Abstract
The layers and sublayers of primary visual cortex, or V1, in primates are easily distinguishable compared to those in other cortical areas, and are especially distinct in anthropoid primates – monkeys, apes, and humans – where they also vary in histological appearance. This variation in primate-specific specialization has led to a longstanding confusion over the identity of layer 4 and its proposed sublayers in V1. As the application of different histological markers relate to the issue of defining and identifying layers and sublayers, we applied four traditional and four more recent histological markers to brain sections of V1 and adjoining secondary visual cortex (V2) in macaque monkeys, chimpanzees, and humans in order to compare identifiable layers and sublayers in both cortical areas across these species. The use of Nissl, neuronal nuclear antigen (NeuN), Gallyas myelin, cytochrome oxidase (CO), acetylcholinesterase (AChE), nonphosphorylated neurofilament H (SMI-32), parvalbumin (PV), and vesicular glutamate transporter 2 (VGLUT2) preparations support the conclusion that the most popular scheme of V1 lamination, that of Brodmann, misidentifies sublayers of layer 3 (3Bβ and 3C) as sublayers of layer 4 (4A and 4B), and that the specialized sublayer of layer 3 in monkeys, 3Bβ, is not present in humans. These differences in interpretation are important as they relate to the proposed functions of layer 4 in primate species, where layer 4 of V1 is a layer that receives and processes information from the visual thalamus, and layer 3 is a layer that transforms and distributes information to other cortical areas.
Keywords: area 17, area 18, cortical layers, histology, immunohistochemistry
Introduction
The neocortex of primates is variably divided into a number of cortical areas of differing functions that can be distinguished by laminar differences in histological appearance.1–11 While early architectonic studies divided cortex into areas based on Nissl stains for cell bodies or myelin stains for neuronal processes, more recent studies have utilized a larger number of histological procedures for processing brain sections to reveal features of different layers within each cortical area. There is a general agreement that the neuronal layers within most of these cortical areas can be characterized by variations on a standard six-layer pattern, which includes sublayers in some cortical areas.11 These subdivisions can be visualized through laminar differences in the distribution of cytochrome oxidase (CO), acetylcholinesterase (AChE), the nonphosphorylated neurofilament H protein (SMI-32), the calcium-binding protein parvalbumin (PV), and the vesicular glutamate transporter 2 protein (VGLUT2).12 Here, we use this battery of histological procedures to characterize laminar and sublaminar patterns in primary (V1 or area 17) and secondary (V2 or area 18) visual cortex of chimpanzees in comparison to these visual areas in macaque monkeys and, to a lesser extent, humans, in order to address a controversy that has persisted since the landmark studies of Brodmann.1
Brodmann famously published a drawing that reproduced the laminar arrangement of cell bodies (neurons) at the junction of area 17 with area 18 in the brain of a human fetus. This depiction seemed to indicate, in a very compelling manner, that, in anthropoid primates, three distinct middle layers in area 17 (V1) merge into the middle layer 4 of area 18 (V2).
The inner granular layer (IV) splits into two dark, cell-dense strips, a superficial inner granular sublayer (IVa) and a deep inner granular sublayer (IVc), while a light cell-poor stripe, the intermediate inner granular sublayer or stria of Gennari (IVb), extends between them.1,13
In the same decade as Brodmann,1 Campbell14 discusses a single layer 4 in V1:
Layer of stellate cells. Known also as the fourth layer, the granular layer, or Kornerschicht (in German). Curiously enough this lamina is best represented in the calcarine cortex, and there lies in association with the accentuated line of Baillarger (Gennari). This observation caused me to start originally with the belief that the layer of stellate cells corresponded in position with the line of Baillarger, and that the two were mutually interdependent. But this was soon proved to be a fallacy, and we now know that if sections of the calcarine cortex stained for nerve cells be superimposed on ones stained for fibers, the main bulk of the Baillarger line will be seen lying above the stellate cell collection; and in other regions of the brain, a faint, pallid zone, situated immediately below the line of Baillarger, usually denotes the position of this lamina.14
Brodmann concluded that layer 4 of V1 in these primates has three main sublayers, 4A, 4B, and 4C, that merge to form a single layer 4 in V2. While this nomenclature has been, and is now, dominant in the field, others have come to the different conclusion that only the layer below the stria of Gennari, layer 4C in Brodmann’s terms,1 is layer 4 in anthropoid primates.15–22 This alternative has been promoted, most notably, by Hässler,2 who evaluated Nissl-stained brain sections from 16 different primate species including humans, Old World monkeys, New World monkeys, and prosimian primates. Hässler concluded that, in all primates, not just anthropoids, only one layer 4 exists in V1, and only this layer continuously merges with layer 4 in V2. Brodmann’s layers 4A and 4B are, instead, sublayers of layer 3.
In some sense, the naming and numbering of layers in cortex is arbitrary, and any system will do. However, different systems of labeling layers across species or across cortical areas can lead to serious misinterpretations of comparative data. When comparing prior studies of lamination patterns in area 17 of humans, apes, Old World monkeys, prosimians, and other taxa, layer 4 is often defined differently across taxa when Brodmann’s system is used.23
In addition, Brodmann’s proposal adds rather unusual sublayers to layer 4 of V1 in anthropoid primates, as sublayers 4A and 4B in his scheme provide major projections to extrastriate cortical visual areas, while the same projections in other taxa arise from layer 3 of V1.24,25 As another problem, layer 3 of V1 across primates, particularly anthropoids, is especially thick due to increasing intra-areal and corticocortical connections from these layers. Under Brodmann’s scheme, anthropoid primates become an exception in the primate Order when a large portion of this layer 3 is designated as part of layer 4. Because of the comparative, evolutionary, and functional implications of the two major laminar schemes for V1 in primates, it seems useful to consider the architectonic evidence further.
To that end, we examined the architecture of V1 and adjoining V2 in the postmortem brains of humans, chimpanzees, and macaque monkeys. These structures have been previously studied with Nissl or myelin stains, but few reports have examined the architectonic characteristics of these areas with other histochemical methods. Chimpanzees (Pan troglodytes) belong to the family of apes within the primate Order and are the closest relatives of humans that remain today. Very few architectonic studies have been conducted on the chimpanzee visual system, largely due to their protected status, but with postmortem cases, scientists have the opportunity to contribute to the existing knowledge on the anatomical organization of the visual system in apes, in relation to other primates. Macaque monkeys (Macaca mulatta) belong to the Old World line of anthropoid primates and are frequently used as a model of primate and human vision. Thus, we compared the better-known anatomy of V1 and V2 in macaques to the lesser-known characteristics of those areas in chimpanzees and humans. Using a battery of histological and immunological markers that identify anatomical characteristics of cortical areas in other primate species, we compared the laminar patterns of primary visual cortex (V1) and secondary visual cortex (V2) in all three species. We found that chimpanzees possess similar laminar characteristics in V1 and V2 to those of other primate species, but also have features that reveal specializations of these areas in apes and humans.
The present results are consistent with those of previous comparative studies of visual structures across the primate lineage, and inform us of conserved anatomical characteristics in early visual areas of the primate brain. They also provide comparative evidence that layers 4A and 4B of Brodmann’s area 17 (V1) are more likely sublayers of layer 3 in all primate species, including humans.
Materials and methods
One chimpanzee hemisphere, one macaque hemisphere, and one human hemisphere were used to examine the laminar characteristics of V1 and V2 across primate species. The human specimen was obtained from an 82 year old female through the Anatomical Request Program at the University of Tennessee Memphis Health Sciences Center (Memphis, TN, USA). The macaque specimen was obtained from a 14 year old male through the Tissue Donation Program at the University of Washington (Seattle, WA, USA), and the chimpanzee specimen was obtained from a 53 year old female at the Texas Biomedical Research Institute (San Antonio, TX, USA).
Tissue acquisition
The human specimen was extracted from the skull postmortem and postfixed for 2 weeks in 10% formalin prior to being shipped overnight to Vanderbilt University (Nashville, TN, USA). Upon arrival, the brain was rinsed briefly in 0.1 M phosphate-buffered saline (PBS) and separated into blocks appropriate for coronal sections through the cortex and subcortical structures. All visual blocks were cryoprotected in 30% sucrose for 2 days prior to histology, and remaining blocks were kept aside for future use.
The chimpanzee specimen was extracted from the skull following postmortem transcardial perfusion with 0.1 M PBS, and was shipped overnight to Vanderbilt University. Upon arrival, the brain was bisected through the corpus callosum and subcortical structures, and the left hemisphere was postfixed in 4% paraformaldehyde (PFA) in 0.1 M PBS for 2 days. The right hemisphere was used in an unrelated study. Following postfixation, the fixed hemisphere was separated into blocks for ease of processing, and cryoprotected in 30% sucrose in 0.1 M PBS for 2 days prior to histology.
The macaque specimen was extracted from the skull following postmortem transcardial perfusion with 0.1 M PBS followed by 1% PFA in 0.1 M PBS, and was shipped overnight to Vanderbilt University. Upon arrival, the cortical hemispheres were bisected through the corpus callosum and separated from the thalamus and brainstem. The right hemisphere was blocked and postfixed in 4% PFA for 6 hours, and then cryoprotected in 30% sucrose for 24 hours prior to histology.
Histology
Cryoprotected blocks from each case were cut into 40–50 μm coronal sections through the extent of V1 and caudal V2, and sections from each block were further separated into ten alternating series for future studies. Two series from the human hemisphere were processed for Nissl substance with thionin and myelinated fibers,26 and the remaining series were stored in cryoprotectant (30% ethylene glycol, 30% sucrose, 1% polyvinyl pyrrolidone in 0.1 M phosphate buffer) at −20°C until further use. Four series from each of the chimpanzee and macaque hemispheres were processed for Nissl substance, CO,27 AChE,28 and myelin.26 All four stains are traditional markers of areal and laminar boundaries in the neocortex. The remaining six series were stored at 4°C in 0.1 M tris-buffered saline with 0.01% sodium azide until processed for immunohistochemistry.
Immunohistochemistry
Alternating series in the chimpanzee and macaque hemispheres were processed for four markers that identify laminar and architectonic differences in mammalian brains29–31 using slight modifications of previously described immunohistochemical techniques.32 Unfortunately, all attempts at immunohistochemistry in the human hemisphere were unsuccessful due to the high level of formalin fixation required for tissue specimens obtained through the donor institution. However, previous anatomical studies of human visual cortex,33–36 which utilized PFA fixed tissue, were instrumental in guiding our interpretations of immunohistochemical differences across monkeys, apes, and humans. Neuronal nuclear antigen (NeuN)36 labels neuronal nuclei and clearly distinguishes laminar patterns in V1 and V2 by morphological differences in labeled neurons. VGLUT237 labels geniculostriate terminations in V1 of primates12,32,34,38 among other glutamatergic visual projections.32 PV39 also identified geniculostriate terminals in V112,40,41 as well as the cell bodies of inhibitory neurons in visual cortex.42–44 SMI-32 labels nonphosphorylated neurofilaments in neurons,45 and preferentially labels subsets of pyramidal neurons in visual cortex.46 Details of all primary antibody characteristics are listed in Table 1. For each immunolabeled series, sections were briefly rinsed in 0.1 M PBS and postfixed in 2% PFA for 20 minutes. Sections were then incubated in 0.1 M citric acid (pH 6) at 75°C for epitope retrieval, and were subsequently quenched in 0.01% hydrogen peroxide in 0.1 M PBS to reduce nonspecific reactivity. Sections were incubated for 2 hours in blocking solution (5% horse serum, 0.05% Triton X-100, 0.1 M PBS) then incubated overnight with the desired primary antibody diluted in fresh blocking solution. The following day, sections were rinsed in 0.01 M PBS with 0.01% Triton X-100 and then incubated for 2 hours in 1:500 horse anti-mouse IgG (Vector Laboratories, Inc., Burlingame, CA, USA) diluted in blocking solution. Antibody signals were amplified overnight using an avidin-biotin conjugate kit (Vector ABC Elite kit; Vector Laboratories, Inc.), and the final product was visualized using 3,3′-diaminobenzidine with 0.02% nickel enhancement. Sections were then mounted on gelatin-subbed slides, dehydrated through a graded alcohol series, rinsed in xylene, and coverslipped with Permount™ (Thermo Fisher Scientific, Waltham, MA, USA).
Table 1.
Details of antibodies used for immunohistochemistry of areas V1 and V2
| Marker | NeuN | VGLUT2 | SMI-32 | Parvalbumin |
|---|---|---|---|---|
| Antibody | Mouse anti-NeuN | Mouse anti-VGLUT2 | Mouse anti-SMI-32 | Mouse anti-parvalbumin |
| Vendor and Catalog Number | EMD Millipore MAB377 (Billerica, MA, USA) |
EMD Millipore MAB5504 (Billerica, MA, USA) |
Covance Inc., SMI32R (Princeton, NJ, USA) |
Sigma-Aldrich P3088 (St Louis, MO, USA) |
| Concentration | 1:5,000 | 1:5,000 | 1:3,000 | 1:2,000 |
Abbreviations: NeuN, neuronal nuclear antigen; SMI-32, nonphosphorylated neurofilament H protein; V1, primary visual cortex; V2, secondary visual cortex; VGLUT2, vesicular glutamate transporter 2 protein.
Image acquisition and analysis
Digital photomicrographs of stained sections were captured using an MBF CX9000 camera mounted on a Nikon E80i microscope with Neurolucida software (MBF Bioscience, Williston, VT, USA). All images for figures were cropped and adjusted for brightness and contrast but were otherwise unaltered. Sections stained for NeuN and VGLUT2 were also serially reconstructed using freely available software (Reconstruct, Boston, MA, USA) to determine the areal extent of V1.
Results
The areal and laminar boundaries of V1 and V2 were easily identified across all three species using the histological and immunohistochemical procedures described above. Staining patterns in the chimpanzee and human brain were more variable compared to those seen in the macaque brain, due to the lower quality of tissue preservation in both species, but major characteristics of both cortical areas were identifiable across all three specimens. V1 in chimpanzees, macaques, and humans is distinctly laminated compared to the rest of the neocortex, with well-defined boundaries between each of the six neocortical layers, as well as multiple subdivisions of layers 3–6 that correlate with distinct patterns of projections to and from V1. In contrast, V2 consists of six basic neo-cortical layers, with few obvious subdivisions within layers, and is histologically similar to cortical visual areas anterior to V1. When compared across species, laminar patterns in V1 and V2 of chimpanzees, macaques, and humans all have specialized as well as conserved characteristics. Descriptions of these features are outlined below.
Areal and laminar characteristics of V1 in chimpanzees, macaques, and humans
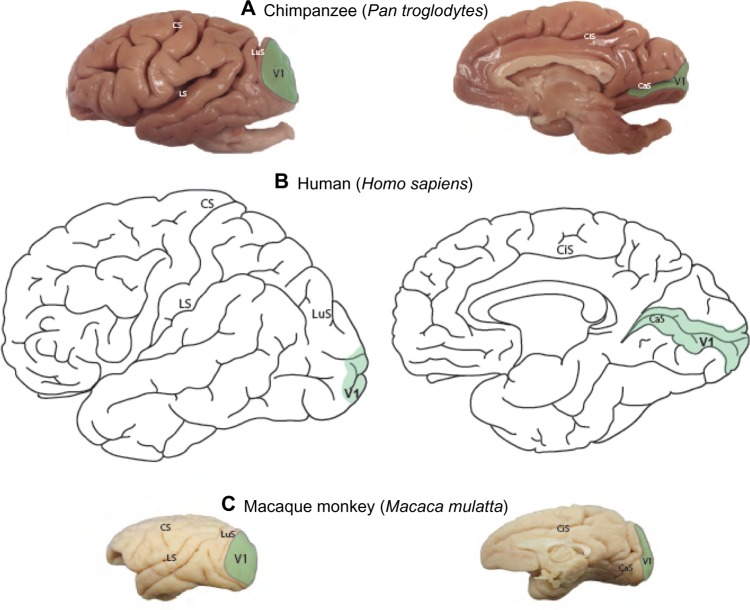
Reconstructions of the boundary of V1 were based on NeuN and VGLUT2-stained sections to identify its areal extent across the cortical surface of the chimpanzee brain (Figure 1A) in comparison to the extent of V1 in humans (Figure 1B) and macaque monkeys (Figure 1C). On the dorsolateral surface, V1 in chimpanzees occupies a large portion of the occipital pole, spanning almost 2 cm at its widest point. On the medial surface, V1 only covers ~1 cm across its dorsoventral span, but continues rostrally for several more centimeters along the banks of the calcarine sulcus. The relative extent and location of V1 in chimpanzee is largely similar to the boundary of V1 identified in humans (Figure 1A), Old World macaque monkeys (Figure 1C), and New World monkeys;3,47,48 although V1 in chimpanzees appears to occupy proportionately less cortical space overall, compared to V1 in monkeys,3 but still more proportional cortical space than V1 in humans.1,3
Figure 1.
Areal extent of V1 on the lateral and medial surfaces of neocortex.
Notes: Areal extent of V1 on the lateral and medial surfaces of neocortex in chimpanzees (A; serial reconstruction from NeuN-stained sections); humans (B; Brodmann areas1); and macaque monkeys (C; Felleman and Van Essen areas71). Scale bar is 10 cm for all species.
Abbreviations: CaS, calcarine sulcus; CiS, cingulate sulcus; CS, central sulcus; LS, lateral sulcus; LuS, lunate sulcus; NeuN, neuronal nuclear antigen; V1, primary visual cortex.
In all primates, V1 is stratified into six distinct layers based on differences in cell distribution (Figures 2 and 3), with some layers divided into sublayers in certain primate species.1,2,4,12,23,49 While several systems of nomenclature exist for the layers of V1, the system of Brodmann dominates in vision research, but the system of Hässler is in widespread use as well. Here, we use Hässler’s laminar scheme to delineate cortical layers in V1 and V2 of chimpanzees, macaque monkeys, and humans.2 Hässler’s scheme identifies six distinct layers, numbered 1–6 through the dorsoventral depth of V1, with three sublayers of layer 3 and two sublayers of layer 4 (Figures 3C, D, G, H, and 4). The distinct sublamination of V1 terminated abruptly at the V1–V2 border (Figures 3G, H, and 5), clearly separating these two areas in cortex.
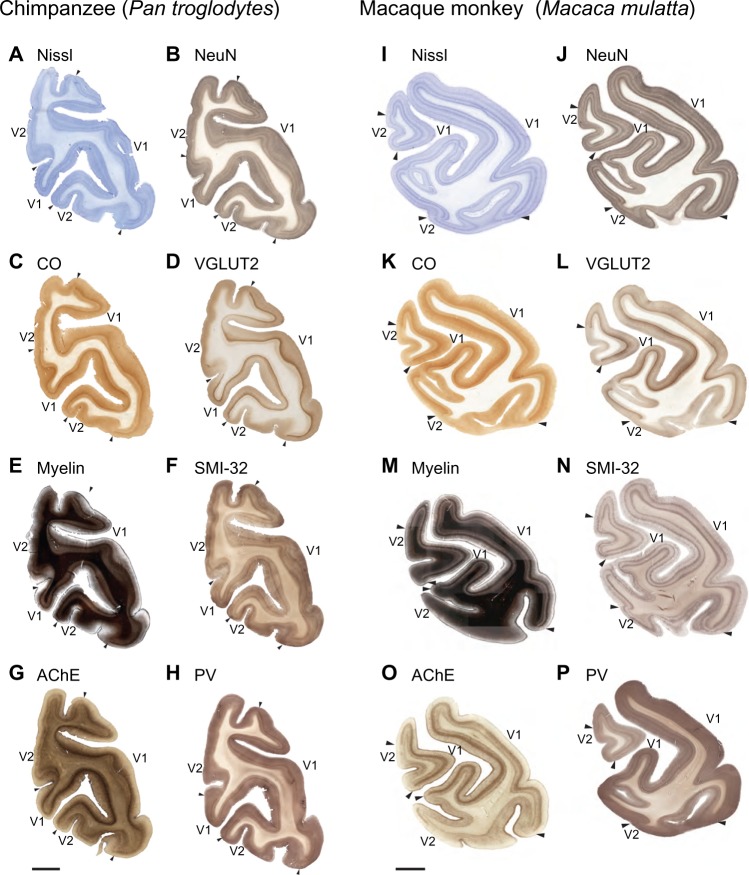
Figure 2.
Low magnification images of coronal sections through the occipital lobe of a chimpanzee brain and a macaque monkey brain.
Notes: Low magnification images of coronal sections through the occipital lobe of a chimpanzee brain (A–H) and a macaque monkey brain (I–P). Each section was processed for a different architectonic marker and, even at low magnification, all markers clearly identify the boundaries of V1. Midline is to the left, scale bar is 1 cm.
Abbreviations: AChE, acetylcholinesterase; CO, cytochrome oxidase; PV, parvalbumin; SMI-32, nonphosphorylated neurofilament H protein; V1, primary visual cortex; V2, secondary visual cortex; VGLUT2, vesicular glutamate transporter 2.
Figure 3.
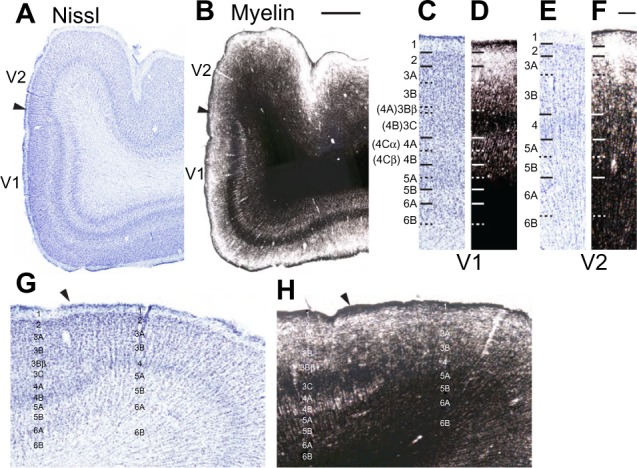
Low and high magnification images of V1 and V2 in the human brain.
Notes: Low (A and B) and high (C–F) magnification images of V1 and V2 in Nissl (A, C, E, G) and myelin (B, D, F, H) stained sections through the human brain. Arrowheads indicate the boundary between V1 and V2 (A, B, G, and H). Hässler’s laminar divisions are listed for panels (C–H), Brodmann’s divisions are listed in parentheses for panels (C–F). Scale bar is 1 cm.
Abbreviations: V1, primary visual cortex; V2, secondary visual cortex.
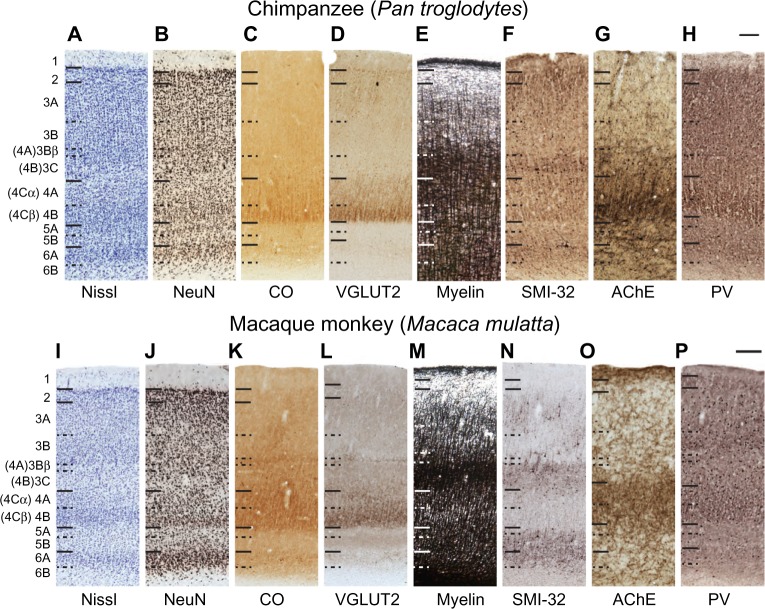
Figure 4.
High magnification images through V1 in chimpanzees and macaque monkeys.
Notes: High magnification images through V1 in chimpanzees (A–H) and macaque monkeys (I–P). Hässler’s laminar divisions are indicated on the left. Brodmann’s divisions for layer 4 are listed in parentheses. Scale bars are 100 μm.
Abbreviations: AChE, acetylcholinesterase; CO, cytochrome oxidase; NeuN, neuronal nuclear antigen; PV, parvalbumin; SMI-32, nonphosphorylated neurofilament H protein; V1, primary visual cortex; VGLUT2, vesicular glutamate transporter 2.
Figure 5.
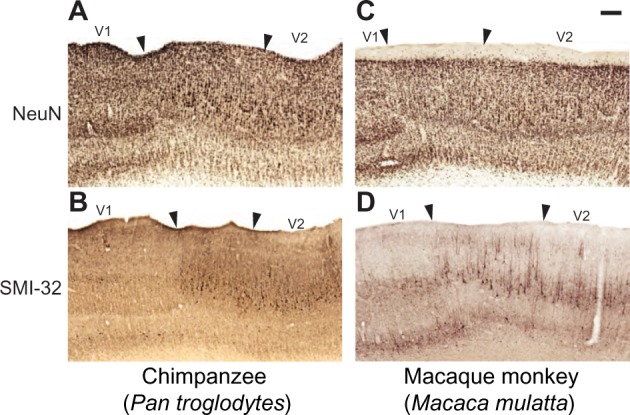
Changes in the lamination pattern at the border of V1 and V2 in chimpanzees (A and B) and macaque monkeys (C and D).
Notes: Arrowheads mark the V1–V2 border and include the portion of V2 specialized for callosal connections. Scale bar is 1 mm.
Abbreviations: NeuN, neuronal nuclear antigen; SMI-32, nonphosphorylated neurofilament H protein; V1, primary visual cortex; V2, secondary visual cortex.
Layer 1 of V1 in all three species is cell-sparse (Figures 2A, B, I, J; 3A, C, G; and 4A, B, I, and J) and largely consists of myelinated fibers running parallel to the brain surface (Figures 2E, M; 3B, D; 4E, and M). In chimpanzees and macaques, layer 1 also showed moderate labeling for AChE and PV (Figures 2; 4G, H, O, and P), but no labeling for CO, SMI-32, or VGLUT2 (Figures 2C, D, F, K, L, and N; 4C, D, F, K, L, and N). Layer 2 of V1 is densely populated with small cells, but is sparsely myelinated, in all three species (Figures 2–4). Cells in this layer stain moderately for CO, VGLUT2, SMI-32, and PV, but weakly for AChE. Layer 2 in chimpanzees also shows more punctate labeling of VGLUT2 terminals compared to layer 2 in macaque monkeys.
Layer 3 of V1 could be divided into three sublayers, 3A, 3B, and 3C, based on differences in cell size and density in chimpanzees, macaques, and humans (Figures 2–4). Layer 3A largely consists of small- and medium-sized cells that are evenly distributed through the layer, some of which stain darkly for VGLUT2, SMI-32, and PV in chimpanzees and humans. At low magnification, discrete patches of SMI-32-positive neurons were visible in layers 3A and 3B of chimpanzee V1 (Figure 2F), similar to the diffuse patches of labeled neurons seen in macaque V1 (Figure 2N). Diffuse neuropil labeling of layer 3A was also apparent in PV preparations of chimpanzee and macaque V1, but also continued throughout the extent of layer 3 in both species. The vast majority of cells in 3A, however, were not identifiable with the markers used in this study. Layer 3B in all species consisted of larger cells with slightly denser myelination compared to 3A. In macaques, cells in 3B were more clustered together compared to cells in 3A, but both layers in chimpanzees and humans appeared equally densely packed. Some cells in 3B of chimpanzees and macaques stained strongly for SMI-32 and AChE, but the majority of cells remained unlabeled in all preparations. The basal portion of layer 3B in macaques, also known as 3Bβ, is a thin layer of small, densely packed granule-like cells that receive projections from parvocellular and koniocellular cells in the lateral geniculate nucleus,4,23,50 and dense terminal labeling of VGLUT2 in this layer of macaque V1 reflects those projections.32,38 In chimpanzees and humans, a similar layer of densely packed granule cells is visible in Nissl and NeuN preparations, but the same region shows no labeling of VGLUT2 in either species.34,38 Lastly, layer 3C in all three species consisted of sparsely distributed large pyramidal cells and medium-sized nonpyramidal cells. Large cells in layer 3C all stained strongly for CO, SMI-32, and AChE, and moderately for VGLUT2 and PV, while smaller cells were only weakly labeled in each preparation. Layer 3C was also densely myelinated compared to layers 3B and 4 in all three species, and appeared as a dark band across the superficial layers of V1.
Layer 4 of V1 could be distinctly subdivided into two layers, 4A and 4B, based on cell density and staining reactivity in all three species (Figures 2–4). Both subdivisions of layer 4 consisted of small granule cells and moderate myelination compared to adjacent layers 3C and 5A. However, layer 4A was less cell dense than 4B, and a distinct band of slightly denser myelin was visible at the border between 4A and 4B. In chimpanzees and macaque monkeys, both subdivisions of layer 4 labeled densely for CO and VGLUT2, and contained scattered cells that stained for SMI-32, AChE, and PV. In both cases, 4A showed weaker labeling for CO, VGLUT2, AChE, and PV, but stronger labeling for SMI-32, compared to 4B.
Layer 5 of V1 could also be divided into two sublayers, based on slight differences in cell density and myelination, in all three species (Figures 2–4). The superficial layer, 5A, was thinner but more densely packed with cells compared to the ventral layer 5B. Cells in this layer stained weakly for CO, SMI-32, and AChE, and showed negligible VGLUT2 or PV activity. Layer 5A was also more myelinated than 5B in all three species. Layer 5B was wider and more sparsely distributed compared to 5A, and cells in this layer showed slightly stronger labeling for SMI-32, AChE, and PV, but almost no labeling for CO or VGLUT2. Diffuse neuropil labeling for VGLUT2 was visible in 5B of macaques, but was not present in the same layer of apes or humans.34
Layer 6 of V1 can be similarly subdivided into two layers based on differences in cell density (Figures 2–4). The superficial layer, 6A, is filled with densely packed medium-sized cells that show moderate labeling for CO, VGLUT2, SMI-32, AChE, and PV in chimpanzees and macaques. The deep layer, 6B, is a thin band of scattered small cells that label weakly for SMI-32 and AChE. Slightly more punctate labeling of VGLUT2 was apparent in layer 6A compared to layer 6B in both chimpanzees and macaques. In all species, cells in 6A occupied most of the width of layer 6 across V1, while cells in 6B occupied only the deepest portion of layer 6 and diffused into the white matter below V1.
Cellular differences at the border of V1 and V2 in chimpanzees, macaques, and humans
The expanded lamination pattern seen in V1 ended abruptly at the border between V1 and V2 (Figures 2, 3, and 5), providing a clear boundary between these two areas in all three species. However, a distinct region immediately adjacent to the edge of V1 and extending several millimeters into V2 displayed unique characteristics compared to both visual areas (Figures 3G, H; and 5). In NeuN preparations, layer 4 of this region appeared slightly dorsal to layer 4 in both V1 and V2 of chimpanzees and macaque monkeys (Figure 5A and B), and showed no SMI-32 immunoreactivity (Figure 5C and D), which contrasted with the evident SMI-32 label in layer 4 of the adjoining areas. The superficial layers of this region, specifically layers 3A and 3C, contained larger pyramidal neurons with elongated dendritic and axonal arborizations that spread across multiple superficial layers. These neurons stained darkly for SMI-32 and were clearly discernible at low magnifications in both SMI-32 and NeuN preparations. The deep layers of this region were slightly cell sparse compared to the deep layers of V1 and V2, and did not display strong immunoreactivity for any marker used in this study. When viewed continuously through the V1–V2 border, especially in NeuN preparations (Figure 5A and B), Brodmann’s layers 4A and 4C, here labeled as Hässler’s 3Bβ and 4, do not appear to merge into a single layer 4 in V2. Instead, Brodmann’s 4C continues into layer 4 of V2, while Brodmann’s 4B appears continuous with the large pyramidal cells in layer 3 of V2, and Brodmann’s 4A ends abruptly at the V1 border with no clear continuation into V2. When considered under Hässler’s scheme, however, Hässler’s layer 4 of V1 is continuous with layer 4 of V2, layer 3C of V1 is continuous with layer 3 of V2, and layer 3Bβ – which is only distinguishable in macaque V1 and appears to be a specialization of Old World monkeys – ends at the V1 border and does not continue into V2. The clear transition of each of these layers in NeuN preparation demonstrates that Brodmann’s laminar scheme, originally based on Nissl stains, misinterprets the transition of neuronal layers from V1 to V2 and inaccurately designates three sublayers of layer 4 in V1 instead of three sublayers of layer 3. Hässler’s laminar scheme more accurately designates individual sublayers of V1 and V2 across primate species.
Laminar characteristics of V2 in chimpanzees, macaques, and humans
In all primates, V2 lies immediately rostral to V1, spans the majority of its lateral and medial boundaries, and contains a systematic representation of the visual field.51–54 Six layers of V2 are consistently identified across primates51,52,55–57 based on differences in cell density between layers (Figures 2, 3, and 6).
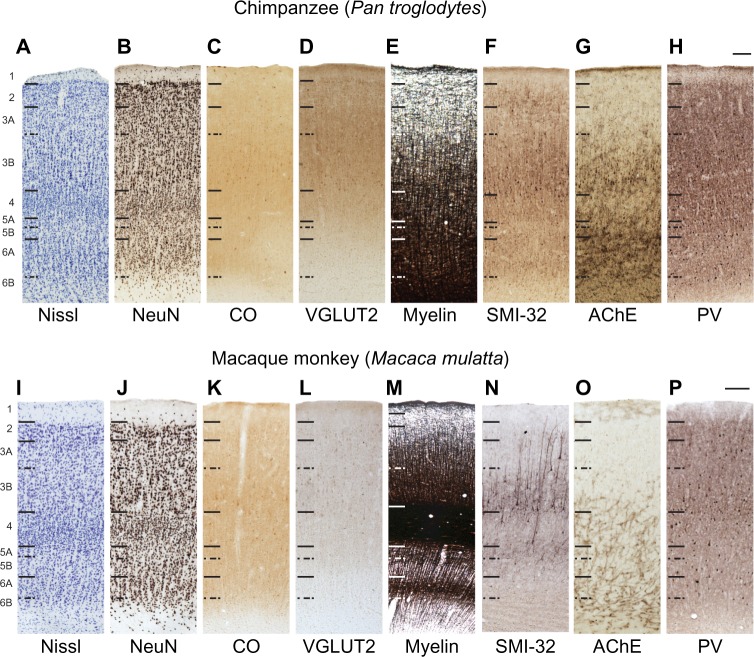
Figure 6.
High magnification images through V2 in chimpanzees (A–H) and macaque monkeys (I–P).
Notes: Hässler’s laminar divisions are indicated on the left. Scale bars are 100 μm.
Abbreviations: AChE, acetylcholinesterase; CO, cytochrome oxidase; NeuN, neuronal nuclear antigen; PV, parvalbumin; SMI-32, nonphosphorylated neurofilament H protein; V2, secondary visual cortex; VGLUT2, vesicular glutamate transporter 2.
Layer 1 of V2 is also cell sparse and densely myelinated (Figures 2A, B, E, I, J, M; 3A, B, E–H; 6A, B, E, I, J and M), similar to that of V1, in all three species. In both chimpanzees and macaques, layer 1 labeled weakly for AChE and PV (Figure 6G, H, O, and P), and did not stain for CO, VGLUT2, or SMI-32 (Figure 6C, D, F, K, L, and N). Layer 2 of V2 (Figures 2, 3, and 6) was sparsely myelinated and consisted of small closely packed cells that did not stain for CO or AChE. Some cells in layer 2 stained weakly for VGLUT2 and PV in both chimpanzees and macaques, and moderately for SMI-32 in chimpanzees, but the vast majority of cells in this layer remained unlabeled with these techniques. In general, more punctate labeling of VGLUT2 and PV was visible in layer 2 of chimpanzees compared to layer 2 of macaque monkeys.
Layer 3 of V2 could be subdivided into two layers, 3A and 3B, based on differences in cell density and myelination (Figures 2, 3, and 6). The superficial layer, 3A, was moderately myelinated and contained small densely distributed cells that only weakly labeled for PV. The deep layer, 3B, was more densely myelinated compared to 3A and contained scattered distributions of variably sized cells that labeled strongly for SMI-32 and PV in both chimpanzees and macaques. Layer 3B also showed dense AChE label in V2 of chimpanzees but not in V2 of macaques.
Layer 4 of V2 appeared as a wide dense band of small granule cells in the middle of V2 with no apparent subdivisions in any preparation (Figures 2, 3, and 6). Layer 4 showed moderate CO and VGLUT2 reactivity, and weak PV reactivity, compared to the other layers of V2, but did not label for SMI-32 or AChE. In macaques, layer 4 appeared densely myelinated compared to the other layers of V2, but was only moderately myelinated in chimpanzees and humans.
Layer 5 of V2 could be separated into two divisions in all three species (Figures 2, 3, and 6): a thin upper layer, 5A, of densely packed cells that showed strong SMI-32 labeling in macaques, but more moderate labeling in chimpanzees; and a wider, more distributed lower layer, 5B, that stained for AChE and PV. Both divisions were clearly distinguishable from each other in macaques by the presence of a dense myelin band between them, but this band was not apparent in chimpanzees or humans. Layer 6 of V2 (Figures 2, 3, and 6) largely consisted of a wide superficial band of distributed medium and large cells, with dense myelination and AChE reactivity as well as weak PV reactivity, and a deeper band of scattered cells that did not label for any marker and largely diffused into the white matter below V2. These two bands were termed 6A and 6B, respectively, and did not show distinct label for CO, VGLUT2, or SMI-32. Overall, laminar patterns across V2 grew progressively less distinct from macaque monkeys to chimpanzees to humans, likely reflecting the altered segregation of afferent and efferent connections through this area in each species.
Discussion
While the areal and laminar organization of V1 and V2 has been extensively studied in Old World and New World monkeys, much less is known about the same levels of organization in present day apes and humans. In both species (apes and humans), architectonic studies are often difficult to conduct, partially due to the scarcity of specimens available for basic neuroscience research, but more often due to the challenge of safely fixing brain tissue to the point where a specimen no longer poses a biohazard threat, but still maintains enough permeability to allow full penetration of any labeling molecules. In the present results, the chimpanzee specimen fulfilled both conditions, but the human specimen was postfixed to a point that no longer allowed the penetration of antibodies used in immunohistochemical labeling. Thus, these findings are somewhat limited by the lack of comparable immunohistochemical labeling in human V1 and V2. However, related findings from other studies on human tissue,33–36,38,46,58–62 as well as the overall Nissl and myelin distributions described here, provide compelling evidence for homologous layers and sublayers in humans and apes. To date, several studies have shed light on individual structural aspects of V1 and V2 in either apes9,63 or humans,59,64–66 but few studies have surveyed differences in cortical structure across the primate lineage in a consistent manner.3,38,60–62,67,68 The present study aimed to compare the areal and laminar structure of V1 and V2 across humans, chimpanzees, and macaque monkeys to determine novel or conserved characteristics of these areas across the primate lineage. We found that, in general, laminar and areal characteristics of V1 and V2 are largely conserved across all three species examined in this study. However, slight shifts in the relative location of V1, as well as shifts in staining densities across V1 and V2, may reflect anatomical changes that arise from functional or connectional differences in these areas.69,70 These characteristics reflect the structural evolution of V1 and V2 from anthropoid primates to apes and humans.
Cortical position and laminar organization of V1 across primates
V1 is one of many visual cortical areas in the primate brain,71,72 but it contains intrinsic specializations that differentiate it from V1 in nonprimate species.4,23,72,73 In all studied primates, V1 contains proportionally more neurons compared to other cortical areas,74–76 devotes proportionally more space to the representation of central vision,47,48,77–79 and typically contains obvious sublayers of layer 4 that reflect segregated classes of thalamic input to this area.23,49,72,73,80–82 Reconstruction of the V1 boundary from serial coronal sections in the chimpanzee brain, when compared to similar reconstructions in macaques,71,83 showed that V1 in chimpanzees occupies less of the lateral surface of the occipital lobe and more of the medial surface instead. Reconstructions in humans shows that V1 also shifts towards the caudal and medial wall of cortex,84 which coincides with the expansion of parietal and temporal visual areas over the primate lineage.72 Thus, the shifted location of V1 in chimpanzees appears to be an intermediate position between humans and macaque monkeys, which is consistent with related studies of brain scaling and cortical organization across primate species.61,72
Histological and immunohistochemical staining distributions from all markers used in this study were largely consistent with previous descriptions of primate V1 and V2, and support related works that utilized other markers in describing laminar subdivisions in these areas.43,85–88 Examinations of cell density via Nissl-stained sections in macaques,49 chimpanzees,4,9 and humans1,65 all demonstrate sparse cell distributions in layer 1 and lower layer 6 of V1, dense cell distributions in layers 4 and upper layer 6, and more variable densities in the remaining layers of V1. NeuN-stained sections confirmed these distributions while making laminar and sublaminar boundaries more pronounced across V1. Additionally, NeuN immunoreactivity in the deep layers of chimpanzee V1 also identified subdivisions of layers 5 and 6 that have been previously reported in macaque monkeys.49,89 These subdivisions of layers 5 and 6 are also visible in Nissl stained sections of human V1, suggesting the conserved evolution of these subdivisions from anthropoid monkeys to humans. The upper subdivision of layer 5 primarily provides intrinsic projections within V1, while the lower division primarily sends projections to subcortical visual structures, and the greater differentiation of these layers likely reflects a further segregation of connected target structures. The same sublayers of layer 5 were also visible in myelin-stained sections of V1, with dense myelination in 5A and sparse myelination in 5B of all three species.
Myelin patterns in the other layers were also instrumental in identifying novel and conserved characteristics of V1. The densely myelinated band in 3C of all three species, known as the outer band of Baillarger, is a conserved feature of V1 in all primates4,23,72 and many nonprimate species.29–31 Similarly, the dense myelin band of layer 5A is termed the inner band of Baillarger and is consistently identified across mammalian species. Slight variations in myelin density within layers 3 and 4 also identified the separation of 3A and 3B, as well as 4A and 4B, in all species.
The dense CO staining seen here in layer 4 of chimpanzees and macaque monkeys, as well as the moderate staining of layers 2 and 6, is consistent with previous descriptions of CO activity in V1.90,91 The denser CO reactivity seen in layer 4B of chimpanzees compared to 4A mirrors the stronger CO staining seen in humans,91 and may reflect an alteration in the relative magnitudes of magnocellular and parvocellular geniculate inputs to 4A and 4B, respectively. When considered alongside the lack of CO staining in layer 3Bβ of chimpanzees61 and humans,92 the evidence suggests that, in these species, parvocellular geniculate projections are reduced or absent in the superficial layers of V1 and are concentrated in layer 4B instead. Magnocellular geniculate projections, however, appear to have expanded in apes and humans, occupying the full extent of layer 4A, and have a modified organization in parts of layer 3.60,61,70 Similarly, dense VGLUT2 label in layer 4 of chimpanzees and macaques, along with moderate label in layers 2 and 6, was consistent with previous descriptions of VGLUT2 in both species, and affirmed that parvocellular geniculate inputs to layer 3Bβ are a specialization of anthropoid monkeys and reduced or absent in great apes and humans.32,34,38
Parvalbumin immunoreactivity has been previously described in macaques and humans,35 and PV labeling in chimpanzee V1 more closely reflects the distributions seen in human V1. Similarly, AChE reactivity has been previously reported for V1 of macaque monkeys93 and humans,59 and the characteristic AChE labeling of layers 1, 4, and 6 in V1 of chimpanzees matches both descriptions. The altered patterns of NeuN and SMI-32 reactivity seen at the border of V1 and V2 in macaques and chimpanzees closely matches previous descriptions of callosally projecting neurons between V1 and V2 of macaque monkeys,94,95 demonstrating that callosal connections in apes and humans likely share the same laminar pattern as those of anthropoid monkeys. Thus, V1 of chimpanzees demonstrates conserved laminar characteristics between monkeys, apes, and humans, as well as intermediate patterns of cell distribution and myelination that reflect the ongoing evolution of V1 across the primate lineage.
Laminar organization of V2 across primates
V2 has been well studied in a range of anthropoid and prosimian primates,12,47,52,55,91,96–98 and all studies suggest that the anatomy of V2 is well conserved across the primate lineage. Chimpanzees and humans are less extensively studied, but some reports have directly compared the laminar characteristics of V2 across primate species,2,67 and the results of the present study correlate closely with the conserved characteristics seen in previous descriptions of V2. Nissl preparations through V2 in all three species identified six distinct layers, with identifiable sublayers of layers 3, 5, and 6, that have been characterized in macaque monkeys.55,57 Our NeuN preparations of V2 identified the same laminar divisions, while making boundaries between 3A and 3B, as well as between 5A and 5B, more distinct in both chimpanzees and macaque monkeys. Both CO and VGLUT2 are discretely expressed in specific layers of V232,52,90 in macaque monkeys, and the same distributions were seen in V2 of chimpanzees. Previous descriptions of SMI-32 in coronal preparations of macaque V2 localized label in the deeper portion of layer 3 and the superficial portion of layer 5,46 which was also apparent in V2 of chimpanzees. Similarly, previous reports of parvalbumin reactivity note its almost uniform appearance across macaque V243 with slightly more PV-positive neurons appearing in layers 2 and 3; this pattern is also present in chimpanzee V2. AChE reactivity in V2 is largely absent in adult monkeys,99 as seen here in macaque V2. However, the marked increase of AChE reactivity seen in specific layers of chimpanzee V2 may reflect alterations in cholinergic input to extrastriate areas in apes and humans. A high level of AChE reactivity in primary auditory cortex of macaques100 has been related to the plasticity of neuronal response characteristics in this area.101
Myelin preparations through V2 in monkeys, chimpanzees, and humans largely identified the same laminar divisions seen in all other preparations, but the relative myelin content in each layer of V2 shifted with each species. In macaques, dense myelination was visible in layer 4 of V2, which has not been described to date, as well as in layer 6A and the boundary between layers 5A and 5B. These densely myelinated bands grew progressively less distinct from monkeys to apes and then humans, to the point where middle and deep layers of V2 in the chimpanzee and human grew indistinguishable from each other. Reasons for the shift in layer 4 myelin content, as well as the decrease in laminar definition across species, are still unclear, but it is likely that changes in the myelin content of V2 across primate species reflect changes in visual processing through this area in apes and humans.
The V1/V2 border and the identification of layer 4
The most important conclusion supported by a detailed examination of how layers and sublayers of V1 merge with those of V2 is that only layer 4C of Brodmann in V1 merges with the broadly recognized layer 4 of V2 in macaques and chimpanzees, as is evident in NeuN preparations through V1 of both species. Layer 4B of Brodmann continues with layer 3 of V2, while layer 4A of Brodmann seems to disappear and, thus, be a specialization of V1 in some primates. Overall, our findings support the use of the alternative laminar scheme of Hässler where layers 4A and 4B of Brodmann are considered sublayers of layer 3. The laminar scheme of Hässler is also supported by comparative studies of V1 lamination that include prosimian primates,12 and studies of laminar connection patterns across species that have layer 4 producing intrinsic connections with other layers and layer 3 providing extrinsic projections to other visual areas. When utilized in chimpanzees, macaques, and humans, Hässler’s laminar scheme provides consistent descriptions of layers and sublayers in V1 and V2.
Acknowledgments
We thank Laura Trice for assistance with tissue processing and histology and Dr Troy Hackett for assistance with image acquisition. This work was funded by NEI R01EY2686 to JHK.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brodmann K. Vergleichende Lokalisationslehre der Gro hirnrinde. Leipzig: Verlag von Johann Ambrosius Barth; 1909. [Google Scholar]
- 2.Hässler R. Comparative anatomy of the central visual system in day-and night-active primates. In: Hässler R, Stephen S, editors. Thieme, Stuttgart: Evolution of the forebrain. Springer; 1967. pp. 419–434. [Google Scholar]
- 3.Passingham RE. Anatomical differences between the neocortex of man and other primates. Brain Behav Evol. 1973;7(5):337–359. doi: 10.1159/000124422. [DOI] [PubMed] [Google Scholar]
- 4.Allman J, McGuinness E. Visual cortex in primates. Comparative Primate Biology. 1988;4:279–326. [Google Scholar]
- 5.Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. Anat Rec A Discov Mol Cell Evol Biol. 2005;287(1):1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- 6.Zilles K, Rehkämper G, Schleicher A. A quantitative approach to cytoarchitectonics. V. The areal pattern of the cortex of microcebus murinus (E Geoffroy 1828), (Lemuridae, primates) Anat Embryol (Berl) 1979;157(3):269–289. doi: 10.1007/BF00304994. [DOI] [PubMed] [Google Scholar]
- 7.Zilles K, Palomero-Gallagher N, Schleicher A. Transmitter receptors and functional anatomy of the cerebral cortex. J Anat. 2004;205(6):417–432. doi: 10.1111/j.0021-8782.2004.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey P, von Bonin G. The isocortex of man. Illinois Monographs in the Medical Sciences. 1951;6:1–2. [Google Scholar]
- 9.Bailey P, Bonin GV, McCulloch WS. The isocortex of the chimpanzee. Urbana: University of Illinois Press; 1950. [Google Scholar]
- 10.Economo Von C, Koskinas GN. Die Cytoarchitectonik der Hirnrinde des erwachsenen Menschen. Berlin: Springer; 1925. [Google Scholar]
- 11.Jones EG. History of cortical cytology. In: Peters A, Jones EG, editors. Cerebral Cortex: Cellular Components of the Cerebral Cortex. New York: Plenum Press; pp. 1–31. [Google Scholar]
- 12.Wong P, Kaas JH. Architectonic subdivisions of neocortex in the Galago (Otolemur garnetti) Anat Rec (Hoboken) 2010;293(6):1033–1069. doi: 10.1002/ar.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garey LJ. Brodmann’s Localisation in the Cerebral Cortex: Translated with editorial notes and an introduction. 1st ed. London: Smith-Gordon Company Limited; 1994. [Google Scholar]
- 14.Campbell AW. Histological Studies on the Localisation of Cerebral Function. Cambridge: Cambridge University Press; 1905. [Google Scholar]
- 15.Kaas JH, Lin CS, Casagrande VA. The relay of ipsilateral and contralateral retinal input from the lateral geniculate nucleus to striate cortex in the owl monkey: a transneuronal transport study. Brain Res. 1976;106(2):371–378. doi: 10.1016/0006-8993(76)91032-5. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick D, Itoh K, Diamond IT. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus) J Neurosci. 1983;3(4):673–702. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spatz WB, Illing RB, Weisenhorn DM. Distribution of cytochrome oxidase and parvalbumin in the primary visual cortex of the adult and neonate monkey, Callithrix jacchus. J Comp Neurol. 1994;339(4):519–534. doi: 10.1002/cne.903390405. [DOI] [PubMed] [Google Scholar]
- 18.Spatz WB, Tigges J, Tigges M. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri) J Comp Neurol. 1970;140(2):155–174. doi: 10.1002/cne.901400203. [DOI] [PubMed] [Google Scholar]
- 19.Florence SL, Conley M, Casagrande VA. Ocular dominance columns and retinal projections in New World spider monkeys (Ateles ater) J Comp Neurol. 1986;243(2):234–248. doi: 10.1002/cne.902430207. [DOI] [PubMed] [Google Scholar]
- 20.Spatz WB. Loss of ocular dominance columns with maturity in the monkey, Callithrix jacchus. Brain Res. 1989;488(1–2):376–380. doi: 10.1016/0006-8993(89)90734-8. [DOI] [PubMed] [Google Scholar]
- 21.Glendenning KK, Kofron EA, Diamond IT. Laminar organization of projections of the lateral geniculate nucleus to the striate cortex in Galago. Brain Res. 1976;105(3):538–546. doi: 10.1016/0006-8993(76)90600-4. [DOI] [PubMed] [Google Scholar]
- 22.Henry GH. Afferent inputs, receptive field properties, and morphological cell types in different laminae of the striate cortex. In: Leventhal AG, editor. Vision and Visual Dysfunction, Vol 4: The Neural Basis of Visual function. Boca Raton: CRC Press; 1989. pp. 223–245. [Google Scholar]
- 23.Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Cerebral Cortex: Primary Visual Cortex of Primates. Vol. 10. 1994. pp. 201–259. [Google Scholar]
- 24.Rosenquist AC. Connections of visual cortical areas in the cat. In: Peters A, Jones EG, editors. Cerebral Cortex: Visual Cortex. Vol. 3. New York: Plenum Press; 1985. pp. 81–117. [Google Scholar]
- 25.Lund JS, Fitzpatrick D, Humphrey AL. The striate cortex of the tree shrew. In: Peters A, Jones EG, editors. Cerebral Cortex: Vol. 3. Visual Cortex. New York: Plenum Press; 1985. pp. 157–205. [Google Scholar]
- 26.Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1(2):203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- 27.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 28.Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- 29.Wong P, Kaas JH. An Architectonic Study of the Neocortex of the Short-Tailed Opossum (Monodelphis domestica) Brain Behav Evol. 2009;73(3):206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis) Anat Rec (Hoboken) 2008;291(10):1301–1333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong P, Kaas JH. Architectonic subdivisions of neocortex in the tree shrew (Tupaia belangeri) Anat Rec (Hoboken) 2009;292(7):994–1027. doi: 10.1002/ar.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balaram P, Hackett TA, Kaas JH. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. J Chem Neuroanat. 2013;50–51:21–38. doi: 10.1016/j.jchemneu.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell MJ, Morrison JH. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol. 1989;282(2):191–205. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Marin V, Ahmed TH, Afzal YC, Hawken MJ. Distribution of vesicular glutamate transporter 2 (VGluT2) in the primary visual cortex of the macaque and human. J Comp Neurol. 2012;521(1):130–151. doi: 10.1002/cne.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blümcke I, Hof PR, Morrison JH, Celio MR. Distribution of parvalbumin immunoreactivity in the visual cortex of Old World monkeys and humans. J Comp Neurol. 1990;301(3):417–432. doi: 10.1002/cne.903010307. [DOI] [PubMed] [Google Scholar]
- 36.Wolf HK, Buslei R, Schmidt-Kastner R, et al. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44(10):1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 37.Aihara Y, Mashima H, Onda H, et al. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74(6):2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- 38.Bryant KL, Suwyn C, Reding KM, Smiley JF, Hackett TA, Preuss TM. Evidence for ape and human specializations in geniculostriate projections from VGLUT2 immunohistochemistry. Brain Behav Evol. 2012;80(3):210–221. doi: 10.1159/000341135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heizmann CW. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JK, Casagrande VA. Distribution of calcium binding proteins within the parallel visual pathways of a primate (Galago crassicaudatus) J Comp Neurol. 1995;356(2):238–260. doi: 10.1002/cne.903560208. [DOI] [PubMed] [Google Scholar]
- 41.Hendrickson AE, Van Brederode JF, Mulligan KA, Celio MR. Development of the calcium-binding protein parvalbumin and calbindin in monkey striate cortex. J Comp Neurol. 1991;307(4):626–646. doi: 10.1002/cne.903070409. [DOI] [PubMed] [Google Scholar]
- 42.Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293(4):599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- 43.DeFelipe J, González-Albo MC, Del Río MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol. 1999;412(3):515–526. doi: 10.1002/(sici)1096-9861(19990927)412:3<515::aid-cne10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Kubota Y, Kawaguchi Y. Three classes of GABAergic interneurons in neocortex and neostriatum. Jpn J Physiol. 1994;44(Suppl 2):S145–S148. [PubMed] [Google Scholar]
- 45.Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hof PR, Morrison JH. Neurofilament protein defines regional patterns of cortical organization in the macaque monkey visual system: a quantitative immunohistochemical analysis. J Comp Neurol. 1995;352(2):161–186. doi: 10.1002/cne.903520202. [DOI] [PubMed] [Google Scholar]
- 47.Allman J, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35(1):89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- 48.Cowey A. Projection of the retina on to striate and prestriate cortex in the squirrel monkey, Saimiri sciureus. J Neurophysiol. 1964;27:366–393. doi: 10.1152/jn.1964.27.3.366. [DOI] [PubMed] [Google Scholar]
- 49.Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- 50.Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol. 1972;6(1):253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- 51.DeYoe EA, Van Essen DC. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317(6032):58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- 52.Tootell RB, Silverman MS, De Valois RL, Jacobs GH. Functional organization of the second cortical visual area in primates. Science. 1983;220(4598):737–739. doi: 10.1126/science.6301017. [DOI] [PubMed] [Google Scholar]
- 53.Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Annu Rev Neurosci. 2005;28:303–326. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- 54.Essen DC, Zeki SM. The topographic organization of rhesus monkey prestriate cortex. J Physiol. 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockland KS. A reticular pattern of intrinsic connections in primate area V2 (area 18) J Comp Neurol. 1985;235(4):467–478. doi: 10.1002/cne.902350405. [DOI] [PubMed] [Google Scholar]
- 56.Van Essen DC, Newsome WT, Maunsell JH, Bixby JL. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J Comp Neurol. 1986;244(4):451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- 57.Lund JS, Hendrickson AE, Ogren MP, Tobin EA. Anatomical organization of primate visual cortex area VII. J Comp Neurol. 1981;202(1):19–45. doi: 10.1002/cne.902020104. [DOI] [PubMed] [Google Scholar]
- 58.Braak E. On the structure of the human striate area. Adv Anat Embryol Cell Biol. 1982;77:1–86. doi: 10.1007/978-3-642-68572-9. [DOI] [PubMed] [Google Scholar]
- 59.Mesulam MM, Geula C. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 1991;306(2):193–220. doi: 10.1002/cne.903060202. [DOI] [PubMed] [Google Scholar]
- 60.Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cereb Cortex. 2002;12(7):671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- 61.Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96(20):11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherwood CC, Raghanti MA, Stimpson CD, et al. Scaling of inhibitory interneurons in areas V1 and V2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain Behav Evol. 2007;69(3):176–195. doi: 10.1159/000096986. [DOI] [PubMed] [Google Scholar]
- 63.Tigges J, Tigges M. Ocular dominance columns in the striate cortex of chimpanzee (Pan troglodytes) Brain Res. 1979;166(2):386–390. doi: 10.1016/0006-8993(79)90224-5. [DOI] [PubMed] [Google Scholar]
- 64.Bridge H, Clare S, Jenkinson M, Jezzard P, Parker AJ, Matthews PM. Independent anatomical and functional measures of the V1/V2 boundary in human visual cortex. J Vis. 2005;5(2):93–102. doi: 10.1167/5.2.1. [DOI] [PubMed] [Google Scholar]
- 65.Braak H. On the striate area of the human isocortex. A Golgi- and pigmentarchitectonic study. J Comp Neurol. 1976;166(3):341–364. doi: 10.1002/cne.901660305. [DOI] [PubMed] [Google Scholar]
- 66.Hockfield S, Tootell RB, Zaremba S. Molecular differences among neurons reveal an organization of human visual cortex. Proc Natl Acad Sci U S A. 1990;87(8):3027–3031. doi: 10.1073/pnas.87.8.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Sousa AA, Sherwood CC, Schleicher A, et al. comparative cytoarchitectural analyses of striate and extrastriate areas in hominoids. Cerebral Cortex. 2010;20(4):966–981. doi: 10.1093/cercor/bhp158. [DOI] [PubMed] [Google Scholar]
- 68.Zilles K, Palomero-Gallagher N, Grefkes C, et al. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol. 2002;12(6):587–599. doi: 10.1016/s0924-977x(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 69.Schoenemann PT. Evolution of the size and functional areas of the human brain. Annu Rev Anthropol. 2006;35:379–406. [Google Scholar]
- 70.Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagosto M, editors. Primate origins: Adaptation and Evolution. Boston, MA: Springer US; 2007. pp. 625–675. [Google Scholar]
- 71.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 72.Kaas JH. The evolution of neocortex in primates. Prog Brain Res. 2012;195:91–102. doi: 10.1016/B978-0-444-53860-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Essen DC. Visual areas of the mammalian cerebral cortex. Annu Rev Neurosci. 1979;2:227–263. doi: 10.1146/annurev.ne.02.030179.001303. [DOI] [PubMed] [Google Scholar]
- 74.Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A. 2010;107(36):15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collins CE, Leitch DB, Wong P, kaas JH, Herculano-Houzel S. Faster scaling of visual neurons in cortical areas relative to subcortical structures in non-human primate brains. Brain Struct Funct. 2013;218(3):805–816. doi: 10.1007/s00429-012-0430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herculano-Houzel S, Collins CE, Wong P, Kaas JH, Lent R. The basic nonuniformity of the cerebral cortex. Proc Natl Acad Sci U S A. 2008;105(34):12593–12598. doi: 10.1073/pnas.0805417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gattass R, Sousa AP, Rosa MG. Visual topography of V1 in the Cebus monkey. J Comp Neurol. 1987;259(4):529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- 79.Pessoa VF, Abrahão JC, Pacheco RA, Pereira LC, Magalhães-Castro B, Saraiva PE. Relative sizes of cortical visual areas in marmosets: functional and phylogenetic implications. Exp Brain Res. 1992;88(2):459–462. doi: 10.1007/BF02259123. [DOI] [PubMed] [Google Scholar]
- 80.Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci. 1998;21(1):47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- 81.Casagrande VA, Boyd KI. The evolution of parallel visual pathways in the brains of primates. In: Preuss TM, Kaas JH, editors. Evolution of the Nervous System. Elsevier Inc; 2007. pp. 87–108. [Google Scholar]
- 82.Billings-Gagliardi S, Chan-Palay V, Palay SL. A review of lamination in Area 17 of the visual cortex of Macaca mulatta. J Neurocytol. 1974;3(5):619–629. doi: 10.1007/BF01097627. [DOI] [PubMed] [Google Scholar]
- 83.Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glasser MF, Goyal MS, Preuss TM, Raichle ME, Van Essen DC. Trends and properties of human cerebral cortex: Correlations with cortical myelin content. Neuroimage. 2013 Apr 6; doi: 10.1016/j.neuroimage.2013.03.060. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fonta C, Négyessy L, Renaud L, Barone P. Areal and subcellular localization of the ubiquitous alkaline phosphatase in the primate cerebral cortex: evidence for a role in neurotransmission. Cereb Cortex. 2004;14(6):595–609. doi: 10.1093/cercor/bhh021. [DOI] [PubMed] [Google Scholar]
- 86.Fonta C, Negyessy L, Renaud L, Barone P. Postnatal development of alkaline phosphatase activity correlates with the maturation of neurotransmission in the cerebral cortex. J Comp Neurol. 2005;486(2):179–196. doi: 10.1002/cne.20524. [DOI] [PubMed] [Google Scholar]
- 87.Hendrickson AE, Tillakaratne NJK, Mehra RD, et al. Differential localization of two glutamic acid decarboxylases (GAD65 and GAD67) in adult monkey visual cortex. J Comp Neurol. 2004;343(4):566–581. doi: 10.1002/cne.903430407. [DOI] [PubMed] [Google Scholar]
- 88.Leuba G, Kraftsik R, Saini K. Quantitative distribution of parvalbumin, calretinin, and calbindin D-28k immunoreactive neurons in the visual cortex of normal and Alzheimer cases. Exp Neurol. 1998;152(2):278–291. doi: 10.1006/exnr.1998.6838. [DOI] [PubMed] [Google Scholar]
- 89.Lund JS. Local circuit neurons of macaque monkey striate cortex: I. Neurons of laminae 4C and 5A. J Comp Neurol. 1987;257(1):60–92. doi: 10.1002/cne.902570106. [DOI] [PubMed] [Google Scholar]
- 90.Horton JC. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984;304(1119):199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- 91.Wong-Riley MT, Hevner RF, Cutlan R, et al. Cytochrome oxidase in the human visual cortex: distribution in the developing and the adult brain. Vis Neurosci. 2009;10(1):41–58. doi: 10.1017/s0952523800003217. [DOI] [PubMed] [Google Scholar]
- 92.Horton JC, Hedley-Whyte ET. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond, B, Biol Sci. 1984;304(1119):255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- 93.Hedreen JC, Uhl GR, Bacon SJ, Fambrough DM, Price DL. Acetylcholinesterase-immunoreactive axonal network in monkey visual cortex. J Comp Neurol. 1984;226(2):246–254. doi: 10.1002/cne.902260208. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy H, Dehay C, Bullier J. Organization of the callosal connections of visual areas V1 and V2 in the macaque monkey. J Comp Neurol. 1986;247(3):398–415. doi: 10.1002/cne.902470309. [DOI] [PubMed] [Google Scholar]
- 95.Hof PR, Ungerleider LG, Adams MM, et al. Callosally projecting neurons in the macaque monkey V1/V2 border are enriched in nonphosphorylated neurofilament protein. Vis Neurosci. 1997;14(5):981–987. doi: 10.1017/s0952523800011688. [DOI] [PubMed] [Google Scholar]
- 96.Collins CE, Stepniewska I, Kaas JH. Topographic patterns of v2 cortical connections in a prosimian primate (Galago garnetti) J Comp Neurol. 2001;431(2):155–167. [PubMed] [Google Scholar]
- 97.Preuss TM, Beck PD, Kaas JH. Areal, modular, and connectional organization of visual cortex in a prosimian primate, the slow loris (Nycticebus coucang) Brain Behav Evol. 1993;42(6):321–335. doi: 10.1159/000114169. [DOI] [PubMed] [Google Scholar]
- 98.Horton JC, Hocking DR. Myelin patterns in V1 and V2 of normal and monocularly enucleated monkeys. Cerebral Cortex. 1997;7(2):166–177. doi: 10.1093/cercor/7.2.166. [DOI] [PubMed] [Google Scholar]
- 99.Barone P, Dehay C, Berland M, Kennedy H. Developmental changes in the distribution of acetylcholinesterase in the extrastriate visual cortex of the monkey. Brain Res Dev Brain Res. 1994;77(2):290–294. doi: 10.1016/0165-3806(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 100.Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335(3):437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- 101.Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97(22):11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]