Abstract
Background:
The neurotransmitter norepinephrine has been implicated in psychiatric and neurodegenerative disorders. Examination of synaptic norepinephrine concentrations in the living brain may be possible with positron emission tomography (PET), but has been hampered by the lack of suitable radioligands.
Methods:
We explored the use of the novel α2C-adrenoceptor antagonist PET tracer [11C]ORM-13070 for measurement of amphetamine-induced changes in synaptic norepinephrine. The effect of amphetamine on [11C]ORM-13070 binding was evaluated ex vivo in rat brain sections and in vivo with PET imaging in monkeys.
Results:
Microdialysis experiments confirmed amphetamine-induced elevations in rat striatal norepinephrine and dopamine concentrations. Regional [11C]ORM-13070 receptor binding was high in the striatum and low in the cerebellum. After injection of [11C]ORM-13070 in rats, mean striatal specific binding ratios, determined using cerebellum as a reference region, were 1.4±0.3 after vehicle pretreatment and 1.2±0.2 after amphetamine administration (0.3mg/kg, subcutaneous). Injection of [11C]ORM-13070 in non-human primates resulted in mean striatal binding potential (BP ND) estimates of 0.65±0.12 at baseline. Intravenous administration of amphetamine (0.5 and 1.0mg/kg, i.v.) reduced BP ND values by 31–50%. Amphetamine (0.3mg/kg, subcutaneous) increased extracellular norepinephrine (by 400%) and dopamine (by 270%) in rat striata.
Conclusions:
Together, these results indicate that [11C]ORM-13070 may be a useful tool for evaluation of synaptic norepinephrine concentrations in vivo. Future studies are required to further understand a potential contribution of dopamine to the amphetamine-induced effect.
Keywords: amphetamine, atipamezole, atomoxetine, monkey, PET
Introduction
The neurotransmitter norepinephrine (NE) has been implicated in several neuropsychiatric disorders. Many currently-used treatments for depression and attention deficit/hyperactivity disorder are based on modulation of synaptic availability of NE, as both classical tricyclic antidepressants and selective NE transporter (NET) inhibitors act by inhibiting the reuptake of NE into presynaptic nerve terminals, and thus increase its concentration in the synapse. Direct measurement of changes in synaptic NE concentrations in the human brain, using microdialysis, is invasive and impractical.
Positron emission tomography (PET) is a relatively non-invasive technique that has, in some cases, been applied to investigate changes in synaptic neurotransmitter concentrations in the living human brain. In the 1990s, it was already demonstrated that receptor binding of dopamine (DA) D2 receptor radioligands can be reduced by 10–30% by amphetamine-induced elevations of synaptic DA concentrations in human subjects (Laruelle et al., 1995; Breier et al., 1997). This application of PET has provided an important understanding of the role of synaptic DA in drug actions (Tiihonen et al., 1996; Brody et al., 2004), in normal neuropsychology (see for review Egerton et al., 2009), as well as in the pathophysiology of addiction (Volkow et al., 1997), schizophrenia (Laruelle et al., 1996), and Parkinson’s disease (Piccini et al., 2003). Recently, encouraging progress has been reported for similar approaches targeted to synaptic serotonin (Finnema et al., 2010) and glutamate (Miyake et al., 2011) concentrations, but PET measurement of synaptic NE has so far remained unexplored in primates.
In comparison to monitoring of synaptic DA with [11C]raclopride, attempts to assess synaptic NE in the brain with PET are complicated by the multitude of adrenoceptor (AR) subtypes present in the mammalian brain. Almost all of the nine AR subtypes are known to be expressed in the central nervous system of humans and other mammals, with different anatomical distributions and variable expression levels. It is not obvious which AR subtype would be the best candidate for purposes of PET imaging. So far, most experience has been gained from attempts to target α2-ARs in the brain (Hume et al., 2000; Jakobsen et al., 2006; Smith, Dyye, et al., 2006; Van der Mey et al., 2006), compared to α1-ARs (Balle et al., 2004) and β-ARs (van Waarde et al., 2008). Application of α2-AR antagonist radioligands for measurement of changes in synaptic NE concentrations has recently been pioneered in pigs (Landau et al., 2012). The subtype non-selective α2-AR antagonist yohimbine was labeled with 11C for PET imaging, and d-amphetamine (10mg/kg) was administered to elevate synaptic NE concentrations. Distribution volumes were reduced by 20–30% in several brain regions known to express α2-ARs, including the thalamus, cerebral cortex, the caudate nucleus, and cerebellum (Landau et al., 2012).
Two limitations of the α2-AR radioligands evaluated so far are that they lack selectivity for α2-AR subtypes and have not been applied in primates. Of the three mammalian α2-AR subtypes, two (α2A-AR and α2C-AR) have widespread expression in the brain (Scheinin et al., 1994; Fagerholm et al., 2008). α2C-ARs are mainly located in the basal ganglia, in contrast with α2A-ARs that are more widely distributed throughout the brain (Scheinin et al., 1994; Grijalba et al., 1996; Fagerholm et al., 2008). Recently, Arponen et al. (2014) described the radiosynthesis of a novel α2C-AR selective antagonist radioligand [11C]ORM-13070 (K i = 0.6nM) and validated the tracer by ex vivo receptor binding studies in α2A- and α2AC-AR knockout mice, demonstrating that [11C]ORM-13070 provides selective and specific binding to α2C-ARs in the striatum and olfactory tubercle. In a subsequent first-in-man study, [11C]ORM-13070 was evaluated for its suitability as a radioligand for quantitative imaging of α2C-ARs in the human brain. It was suggested that striatal [11C]ORM-13070 receptor binding is sufficient for quantitation in human subjects and that the cerebellum is a suitable reference region for data analysis (Lehto et al. 2014; Luoto et al., 2014).
In the current study, we explored the suitability of [11C]ORM-13070 for measurement of acute changes in synaptic NE concentrations. We used amphetamine to stimulate NE release in the brain and to elevate its synaptic concentrations in rats and in non-human primates. Microdialysis experiments were performed to relate the observed changes in [11C]ORM-13070 binding to the amphetamine-induced elevations in synaptic NE and DA concentrations.
Materials and Methods
Preparation of [11C]ORM-13070
The reference compound ORM-13070 (1-[(S)-1-(2,3-dihydrobenzo[1,4]dioxin-2-yl)methyl]-4-(3-methoxymethylpyridin-2-yl)-piperazine) and its O-desmethyl precursor (ORM-13333) were provided by Orion Pharma. All other chemicals were obtained from commercial sources. The radiopharmaceutical procedures were performed as described earlier (Arponen et al., 2014).
Receptor Autoradiography
Autoradiographic receptor binding experiments were performed using [11C]ORM-13070 and cryosections of cynomolgus monkey (Macaca fascicularis) and human brains (source: Department of Forensic Medicine, Semmelweis Medical University, Budapest, Hungary; ethical approval: Semmelweis University Human Ethical Committee, no. 113/1995, 180/2001), following established protocols (Hall et al., 1998). Non-specific binding was determined by co-incubation with the subtype non-selective α2-AR antagonist atipamezole (10 µM). Radioactivity was detected with a phosphorimager (BAS-5000, Fuji Photo Film Co.).
Ex Vivo Binding in Rats
Ex vivo experiments in rats were approved by the Animal Experiment Board of the Province of Southern Finland. Animal care complied with the guidelines of the International Council of Laboratory Animal Science (ICLAS).
Ten male Sprague-Dawley rats (weighing 298±23g, mean ± standard deviation) were pretreated (subcutaneous [s.c.], 1mL/kg) with either vehicle (saline, n = 5) or d-amphetamine sulphate (0.3mg/kg as the free base, n = 5; Sigma Aldrich). Fifteen min later, they were sedated by inhalation of isoflurane (3–4%) and cannulated. Approximately 20min after the vehicle or amphetamine injection, [11C]ORM-13070 was injected into a tail vein at doses ranging from 70 MBq to 99 MBq (86±10 MBq; 400–850 μL, corresponding to molecular masses of 44–353ng of ORM-13070) under 2% isoflurane anesthesia. At 30min post injection of [11C]ORM-13070, the rats were sacrificed by cardiac puncture. Their brains were frozen and 40 μm cryosections were cut, thaw-mounted onto glass slides, and immediately opposed to imaging plates (BAS-TR2025, Fuji) for at least 40min.
The imaging plates were scanned with a Fuji BAS-5000 Analyzer and analyzed for count densities (photon-stimulated luminescence per unit area, PSL/mm2) using the Aida 2D densitometry program (Raytest Isotopenmessgeräte GmbH). Regions of interest were drawn for the olfactory tubercle, striatum (including the caudate and putamen) and cerebellum (cerebellar cortex). The cerebellum is known to have a negligible density of α2C-ARs, and was here used to estimate the free and non-specifically bound radioligand concentration in the brain. The binding of [11C]ORM-13070 was quantified using specific binding ratios (SBRs), obtained by first subtracting the cerebellum pixel density from the regional pixel density and then dividing the result with the cerebellum pixel density. The SBRs of the vehicle and amphetamine pretreatment groups were compared using two-tailed t-tests.
PET Measurements in Monkeys
Experiments with non-human primates were approved by the Animal Research Ethical Committee of the Northern Stockholm region, and were performed according to the ‘‘Guide for the Care and Use of Laboratory Animals’’ (Clark et al., 1996). Four adult cynomolgus monkeys (three females and one male), weighing 4.0–6.2kg, were included in the study. PET measurements were conducted with a High Resolution Research Tomograph (Siemens Molecular Imaging), and were performed under sevoflurane anesthesia using previously described protocols (Finnema et al., 2012).
In each PET measurement, a sterile phosphate buffered saline (PBS, pH = 7.4) solution containing 153±4 MBq (range: 148–160 MBq) [11C]ORM-13070 was injected as a bolus into a sural vein during five seconds with the simultaneous start of PET data acquisition. The average specific radioactivity at the time of injection of [11C]ORM-13070 was 569 GBq/μmol (range: 186–1104 GBq/μmol), corresponding to a mean injected mass of 0.12 μg (range: 0.05–0.28 μg) per injection. The radiochemical purity of [11C]ORM-13070 always exceeded 98%.
Study Design
A total of 20 PET measurements were performed on nine experimental days. The PET measurements were conducted two and an half hours apart. First, experiments were conducted to test the receptor specificity of [11C]ORM-13070 binding. On two days, a baseline PET measurement with [11C]ORM-13070 was followed by two PET measurements preceded by escalating doses of atipamezole. Atipamezole was infused i.v. over 15min, starting 25min before the [11C]ORM-13070 injection. The examined doses of atipamezole were 2.5 and 10 μg/kg on one day (NHP1) and 50 and 500 μg/kg on another day (NHP2). Atipamezole hydrochloride (Orion Pharma) was diluted with saline and the reported doses refer to the salt.
A second set of PET examinations was performed to evaluate the sensitivity of [11C]ORM-13070 binding to changes in synaptic NE concentrations. On four experimental days, a baseline PET measurement with [11C]ORM-13070 was followed by a measurement after pretreatment with amphetamine (0.5 or 1.0mg/kg as salt; NHP1–3). Dextroamphetamine sulphate (Apoteket) was formulated in PBS and was infused i.v. over 15min, starting 25min before the [11C]ORM-13070 injection. On another two experimental days, baseline PET measurements with [11C]ORM-13070 were followed by measurements after pretreatment with atomoxetine (0.3mg/kg as free base; NHP1 and NHP3). Atomoxetine (tomoxetine hydrochloride; Tocris) was formulated in saline and was infused i.v. using a high dose continuous pseudo-steady-state infusion protocol until the end of the PET measurement, starting 60min before the [11C]ORM-13070 injection (Seneca, Gulyas, et al., 2006; Takano et al., 2009). On one final experimental day, two control baseline PET measurements were performed (NHP4). (Table 1).
Table 1.
Striatal BP ND and Cerebellar V T AUC Values During Baseline and After i.v. Administration of Atipamezole (2.5, 10, 50, and 500 µg/kg), Amphetamine (0.5 and 1.0mg/kg), or Atomoxetine (0.3 mg/kg) in Cynomolgus Monkeys.
| Day | Monkey | Study | BP ND | %Change | V T AUC | %Change |
|---|---|---|---|---|---|---|
| 1 | NHP1 | Baseline | 0.46 | 3.9 | ||
| Atipamezole 2.5 µg/kg | 0.42 | -9 | 4.1 | 6 | ||
| Atipamezole 10 µg/kg | 0.26 | -43 | 5.4 | 39 | ||
| 2 | NHP2 | Baseline | 0.64 | 5.2 | ||
| Atipamezole 50 µg/kg | 0.17 | -73 | 5.0 | -3 | ||
| Atipamezole 500 µg/kg | 0.07 | -89 | 5.1 | -2 | ||
| 3 | NHP2 | Baseline | 0.75 | 4.4 | ||
| Amphetamine 0.5mg/kg | 0.49 | -35 | 4.3 | -2 | ||
| 4 | NHP3 | Baseline | 0.51 | 4.4 | ||
| Amphetamine 0.5mg/kg | 0.33 | -35 | 4.6 | 4 | ||
| 5 | NHP1 | Baseline | 0.55 | 4.5 | ||
| Amphetamine 1.0mg/kg | 0.38 | -31 | 4.3 | -6 | ||
| 6 | NHP2 | Baseline | 0.84 | 4.3 | ||
| Amphetamine 1.0mg/kg | 0.42 | -50 | 4.0 | -6 | ||
| 7 | NHP1 | Baseline | 0.70 | 3.9 | ||
| Atomoxetine 0.3 mg/kg | 0.61 | -13 | 4.6 | 16 | ||
| 8 | NHP3 | Baseline | 0.68 | 3.8 | ||
| Atomoxetine 0.3 mg/kg | 0.71 | 4 | 3.6 | -4 | ||
| 9 | NHP4 | Baseline | 0.72 | 4.3 | ||
| Baseline | 0.73 | 1 | 4.8 | 12 |
BP ND, binding potential; V T AUC, surrogate distribution volume
Plasma Radiometabolite Analysis
Venous blood samples were collected at 4, 15, 30, 45, and 60min after i.v. injection of [11C]ORM-13070. Plasma radioactivity concentrations were determined in a well counter. The proportions of unchanged [11C]ORM-13070 and radioactive metabolites in plasma were determined with high-performance liquid chromatography (HPLC) (Halldin et al., 1995; Arponen et al., 2014).
PET Quantitation
Regional time-radioactivity curves were generated with individual template magnetic resonance images (Finnema et al., 2012). Specific receptor binding was defined as the difference between the total radioactivity concentration in the striatum and cerebellum. BP ND estimates were calculated with the simplified reference tissue model (SRTM; with time interval 0–33min; Lammertsma and Hume, 1996) and the interval method (with time interval of 4–33min; Ito et al., 1998) with the cerebellum as reference region. Surrogate distribution volumes (V T AUC) were calculated using the area under the regional time-radioactivity curve (4–63min) divided by the area under the curve of the venous plasma concentration of [11C]ORM-13070 (4–60min).
Determination of Atipamezole and Atomoxetine Concentrations
Blood samples were collected before (5min) and during (20, 40, and 60min) the PET measurements for determination of concentrations of atipamezole and atomoxetine in plasma. Analysis was with HPLC coupled to tandem mass spectrometry detection, using an API4000 Q TrapTM tandem mass spectrometer equipped with an atmospheric pressure Turbo VTM ion source (AB SCIEX). For both analytes, the lower limit of quantitation was 1ng/mL, and the intra-assay CV was <10%.
Rat Brain Microdialysis
All procedures were in accordance with the Pfizer Laboratory Animal Care Program and were approved by the Institutional Animal Care and Use Committee of Pfizer Global Research and Development. Rats were individually housed, had free access to food and water, and were maintained on a 12:12h light/dark cycle. In vivo microdialysis was performed essentially as described before (Rollema et al., 2000). Briefly, microdialysis guide cannulae were implanted above the striatum (AP +0.2mm, ML +3.2mm, DV -3.5mm from bregma) of isoflurane-anesthetized adult male Sprague-Dawley rats (Charles River). One day after surgery, microdialysis probes (BR-4, Bioanalytical Systems Inc.; 4mm polyacrylonitrile membrane) were inserted and perfused with artificial cerebrospinal fluid at 0.3 μL/min overnight. On the day of the experiment, probes were perfused at 2 μL/min and dialysate samples were collected in 30min fractions. After baseline sampling (3 x 30min), rats were dosed s.c. with d-amphetamine sulphate (0.3mg/kg as the free base, n = 6) or vehicle (saline, 2mL/kg, n = 4) in experiment 1 or i.p. with atomoxetine hydrochloride (1mg/kg as the free base, n = 5) or vehicle (saline, 2mL/kg, n = 5) in experiment 2, and samples were collected for another 3h. In order to evaluate the possible impact of isoflurane anesthesia on the PET study results, as part of experiment 1, after collection of the three baseline samples, one group of rats was anesthetized with isoflurane (3.5%) and anesthesia was maintained with 2% isoflurane for the remainder of the experiment. New baseline samples were collected under anesthesia, and the rats were then dosed with amphetamine (n = 8) or vehicle (n = 6) as indicated above. Concentrations of NE and DA in the dialysate samples were measured using HPLC with electrochemical detection (Rollema et al., 2000).
Data Analysis
The effect of anesthesia on basal concentrations of NE and DA was determined by comparing results from anesthetized rats to those from freely-moving animals using Mann-Whitney’s U test. The effects of the drug treatments were determined by normalizing all NE and DA concentration data to baseline. Repeated measures analysis of variance (main effects: time and treatment) was employed to compare the drug treatments with vehicle treatment.
In Vitro Receptor Binding
The binding affinity of amphetamine, atipamezole, and atomoxetine to human α2A-, α2B-, and α2C-ARs was determined with transfected Chinese hamster ovary cells and the α2-AR antagonist radioligand [ethyl-3H]RS79948-197 (0.3nM; Amersham Biosciences), as described previously (Laurila et al., 2007).
Results
Receptor Autoradiography
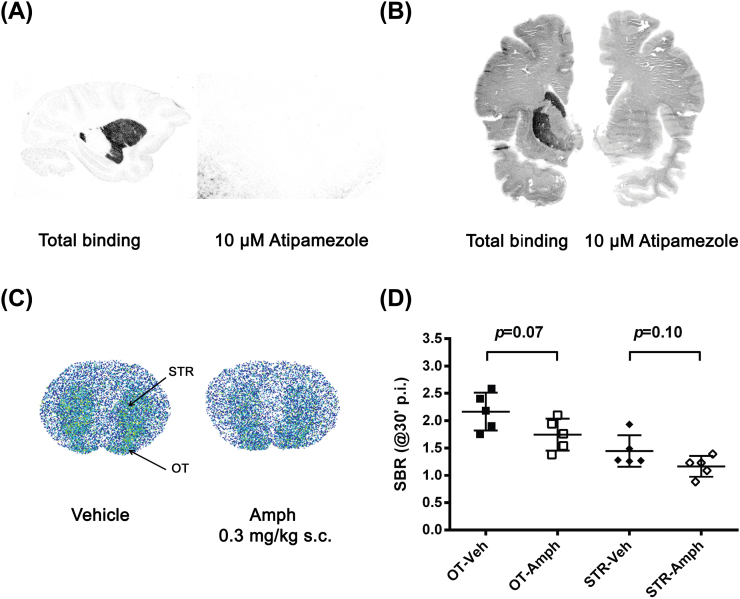
In cryosections of monkey and human brain, the binding of [11C]ORM-13070 was highest in the striatum, both in the caudate nucleus and the putamen, and low in the cerebellum. The striatal binding of [11C]ORM-13070 was blocked by co-incubation with the subtype non-selective α2-AR antagonist atipamezole (10 µM; Figure 1A and B).
Figure 1.
Representative whole-hemisphere autoradiograms showing the distribution of [11C]ORM-13070 binding to sagittal sections of a monkey brain (A), coronal sections of a human brain (B), and coronal sections of two rat brains (C). The rat sections were obtained from animals euthanized 30min post [11C]ORM-13070 injection. Amphetamine (‘Amph’, 0.3mg/kg subcutaneous [s.c.], 20min before i.v. injection of [11C]ORM-13070) tended to reduce the specific binding ratios (mean ± standard deviation, n = 5) in the olfactory tubercle (OT) and striatum (STR) of rats (D). In the primate brain sections, specific binding was demonstrated by co-incubation with a 10 µM concentration of the subtype non-selective α2-adrenoceptor antagonist atipamezole. SBR, specific binding ratio.
Ex Vivo Binding in Rats
Thirty min after i.v. injection of [11C]ORM-13070, the radioactivity concentration was high in the olfactory tubercle and striatum of rats and low in the cerebellum (Figure 1C). Following pretreatment with vehicle (n = 5), the SBRs were 2.2±0.3 for the olfactory tubercle and 1.4±0.3 for the striatum (Figure 1D). After amphetamine pretreatment (n = 5), the SBRs were 1.7±0.3 for the olfactory tubercle and 1.2±0.2 for the striatum, indicating reductions in radioligand binding, but statistical significance was not reached for the effect of amphetamine on SBRs (-19%, p = 0.07 for the olfactory tubercle; -20%, p = 0.10 for the striatum; Figure 1D).
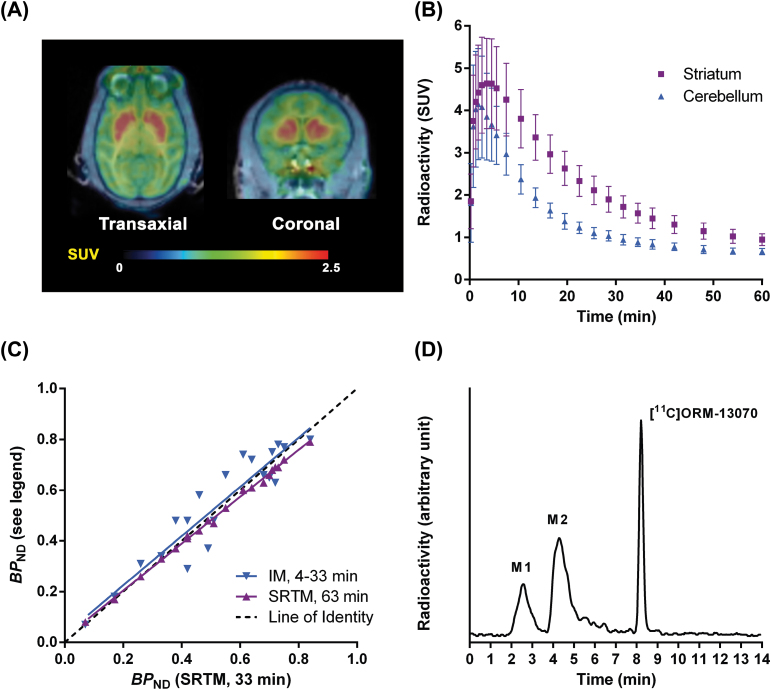
PET Measurements in Monkey
After i.v. injection of [11C]ORM-13070 in monkey, the radioactivity concentration was high in the striatum and low in the cerebellum (Figure 2A and B). Using the cerebellum as a reference region, the peak in striatal specific binding was observed after 10–15min. Regional time activity curves were fitted using the SRTM. Striatal BP ND values estimated using only the first 33min of the PET acquisition data were in good agreement with longer, 63min data collection. Striatal BP ND values estimated with the SRTM also agreed well with corresponding values obtained with the interval method (Figure 2C). Radiometabolite analysis of monkey plasma demonstrated the presence of significant amounts of radioactivity in two distinct metabolite fractions (termed M1 and M2 with tR of 2.6min and 4.3min, respectively), both more polar than the parent [11C]ORM-13070 (tR = 8.2min; Figure 2D). The proportion of intact [11C]ORM-13070 decreased over time from 66±7% at 4min to 24±3% at 60min (n = 9).
Figure 2.
Evaluation of the utility of [11C]ORM-13070 as a positron emission tomography (PET) tracer in cynomolgus monkeys. (A) PET summation images represent mean radioactivity levels in time frames of 9–63min after injection of [11C]ORM-13070. (B) Mean regional brain radioactivity in the striatum (■) and cerebellum (▲) during baseline conditions (mean ± standard deviation, n = 9). (C) Correlation between striatal BP ND (simplified reference tissue model [SRTM], 33min) and mean striatal binding potential (BP ND) estimates calculated by SRTM (63min) and interval method (IM) (n = 20). (D) Radiochromatogram of a plasma sample obtained 30min after i.v. injection of [11C]ORM-13070. SUV, standard uptake value.
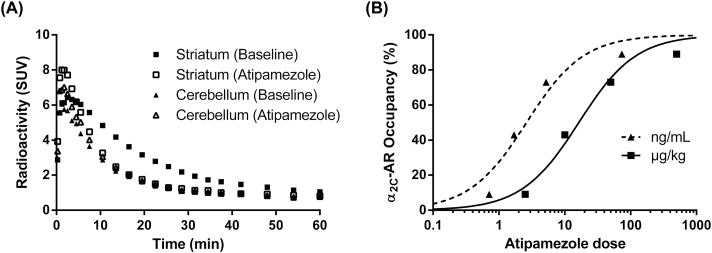
Pretreatment with atipamezole was associated with dose-dependent decreases in striatal [11C]ORM-13070 binding, but did not affect tracer uptake in the cerebellum (Figure 3A and B, Table 1). A constrained one-site binding hyperbole described the relationship between dose and striatal α2C-AR occupancy well (R 2 = 0.97), with an ID 50 of 16±3 μg/kg. Similarly, the relationship between atipamezole concentrations in plasma (mean of the values at 20–60min) and α2C-AR occupancy was also well described by a constrained one-site binding hyperbole (R 2 = 0.93), with an IC 50 of 3±1ng/mL.
Figure 3.
(A) Time activity course for regional radioactivity (SUV) in the striatum and cerebellum during baseline and pretreatment with atipamezole (500 µg/kg) before i.v. injection of [11C]ORM-13070 in a representative cynomolgus monkey. (B) Relative decrease in mean striatal binding potential as a function of atipamezole dose and atipamezole concentration in plasma. The lines represent a constrained one-site binding fit.
Pretreatment with amphetamine was associated with dose-dependent decreases in the striatal BP ND estimates (Table 1). The decrease in striatal BP ND was 35% after 0.5mg/kg (n = 2) and 40% after 1.0mg/kg (n = 2) of amphetamine. Pretreatment with atomoxetine (0.3mg/kg) did not markedly influence the striatal binding of [11C]ORM-13070 (-4%, n = 2; Table 1). In the final test-retest experiment, the striatal BP ND estimates were almost identical, at 0.72 and 0.73 (Table 1).
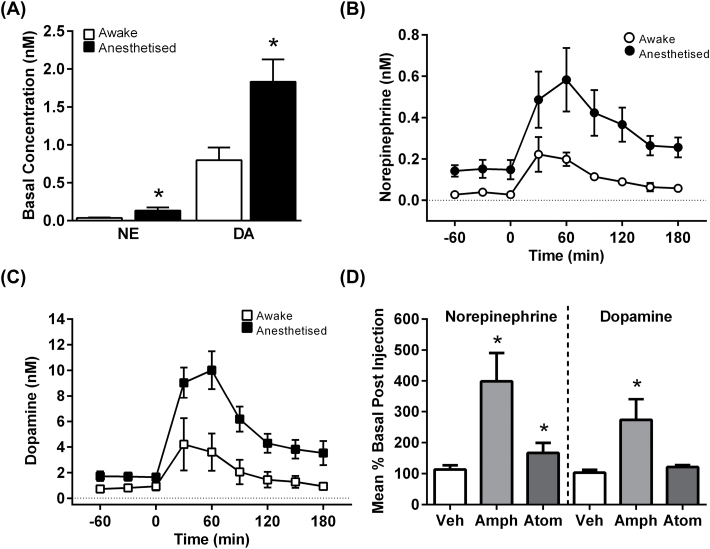
Rat Brain Microdialysis
Baseline striatal NE concentrations were consistently lower than DA concentrations (nM: 0.09±0.03 vs 1.39±0.23; p < 0.0001). Concentrations of both neurotransmitters were significantly higher under isoflurane anesthesia (DA: p = 0.008; NE: p = 0.015; Figure 4A). Administration of amphetamine (0.3mg/kg, s.c.) produced significant increases in NE and DA concentrations in anesthetized and freely-moving rat striatum (Figure 4B and C). In freely-moving rats (n = 4–6), the absolute increase in DA (peak change ≈ 3.4nM) far exceeded the change in NE (peak change ≈ 0.2nM), but if expressed in terms of percent of baseline, the effect on NE was greater (Figure 4D). In anesthetized rats (n = 6–8), the absolute increase in DA (peak change ≈ 7.1nM) also far exceeded the change in NE (peak change ≈ 0.4nM). Administration of atomoxetine (1mg/kg, i.p.; n = 5) to freely-moving rats produced almost two-fold increases in striatal NE (p = 0.037) with no significant effects on DA (p = 0.312; Figure 4D).
Figure 4.
(A) Extracellular concentrations of norepinephrine (NE) and dopamine (DA) in the striatum of awake or anesthetized rats. The effects of amphetamine (0.3mg/kg s.c., administered at t = 0) on concentrations of NE (B) and DA (C). The mean effects of amphetamine (Amph; 0.3mg/kg s.c.) and atomoxetine (Atom; 1mg/kg i.p.) on NE and DA in freely-moving rats expressed as percent of basal concentrations (D). Data are means ± standard error of the mean, *p < 0.05. Veh, vehicle.
In Vitro Receptor Binding
Atipamezole was bound with high affinity (K i < 5nM) to all three α2-AR subtypes, while amphetamine and atomoxetine displayed low binding affinities (K i > 1 µM; Table 2).
Table 2.
Results of in vitro receptor binding assay with recombinant human α2-AR subtypes and the subtype non-selective antagonist radioligand [3H]RS 79948-197.
| K i [nM] (CI) | α2A-AR | α2B-AR | α2C-AR |
|---|---|---|---|
| Amphetamine | 1244 (986–1570) | 1578 (1148–2170) | 4919 (3889–6221) |
| Atipamezole | 1.1 (0.91–1.3) | 2.2 (1.8–2.6) | 4.9 (3.8–6.4) |
| Atomoxetine | 4626 (3780–5661) | 1387 (1046–1840) | 8735 (7048–10830) |
AR, adrenoceptor; CI, 95% confidence interval of the K i estimate; K i, binding affinity estimate.
Discussion
We explored the use of the novel α2C-AR antagonist PET radioligand [11C]ORM-13070 for measurement of changes in synaptic NE concentrations in the brain. In vivo PET measurements in monkeys demonstrated that administration of amphetamine caused dose-dependent decreases in striatal [11C]ORM-13070 binding. Ex vivo experiments in rats provided support for this notion. Striatal microdialysis confirmed amphetamine-induced elevations in extracellular NE, both in awake and in isoflurane-anesthetized rats. Taken together, these results indicate that [11C]ORM-13070 may be useful as a tool to investigate alterations in synaptic NE concentrations in vivo.
The need for methods to monitor changes in synaptic NE in the living human brain has been recognized in clinical neuroscience. With post-mortem autoradiography, we demonstrated that the regional pattern of [11C]ORM-13070 binding is conserved across species, and is consistent with the known α2C-AR distribution in mammalian brains (Holmberg et al., 2003; Fagerholm et al., 2008). Validation of measurement of changes in synaptic NE concentrations using [11C]ORM-13070 in rodents and monkeys thus provides a cross-species translational method suitable for application in human subjects.
This is the first study to evaluate [11C]ORM-13070 binding in non-human primates. After injection of [11C]ORM-13070, the radioactivity concentration was high in the striatum and the cerebellar radioactivity concentration was low, in agreement with the cerebellum having a negligible density of α2C-ARs. The lack of specific binding in the cerebellum was confirmed in experiments with atipamezole pretreatment, as atipamezole did not affect cerebellar tracer uptake (Figure 3A and Table 1). The relatively fast receptor kinetics and adequate specific binding ratios further support that [11C]ORM-13070 may be suitable for quantitative assessment of α2C-ARs in the non-human primate brain.
After injection of [11C]ORM-13070, two radioactive metabolites were observed in monkey blood. A similar pattern of metabolites has been seen in rodent studies, and M1 but not M2 could be detected in the rat brain (Arponen et al., 2014). The molecular identities of M1 and M2 have not been resolved, in spite of efforts involving stable isotopes and mass spectroscopy, but they have been inferred to be very small molecular weight fragments with no affinity for α2C-ARs (Arponen et al., 2014). Additional studies are thus required to further understand the potential influence of M1 on the estimates of non-specific [11C]ORM-13070 binding in brain during the later phase of data acquisition.
PET measurements, combined with amphetamine administration, have previously been used to investigate changes in synaptic DA concentrations in humans (Laruelle, 2000). Amphetamine, however, elevates synaptic monoamine concentrations in a non-selective manner, in particular NE and DA, predominantly through the redistribution of catecholamines from synaptic vesicles to the cytosol and the induction of reverse transport of transmitters through plasma membrane uptake carriers (Sulzer et al., 2005). In the current monkey study, amphetamine dose-dependently decreased striatal BP ND estimates, with a reduction of about 40% in BP ND after 1.0mg/kg. A smaller dose of amphetamine (0.3mg/kg) caused a trend-level decrease in [11C]ORM-13070 binding in rats (20%, p = 0.10), which was in accordance with the anticipated decrease based on the results in monkeys. Thus, amphetamine dose-dependently reduced striatal [11C]ORM-13070 binding, which was consistent across species and in line with amphetamine-enhanced synaptic NE concentrations.
Microdialysis experiments were performed in rats to further elucidate the amphetamine-induced changes in [11C]ORM-13070 binding. Striatal extracellular DA concentrations were 15-fold higher than those of NE at baseline, and anesthesia increased the extracellular concentrations of NE and DA, as already reported by others (Chave et al., 1996; Anzawa et al., 2001). Importantly, the amphetamine-induced relative increases in NE and DA were similar in awake and anesthetized rats. The use of anesthesia in the non-human primate PET experiments can thus be considered not to significantly confound the evaluation of the suitability of [11C]ORM-13070 binding to reflect synaptic NE levels.
When attempting to monitor drug-induced alterations of synaptic neurotransmitter concentrations with a competition binding approach, it is important to consider a possible direct effect of the challenge compound on radioligand binding to the target receptor. Amphetamine was found to have very low α2C-AR affinity (K i ≈ 5 µM), suggesting that the reduction observed in [11C]ORM-13070 binding is likely to represent an indirect effect, e.g. changes in monoamine concentrations at the target receptors.
The amphetamine-induced decreases observed in [11C]ORM-13070 binding in monkey striatum were larger than those previously reported for the D2 receptor radioligand [11C]raclopride (23% after 1.0mg/kg of amphetamine; Seneca, Finnema, et al., 2006). In microdialysis experiments in rat striatum, the relative amphetamine-evoked increase in NE concentrations was greater than the DA increase. Still, DA also binds to α2C-ARs, albeit with five-fold lower affinity than NE (Ruuskanen et al., 2005). From the current study, it cannot be ruled out that the decreased [11C]ORM-13070 binding was partly related to increased DA, and future studies are required to further understand a potential contribution of DA to the amphetamine effect.
A possible limitation of using [11C]ORM-13070 is that α2C-ARs are predominantly expressed in the striatum, a brain region with relatively scant NE innervation. Striatal NE projections have, however, been demonstrated in the nucleus accumbens, using immunohistochemical staining of dopamine beta-hydroxylase in rats (Berridge et al., 1997; Delfs et al., 1998) and autoradiography of NET in monkeys (Smith, Beveridge, et al., 2006). Interestingly, a previous PET study in pigs, performed with the subtype non-selective α2-AR tracer [11C]yohimbine, also demonstrated substantial amphetamine-evoked reductions in tracer uptake in the striatum, as well as in other brain regions in which NE predominates over DA (Landau et al., 2012). These studies provide support for the notion that the observed reduction in [11C]ORM-13070 binding after amphetamine could relate to elevated extracellular concentrations of NE.
In an attempt to selectively elevate synaptic NE concentrations, we performed experiments with the potent NET inhibitor atomoxetine. Administration of atomoxetine to freely-moving rats increased striatal extracellular NE concentrations almost two-fold, without significant effects on DA, which agrees with previous results (Bymaster et al., 2002). In preliminary PET studies in two monkeys, atomoxetine did not markedly modify the striatal binding of [11C]ORM-13070: the observed changes were -13% and +4%. This is not surprising, since atomoxetine increased extracellular NE to a smaller extent than amphetamine. However, insufficient NET occupancy in the monkey brain can be excluded as an explanation because this dose of atomoxetine almost saturates the NET capacity (Seneca, Gulyas, et al., 2006; Takano et al., 2009). Future studies with atomoxetine should thus be performed in a larger number of subjects. Preliminary results in humans indicate that atomoxetine, as well as sympathetic activation by ketamine and the cold pressor test (Marasini et al., 1991) reduce [11C]ORM-13070 binding by 10–15% in human subjects (Lehto et al., 2014; Scheinin et al., 2013).
It should be noted that α2C-ARs are currently considered as potential drug targets for several central nervous system disorders, including schizophrenia and Alzheimer’s disease (Sallinen et al., 2013). A number of established antipsychotics, in particular clozapine, have relative high α2C-AR affinity (Kalkman and Loetscher, 2003). The possible interaction of DA with α2C-ARs may also be clinically relevant and needs further evaluation. Characterization of the DA contribution in the effect of amphetamine on [11C]ORM-13070 binding in dopamine beta-hydroxylase knockout mice that are deficient of NE may help determine the extent to which DA is a co-transmitter of striatal α2C-ARs.
We evaluated the use of the novel α2C-AR antagonist PET radioligand [11C]ORM-13070 for measurement of changes in synaptic NE concentrations. Amphetamine caused dose-dependent decreases in [11C]ORM-13070 binding in rat and monkey striatum. It is concluded that [11C]ORM-13070 may be a useful tool for investigations on alterations in synaptic NE concentrations in vivo. Future studies are required to further understand a potential contribution of DA to the amphetamine-evoked effect.
Statement of Interest
The laboratories of Mika Scheinin and Merja Haaparanta-Solin have contract research relationships with the Orion Corporation. Mika Scheinin has received speaker’s fees and research support from the Orion Corporation. Jukka Sallinen is employed by the Orion Corporation. Jukka Sallinen and Mika Scheinin are listed as inventors on US Patent no. 5,902,807: “Method for the treatment of mental illness in mammals and a composition therefor” (1999). The Orion Corporation is a marketer of atipamezole and is actively pursuing drug development related to α2C-adrenoceptors.
Sjoerd Finnema and Andrea Varrone have received compensation as members of a scientific advisory board of F. Hoffmann-La Roche. Zoë Hughes, Sarah Grimwood, and Phebian Babalola are all current or past employees of Pfizer Inc. Lars Farde is employed by AstraZeneca Pharmaceuticals. The other authors have no conflicts of interest to declare.
Acknowledgments
We gratefully acknowledge the excellent assistance of Dr Marie Svedberg and Siv Eriksson in the autoradiography experiments and of Gudrun Nylén in the non-human primate PET experiments. The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no 115008 of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013).
References
- Anzawa N, Kushikata T, Ohkawa H, Yoshida H, Kubota T, Matsuki A. (2001). Increased noradrenaline release from rat preoptic area during and after sevoflurane and isoflurane anesthesia. Can J Anaesth 48:462–465. [DOI] [PubMed] [Google Scholar]
- Arponen E, Helin S, Marjamaki P, Gronroos T, Holm P, Loyttyniemi E, Nagren K, Scheinin M, Haaparanta-Solin M, Sallinen J, Solin O. (2014). A PET Tracer for Brain alpha2C Adrenoceptors, 11C-ORM-13070: Radiosynthesis and Preclinical Evaluation in Rats and Knockout Mice. J Nucl Med 55:1171–1177. [DOI] [PubMed] [Google Scholar]
- Balle T, Halldin C, Andersen L, Alifrangis LH, Badolo L, Jensen KG, Chou YW, Andersen K, Perregaard J, Farde L. (2004). New alpha(1)-adrenoceptor antagonists derived from the antipsychotic sertindole-carbon-11 labelling and pet examination of brain uptake in the cynomolgus monkey. Nucl Med Biol 31:327–336. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. (1997). Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27:230–241. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. (1997). Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 94:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. (2004). Smoking-induced ventral striatum dopamine release. Am J Psych 161:1211–1218. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711. [DOI] [PubMed] [Google Scholar]
- Chave S, Kushikata T, Ohkawa H, Ishiara H, Grimaud D, Matsuki A. (1996). Effects of two volatile anesthetics (sevoflurane and halothane) on the hypothalamic noradrenaline release in rat brain. Brain Res 706:293–296. [DOI] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggerda J-AD, VandeBergh JL. (1996). Guide for the care and use of laboratory animals. Washington DC: The National Academies Press. [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. (1998). Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806:127–140. [DOI] [PubMed] [Google Scholar]
- Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, Cunningham VJ, Grasby PM. (2009). The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev 33:1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm V, Rokka J, Nyman L, Sallinen J, Tiihonen J, Tupala E, Haaparanta M, Hietala J. (2008). Autoradiographic characterization of alpha(2C)-adrenoceptors in the human striatum. Synapse 62:508–515. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Gulyas B, Pierson ME, Halldin C, Farde L. (2010). Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse 64:573–577. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Halldin C, Farde L. (2012). Confirmation of fenfluramine effect on 5-HT(1B) receptor binding of [(11)C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab 32:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalba B, Callado LF, Javier Meana J, Garcia-Sevilla JA, Pazos A. (1996). Alpha 2-adrenoceptor subtypes in the human brain: a pharmacological delineation of [3H]RX-821002 binding to membranes and tissue sections. Eur J Pharmacol 310:83–93. [DOI] [PubMed] [Google Scholar]
- Hall H, Halldin C, Farde L, Sedvall G. (1998). Whole hemisphere autoradiography of the postmortem human brain. Nucl Med Biol 25:715–719. [DOI] [PubMed] [Google Scholar]
- Halldin C, Swahn CG, Farde L, Sedvall G. (1995). Radioligand disposition and metabolism. In: PET for drug development and evaluation (Comar D, ed), pp 55–65. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Holmberg M, Fagerholm V, Scheinin M. (2003). Regional distribution of alpha(2C)-adrenoceptors in brain and spinal cord of control mice and transgenic mice overexpressing the alpha(2C)-subtype: an autoradiographic study with [(3)H]RX821002 and [(3)H]rauwolscine. Neuroscience 117:875–898. [DOI] [PubMed] [Google Scholar]
- Hume SP, Hirani E, Opacka-Juffry J, Osman S, Myers R, Gunn RN, McCarron JA, Clark RD, Melichar J, Nutt DJ, Pike VW. (2000). Evaluation of [O-methyl-C-11]RS-15385-197 as a positron emission tomography radioligand for central alpha(2)-adrenoceptors. Eur J Nucl Med 27:475–484. [DOI] [PubMed] [Google Scholar]
- Ito H, Hietala J, Blomqvist G, Halldin C, Farde L. (1998). Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab 18:941–950. [DOI] [PubMed] [Google Scholar]
- Jakobsen S, Pedersen K, Smith DF, Jensen SB, Munk OL, Cumming P. (2006). Detection of alpha2-adrenergic receptors in brain of living pig with 11C-yohimbine. J Nucl Med 47:2008–2015. [PubMed] [Google Scholar]
- Kalkman HO, Loetscher E. (2003). alpha2C-Adrenoceptor blockade by clozapine and other antipsychotic drugs. Eur J Pharmacol 462:33–40. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Landau AM, Doudet DJ, Jakobsen S. (2012). Amphetamine challenge decreases yohimbine binding to alpha2 adrenoceptors in Landrace pig brain. Psychopharmacology (Berl) 222:155–163. [DOI] [PubMed] [Google Scholar]
- Laruelle M. (2000). Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SZ, Baldwin RM, Charney DS, Hoffer PB, Kung HF, Innis RB. (1995). SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med 36:1182–1190. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. (1996). Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila JM, Xhaard H, Ruuskanen JO, Rantanen MJ, Karlsson HK, Johnson MS, Scheinin M. (2007). The second extracellular loop of alpha2A-adrenoceptors contributes to the binding of yohimbine analogues. Br J Pharmacol 151:1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto J, Virta JR, Oikonen V, Roivainen A, Luoto P, Arponen E, Helin S, Hietamäki J, Holopainen A, Kailajärvi M, Peltonen JM, Rouru J, Sallinen J, Virtanen K, Volanen I, Scheinin M, Rinne JO (2014) Test-retest reliability of 11C-ORM-13070 in PET imaging of α2C-adrenoceptors in vivo in the human brain. Eur J Nucl Med Mol Imaging 42:120–127. [DOI] [PubMed]
- Luoto P, Suilamo S, Oikonen V, Arponen E, Helin S, Herttuainen J, Hietamaki J, Holopainen A, Kailajarvi M, Peltonen JM, Rouru J, Sallinen J, Scheinin M, Virta J, Virtanen K, Volanen I, Roivainen A, Rinne JO. (2014). 11C-ORM-13070, a novel PET ligand for brain alpha-adrenoceptors: radiometabolism, plasma pharmacokinetics, whole-body distribution and radiation dosimetry in healthy men. Eur J Nucl Med Mol Imaging 41:1947–1956. [DOI] [PubMed] [Google Scholar]
- Marasini B, Biondi ML, Mollica R, Del Santo A, Agostoni A. (1991). Cold-induced changes in plasma norepinephrine, epinephrine and dopamine concentrations in patients with Raynaud’s phenomenon. Eur J Clin Chem Clin Biochem 29:111–114. [DOI] [PubMed] [Google Scholar]
- Miyake N, Skinbjerg M, Easwaramoorthy B, Kumar D, Girgis RR, Xu X, Slifstein M, Abi-Dargham A. (2011). Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry 69:822–824. [DOI] [PubMed] [Google Scholar]
- Piccini P, Pavese N, Brooks DJ. (2003). Endogenous dopamine release after pharmacological challenges in Parkinson’s disease. Ann Neurol 53:647–653. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. (2000). 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry 48:229–237. [DOI] [PubMed] [Google Scholar]
- Ruuskanen JO, Peitsaro N, Kaslin JV, Panula P, Scheinin M. (2005). Expression and function of alpha-adrenoceptors in zebrafish: drug effects, mRNA and receptor distributions. J Neurochem 94:1559–1569. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Holappa J, Koivisto A, Kuokkanen K, Chapman H, Lehtimaki J, Piepponen P, Mijatovic J, Tanila H, Virtanen R, Sirvio J, Haapalinna A. (2013). Pharmacological characterisation of a structurally novel alpha2C-adrenoceptor antagonist ORM-10921 and its effects in neuropsychiatric models. Basic Clin Pharmacol Toxicol 113:239–249. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr. (1994). Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res 21:133–149. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Hirvonen MM, Johansson J, Kemppainen J, Lehto J, Lovro Z, Luoto P, Oikonen V, Naukkarinen T, Rouru J, Sallinen J, Scheinin H, Vuorilehto L, Finnema SJ, Halldin C, Rinne JO. (2013). Evaluation of [11C]ORM-13070 as a PET tracer for α2C-adrenoceptors in the human brain. In: Catecholamine Research in the 21st Century—Abstracts and Graphical Abstracts, 10th International Catecholamine Symposium, 2012 (Eiden LE, ed), p 162. London, UK: Elsevier. [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C, Innis RB. (2006). Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59:260–269. [DOI] [PubMed] [Google Scholar]
- Seneca N, Gulyas B, Varrone A, Schou M, Airaksinen A, Tauscher J, Vandenhende F, Kielbasa W, Farde L, Innis RB, Halldin C. (2006). Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: a PET study in nonhuman primate brain using (S,S)-[18F]FMeNER-D2. Psychopharmacology (Berl) 188:119–127. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. (2006). Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience 138:703–714. [DOI] [PubMed] [Google Scholar]
- Smith DF, Dyve S, Minuzzi L, Jakobsen S, Munk OL, Marthi K, Cumming P. (2006). Inhibition of [C-11]mirtazapine binding by alpha(2)-adrenoceptor antagonists studied by positron emission tomography in living porcine brain. Synapse 59:463–471. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. (2005). Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433. [DOI] [PubMed] [Google Scholar]
- Takano A, Gulyas B, Varrone A, Maguire RP, Halldin C. (2009). Saturated norepinephrine transporter occupancy by atomoxetine relevant to clinical doses: a rhesus monkey study with (S,S)-[(18)F]FMeNER-D (2). Eur J Nucl Med Mol Imaging 36:1308–1314. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppamaki M, Nagren K, Bergman J, Eronen E, Syvalahti E, Hietala J. (1996). Serotonergic modulation of striatal D2 dopamine receptor binding in humans measured with positron emission tomography. Psychopharmacology (Berl) 126:277–280. [DOI] [PubMed] [Google Scholar]
- Van der Mey M, Windhorst AD, Klok RP, Herscheid JDM, Kennis LE, Bischoff F, Bakker M, Langlois X, Heylen L, Jurzak M, Leysen JE. (2006). Synthesis and biodistribution of [C-11]R107474, a new radiolabeled alpha(2)-adrenoceptor antagonist. Bioorg Med Chem 14:4526–4534. [DOI] [PubMed] [Google Scholar]
- van Waarde A, Doorduin J, de Jong JR, Dierckx RA, Elsinga PH. (2008). Synthesis and preliminary evaluation of (S)-[C-11]-exaprolol, a novel beta-adrenoceptor ligand for PET. Neurochem Int 52:729–733. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833. [DOI] [PubMed] [Google Scholar]