Abstract
Background
Cervical cancer has been shown to be highly associated with human papillomavirus (HPV) infection. The viral oncogenes E6 and E7 are constantly expressed by the tumor cells and are therefore potent targets for therapeutic genetic vaccination. In the present study, it was investigated the potential effect of HPV-16 E6, E7 and L1 co-administration to activate specific cytotoxic T lymphocytes in tumor mice models.
Methods
The HPV-16 E6, E7 and L1 genes from Iranian isolate were separately inserted into the mammalian expression vector, pcDNA3, to construct the DNA vaccine candidates. Tumor-bearing Animals (C57BL/6 mice) were immunized with the vaccine candidate; then, Lymphocyte Proliferation Assay (LPA) and relative tumor volume measurements were carried out in order to examine the immunological effects of the vaccine.
Results
Obtained results showed that co-administration of the HPV-16 E6, E7 and L1 DNA induced HPV-16 specific cellular immune responses and also protected against TC-1-induced tumor in vivo compared with negative controls.
Conclusion
The results showed that mixed delivery systems might be valuable to improve the magnitude of the induced immune responses and confirmed therapeutic effects of HPV-16 E6, E7 through cytotoxic T lymphocyte induction and illustrate the new promising role for HPV-16 L1 CTL epitopes as a suitable CTL inducer.
Keywords: pcDNA3/E6, pcDNA3/E7, pcDNA3/L1, immunocellular responses
Introduction
Worldwide, cervical cancer, with approximately 470,000 new cases and 233,000 deaths each year is the second most common cancer in women [1]. Approximately 70% of cervical cancers occur in developing countries [2, 3]. Today, the causal role of high-risk strains of HPV (especially types 16 and 18) in cervical cancer has been documented [4, 5].
Since, some of the HPV early proteins, such as E6 and E7 are constantly expressed in cervical cancer cells as oncogenes that promote tumor growth and malignant transformation <http://en.wikipedia.org/wiki/Malignant> [3], they represent ideal targets for immunotherapy of cervical cancer [6]. E6 can bind to a C-terminal region of p53 and degrade it [7]. Also, E7 proteins is a small nuclear proteins that binds to hypo-phosphorylated form of the retinoblastoma protein (Rb), causing that to be disabled [8]. In this situation, cell cycle proceeds to S phase and ultimately cellular DNA synthesis and cell proliferation occur [3, 9]. In some studies, this has been reported that memory T cells specific for an E7-derived cytotoxic T cell (CTL) epitope were generated in Cervical Intraepithelial Neoplasia (CIN) and cervical cancer patients [9, 10]. So, E6, E7 can be considered the logical targets for therapeutic HPV vaccines.
In order to reduce the number of deaths from cervical cancer, prophylactic vaccines which protect against primary HPV infection in naïve individuals would be of unquestionable value. Anti L1 and L2-directed antigens are efficient in this role [11]. Prophylactic vaccines containing L1 as DNA or VLP have shown significantly effective in clinic [11]. Also today, some studies have shown the therapeutic potential of L1-specific CD4(+) and CD8(+) T lymphocytes responses in cervical cancer patients [12, 13]. A study indicated that HPV-16 L1 DNA vaccine stimulates the vaginal CD8(+) T lymphocytes which are essential to eliminate virus-infected cells [14]. It seems to be required more studies about the ability of L1 gene to stimulate immune cells.
In the current study, DNA-based immunization was examined for induction of cell-mediated immune responses of L1. Also, the effect of co-administration of HPV-16 E6, E7 and L1 genes on the protection and cellular immune system was evaluated and discussed in C57BL/6 mice model.
Materials and Methods
The TC-1 cells were purchased from Pasteur Institute , Tehran, Iran. They were derived from the primary lung epithelial cells of C57BL/6 mice and were immortalized with the amphotropic retrovirus vector, containing E6 and E7 genes and subsequently transformed with the plasmid expressing the activated human c-Ha-ras oncogene [15]. The cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 cell-culture medium (Gibco Invitrogen; Paisley, Scotland, UK), supplemented with 10% fetal calf serum (FCS) (Gibco BRL), 100 IU/ml penicillin,100 μg/ml streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, 2 mM nonessential amino acids, at 37°C with 5% CO2.
Female C57BL/6 mice (3-4 weeks old) were purchased from the Pasteur Institute, Tehran, Iran (n=63). Given free access to food and water, the mice were housed for one week before the experiment, and maintained in a good standard condition. All experiments were done according to the guidelines for the care and use of laboratory animals by the Ethical Commission of the Tarbiat Modares University.
Tumor Monitoring
For the therapeutic experiments, C57BL/6 mice were challenged by a subcutaneous (s.c) injection of a suspension of 100 μl PBS containing 106 TC-1 cells/mouse in the left flank and then, were grouped into nine cages (seven for each group).
After two weeks, the resulting tumors were obvious and palpable in mice. The neoplastic masses were measured with calipers every other day and tumor volume was estimated according to Carlsson’s formula [16, 17]. The average tumor size in millimeter was reported as the average of all dimensions measured. Smallest diameter (a) and biggest diameter (b) were measured and tumor volume was calculated using the following formula: V= (a2b)/2 (data not shown).
Vectors Preparation
The HPV-16 E7 gene was isolated by PCR, cloned in pTZ57R/T, confirmed by sequencing and subcloned into the unique EcoRI and XbaI cloning sites of the pcDNA3 expression vector (Invitrogen, Burlington, Canada), down-stream of the cytomegalovirus promoter as described previously by Meshkat et al., [18]. Also, in the study that was conducted by Mirshahabi et al., the HPV-16 E6 gene was isolated by PCR, and cloned in the pTZ57R/T. Cloning was confirmed by sequencing. Then, this gene was subcloned into the unique BamHI and HindIII cloning sites of the pcDNA3 expression vector (Invitrogen, Burlington, Canada), down-stream of the cytomegalovirus promoter [19].
Also, pET/L1 that was produced by Teimoori et al. previously [20] and subcloned into the unique site of XhoI and NheI cloning sites of the pcDNA3 expression vector (Invitrogen, Burlington, Canada), down-stream of the cytomegalovirus promoter.
Competent cells of DH5α strain of E. coli were transformed with confirmed recombinant pcDNA3/E6, pcDNA3/E7 and pcDNA3/L1 vectors in Luria-Bertani medium. The presence of the HPV-16 E6, E7 and L1 genes in the constructed vectors was determined using restriction enzyme analysis. Large-scale preparation of the plasmids were performed according to standard polyethylene glycol (PEG) precipitation method [21].
Mice immunization and in vivo tumor rejection assay
Two weeks after TC-1 cells injection, mice were immunized intramuscularly (IM) twice at a two weeks interval with 100 ml phosphate buffered saline (PBS; negative control), 100 mg naked DNA vaccine encoding pcDNA3 (negative plasmid control), pcDNA3/L1, pcDNA3/E6, pcDNA3/E7, pcDNA3/E6 & pcDNA3/E7, pcDNA3/E6 & pcDNA3/L1, pcDNA3/E7 & pcDNA3/L1 and pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1 in PBS. Negative control mice groups were used for elimination of none specific responses and other environmental interferences. Finally, three animals per group were sacrificed randomly on day 14 after the final immunization and their spleens were isolated aseptically for in vitro splenocytes culture. Remained mice followed up to 60 days for tumor appearance and size of tumors. The tumor volume was estimated according to Carlsson’s formula as described in previous [16].
Lymphocyte Proliferation Assay (LPA)
The CTL assay was performed as described earlier [22]. Briefly, the suspension of isolated spleen cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 1% L-glutamine, 1% HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)], 0.1 mM minimal essential medium with nonessential amino acids, 0.1% penicillin/streptomycin and incubated in the presence of 4´105 inactivated treated TC-1 cells per well. A total of 100 µl of medium alone or 5 µg/well phytohemoagglutinin (PHA) (Sigma Chemical Co, St Louis, MO, USA) was added in triplicate wells as negative and positive control respectively. All of the plates were incubated at 37°C for 72 hours in a humidified atmosphere containing 5% CO2. A 100 µl aliquot of supernatant was removed and 20 µl of MTT (3-(4, 5-dimethyl tetrazolyl-2) 2, 5 diphenyl) tetrazolium bromide (Sigma Chemical Co, St Louis, MO, USA) in concentration of 5 µg/ml was added per well and incubated for additional 5 h at 37°C in 5% CO2. DMSO (dimethyl sulfoxide) (100 µl) was added to dissolve produced formazan crystals by proliferating cells.
Plates were incubated for 15 minutes at 37°C and read at 540 nm. The results were expressed as stimulation index (SI). The SI was calculated as follows: OD values of stimulated cells (Cs) minus relative cell numbers of unstimulated cells (Cu) by relative OD values of unstimulated cells [23].
SI = (Cs _ Cu)/Cu
All tests were performed in triplicate for each mouse.
Statistical Analysis
The SPSS version 21 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis .The significance of statistical comparisons was calculated using one way ANOVA. The p-values less than 0.05 were considered statistically significant. All values were expressed as means±SD.
Results
In vivo Tumor Challenge Assays
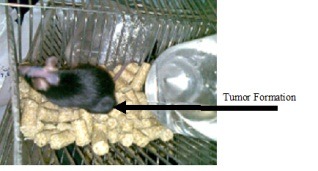
The TC-1 cells were used as a tumor cell model in this study (Figure 1). As shown in figure 2, tumor was appeared rapidly in mice after s.c inoculation of 106 TC-1 cells. To determine whether produced plasmids can induce regression of E7 & E6-related tumors, mice were immunized intramuscularly with pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1 after TC-1 inoculation and were followed for tumor development.
Figure 1.

TC-1 cell line in media culture before counting, and injecting into the left flank of C57BL/6.
Figure 2.
Observation of tumor in mice that were inoculated with 106 TC-1 cells.
Figure 3.
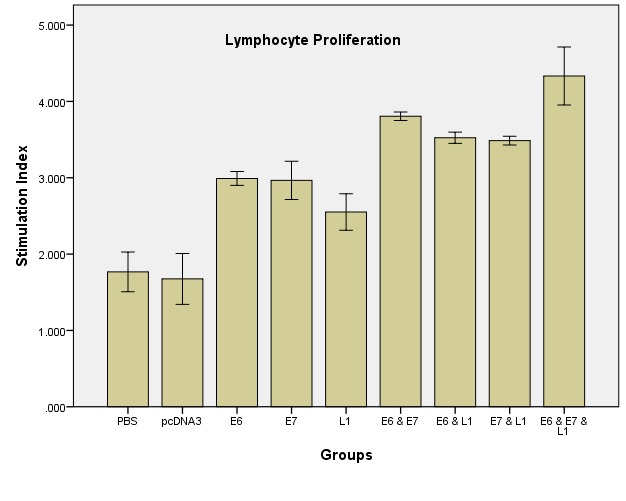
The stimulation calculated indexes for different vaccinated groups; C57BL/6 mice were injected subcutaneously with TC-1 cells. After two week, the mice were immunized intramuscularly (IM) twice at a two weeks interval with 100 ml phosphate buffered saline (PBS; negative control), 100 mg naked DNA vaccine encoding pcDNA3 (negative plasmid control), pcDNA3/L1, pcDNA3/E6, pcDNA3/E7, pcDNA3/E6 & pcDNA3/E7, pcDNA3/E6 & pcDNA3/L1, pcDNA3/E7 & pcDNA3/L1 and pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1 in PBS. Two weeks after final immunization, spleen of individual mice (three/group) was removed and lymphocyte proliferation was evaluated using the MTT method. Formazan crystal formation after incubation with MTT was determined by solving the crystals in DMSO, and the OD was read at 540 nm. Lymphocyte proliferation in the pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1 group was significantly higher than in the other groups especially negative control group (p<0.05).
Figure 4.
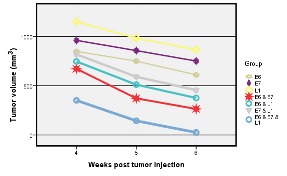
Assessment of therapeutic vaccine on tumor size between the 4th and 6th weeks after the tumor inoculation
Determination of the T Cells Proliferation Following Vaccination
Lymphocyte proliferation was measured as the Stimulation Index (SI) for vaccinated mice. The results showed a significantly enhanced Ag-specific splenocytes proliferation in the pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1 vaccinated mice group compared with other groups using MTT cell proliferation assay (p<0.005).
In vivo Tumor Rejection Assays
In this study, we evaluated the effect of DNA vaccination on the progression of tumor in the TC-1 tumor-bearing mice. The inhibition rate of tumor growth was significantly different (p<0.05) in the vaccinated group with all test groups in compare to the control groups. The efficacy of DNA vaccination appeared to be in order of pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1>pcDNA3/E6 & pcDNA3/E7> pcDNA3/E6 & pcDNA3/L1> pcDNA3/E7 & pcDNA3/L1> pcDNA3/E6> pcDNA3/E7> pcDNA3/L1. The enhanced inhibition of tumor growth was shown in pcDNA3/E6 & pcDNA3/E7 & pcDNA3/L1.
Discussion
In this study, we provides evidences that E6, E7 and L1 genes of HPV-16 cloned in mammalian expression vector are able to produce immune response against cervical cancer model in term of reduction in tumor size and increased cell proliferation in comparison to control groups.
Cervical cancer that is the second-leading cause of cancer death in women usually seems to occur by exposure to the high risk papillomaviruses. So far, two types of preventive vaccines against cervical cancer have been approved by the Food and Drug Administration (FDA). However, nowadays, due to the cost of these vaccines in developing countries, they are considered as inappropriate strategies. Therapeutic vaccines may be effective in inducing cellular immunity against cells expressing viral antigens and improvement of the HPV lesions. Also today, researches demonstrated DNA vaccines have significant potential, compared to traditional protein vaccines, in terms of priming CTL responses and generating memory CD8+ T-cell responses [11, 24, 25].
The HPV-16 E6 and E7 proteins have been shown to be continuously expressed in cervical carcinoma cells [26]; therefore they are logical targets for therapeutic HPV-16 vaccination in patients with cervical dysplasia and cancer. Studies showed that endothelial cells can take up the purified E7 oncoprotein added to the culture medium [27]. Also, the HPV-16 E6/E7+ tumor cells could be genetically modified with DNA coding for immunostimulatory cytokines or co-stimulatory molecules and used for vaccination. The genetically modified tumor cells were found to be substantially more effective than the parental tumor cell vaccines. Immunological examination had revealed an important role for CD8+ lymphocytes in eradication of an established and actively growing carcinoma [28].
Recent studies have shown that HPV-16 L1 gene has an effect on cellular immunity, so it can be used in the production of therapeutic vaccines [13]. Cheung et al. (2004) engineered L1E7hpSCA1 plasmid encoding the L1 and E7 genes with the codon usage optimized for mammalian cell expression. After injection into a muscle, they showed that this plasmid had efficiently induced T cell cytotoxicity and also can lyse the TC-1 cells expressing E7; also, tumor growth was stopped in vaccinated mice [29]. The protection against TC-1 tumor challenge was also observed after vaccination with chimeric papillomavirus-like particles consisting of the HPV-16 L1/L2 viral capsid proteins plus the entire E7 non-structural protein [30].
Since CD8+ T cells exert a major immunological role in tumor rejection, we compared in vitro cytotoxic function of the splenic cells from the various vaccinated animals. Our results indicated an increase CTL activity of the mice treated with E6, E7 and L1 as a DNA vaccine. In the interpretation of this experiment, we considered that enhancement of E6 and E7 effects as stimulators of cellular immunity in the presence of L1 is probably due to specific epitopes on it. Bellone et al. (2009) showed that the HPV-16 L1 DNA vaccine strongly promotes the stimulation of vaginal CD8+ T cells, which are essential for the elimination of virus-infected cells [13].
In our setting, the amount of CTL response was lower than previous studies [13, 14] which may be due to several reasons such as the stage of tumor, the method of CTL assay, the mouse model and cell line type. On the other hand, pro-inflammatory cytokines can activate the natural killer (NK) cell, and the NK cells have important role in rejecting tumors [31]. Although, we did not assay NK cell infiltration in our established tumor model, but the role of NK cells in tumor resistance and development cannot be ignored. Since modulation of T helper subsets as effector cell populations is responsible for protective immunity, therefore, directing immune responses is needed for considerable protection [32].
Conclusion
We have studied HPV16 DNA vaccines containing HPV-16 E6, E7 and L1 genes as a therapeutic vaccine. Based on the obtained data, they are ideal targets for HPV vaccine development and tumor rejection. Since, whole genomes of E6 and E7 are toxic for human cells and given the strong antitumoric effects of them, it seems that further examinations of these approaches are acquired.
Acknowledgments
This study was performed as part of Ph.D thesis of Maryam Fazeli, and it was financially supported by the grant No. 52127827, provided from the Research Deputy of Tarbiat Modares University, Faculty of Medical Sciences, Tehran, Iran.
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet oncol. 2001;2(9):533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Czegledy J, Rogo KO, Evander M, Wadell G. High-risk human papillomavirus types in cytologically normal cervical scrapes from Kenya. Med microbiol immunol. 1992;180(6):321–6. doi: 10.1007/BF00191552. [DOI] [PubMed] [Google Scholar]
- 4.Castellsagué X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol oncol. 2009;115(3):15–23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Bosch F, Lorincz A, Munoz N, Meijer C, Shah K. The causal relation between human papillomavirus and cervical cancer. J Clin Path. 2002;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellone S, Pecorelli S, Cannon MJ, Santin AD. Advances in dendritic cell-based therapeutic vaccines for cervical cancer. 2007 doi: 10.1586/14737140.7.10.1473. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70(7):4509–16. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh K-J, Kalinina A, Bagchi S. Destabilization of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis. Virology. 2010;396(1):118–24. doi: 10.1016/j.virol.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann A, Nieland J, Schinz M, Nonn M, Gabelsberger J, Meissner H, et al. HPV16 L1E7 chimeric virus‐like particles induce specific HLA‐restricted T cells in humans after in vitro vaccination. Int J Cancer. 2001;92(2):285–93. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1181>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Evans EM-l, Man S, Evans AS, Borysiewicz LK. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Research. 1997;57(14):2943–50. [PubMed] [Google Scholar]
- 11.Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27(3):431–40. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188(2):327–38. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 13.Bellone S, El-Sahwi K, Cocco E, Casagrande F, Cargnelutti M, Palmieri M, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J virol. 2009;83(13):6779–89. doi: 10.1128/JVI.02443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuy C, Buzoni-Gatel D, Touzé A, Bout D, Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J virol. 1999;73(11):9063–71. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin K-Y, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56(1):21–6. [PubMed] [Google Scholar]
- 16.Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas-an experimental study in rats. J Cancer Res Clin Oncol. 1983;105(1):20–3. doi: 10.1007/BF00391826. [DOI] [PubMed] [Google Scholar]
- 17.Fazeli M, Soleimanjahi H, Ghaemi A, Farzanepour M, Amanzadeh A, Hashemi SR. Efficacy of HPV-16 E7 based vaccine in a TC-1 tumoric animal model of cervical cancer. Cell J. 2011;12(4):483–8. [Google Scholar]
- 18.Meshkat Z, Soleimanjahi H, Mahmoudi M, Hassan ZM, Mirshahabi H, Meshkat M, et al. CTL responses to a DNA vaccine encoding E7 gene of human papillomavirus type 16 from an Iranian isolate. Iran J Immunol. 2008;5(2):82–91. [PubMed] [Google Scholar]
- 19.Mirshahabi H, Soleimanjahi H, Pourpak Z, Meshkat Z, Hassan ZM. Production of Human Papilloma Virus Type 16 E6 Oncoprotein as a Recombinant Protein in Eukaryotic Cells. Iranian J Cancer Prevent. 2012;5(1):16–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Teimoori A, Soleimanjahi H, Fotouhi F, Meshkat Z. Isolation and cloning of human papillomavirus 16 L1 gene from Iranian isolate. Saudi Med J. 2008;29(8):1105–8. [PubMed] [Google Scholar]
- 21.Sambrook J, Russell D. Cold Spring Habor Laboratory Press; Cold Spring Habor, NJ, USA: 2001. Molecular Cloning; pp. 19–24. [Google Scholar]
- 22.Farzanehpour M, Soleimanjahi H, Hassan Z, Amanzadeh A, Ghaemi A, Fazeli M. HSP70 modified response against HPV based tumor. Eur Rev Med Pharmacol Sci. 2013;17(2):228–34. [PubMed] [Google Scholar]
- 23.Jazayeri M, Soleimanjahi H, Fotouhi F, Bamdad T, Jamali A. Evaluation of cross immune response in DNA based vaccinated mice against HSV-1 and HSV-2. Genes Ther Mol Biol. 2007;11:263–8. [Google Scholar]
- 24.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annual review of immunology. 2000;18(1):927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 25.Kianmehr Z, Soleimanjahi H, Ardestani SK, Fotouhi F, Abdoli A. Influence of Brucella abortus lipopolysaccharide as an adjuvant on the immunogenicity of HPV-16 L1VLP vaccine in mice. Med Microbiol Immunol. 2014 Sep 4. Med Microbiol Immunol. 2014 doi: 10.1007/s00430-014-0356-z. [DOI] [PubMed] [Google Scholar]
- 26.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J virol. 2003;77(2):1551–63. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Anna R, Le Buanec H, Alessandri G, Caruso A, Burny A, Gallo R, et al. Selective activation of cervical microvascular endothelial cells by human papillomavirus 16-e7 oncoprotein. J Natl Cancer Inst. 2001;93(24):1843–51. doi: 10.1093/jnci/93.24.1843. [DOI] [PubMed] [Google Scholar]
- 28.Kondo K. [Development of an HPV vaccine--remaining issues and perspective]. Nippon Rinsho Jpn J Clin Med. 2009;67(1):62–8. [PubMed] [Google Scholar]
- 29.Cheung YK, Cheng SC, Sin FW, Xie Y. Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23(5):629–38. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Greenstone HL, Nieland JD, De Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci. 1998;95(4):1800–5. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Mol. 2009;9(8):568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70(4):326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


