2 | 10.0 (8.4–13.6), n=9 | 10.8 (8.8–14.0), n=18 | 11.3 (9.0–14.4), n=14 | 0.2 |
| Leukocytes, × 109/l; median (range) | 12.3 (1.5–232.0) | 15.8 (2.1–232.0) | 7.5 (1.5–23.7) | 13.4 (2.5–31.2) | 3.4 (3.0–6.2) | 0.005 | 7.5 (1.5–23.7) | 10.1 (2.1–94.7) | 21.8 (2.5–122.4) | 0.006 |
| Platelets, × 109/l; median (range) | 162 (50–738) | 159 (50–738) | 184 (65–596) | 310 (105–657) | 110 (73–291) | 0.3 | 184 (65–596) | 140 (50–691) | 161 (51–521) | 0.9 |
| Circulating blast % median (range) | 1 (0–14) | 1 (0–13) | 1 (0–4) | 1 (0–14) | 1 (0–5) | 0.8 | 1 (0–4) | 1 (0–14) | 1 (0–8) | 1.0 |
| DIPSS-plus risk group | 0.3 | 0.2 | ||||||||
| Intermediate- 1 | 1 (1%) | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Intermediate- 2 | 36 (36%) | 26 (36%) | 9 (56%) | 0 | 1 (25%) | 9 (56%) | 12 (29%) | 10 (37%) | ||
| High | 63 (63%) | 46 (63%) | 7 (44%) | 7 (100%) | 3 (75%) | 7 (44%) | 29 (71%) | 17 (63%) | ||
| Constitutional symptoms; n (%) | 58 (58%) | 47 (64%) | 7 (44%) | 2 (29%) | 2 (50%) | 0.2 | 7 (44%) | 25 (61%) | 14 (52%) | 0.5 |
| Circulating blasts ⩾1% n (%) | 72 (72%) | 49 (67%) | 14 (88%) | 6 (86%) | 3 (75%) | 0.3 | 14 (88%) | 25 (61%) | 22 (81%) | 0.06 |
| Hemoglobin <10 g/dl; non-transfusion dependent at baseline=51; n (%) | 19 (37%), n=51 | 13 (33%), n=40 | 4 (44%), n=9 | 1 (100%), n=1 | 1 (100%), n=1 | 0.3 | 4 (44%), n=9 | 6 (33%), n=18 | 4 (29%), n=14 | 0.7 |
| Transfusion requiring; n (%) | 49 (49%) | 33 (45%) | 7 (44%) | 6 (86%) | 3 (75%) | 0.1 | 7 (44%) | 23 (56%) | 13 (48%) | 0.7 |
| Leukocytes >25 × 109/l; n (%) | 28 (28%) | 26 (36%) | 0 | 2 (29%) | 0 | 0.02 | 0 | 11 (27%) | 12 (44%) | 0.007 |
| Platelets <100 × 109/l; n (%) | 27 (27%) | 22 (30%) | 3 (19%) | 0 | 2 (50%) | 0.2 | 3 (19%) | 13 (32%) | 8 (30%) | 0.6 |
| ASXL1-mutated; n (%); n=84 | 35 (42%) | 25 (43%), n=58 | 8 (50%), n=16 | 2 (33%), n=6 | 0, n=4 | 0.3 | n/a | n/a | n/a | n/a |
| SRSF2-mutated; n (%); n=78 | 14 (17%) | 9 (17%), n=54 | 1 (7%), n=15 | 2 (40%), n=5 | 2 (50%), n=4 | 0.1 | 1 (7%), n=15 | 8 (22%), n=37 | 5 (19%), n=26 | 0.4 |
| IDH1/2-mutated; n (%); n=53 | 2 (4%) | 1 (3%), n=39 | 0, n=11 | 0, n=1 | 1 (50%), n=2 | 0.006 | 0, n=11 | 2 (8%), n=24 | 0, n=18 | 0.3 |
| U2AF1-mutated; n (%); n=46 | 3 (7%) | 3 (11%), n=27 | 0, n=13 | 0, n=3 | 0, n=3 | 0.5 | 0, n=13 | 1 (5%), n=22 | 2 (18%), n=11 | 0.2 |
| Cytogenetic categories (revised); n (%); n=100 | 0.054 | 0.09 | ||||||||
| Favorable risk | 83 (83%) | 59 (81%) | 16 (100%) | 4 (57%) | 4 (100%) | 16 (100%) | 33 (80%) | 20 (74%) | ||
| Unfavorable risk | 17 (17%) | 14 (19%) | 0 | 3 (43%) | 0 | 0 | 8 (20%) | 7 (26%) | ||
| Transfusion-independence response (evaluable=49); n (%) | 25 (51%) | 15 (45%), n=33 | 4 (57%), n=7 | 4 (66%), n=6 | 1 (33%), n=3 | 0.7 | 4 (57%), n=7 | 12 (52%), n=23 | 4 (31%), n=13 | 0.4 |
| Anemia response (evaluable=68); n (%) | 30 (44%) | 20 (43%), n=46 | 4 (36%), n=11 | 4 (57%), n=7 | 2 (50%), n=4 | 0.8 | 4 (36%), n=11 | 14 (48%), n=29 | 4 (24%), n=17 | 0.2 |
| Spleen responsea (evaluable=91); n (%) | 39 (43%) | 23 (34%), n=67 | 11 (73%), n=15 | 3 (60%), n=5 | 2 (50%), n=4 | 0.04 | 11 (73%), n=15 | 16 (43%), n=37 | 6 (25%), n=24 | 0.01 |
| Deaths; n (%) | 57 (57%) | 46 (63%) | 4 (25%) | 4 (57%) | 3 (75%) | n/ab | 4 (25%) | 23 (56%) | 20 (74%) | n/ab |
| Documented leukemic transformation; n (%) | 12 (12%) | 7 (10%) | 1 (6%) | 2 (29%) | 2 (50%) | n/ab | 1 (6%) | 8 (20%) | 2 (7%) | n/ab |
Abbreviations: ASXL1, Additional Sex Combs Like 1 mutations; CALR, CALR exon 9 indel mutations; DIPSS, Dynamic International Prognostic Scoring System; IDH-1/2, isocitrate dehydrogenase 1 and 2 mutations; JAK2, JAK2V617F mutation; MPL, MPL exon 10 mutations; n, number of patients; n/a, not applicable; SRSF2, serine/arginine-rich splicing factor 2 mutations; U2AF1, U2 small nuclear RNA auxiliary factor mutations.
Includes four previously splenectomized patients who were evaluable based on treatment-related reduction in palpable liver size.
Time-dependent variable; see Kaplan–Meier and Cox proportional hazards analysis.
Eighty-seven patients were evaluable for spleen response and in addition four splenectomized patients were evaluable for liver response; the median (range) palpable spleen size at baseline was 19 cm (6–32). Sixty-eight patients (68%) were evaluable for anemia response; of these 49 patients (49%) were RBC transfusion-dependent at study enrollment. Thirty-eight patients achieved a spleen response and one of four splenectomized patients a liver response, for an overall response rate of 43%. Thirty patients (44%) achieved an anemia response, including 25 who were RBC transfusion-dependent at baseline.
Spleen response was correlated with JAK2/MPL/CALR and CALR/ASXL1 mutational status, with the greatest benefit conferred by CALR mutated status (Table 1), and, additionally, by smaller palpable spleen (57% vs ⩾30% response rate for less or more than the median spleen size; P=0.02) and constitutional symptoms (absent 57% vs present 34% P=0.049). On multivariable analysis, only CALR mutated (hazard ratio (HR)=0.2, 95% confidence interval (CI)=0.04–0.6) and ASXL1 unmutated (HR=0.3, 95% CI=0.1–0.8) status were independently associated with spleen response. Anemia response was not correlated with mutational status (Table 1), baseline karyotype or other identifiable clinical parameter. However, anemia response in RBC transfusion-dependent patients (n=49) was correlated with DIPSS-plus status (intermediate-2 100% vs high 41% P=0.004), baseline karyotype (normal 70% vs abnormal 38% P=0.04) and platelet count (⩾100 × 109/l 59% vs <100 × 109/l 25% P=0.05).
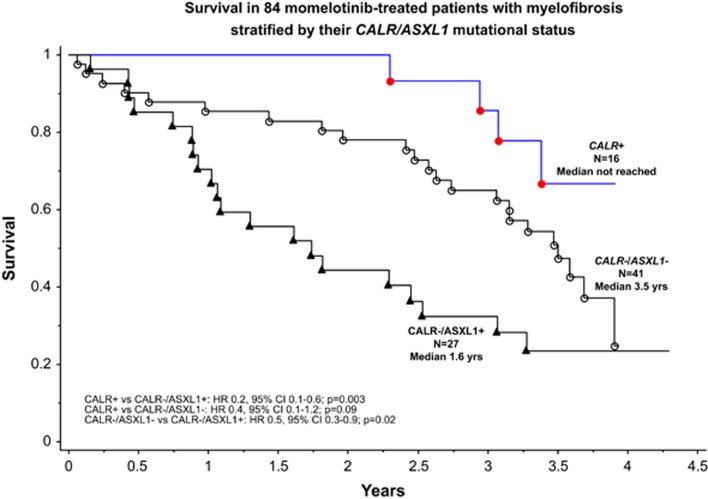
Absence of CALR (HR=3.4, 95% CI=1.2–9.4), and presence of ASXL1 (HR=2.0, 95% CI=1.1–3.5) or SRSF2 (HR=2.6, 95% CI=1.3–5.0) mutations was associated with inferior survival, independent of age >65 years or DIPSS-plus risk status. Patients who were CALR+ (HR=0.2, 95% CI=0.07–0.6) or CALR-/ASXL1− (HR=0.5, 95% CI=0.3–0.9) lived longer than those with CALR-/ASXL1+ mutational status (Figure 1). This relationship was independent of SRSF2 mutation status (HR=0.2, 95% CI=0.1–0.7 and HR=0.5, 95% CI=0.3–0.96, respectively), DIPSS-plus risk status (HR=0.2, 95% CI=0.1–0.7 and HR=0.5, 95% CI=0.3–0.9, respectively) or momelotinib dose (HR=0.3, 95% CI=0.1–0.9 and HR=0.5, 95% CI=0.3–0.99, respectively). CALR/ASXL1 mutational status did not correlate with leukemia-free survival, which was, however, inferior in ‘triple-negative (JAK2-/CALR-/MPL−)' vs CALR+ patients (HR=13.4, 95% CI=1.2–153).
Figure 1.
Kaplan–Meier survival curves of 84 myelofibrosis patients treated with single agent momelotinib stratified by CALR/ASXL1 mutation status. Survival was calculated from the time of study entry.
Our observations on survival are consistent with recent reports in the general population of patients with MF.1, 7, 8 In other words, momelotinib treatment was unable to overcome the negative prognostic impact of mutational status in MF. This was also the case with ruxolitinib, another JAK-1/2 inhibitor.9 However, in contrast to our observation with momelotinib, ruxolitinib-induced spleen response in the latter study, which predated the discovery of CALR mutations, was reported to be independent of mutational status.9 Regardless, the data from the current study require confirmation from the ongoing larger phase-3 studies of momelotinib therapy in MF. Similarly, it remains to be seen whether our observations are generalizable to other JAK inhibitors. Finally, the basis for the discordance in terms of spleen versus anemia response vis-à-vis CALR mutation status in the current study suggests distinct pathogenetic mechanisms of response.
Acknowledgments
The sponsors (Cytopia, YM Biosciences, Gilead) funded the aforementioned clinical trials and provided the study drug, but were not involved in the current substudy or in manuscript preparation.
Author contributions
AP and AT designed the study, analyzed the data and wrote the first draft of the manuscript; RAA abstracted the clinical data; CF and TTL performed the molecular analysis; KHB, AA-K, WJH and MRL contributed patients/patient samples to the study; CAH reviewed bone marrow histology data; and RPK reviewed the cytogenetic data. All authors reviewed, provided input and approved the final draft of the manuscript.
AT was Principal Investigator for studies CCL09101 and CCL09101E for the Mayo Clinic (Rochester) site. AP and AT are/have served as Principal Investigator for other JAK inhibitor clinical trials for myelofibrosis treatment. The remaining authors declare no conflict of interest.
References
- Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014;124:2507–2513. doi: 10.1182/blood-2014-05-579136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Gotlib J, Gupta V, Roberts A, Wadleigh M, Sirhan S, et al. Update on the long-term efficacy and safety of momelotinib, a JAK1 and JAK2 inhibitor, for the treatment of myelofibrosis. Blood. 2013;122 doi: 10.1038/leu.2017.330. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–1500. doi: 10.1038/leu.2014.57. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Lasho TL, Rotunno G, Score J, Mannarelli C, Pancrazzi A, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014;28:1804–1810. doi: 10.1038/leu.2014.76. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Biamonte F, Rotunno G, Artusi V, Artuso L, Bernardis I, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123:2157–2160. doi: 10.1182/blood-2013-11-536557. [DOI] [PubMed] [Google Scholar]