Abstract
Only one out of four arrestin subtypes expressed in mammals, arrestin-3, facilitates the activation of JNK family kinases. Here we describe two different paradigms that allow the elucidation of the mechanisms involved. One is based on reconstitution of signaling modules from purified proteins: arrestin-3, MKK4, MKK7, JNK1, JNK2, and JNK3. The main advantage of this method is that it can unambiguously establish which effects are direct, because only intended purified proteins are present in these assays. The key drawback is that the upstream-most kinases of these cascades, ASK1 or other MAPKKKs, are not available in purified form, limiting reconstitution to incomplete two-kinase modules. The other set of methods analyzes the effects of arrestin-3 on JNK activation in intact cells. In this case, signaling modules include ASK1 and/or other MAPKKKs. However, every cell expresses thousands of different proteins, and their possible effects on the readout cannot be excluded. However, the combination of in vitro reconstitution from purified proteins and cell-based assays enables comprehensive elucidation of the mechanisms of arrestin-3-dependent activation of JNK family kinases.
Keywords: c-Jun N-terminal kinase (JNK), scaffold, activation, arrestin, biphasic dependence
INTRODUCTION
Mitogen-activated protein kinases (MAPKs) are expressed in virtually all eukaryotes, and the principles of the organization of MAP cascades are conserved from yeast to mammals (Widmann et al., 1999). MAPK signaling modules consist of at least three kinases, MAP kinase kinase kinase (MAPKKK), MAP kinase kinase (MAPKK), and effector MAP kinase, that sequentially phosphorylate and activate each other (Widmann et al., 1999). Mammals express 21 MAPKKKs, 7 MAPKKs, and 11 MAPKs (Johnson, 2011). Kinases of all levels demonstrate certain substrate specificity, which is further enhanced by scaffold proteins that bind particular kinases to organize specific signaling modules and localize them to appropriate cellular compartments (Burack and Shaw, 2000; Dhanasekaran et al., 2007). Considering the fairly high protein phosphatase activity in the cytoplasm, without scaffolds to bring together three matching kinases of a particular cascade, activation of effector MAP kinases would be rarely achieved.
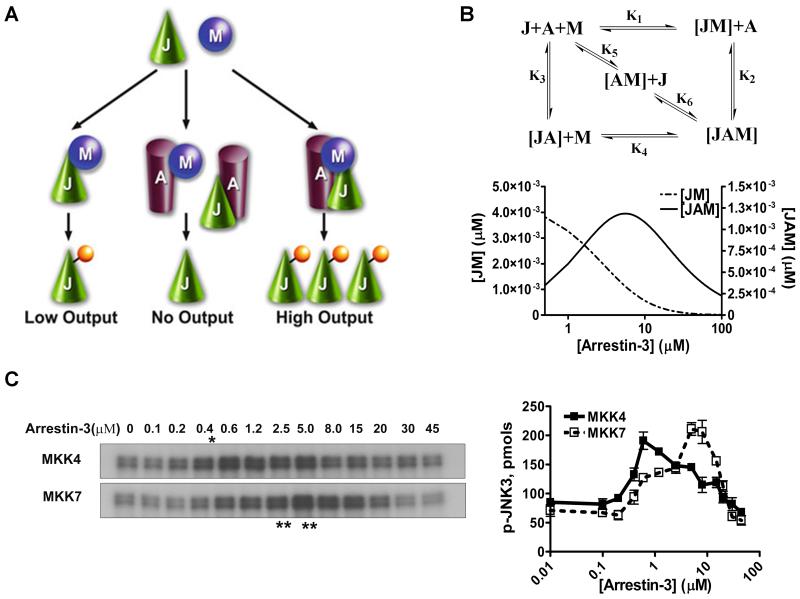
In the case of simple scaffolds that do not activate kinases or their substrates, but act by bringing them into proximity, optimal signaling is achieved at a certain level of expression, whereas both lower and higher scaffold levels result in lower signal output (Levchenko et al., 2000; Levchenko et al., 2004). Thus, the dependence of signaling on scaffold concentration is biphasic or bell-shaped: when the scaffold level is low, its amount is insufficient to organize all kinases into signaling modules, whereas when the concentration of scaffold exceeds optimal, it promotes the formation of incomplete and unproductive complexes containing only a single kinase (Fig. 1A).
Fig. 1. Arrestin-3-mediated JNK3α2 activation by MKK4/7.
A. A three-state model showing the scaffolding mechanism of the two-kinase signaling module. A, J, and M designate arrestin-3, JNK3α2, and upstream kinases MKK4/7, respectively. Kinases can exist in three states: (a) interacting in solution, (b) bound to the scaffold to form incomplete complexes containing a single kinase, and (c) simultaneously assembled by arrestin-3 to form a complete signaling complex. B. Six affinity constants (k1 through k6) describe the indicated binding equilibria. Calculated concentrations of JM (dotted line, left y axis) and JAM (solid line, right y axis) complexes at different arrestin-3 concentrations (KinTek Explorer 3.0; all six Kd values were set at 5 μM). C. Representative autoradiograms showing JNK3α2 phosphorylated by MKK4 (upper panel) or MKK7 (lower panel) at the indicated concentration of arrestin-3 (10s incubation). The optimal arrestin-3 concentrations are indicated (*, for MKK4; **, for MKK7). D. The effect of arrestin-3 concentration on JNK3α2 phosphorylation by both MKK4 and MKK7 is biphasic. The bands from the gels were cut out, and the radioactivity was measured in a Tri-Carb liquid scintillation counter to quantify the incorporation of [32P]phosphate from [ɤ-32P]ATP into JNK3α2. Data from (Zhan et al., 2013b).
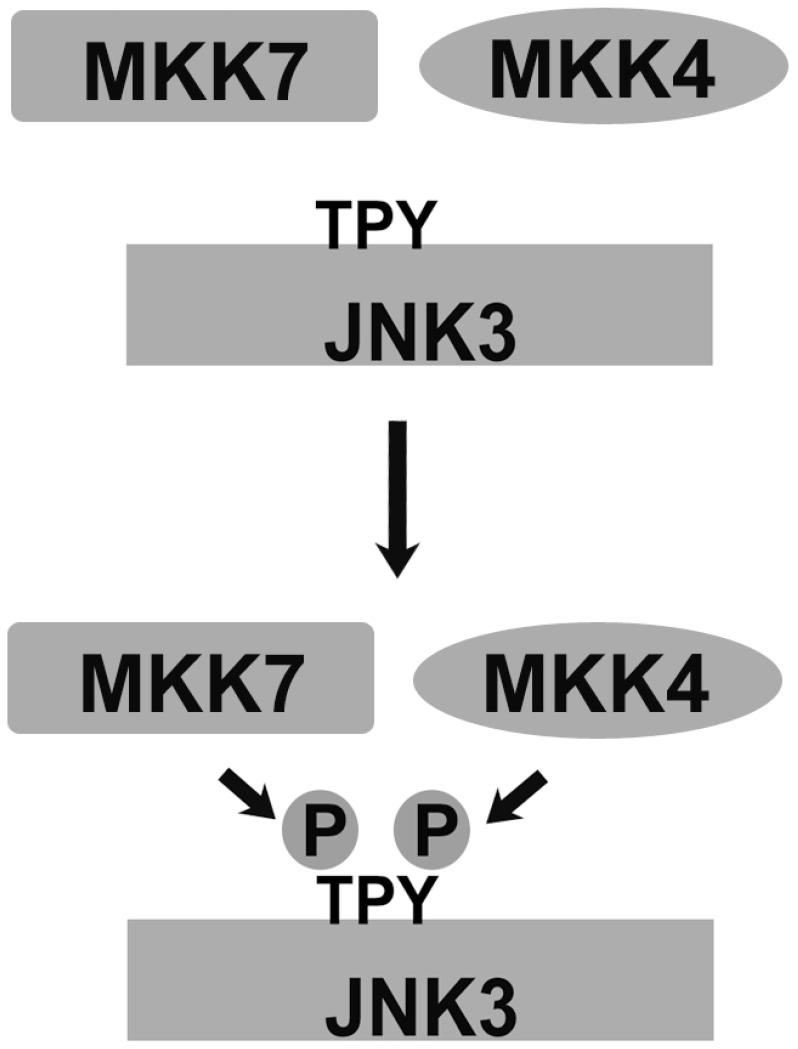
The activation of MAPKs of c-Jun N-terminal kinase (JNK) family is regulated by several specialized scaffolds, including JIP [JNK-interacting proteins (Willoughby et al., 2003)]. One of two vertebrate non-visual arrestins, arrestin-3 (a.k.a. β-arrestin2 or hTHY-ARRX), acts as a receptor-regulated scaffold for the activation of JNK3 (McDonald et al., 2000). The original study suggested that only receptor-bound arrestin-3 is active, and that only JNK3, an isoform with limited expression in the nervous system and the heart, but not the ubiquitous JNK1 and JNK2 isoforms, is activated via arrestin-3-dependent mechanism. The original study also suggested that arrestin-3 directly binds only two kinases in this cascade, the upstream-most MAPKKK ASK1, and down-stream MAPK JNK3, and that out of two MAPKKs necessary to activate JNK3, only MKK4 associates with this complex via its interactions with ASK1 and JNK3 (McDonald et al., 2000). However, full activation of any JNK family kinase requires dual phosphorylation in the activation loop: tyrosine by MKK4 and threonine by MKK7 (Fig. 2) (Gupta et al., 1996; Lawler et al., 1998).
Fig. 2. Full activation of JNKs requires dual phosphorylation.
For full activity, JNK family kinases must be phosphorylated at Thr (by MKK7) and Tyr (by MKK4). The cartoon is from (Zhan et al., 2013a).
Subsequent studies (Miller et al., 2001); (Breitman et al., 2012; Seo et al., 2011; Song et al., 2009; Zhan et al., 2011b) showed that free arrestin-3 can facilitate JNK3 activation, that GPCR binding-deficient form of arrestin-3 facilitates JNK3 activation in cells (Song et al., 2009), and that arrestin-3 directly binds to MKK4 (Zhan et al., 2011b) and MKK7 (Zhan et al., 2013a). All JNK isoforms are activated via phosphorylation of conserved Tyr and Thr residues by MKK4 and MKK7, respectively (Gupta et al., 1996; Lawler et al., 1998). Thus, the finding that arrestin-3 facilitated activation of JNK1 and JNK2 in vitro and in intact cells (Kook et al., 2014), acting in all cases as a simple scaffold (Kook et al., 2014), was not unexpected. Interestingly, arrestin-3 demonstrates different affinities for MKK4 and MKK7 (Zhan et al., 2013a),and for different JNK isoforms (Kook et al., 2014). Arrestin-3 has higher affinity for MKK4 than for MKK7, and JNK3 binding further increases arrestin-3 affinity for MKK4, while decreasing the affinity for the more abundant MKK7 (Zhan et al., 2013a). This might serve to equalize the chances of these two MKKs to phosphorylate JNK3. Higher affinity for JNK2 than JNK1 (Kook et al., 2014) suggests that arrestin-3 plays greater role in regulation of the former of the two ubiquitous JNK subtypes. Arrestin-3 affinity for the kinases of different pathways determines its optimal concentration for scaffolding particular signaling modules (Zhan et al., 2013a), suggesting that arrestin-3 expression level can direct signaling in this group of cascades to specific modules, leading to the activation of some JNK isoforms to a greater extent than others (Kook et al., 2014).
In the present unit we describe methods for the purification of MKK4 and MKK7 in active and inactive forms, as well as purification of JNK1, JNK2, and JNK3. In Basic Protocol 1, we describe assays where individual MKK-JNK signaling modules can be reconstituted from purified proteins in the absence and presence of pure arrestin-3. We also describe the assays in intact cells that can be used to characterize arrestin-3 dependent activation of particular JNK isoforms by individual upstream kinases (Basic Protocol 2), as well as methods to test whether arrestin-3 functions as a simple scaffold and therefore yields a biphasic dose-response curve (Basic Protocol 3). In Support Protocols 1, 2, 3, and 4 we describe methods of expression, purification, and activation of tagged and tag-free MKK4, MKK7, JNK1α1, and JNK2α2, respectively.
Both in vitro and cell-based assays described here can be used to identify and test small molecule inhibitors of particular kinase cascades leading to the activation of different JNK isoforms. These methods do not have high enough throughput for screening large numbers of compounds, but are suitable for lead validation and structure-activity relationship studies.
BASIC PROTOCOL 1
Reconstruction of arrestin3-scaffolded MKK4/7-JNK1/2/3 signaling modules in vitro using purified proteins
Most cells express thousands of different proteins, so any effect of one protein on another in the context of intact cell could be mediated by intermediaries, the number and nature of which is not known. Even co-immunoprecipitation of two proteins only shows that they can be present in the same complex, but does not prove their interaction. Direct protein-protein interactions and their effects on the activity of the proteins involved can only be proven by demonstration that a particular pair of purified proteins bind, and that this binding affects functional properties of one or both partners. Here we describe the assays where the ability of purified MKK4 and MKK7 to phosphorylate individual purified JNK isoforms in the absence and presence of pure arrestin-3 is tested.
Materials
Purified proteins
Purified arrestin-3, GST-MKK4, GST-MKK7, His-JNK1α1, His-JNK2α2, and His-JNK3α2; the protocol for arrestin-3 purification was previously described (Vishnivetskiy et al., 2014; Zhan et al., 2011a), protocols for the purification of other proteins are described here as supporting protocols.
The protocols for activating MKK4 (p-MKK4) and MKK7 (p-MKK7) are also presented as supporting protocols.
Solutions and Reagents
10× Kinase assay buffer:
100 mM Hepes, pH 7.4;
50 mM MgCl2;
1M NaCl;
Tris Buffer:
10 mM Tris-HCl, pH 7.5;
100 mM NaCl;
10 mM ATP (solution in distilled water, A2383, Sigma)
Scintillation cocktail (Scintisafe Econo 2, Fisher)
ATP, [γ-32P] (PerkinElmer)
Antibodies: phospho-JNK antibody (rabbit, #9251, Cell Signaling Technology); total JNK antibody (rabbit, #9252, Cell Signaling Technology)
Arrestin-3 mediated JNK3α2 activation by MKK4/7*
*Note: We have employed both [γ-32P]-ATP (quantified by autoradiography and scintillation counting) and Western blot (using phospho-JNK antibody) to measure JNK phosphorylation. Here we show the [γ-32P]-ATP measurement in JNK3 assays, as well as Western blot detection of the activation of JNK1/2.
Measurements of JNK3 phosphorylation using the incorporation of 32P
All purified proteins were stored at −80°C and thawed on ice before use. After protein aliquots were completely thawed, the samples were ultra-centrifuged at 100,000 rpm in a TLA120 rotor in a Beckman TLA centrifuge (356,000 ×g) for 1 h to at 4°C to remove aggregated proteins. Supernatants were carefully transferred into clean tubes. The protein concentration was determined using the Bradford assay (Bio-Rad).
Preparing the kinase mix. The kinase reactions were performed in a volume of 20 μl.. To avoid pipetting small volumes, a stock tube was used to premix the kinases with buffer. Table 1 shows the recipe for the kinase mix for 20 reactions.
Preparation of arrestin-3 solutions. Dilute arrestin-3 protein with Tris buffer as shown in Table 2.
Add 3.3 μl kinase mix to each tube (18 μl total volume at this point), mix well by gentle vortexing. Then incubate samples at 30°C in water bath for 15 min.
Meanwhile, prepare 1 mM ATP mixture containing [γ-32P]-ATP. For 20 reactions, add 4 μl of ATP (10 mM) and 4 μl of [γ-32P]-ATP to 32 μl of distilled water.
Initiate reaction by adding 2 μl of 1 mM ATP mixture containing [γ-32P]-ATP (prepared in step 5), and stop each reaction by the addition of 20 μl SDS buffer. We found that at these concentrations of kinases and at 30°C the reactions are linear for 10-15 s.
Load 10 μl samples from each reaction onto 10% SDS-PAGE gel. After electrophoresis stain gels with Coomassie blue.
Autoradiography. Dry SDS-PAGE gel in a Gel Dryer (Bio-Rad), then place dry gel into autoradiography cassette, and expose overnight. Adjust the exposure time if the signal is too strong or too weak to achieve clear differentiation between band intensities in various lanes (Fig. 1C).
Quantify using scintillation counting. Cut the JNK3 bands from the SDS-PAGE gel, and put them into scintillation vials. Add 5 ml scintillation cocktail and incubate on a shaker for 30 min. Quantify radioactivity in vials using scintillation counter. Dilute the ATP mix containing [γ-32P]-ATP to a final concentration of 10 nM/μl as the standards (5 μl) to calculate the specific activity of ATP mix (Fig. 1). Based on specific activity of ATP used, calculate absolute amounts of phosphate incorporation into JNK bands (pmols in the lower panel of Fig. 1B)
Table 1. Preparing the kinase mix.
| Volume (each) | Volume (20 ×) | |
|---|---|---|
| 10× Kinase assay buffer | 2 μl | 40 μl |
| 100 mM DTT | 0.4 μl | 8 μl |
| JNK3α2 * | 0.8 μl | 16 μl |
| p-MKK4 or p-MKK7 # | 0.1 μl | 2 μl |
| Total | 3.3 μl | 66 μl |
JNK3α2 stock concentration: 12 μM;
p-MKK4 and p-MKK7 stock concentrations: 10 μM.
Table 2. Preparing arrestin-3 solutions.
| Tube Number | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 | A12 | A13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Arrestin-3
concentration (μM) |
0 | 0.1 | 0.2 | 0.4 | 0.6 | 1.2 | 2 | 4 | 8 | 12 | 20 | 30 | 45 |
|
Arrestin-3 (μl) |
0 | 2* | 4 | 8 | 12 | 2.4 | 4 | 8 | 2.4 | 3.6 | 6 | 9.2 | 13.2 |
|
Tris buffer
(μl) |
14.7 | 12.7 | 10.7 | 6.7 | 2.7 | 12.3 | 10.7 | 6.7 | 12.3 | 11.1 | 8.7 | 5.5 | 1.5 |
Arrestin stock concentrations: For A2-A5, 1 μM; For A6-A8, 10 μM; For A9-A13, 65 μM.
Arrestin-3 mediated JNK1/2 activation by MKK4/7. Measurements of JNK1/2 phosphorylation using Western blotting with phospho-JNK antibodies
Prepare kinase mix according to Table 3 (10 reactions).
Prepare arrestin solutions according to Table 4.
Add 3.3 μl kinase mix to each tube (18 μl total volume at this point), mix well by gentle vortexing. Then incubate samples at 30°C in water bath for 15 min.
Meanwhile, prepare 1 mM ATP mixture. For 10 reactions, add 2 μl of ATP (10 mM) to 18 μl of distilled water.
Initiate reaction by adding 2 μl of 1 mM ATP solution, and stop each reaction by the addition of 20 μl SDS buffer. We found that at 30°C the reactions are linear for 10-15 s.
Load 5 μl and 10 μl of reaction samples onto 10% SDS-PAGE gels for Western blot with pan-JNK and phospho-JNK antibodies, respectively.
Transfer to PVDF membrane (Millipore) and develop with pan-JNK and phospho-JNK antibodies (both from Cell Signaling Technology) to check for equal input of JNK proteins and the phosphorylation of JNK, respectively.
Develop Western blots with enhanced chemiluminescence reagent (e.g., SuperSignal West Pico from Pierce), following the manufacturer’s instructions. Adjust the exposure time if the signal is too strong or too weak to achieve clear differentiation between band intensities in various lanes, while avoiding saturation (all bands should be different shades of gray).
Quantify bands on the X-ray film using Quantity One software (Bio-Rad). Plot phospho-JNK band intensity (arbitrary units) as a function of arrestin-3 concentration (Fig. 3)
Table 3. Preparing the kinase mix (JNK1 or JNK2).
| Volume (each) | Volume (10 ×) | |
|---|---|---|
| 10× Kinase assay buffer | 2 μl | 20 μl |
| 100 mM DTT | 0.4 μl | 4 μl |
| JNK1α1 or JNK2α2 * | 0.8 μl | 8 μl |
| p-MKK4 or p-MKK7 # | 0.1 μl | 1 μl |
| Total | 3.3 μl | 33 μl |
Stock concentration: 12 μM
Table 4. Preparing arrestin-3 solutions.
| Tube Number | A1 | A2 | A3 | A4 | A5 | A6 | A7 |
|---|---|---|---|---|---|---|---|
|
Arrestin-3
concentration (μM) |
0 | 0.2 | 0.5 | 1 | 5 | 10 | 20 |
|
Arrestin-3
(μl) |
0 | 4* | 10 | 1 | 5 | 10 | 10 |
|
Tris buffer
(μl) |
14.7 | 10.7 | 4.7 | 13.7 | 9.7 | 4.7 | 4.7 |
Arrestin stock concentrations: For A2-A3, 1 μM; For A4-A6, 20 μM; For A7, 40 μM.
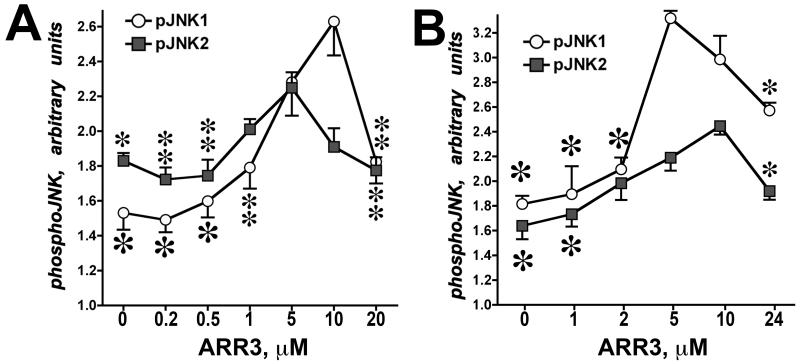
Fig. 3. Arrestin-3 mediated JNK1/2 activation by MKK4/7.
In vitro phosphorylation of JNK1α1 and JNK2α2 in the presence of MKK4 (A) or MKK7 (B) yielded bell-shaped curves as functions of arrestin-3 concentration. Means ± SD of three independent experiments are shown. ANOVA analysis with arrestin-3 as main factor demonstrated significance of arrestin-3 concentration in the presence of MKK4 and MKK7 for both JNK1α1 and JNK2α2 (p<0.001). * - p<0.001, ** - p<0.01, * - p<0.05 to maximal values (at 5 or 10 μM of arrestin-3, respectively) according to Bonferroni/Dunn post-hoc test with correction for multiple comparisons. Data from (Kook et al., 2014).
BASIC PROTOCOL 2
BASIC PROTOCOL TITLE: The role of arrestin-3 in JNK activation in intact cells
While direct interactions between proteins and their functional effects can only be proven using pure proteins (Basic protocol 1), the occurrence of these interactions and their biological role must be studied in intact cells. Here we present the protocols for cell-based assays that enable the study of arrestin-3-dependent activation of JNK family kinases.
Materials
Solutions and Reagents
Dulbecco’s Modification of Eagle’s Medium (DMEM) with 4.5g/L glucose, L-glutamine and sodium pyruvate (10-013-CV, CORNING cellgro)
Fetal Bovine Serum, Qualified (2017-07, Gibco)
Penicillin/Streptomycin (15140-122, Gibco)
0.05% Trypsin-EDTA (25300-054, Gibco)
Dulbecco’s Phosphate Buffered Saline (DPBS) without calcium and magnesium (21-031-CV, CORNING cellgro)
FuGENE HD Transfection Reagent (E231A, Promega)
Opti-MEM I Reduced Serum Medium, no phenol red (11058-021, Invitrogen)
Sodium dedecyl sulfate (SDS) Solution 10% (w/v) (161-0416, BIO-RAD)
1.0 M Tris-HCl buffer solution, pH 7.5 (Amresco)
1.0 M Benzamidine (BA) stock solution: store at −80 °C
100 mM Phenylmethanesulfonyl fluoride (PMSF): fresh solution in DMSO
500 mM Sodium fluoride (NaF) stock solution
100 mM Sodium orthovanadate (Na3VO4) solution: store at −20°
Bio-Rad Protein Assay Dye Reagent Concentrate (500-0006, BIO-RAD)
2× SDS Sample buffer (Sigma)
Additional reagents and equipment for performing SDS-PAGE electrophoresis and Western Blotting (described in (Zhan et al., 2011b; Zhan et al., 2013a)).
Antibodies for Western blot:
F4C1 - Mouse monoclonal antibody that detects the epitope DGVVLVD, a sequence that is present in all known mammalian arrestins (Donoso et al., 1990) or F431 - Rabbit polyclonal antibody that detects the same epitope (Song et al., 2011)
Phospho-SAPK/JNK (Thr183/Tyr185) antibody (9251, Cell Signaling Technology), SAPK/JNK antiobody (9252, Cell Signaling Technology), HA-Tag (6E2) Mouse antibody (2367, Cell Signaling Technology), Monoclonal anti-Flag M2 antibody (F3165, Sigma).
Lysis Buffer: 1% SDS, 10 mM Tris-HCl, pH 7.5, 10 mM NaF, 100 μM Na3VO4, 2 mM EDTA, 2 mM benzamidine and 1 mM PMSF.
*Note: Add all protease inhibitors immediately before use, especially PMSF, which is unstable in aqueous solutions.
COS-7 (african green monkey fibroblast) cells were obtained from ATCC
ASK-1/MKK7 induced JNK activation
To measure arrestin-3 effects on ASK1/MKK7/MKK4-induced activation of JNK family kinases in COS-7 cells, increasing amounts of HA-ASK1, Flag-MKK7β1, or HA-MKK4 were co-transfected into COS-7 cells with or without constant amount of pcDNA3-arrestin-3.
Cell Culture & Transfection
-
1
COS-7 african green monkey cells were maintained in DMEM supplemented with 10% heat-inactivated FBS, penicillin, and streptomycin at 37°C in a humidified incubator with 5% CO2.
-
2
Plate 2.5×105 COS-7 cells in 2 ml per well of 6-well plate one day before transfection so that cells will be 80-90% confluent at the time of transfection.
-
3
To a sterile 1.5 ml microcentrifuge tube, add 125 μl of Opti-MEM pre-warmed to 37°C.
-
4.1
For ASK-1 induced JNK activation, mix different amounts of pcDNA3-HA-ASK1 and empty pcDNA3 (to make total DNA concentration 2 μg per transfection) with Opti-MEM (Table 5).
-
4.2
For Arrestin-3 effect on ASK-1 induced JNK activation, mix each concentration of pcDNA3-HA-AKS1 and pcDNA3 (total DNA 1.5 g) with Opti-MEM, and then add 0.5 μg of pcDNA3-Arrestin-3 to each tube (Table 6).
-
5
For a 3:1 FuGENE HD: DNA ratio, add 6 μl of FuGENE HD directly to diluted DNA.
-
6
Incubate the FuGENE HD and DNA mixture for 15 minutes at room temperature.
-
7
Add each DNA-FuGENE HD complex to a separate well in a 6-well plate containing 2 ml of cells in growth medium.
-
8
Mix gently by rocking the plate back and forth.
-
9
Return cells to a CO2 incubator and incubate for 24 hrs.
-
10
Replace growth medium with serum-free medium and incubate overnight.
Table 5. Transfection to determine ASKl-dependent JNK activation.
| pcDNA3-HA-ASK1 | 0 μg | 0.05 μg | 0.1 μg | 0.25 μg | 0.5 μg | 1 μg | 1.5 μg |
| pcDNA3 | 2.0 μg | 1.95 μg | 1.9 μg | 1.75 μg | 1.5 μg | 1 μg | 0.5 μg |
| FuGENE HD | 6μl | 6μl | 6μl | 6μl | 6μl | 6μl | 6μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
Note: For MKK7-induced JNK activation, add pcDNA3-Flag-MKK7β1 instead of pcDNA3-HA-ASK1.
Table 6. Transfection to determine ASK1- and arrestin-3-dependent JNK activation.
| pcDNA3-HA-ASK1 | 0 μg | 0.05 μg | 0.1 μg | 0.25 μg | 0.5 μg | 1 μg | 1.5 μg |
| pcDNA3 | 1.5 μg | 1.45 μg | 1.4 μg | 1.25 μg | 1.0 μg | 0.5 μg | 0 μg |
| pcDNA3-arrestin-3 | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg |
| FuGENE HD | 6μl | 6μl | 6μl | 6μl | 6μl | 6μl | 6μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
Note: For MKK7 induced JNK activation, add pcDNA3-Flag-MKK7β1 instead of pcDNA3-HA-ASK1.
Sample Preparation
-
11
Take 6-well culture plate from incubator and put on ice.
-
12
Remove medium and wash transfected COS-7 cells in each well with 1 ml of cold PBS twice.
-
13
Add 150 μl of lysis buffer per well in a 6-well plate and collect lysates using cell scraper.
-
14
Heat cell lysates at 95°C for 10 min and cool down to room temperature.
-
15
Measure protein concentration using Bio-Rad Protein Assay Dye Reagent Concentrate.
Western Blot
-
16
Use 10 μg of cell lysate protein for Western blot with phospho-JNK and HA antibody and 5 μg for Western blot with arrestin-3 antibody. Mix cell lysates with 2× SDS-sample buffer (Sigma) at a 1:1 ratio.
-
17
Load samples onto 8% SDS-PAGE gel, run electrophoresis until the dye reaches the bottom of the gel.
-
18
Transfer to PVDF membrane (Millipore) and develop with phospho-JNK and JNK antibody to check the activation of endogenous JNKs and HA-tag, F4C1 or F431 antibody (or any other arrestin-specific antibody) to check the expression of HA-ASK1 and arrestin-3, respectively (Figs. 4,5).
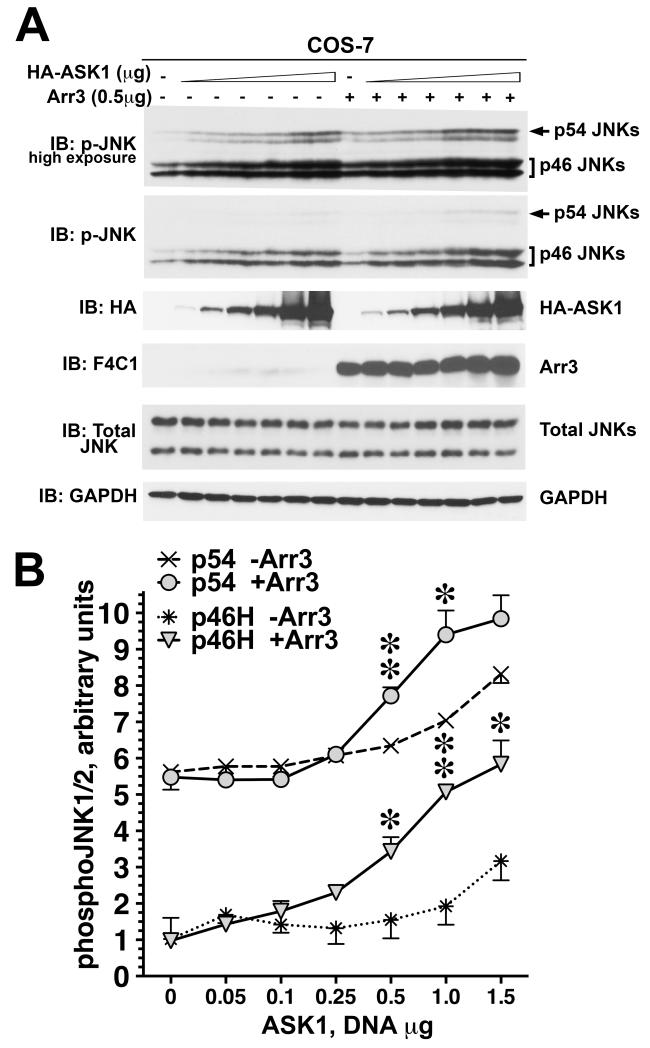
Fig. 4. Arrestin-3 promotes JNK1/2 activation induced by the expression of ASK1 in intact cells.
A. Representative Western blot showing phosphorylation of endogenous JNK1/2 isoforms with or without arrestin-3 in COS-7 cells expressing varying amounts of ASK1. Upper p-JNK blot is the same as lower blot exposed for longer time to visualize p54 isoforms. B. Quantification of phosphorylation of JNK p46H and p54 isoforms with or without arrestin-3. ANCOVA with arrestin-3 as factor and ASK1 concentration as co-variate showed significant effect of ASK1 concentration on the level of p46H and p54 phosphorylation (p<0.0001). * - p<0.05, ** - p<0.01 to - Arr3, Student’s t-test for individual points. Data from (Kook et al., 2014)
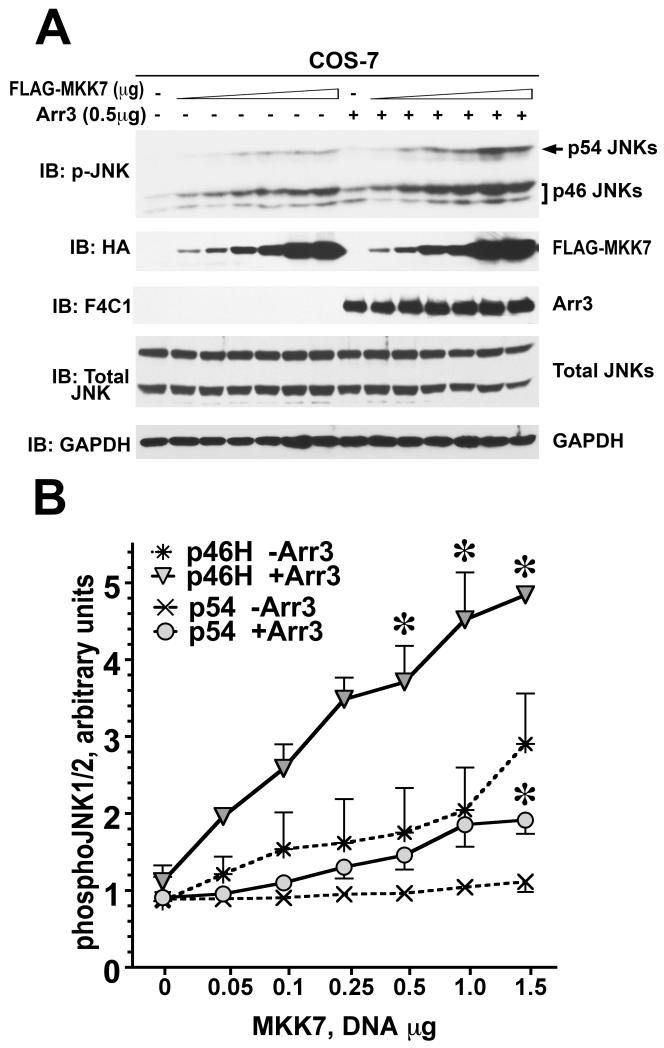
Fig. 5. Arrestin-3 enhances MKK7-dependent phosphorylation of endogenous JNK1/2 in intact cells.
A. Representative Western blot showing phosphorylation of endogenous JNK1/2 isoforms with or without arrestin-3 in COS-7 cells expressing varying amounts of MKK7. B. Quantification of phosphorylation of JNK p46H and p54 isoforms with or without arrestin-3. ANCOVA with arrestin-3 as factor and MKK7 concentration as co-variate showed significant effect of MKK7 concentration of the level of p46H and p54 phosphorylation (p<0.0001). The presence of arrestin-3 significantly affected the level of p46H, but not p54, phosphorylation across MKK7 concentrations (F(1,38)=8.592 p=0.0057). * - p<0.05 to -Arr3, Student’s t-test for individual points. Data from (Kook et al., 2014).
MKK4-induced JNK activation
pcDNA3-HA-MKK4 tends to express lower amount of protein compared with pcDNA3-HA-ASK1 in COS-7 cells. Therefore, to match expression level, more pcDNA3-MKK4-HA is used for transfection.
Transfection
All procedures are the same as with ASK1-induced JNK activation except as indicated in tables below.
Alternative step 4.1 For MKK4-induced JNK activation, mix different amounts of pcDNA3-HA-MKK4 with empty pcDNA3 (total DNA 4.5 μg) with Opti-MEM (Table 7).
Table 7. Transfection to determine MKK4-dependent JNK activation.
| pcDNA3-HA-MKK4 | 0 μg | 0.2 μg | 0.5 μg | 1.0 μg | 2.0 μg | 4.0 μg |
| pcDNA3 | 4.5 μg | 4.3 μg | 4.0 μg | 3.5 μg | 2.5 μg | 0.5 μg |
| FuGENE HD | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
4.2 For arrestin-3 effect on MKK4-induced JNK activation, mix different amounts of pcDNA3-HA-MKK4 and empty pcDNA3 (total DNA 4.0 μg) with Opti-MEM, and then add 0.5 μg of pcDNA3-arrestin-3 to each tube (Table 8).
Table 8. Transfection to determine MKK4- and arrestin-3-dependent JNK activation.
| pcDNA3-HA-MKK4 | 0 μg | 0.2 μg | 0.5 μg | 1.0 μg | 2.0 μg | 4.0 μg |
| pcDNA3 | 4.0 μg | 3.8 μg | 3.5 μg | 3.0 μg | 2.0 μg | 0 μg |
| pcDNA3-arrestin-3 | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg |
| FuGENE HD | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
Basic protocol 3. Biphasic effect of arrestin-3 on ASK1- and MKK7-stimulated JNK phosphorylation in cells
To examine the concentration-dependence of arrestin-3 expression level on ASK1- and MKK7-stimulated JNKs phosphorylation in COS-7 cells, the expression of ASK1 or MKK7 is kept constant, whereas the expression of arrestin-3 is varied.
SUPPORT PROTOCOLS 1-4 Expression, purification and activation of GST-MKK4, GST-MKK7-His6, tag-less JNK1α1 and tag-less JNK2α2
Materials and buffers
Materials
TEV (Tobacco Etch Virus) protease (Express and purify TEV protease as previously described (Abramczyk et al., 2011). Plasmid is available in Addgene, plasmid 8827: pRK793, TEV protease, S219V mutant.
Thrombin protease (Novagen)
BL21 (DE3) (Novagen). Prepare electro-competent cells as previously described (Gonzales et al., 2013).
Econo-Column® Chromatography glass columns, 2.5 × 20 cm (98 mL) (Biorad)
Glutathione Sepharose™ High Performance (Amersham Biosciences). On the day of the experiment, pack the required beads in a glass Econo-Column, wash and equilibrate in buffer A. Ni-NTA beads (Qiagen). On the day of the experiment, pack the required beads in a glass Econo-Column, wash and equilibrate in buffer E.
120 mL HiLoad 16/60 Superdex 200 prep grade column (Amersham)
Mono Q HR 10/10 anion exchange column (Amersham)
Luria Broth (LB) medium (Sigma) containing 50 μg/mL ampicillin sodium (Sigma)
Luria Broth (LB) medium containing 30 μg/mL kanamycin (Sigma)
Terrific Broth (TB) medium (Sigma) containing 50 μg/mL ampicillin sodium (Sigma)
1 M Isopropyl β-D-1-thiogalactopyranoside (IPTG) solution in sterile water (Sigma)
1M MgCl solution (Sigma)
Lysozyme lyophilized enzyme (MP Biomedicals)
100 % Glycerol
10% (v/v) Triton in water (Sigma)
50 mM ATP (Roche) in 25 mM HEPES buffer (pH 7.5)
NaCl (Biological grade MP Biomedical)
Imidazole
CaCl2
Bradford reagent (Biorad)
GST-MEKK1c (C terminal 320 amino acids corresponding to the catalytic domain) is necessary for the activation of GST-MKK4, GST-MKK7-His6. The protocol for GST-MEKK1c vector construction and protein purification has been previously described (Gallagher et al., 2002; Khokhlatchev et al., 1997). This plasmid is available upon request from Dr. K.N. Dalby.
Buffer A (pH 7.3)
10 mM Na2HPO4,
1.8 mM KH2PO4,
140 mM NaCl,
2.7 mM KCl,
0.1% (v/v) β-mercaptoethanol,
0.1 mM TPCK,
0.1 mM PMSF and
1 mM benzamidine
Buffer B (pH 7.5)
50 mM Tris HCl,
20 mM reduced glutathione,
0.1% (v/v) β-mercaptoethanol,
0.1 mM TPCK,
0.1 mM PMSF and
1 mM benzamidine
Buffer C (pH 7.5)
25 mM Hepes,
20 mM MgCl2,
0.1 mM EDTA,
0.1 mM EGTA and
2 mM TCEP
Buffer D (pH 7.5)
25 mM HEPES,
100 mM KCl,
0.1 mM EDTA,
0.1 mM EGTA and
2 mM TCEP
Buffer E (pH 8)
20 mM Tris-HCl,
0.03% Brij-30,
0.1% (v/v) β-mercaptoethanol,
5 mM imidazole,
1 mM benzamidine,
0.1 mM PMSF and
0.1 mM TPCK
Buffer F (pH 8.0)
20 mM Tris-HCl,
0.03% (v/v) Brij-30 and
0.1% (v/v) β-mercaptoethanol
Buffer G (pH 8.4)
20 mM Tris-HCl,
150 mM NaCl and
0.1 % (v/v) β-mercaptoethanol
Buffer S (pH 7.5)
25 mM HEPES,
50 mM KCl,
0.1 mM EDTA,
0.1 mM EGTA and
2 mM DTT
SUPPORT PROTOCOL 1. Expression, purification and activation of GST-MKK4
Plasmid: pGEX4T1-MKK4 vector: a construct encoding full-length wild type Homo sapiens mitogen-activated protein kinase kinase 4 (MAP2K4) (GenBank accession number NM_003010) with an N-terminal cleavable GST-tag was previously described (Yan et al., 2011; Zhan et al., 2011c). This plasmid is available upon request from Dr. K.N. Dalby.
Expression of GST-MKK4
Day 1: Transform the pGEX4T1-MKK4 vector into BL21 (DE3) electro-competent cells following the previously described protocol of DNA electroporation (House et al., 2004).
Day 2: Inoculate a single colony of freshly transformed cells in a 30 mL culture of Luria Broth (LB) containing 50 μg/mL ampicillin, then incubate with shaking overnight at 37°C.
Day 3: Dilute the culture 100-fold into TB (Terrific Broth) containing 50 μg/mL ampicillin, and incubate with shaking at 37°C until the OD600 reaches 0.6–0.7. Induce GST-MKK4 expression by adding 25-50 μM IPTG, and continue shaking at 25°C for 20 hours.
Day 4: Pellet the cells (8000 × g, 15 min), immediately freeze in liquid nitrogen and store at −80°C.
Purification of GST-MKK4
Day 5:
-
5
Resuspend frozen wet cells in 150 mL of Buffer A containing 0.2 mg/mL lysozyme, 1 mM MgCl2, and 20% (v/v) glycerol. Incubate the mixture at 4°C for 30 minutes, then add Triton-100 to a final concentration of 1% (v/v) and incubate at 4°C for another 30 minutes.
-
6
Sonicate the cell lysate (power level 5, 50% duty cycle) at 4°C for 5–10 minutes (at 5 s pulses with 5 s intervals and with careful monitoring of the temperature using a temperature probe). If the cell lysate is unusually viscous, the sonication time can be extended with careful monitoring of the temperature (do not to exceed 4°C) until it becomes more fluid.
-
7
Centrifuge the lysate for 30 minutes at 12,000 × g at 4°C and mix the supernatant with 10 mL of Glutathione Sepharose™ High Performance resin (Amersham Biosciences) equilibrated with Buffer A, and shake gently for 1.5 h at 4°C.
-
8
In a glass Econo-column (98 mL), wash the beads with 150 mL of Buffer A.
-
9
Elute the GST-tagged proteins with 20 mL buffer B containing 20% (v/v) glycerol. Adjust the pH of the GST elution buffer to 7.5 as glutathione decreases the pH to ~2.0. Also, adjust the pH of all the buffers after adding glycerol. Collect the eluted protein and dialyze overnight into buffer S containing 20% glycerol.
-
10
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol. Immediately freeze (100 μl aliquots at around 20 μM concentration) in liquid nitrogen and store at −80°C for later activation.
-
11
The estimated yield is around 20–30 mg of pure GST-MKK4 per 1L of cells. The estimated purity by 12% SDS/PAGE is around 85% (Zhan et al., 2011b; Zhan et al., 2013b).
Activation of GST-MKK4
Day 6:
-
12
Incubate 4 μM GST-MKK4 and 2 μM GST-MEKK1c at 30°C for 60 min in the presence of 4 mM ATP in 10 mL of activation buffer C.
-
13
Concentrate the 10 mL reaction mixture to 2–3 mL using an Amicon Ultra-15 concentrator (10,000 molecular weight cutoff). Centrifuge for 10-15 minutes at 4400 rpm.
-
14
Purify the concentrated reaction mixture using a gel filtration column (120 mL HiLoad 16/60 Superdex 200 prep grade column) that has been equilibrated with buffer D containing 10% glycerol. This column purifies the active GST-MKK4 away from the constitutively active GST-MEKK1c.
-
15
The pure activated GST-MKK4 fractions that emerge from the gel filtration column will not require further dialysis and can be frozen directly after increasing the glycerol concentration to 20%. GST-MKK4 activation, purification and freezing needs to be done in one day to decrease the possibility of GST tag cleavage. The high tendency of GST-MKK4 to precipitate makes it essential to keep the concentration of active and inactive GST-MKK4 at less than 20 μM. Store the activated GST-MKK4 in buffer D containing 20% glycerol in aliquots at −80 °C until further use.
-
16
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol.
-
17
At least 65% of GST-MKK4 should be recovered as pure activated GST-MKK4. The estimated purity by 12% SDS/PAGE is around 85% (Zhan et al., 2011b; Zhan et al., 2013b).
SUPPORT PROTOCOL 2. Expression, purification and activation of GST-MKK7-His6
Plasmid: pGEX-MKK7-His6 vector: a construct encoding full length wild type Mus musculus mitogen-activated protein kinase kinase 7 (GenBank accession number NM_011944) with N-terminal cleavable GST–tag and C-terminal cleavable His-tag was previously described (Madsen et al., 2011; Zhan et al., 2013b). This plasmid is available upon request from Dr. K.N. Dalby.
Expression of GST-MKK7-His
Day 1: Transform the pGEX 4T1 vector containing DNA encoding full length MKK7 into BL21 (DE3) electro-competent cells, following previously described protocol of DNA electroporation (House et al., 2004).
Day 2: From a single colony of freshly transformed cells, inoculate a 30 mL culture of Luria Broth (LB) containing 50 μg/mL ampicillin and incubate with shaking at 250 rpm overnight at 37°C.
Day 3: Dilute the culture 100-fold into TB (Terrific Broth) media containing 50 μg/mL ampicillin, incubate at 37 °C with shaking at 250 rpm. Once the OD600 of the culture reaches 0.6, induce the expression by adding 25 μM IPTG, and continue shaking at 25°C for 20 hours.
Day 4: Pellet the cells (7000g, 12 min) and freeze pellets immediately in liquid nitrogen and store at −80°C.
Purification of GST-MKK7-His
Day 5:
-
5
Resuspend the frozen wet cells in 150 mL of Buffer E containing 0.5 M NaCl, 0.2 mg/mL lysozyme, 1 mM MgCl2, and 20% glycerol. Incubate the mixture at 4°C for 30 minutes, then add Triton-100 to a final concentration of 1% (v/v) and incubate at 4°C for another 30 minutes.
-
6
Sonicate the cell lysate at 4°C for 5–10 minutes (at 5 s pulses with 5 s intervals and careful monitoring of the temperature using a temperature probe). If the cell lysate is unusually viscous, the sonication time can be extended with careful monitoring of the temperature (do not to exceed 4°C) until it becomes more fluid. Remove cell debris by centrifugation at 15,000 rpm for 30 min.
-
7
Agitate the supernatant with Ni-NTA beads for 1 h at 4°C. After washing the beads with 150 mL of buffer E containing 10 mM imidazole and 20% glycerol, elute the GST-MKK7-His6 tagged proteins with 25 mL buffer E (pH 8.0) containing 200 mM imidazole and 20% glycerol.
-
8
Collect the eluted protein and dialyze overnight at 4°C into buffer S containing 20% glycerol.
-
9
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol. Immediately freeze in liquid nitrogen (100 μl aliquots of around 20 μM concentration) and store at −80°C for later activation.
-
10
The estimated yield is around 20 mg of pure GST-MKK7-His6 per 1 L of culture and the estimated purity by 12% SDS/PAGE is more than 95% (Madsen et al., 2011; Zhan et al., 2013b). To enhance the solubility and stability of GST-MKK7-His6, it can be dialyzed at 4°C into storage buffer containing 590 mM sucrose instead of 20% glycerol, but the sucrose needs to be removed before activation by dialyzing it back into buffer S containing 20% glycerol.
Activation of GST-MKK7-His6
Day 6:
-
11
Incubate 4 μM GST-MKK7-His and 2 μM GST-MEKK1 for 60 min at 30°C in the presence of 4 mM ATP in 10 mL activation buffer C.
-
12
Purify the reaction mixture using a Ni affinity column that purifies the active GST-MKK7-His6 from GST-MEKK1. Dilute the reaction mixture in 100 mL buffer E containing 20% glycerol, and agitate the diluted reaction mixture with Ni-NTA beads for 1 h at 4°C. After washing the beads with 100 mL of buffer E containing 10 mM imidazole and 20% glycerol, elute the activated GST-MKK7-His6 tagged proteins with 10 mL buffer E (pH 8.0) containing 200 mM imidazole and 20% glycerol.
-
13
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol.
-
14
Store the activated GST-MKK7-His6 in buffer S containing 20% glycerol at −80°C until further use.
-
15
At least 60% of GST-MKK7-His6 should be recovered as pure active GST-MKK7-His6 and the estimated purity by 12% SDS/PAGE is more than 95% (Madsen et al., 2011; Zhan et al., 2013b). The high tendency of GST-MKK7-His6 to precipitate makes it essential to keep the concentration of active and inactive GST-MKK7-His6 at less than 20 μM.
SUPPORT PROTOCOL 3. Expression, purification and activation of tag-less JNK1α1
Plasmid: pET28a(+)Tev-JNK1α1 vector: a construct encoding full length wild type Homo sapien mitogen-activated protein kinase 8; (GenBank accession number NM_002750) with N-terminal Tev (Tobacco Etch Virus) cleavable His-tag was previously described (Yan et al., 2011). This plasmid is available upon request from Dr. K.N. Dalby.
Expression of His6-Tev-JNK1α1
Day 1: Electroporate pET28a(+)Tev-JNK1 into the E.coli strain BL21(DE3) following previously described protocol of DNA electroporation (House et al., 2004).
Day 2: Use cells from a single colony to inoculate 30 mL of Luria Broth (LB) media containing 30 μg/mL kanamycin and grow overnight at 37°C.
Day 3: Dilute the culture 100-fold into Luria Broth media containing 30 μg/mL kanamycin and grow at 37 °C to an O.D600 of 0.6, then induce the cells by 500 μM IPTG (??? Not in list of materials) and culture them for 5 hours at 30°C. Pellet the cells (7000g, 12 min), freeze pellets immediately in liquid nitrogen and store at −80°C.
Purification of His6-Tev-JNK1α1
Day 4:
-
4
Lyse the cells in 150 mL of buffer E containing 0.5 M NaCl and 0.2 mg/ml lysozyme. Incubate the mixture at 4°C for 30 minutes, then add Triton X-100 to a final concentration of 1% (v/v) and incubate at 4°C for another 30 minutes.
-
5
Sonicate the cell lysate (power level 5, 50% duty cycle) at 4°C for 5–10 minutes (at 5 s pulses with 5 s intervals and careful monitoring of the temperature using a temperature probe). If the cell lysate is unusually viscous, the sonication time can be extended with careful monitoring of the temperature (do not to exceed 4°C) until it becomes more fluid. Remove cell debris by centrifugation at 15,000 rpm at 4°C for 30 min.
-
6
Agitate the supernatant with Ni-NTA beads for 1 h at 4°C. After washing the beads with 150 mL of buffer E containing 10 mM imidazole, elute the His6-tagged proteins with 25 mL buffer E containing 200 mM imidazole.
-
7
Apply the eluted proteins to a Mono Q HR 10/10 anion exchange column equilibrated with buffer F. Develop the column over 15-17 column volumes with a linear gradient of 0–0.5 M NaCl.
-
8
Collect eluted fractions of His6-JNK1α1 and either process to cleave the His6-tag or dialyze into buffer S containing 10% glycerol.
-
9
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol. Immediately freeze in liquid nitrogen and store at −80°C for later usage.
-
10
The estimated yield of is around 13 mg of pure His tagged JNK1α1 per 1 L of culture. The estimated purity by 12% SDS/PAGE is more than 95% (Yan et al., 2011).
TEV cleavage of His6-Tev-JNK1α1
-
11
Dialyse collected fractions of His6-Tev-JNK1α1 after Mono Q HR 10/10 anion exchange chromatography, against Tev cleavage buffer G overnight.
Day 5:
-
12
Incubate the dialyzed protein with TEV enzyme (1.5 %) and 2.5 mM CaCl 2for 2 hours at 30°C, estimate the cleavage by 10 % SDS/PAGE and extend the incubation time until 100% cleavage is achieved.
-
13
After cleavage, dilute the reaction mixture (10 mL) 5-fold with buffer F or desalt the reaction mixture by using any suitable desalting column.
-
14
Filter the protein and load on a Mono Q HR 10/10 anion exchange column equilibrated in buffer F. Develop the column over 15–17 column volumes with a linear gradient of 0–0.5 M NaCl.
-
15
Collect the eluted fractions of tagless JNK1α1 and dialyze into buffer S.
-
16
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol. Immediately freeze in liquid nitrogen and store at −80°C for later use.
-
17
Typically 80% of the tagless JNKα1 is recovered after cleavage. The estimated purity by 12% SDS/PAGE is more than 95% (Yan et al., 2011).
Activation of tag-less JNK1α1
Day 6:
-
18
Incubate 2 μM tag-less JNK1α1, 100 nM active GST-MKK4 and 400 nM active GST-MKK7 for 60 min at 30°C in the presence of 4 mM ATP in 10 mL activation buffer C.
-
19
Concentrate the 10 mL reaction mixture to 2-3 mL using an Amicon Ultra-15 concentrator (10,000 molecular weight cutoff). Centrifuge for 10-15 minutes at 4400 rpm.
-
20
Purify the concentrated reaction mixture using a gel filtration column (120 mL HiLoad 16/60 Superdex 200 prep grade column) that has been equilibrated with buffer D.
-
21
Measure the protein concentration using Bradford reagent following manufacturer’s protocol.
-
22
Store the activated, tag-less JNK1α1 in buffer S containing 10% glycerol at −80°C until further use.
-
23
Typically 60% of the activated JNK1α1 is recovered after activation. The estimated purity by 12% SDS/PAGE is more than 95% (Yan et al., 2011). The high tendency of JNK1α1 to precipitate makes it essential to keep the concentration of active and inactive JNK1α1 at less than 20 μM.
SUPPORT PROTOCOL 4. Expression, purification and activation of tag-less JNK2α2
Plasmid: pET28a(+)-JNK2α2 vector:a construct encoding full length wild type Homo sapien mitogen-activated protein kinase 9; (GenBank accession number NM_002752) with N-terminal Thr (Thrombin) cleavable His-tag was previously described (Madsen et al., 2011). This plasmid is available upon request from Dr. K.N. Dalby.
Expression of His6-Thr-JNK2α2
Day 1 Electroporate pET28a(+)-JNK2 into the E.coli strain BL21(DE3) following the previously described protocol of DNA electroporation (House et al., 2004).
Day 2: Use cells from single colony to inoculate 30 ml Luria-Broth media containing 30 μg/mL kanamycin and grow overnight at 37°C.
Day 3: Dilute the culture 100-fold into Luria-Broth media containing 30 μg/mL kanamycin and grow at 37 °C to an O.D600 of 0.6. Then induce the cells by 500 μM IPTG and culture them for 3-5 hours at 30°C. Pellet the cells (7000g, 12 min) and freeze pellets immediately in liquid nitrogen and store at −80°C.
Purification of His6-Thr-JNK2α2
Day 4:
-
4
Lyse the cells in 150 mL of buffer E containing 0.5 M NaCl and 1% Triton X-100.
-
5
Sonicate cell lysate (power level 5, 50% duty cycle) for 20 min at 4°C (at 5 s pulses with 5 s intervals with careful monitoring of the temperature using a temperature probe).
-
6
Centrifuge the lysate for 30 minutes at 16,000 rpm and agitate the supernatant with Ni-NTA beads for 1 h at 4°C.
-
7
After washing the beads in a glass Econo-column (98 mL) with 150 mL of buffer E containing 10 mM imidazole, elute the His6-tagged proteins with 25 mL buffer E (pH 8.0) containing 200 mM imidazole.
-
8
Apply the eluted proteins to a Mono Q HR 10/10 anion exchange column equilibrated in buffer F. Develop the column over 15-17 column volumes with a linear gradient of 0-0.5 M NaCl.
-
9
Collect the eluted fractions of His6-Thr-JNK2α2 that correspond to the middle area of the Mono Q protein chromatogram and either process to cleave the His6-tag or dialyze into buffer S containing 10 % glycerol. Measure the protein concentration using Bradford reagent following manufacturer’s protocol.
-
10
Immediately freeze in liquid nitrogen and store at −80°C for later use.
-
11
Assess the purity by 12% SDS/PAGE or by LC/ESI.
Thrombin cleavage of His6-Thr-JNK2α2
-
12
Dialyse the collected fractions of His6-Thr-JNK2α2 after Mono Q HR 10/10 anion exchange chromatography, against thrombin cleavage buffer G overnight.
Day5:
-
13
Incubate the dialysed protein with thrombin (1 unit thrombin/mg protein) and 2.5 mM CaCl 2 for 3-5 hours at room temperature with mild agitation.
-
14
Estimate the cleavage by 10 % SDS/PAGE and extend the incubation time until 100% cleavage is achieved.
-
15
After cleavage, dilute the reaction mixture (10 mL) five-fold with buffer F or desalt the reaction mixture using any suitable desalting column.
-
16
Filter the protein and load onto a Mono Q HR 10/10 anion exchange column equilibrated in buffer F. Develop the column over 15–17 column volumes with a linear gradient of 0–0.5 M NaCl.
-
17
Collect the eluted fractions of tagless JNK2α2 and dialyze them into buffer S containing 10% glycerol. Measure the protein concentration using Bradford reagent following the manufacturer’s protocol.
-
18
Immediately freeze in liquid nitrogen and store at −80°C for later use.
-
19
The estimated yield is around 35 mg of pure tagless JNK2α2 per 1 L of culture. The estimated purity by 12% SDS/PAGE is more than 95% (Madsen et al., 2011).
Activation of tag-less JNK2α2
Day 6:
-
20
Incubate 2 μM tag-less JNK2α2, 100 nM active GST-MKK4 and 400 nM active GST-MKK7 for 60 min at 30°C in the presence of 4 mM ATP in 10 mL activation buffer C.
-
22
Concentrate the 10 mL reaction mixture to 2–3 mL using an Amicon Ultra-15 concentrator (10000 molecular weight cutoff). Centrifuge for 10-15 minutes at 4400 rpm.
-
23
Purify the concentrated reaction mixture using a gel filtration column (120 mL HiLoad 16/60 Superdex 200 prep grade column) that has been equilibrated with buffer D.
-
24
Measure the protein concentration using Bradford reagent following the manufacturer’s protocol.
-
25
Store the activated, tagless JNK2α2 in buffer S containing 10% glycerol at −80°C until further use.
-
26
At least 50% of tagless JNK2α2 should be recovered as active tagless JNK2α2. The estimated purity by 12% SDS/PAGE is more than 95% (Madsen et al., 2011). After activation, the gel filtration chromatography may show two different species of activated JNK2α2. It is advisable to collect these two species separately, and test their activity against any known JNK substrate to determine the activity of each fraction.
Commentary
a. Background Information
Two types of experiments necessary to study arrestin-3-dependent activation of JNK family kinases are described. One is performed in intact cells (Basic protocol 1), the other involves reconstitution of signaling modules from purified proteins (Basic protocol 2). Arrestin-3-dependent JNK activation was first reported in intact cells over-expressing JNK3 and other components of the Cascade (ASK1 and/or MKK4) (McDonald et al., 2000) and was extensively studied in the same cell-based paradigm with kinase and arrestin-3 over-expression (Breitman et al., 2012; Seo et al., 2011; Song et al., 2009). The key drawback of this approach is that it cannot prove that protein-protein interactions detected by co-immunoprecipitation or functional effects of expressed arrestin-3 and/or kinases involved are direct, rather than mediated by unknown intermediaries among the thousands of proteins expressed in every cell.
The reconstruction of MKK-JNK signaling module with pure proteins is perfectly suited for the identification of direct interactions and effects, but it became feasible only recently, when methods necessary for the purification and activation MKK4, MKK7, and different JNK isoforms were developed (Support protocols 1-4). As the most upstream kinase ASK1 is unavailable in purified form, only the two-kinase part of the cascade can be reconstructed. Despite clear limitations, this approach has already yielded several novel findings. First, it proved direct binding of MKK4 (Zhan et al., 2011b) and then MKK7 (Zhan et al., 2013b) to arrestin-3. Second, free arrestin-3 in the absence of any GPCRs was shown to act as a scaffold for the MKK4-JNK3 (Zhan et al., 2011b) and MKK7-JNK3 (Zhan et al., 2013b) modules. Third, JNK3 binding affected the affinity of arrestin-3 for MKKs, increasing binding of MKK4 and decreasing that of MKK7 (Zhan et al., 2013b). Fourth, in vitro reconstitution showed that arrestin-3 binds not only JNK3 with limited expression in a few cell types, but also ubiquitous JNK1 and JNK2 (Kook et al., 2014). Fifth, experiments with pure proteins allowed the identification of the main JNK3-binding site in the N-domain of arrestin-3, as well as supporting sites in its C-domain (Zhan et al., 2014). Importantly, in vitro data were used to design experiments in intact cells to prove that these phenomena occur in native cell environment (Kook et al., 2014; Zhan et al., 2013b).
b. Critical Parameters
Purification of MKK4 and MKK7
The GST tag can be cleaved on the resin or in solution. It is imperative to finish the purification process in one day using efficient protease inhibitors and perform all the purification steps in the cold room at 4°C.
Reconstitution in vitro
For the reconstructed MKK-JNK cascades in vitro, the concentration of each kinase should be kept constant at 50 nM for MKKs and 0.5 μM for JNKs, respectively. Changes in kinase concentrations would shift of the optimum concentration of arrestin-3.
Purified arrestin-3, MKK4/7, and JNK1/2/3 survive freeze-thaw cycles fairly well. However, after thawing protein samples on ice, the aggregated inactive proteins should be removed by ultra-centrifugation at 100,000 rpm (356,000 μg) for 60 min at 4°C (TLA 120.1 rotor, TL-100 tabletop ultracentrifuge, Beckman). After carefully transferring the supernatants to new tubes to avoid any traces of pellet, re-measure protein concentrations.
The role of arrestin-3 in JNK activation in intact cells
The most critical parameter of the methods described is the quality of starting cell cultures and DNA for transfection. The purified DNA should be transfection-quality, which most DNA purification kits yield. Sometimes after long storage at −20°C and/or multiple freeze-thaw cycles the DNA yields lower than expected (based on previous experiments) expression levels. In this case it is advisable to grow and purify new batch of DNA. For reproducible results, cell cultures must have the same cell numbers to maintain a consistent cell density at the time of transfection for each experiment. All tables for transfection above are based on 6-well cell culture plate. To transfect cells in different formats, the number of cells and the amounts of medium, DNA, and FugeneHD should be scaled up or down in proportion to the difference in surface area of culture plate. To keep the amount of DNA and FugeneHD for transfection constant in all experiments, an empty vector must be used to balance the total DNA amount.
Since the activation of JNKs is detected by phopho-JNK antibody, the addition of phosphatase inhibitors (NaF and Na3VO4) to the cell lysis buffer is critical to prevent JNK dephosphorylation.
c. Troubleshooting
Purification of MKK4, MKK7, JNK1, and JNK2
The manufacturer’s protocol for the Glutathione Sepharose™ High Performance (Amersham Biosciences), Ni-NTA beads (Qiagen), 120 mL HiLoad 16/60 Superdex 200 prep grade column (Amersham) and Mono Q HR 10/10 anion exchange column (Amersham) contain comprehensive troubleshooting instructions for these purification methods.
Reconstitution in vitro
Due to the modest binding affinities (in micromolar range (Zhan et al., 2011b)) of arrestin-3 for the kinase components, arrestin-dependent increase in JNK activation is also modest (~2-3-fold). If the protein concentrations in the reconstructed system are miscalculated, the observed effects of arrestin could be even smaller. In fact, arrestin-3 acts as a simple scaffold by bringing the two kinases together without activating either of them (Kook et al., 2014; Zhan et al., 2011b; Zhan et al., 2013b). Therefore, while lower arrestin-3 concentrations facilitate JNK phosphorylation by MKKs, higher concentrations inhibit it, apparently by shifting the equilibrium to incomplete (and therefore unproductive) complexes arrestin-3-MKK and arrestin-3-JNK (Kook et al., 2014; Zhan et al., 2011b; Zhan et al., 2013b). To solve this problem, calculate protein concentrations carefully and adjust arrestin concentration to optimal, which is much lower for MKK4 than for MKK7 (Zhan et al., 2013b).
Another common problem is the strong background in the [ɤ-32P] –ATP assay. We have found that the addition of 1-2 mM EDTA into the SDS-PAGE buffer and gel can substantially decrease the noise caused by free [ɤ-32P] –ATP.
The role of arrestin-3 in JNK activation in intact cells
Expression of HA-ASK1, HA-MKK4, Flag-MKK7 and arrestin3 in COS-7 cells may vary depending on the state of cells (confluence, passage number, etc), the quality of DNA, and the number of co-expressed proteins. The researcher should therefore test the expression of each protein with different amounts of DNA to determine the optimal range of each DNA for transfection, using the Tables above as a guide.
d. Anticipated Results
Reconstitution in vitro
The strategy used to reconstruct MAPK cascades can be easily applied to other signaling systems involving three to four protein components interacting with each other (Fig. 1). The biphasic dependence of the output on scaffold concentration should be observed if the assays are carefully designed and evaluated. The optimum concentration of the scaffold protein is mainly determined by the affinities of the binding partners for each other. Therefore, this type of assay can be used to compare the binding affinities of different scaffold proteins for the kinases. It also can be used to evaluate the effects of dynamic movements (conformations) of scaffold proteins in signaling pathways.
The role of arrestin-3 in JNK activation in intact cells
Phospho-JNK (Thr183/Tyr185) antibody is used to detect endogenous levels of p46 and p54 JNK isoforms dually phosphorylated at threonine 183 and tyrosine 185. The level of the phosphorylation of endogenous JNK1/2 isoforms (p46 and p54) in cells transfected with empty pcDNA3 vector can be used as a control for the basal JNK activity. Fig. 4 shows the gradual increase of HA-ASK1 expression in COS-7 cells with the increase of DNA concentration to be used for transfection procedure. Increasing amounts of HA-ASK1 (Fig. 4) or Flag-MKK7 (Fig. 5) result in a dose-dependent increase of the phosphorylation levels of p46 and p54 JNK isoforms. Serial dilutions of anisomycin (1 μg/ml)-stimulated HEK-A cell lysates can be used to ensure that the intensity of ppJNK bands in all samples are in the linear range. To evaluate the arrestin-3 effect, the phosphorylation of p46 and p54 JNK isoforms induced by increasing amounts of HA-ASK1 (Fig. 4) or Flag-MKK7 (Fig. 5) can be quantified and compared without or with arrestin3 co-expresssion.
e. Time Considerations
Reconstitution in vitro
With purified proteins in hand, these experiments can be set up quickly. However, it is necessary to allow an extra hour for high-speed centrifugation of thawed proteins to eliminate aggregates (see critical parameters) and subsequent measurements of protein concentrations in the supernatants. A 10 second reaction time was suggested in the protocol. Therefore, the reactions must be started and stopped individually, one after another. Appropriate reaction times can be adjusted based on the activity of upstream kinases. A 5-60 second time scale should work in similar assay settings for JNKs and likely other MAP kinases. After premixing the kinases with arrestin it is important to incubate the mixtures for at least 15 min at 30°C to allow for equilibration of free proteins and complexes before the addition of ATP.
The role of arrestin-3 in JNK activation in intact cells
The experiment should be planned to allow plating of the cells 1 day before transfection. The entire process of transfection, subsequent culture to express proteins in cells and sample preparation takes 4 days. The process can be stopped after cell lysis. Samples should be stored at −20°C or −80°C and used for Western blotting later. The quality of frozen samples is maintained for many days, but could deteriorate after prolonged storage.
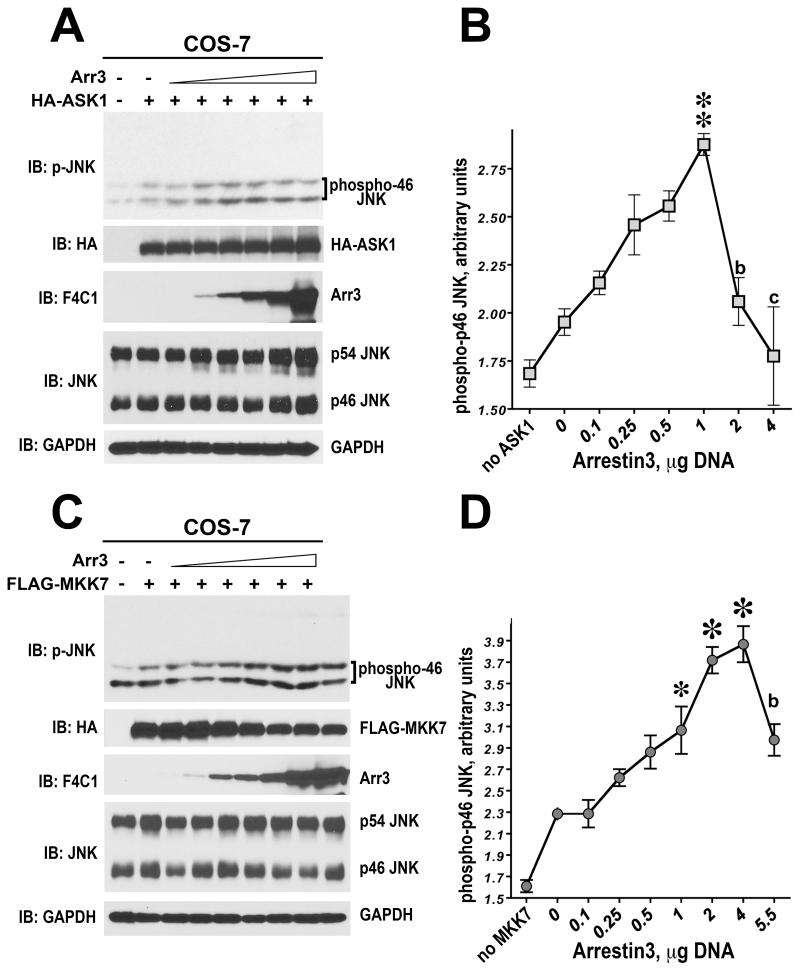
Fig. 6. Arrestin-3 enhances ASK1- and MKK7-dependent phosphorylation of endogenous JNK1/2 in intact cells.
A,C. Representative Western blots showing phosphorylation of endogenous JNK1/2 isoforms in cells expressing ASK1 (A) or MKK7 (C) in the presence of increasing concentrations of arrestin-3. B,D. Quantification of the levels of p46H JNK isoform phosphorylation. One-way ANOVA analysis with arrestin-3 concentration as factor yielded significant effect of arrestin-3 on JNK phosphorylation both in the presence of ASK1 and MKK7 (p<0.0001). ** - p<0.01, * - p<0.05 to the value at 0 arrestin-3; b - p<0.01, c - p<0.001 to the maximal value (at 1 or 4 μg of arrestin-3 DNA), according to Bonferroni/Dunn post-hoc test. Data from (Kook et al., 2014).
Table 9. Transfection to detect biphasic effect of arrestin-3 on ASK1-stimulated JNK phosphorylation (Fig. 6A,B).
| pcDNA3-Arrestin-3 | 0 μg | 0 μg | 0.1 μg | 0.25 μg | 0.5 μg | 1.0 μg | 2.0 μg | 4.0 μg |
| pcDNA3-HA-ASK1 | 0 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg | 0.5 μg |
| pcDNA3 | 4.5 μg | 4.0 μg | 3.9 μg | 3.75 μg | 3.5 μg | 3.0 μg | 2.0 μg | 0 μg |
| FuGENE HD | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl | 13.5μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
Note: These DNA amounts were calculated for 6-well culture plate (equivalent to 35 mm dish). If different size plates are used, the amount of total DNA should be changed proportionally to plate area.
Table 10. Transfection to detect biphasic effect of arrestin-3 on MKK7-stimulated JNK phosphorylation (Fig. 6C,D).
| pcDNA3- Arrestin-3 |
0 μg | 0 μg | 0.1 μg | 0.25 μg | 0.5 μg | 1.0 μg | 2.0 μg | 4.0 μg | 5.5 μg |
| pcDNA3-Flag- MKK7 |
0 μg | 0.08 μg | 0.08 μg | 0.08 μg | 0.08 μg | 0.08 μg | 0.08 μg | 0.08 μg | 0.08 μg |
| pcDNA3 | 5.58 μg | 5.5 μg | 5.4 μg | 5.25 μg | 5.0 μg | 4.5 μg | 3.5 μg | 1.5 μg | 0 μg |
| FuGENE HD | 18μl | 18μl | 18μl | 18μl | 18μl | 18μl | 18μl | 18μl | 18μl |
| Opti-MEM | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl | 125μl |
Note: See note to Table 9 above..
ACKNOWLEDGEMENTS
NIH grants EY011500, GM077561, and GM081756 (VVG); NS065868 and DA030103 (EVG); GM059802 and Welch Foundation (F-1390) (KND); TSK is supported by a postdoctoral trainee fellowship from Cancer Prevention Research Institute of Texas (CPRIT).
References
- Abramczyk O, Tavares CD, Devkota AK, Ryazanov AG, Turk BE, Riggs AF, Ozpolat B, Dalby KN. Purification and characterization of tagless recombinant human elongation factor 2 kinase (eEF-2K) expressed in Escherichia coli. Protein expression and purification. 2011;79:237–244. doi: 10.1016/j.pep.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman M, Kook S, Gimenez LE, Lizama BN, Palazzo MC, Gurevich EV, Gurevich VV. Silent scaffolds: inhibition of c-Jun N-terminal kinase 3 activity in the cell by a dominant-negative arrestin-3 mutant. J Biol Chem. 2012;287:19653–19664. doi: 10.1074/jbc.M112.358192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack WR, Shaw AS. Signal transduction: hanging on a scaffold. Curr Opin Cell Biol. 2000;12:211–216. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- Donoso LA, Gregerson DS, Smith L, Robertson S, Knospe V, Vrabec T, Kalsow CM. S-antigen: preparation and characterization of site-specific monoclonal antibodies. Curr Eye Res. 1990;9:343–355. doi: 10.3109/02713689008999622. [DOI] [PubMed] [Google Scholar]
- Gallagher ED, Xu S, Moomaw C, Slaughter CA, Cobb MH. Binding of JNK/SAPK to MEKK1 is regulated by phosphorylation. J Biol Chem. 2002;277:45785–45792. doi: 10.1074/jbc.M207702200. [DOI] [PubMed] [Google Scholar]
- Gonzales MF, Brooks T, Pukatzki SU, Provenzano D. Rapid protocol for preparation of electrocompetent Escherichia coli and Vibrio cholerae. Journal of visualized experiments: JoVE. 2013 doi: 10.3791/50684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- House BL, Mortimer MW, Kahn ML. New recombination methods for Sinorhizobium meliloti genetics. Appl Environ Microbiol. 2004;70:2806–2815. doi: 10.1128/AEM.70.5.2806-2815.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL. Defining MAPK interactomes. ACS Chem Biol. 2011;6:18–20. doi: 10.1021/cb100384z. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev A, Xu S, English J, Wu P, Schaefer E, Cobb MH. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J Biol Chem. 1997;272:11057–11062. doi: 10.1074/jbc.272.17.11057. [DOI] [PubMed] [Google Scholar]
- Kook S, Zhan X, Kaoud TS, Dalby KN, Gurevich VV, Gurevich EV. Arrestin-3 binds JNK1α1 and JNK2α2 and facilitates the activation of these ubiquitous JNK isoforms in cells via scaffolding. J Biol Chem. 2014;289 doi: 10.1074/jbc.M113.510412. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S, Fleming Y, Goedert M, Cohen P. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol. 1998;8:1387–1390. doi: 10.1016/s0960-9822(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Levchenko A, Bruck J, Sternberg PW. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci U S A. 2000;97:5818–5823. doi: 10.1073/pnas.97.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko A, Bruck J, Sternberg PW. Regulatory modules that generate biphasic signal response in biological systems. Syst Biol (Stevenage) 2004;1:139–148. doi: 10.1049/sb:20045014. [DOI] [PubMed] [Google Scholar]
- Madsen JA, Kaoud TS, Dalby KN, Brodbelt JS. 193-nm photodissociation of singly and multiply charged peptide anions for acidic proteome characterization. Proteomics. 2011;11:1329–1334. doi: 10.1002/pmic.201000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Miller WE, McDonald PH, Cai SF, Field ME, Davis RJ, Lefkowitz RJ. Identification of a motif in the carboxyl terminus of beta -arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–27777. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- Seo J, Tsakem EL, Breitman M, Gurevich VV. Identification of arrestin-3-specific residues necessary for JNK3 activation. J Biol Chem. 2011;286:27894–27901. doi: 10.1074/jbc.M111.260448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Coffa S, Fu H, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem. 2009;284:685–695. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Vishnivetskiy SA, Seo J, Chen J, Gurevich EV, Gurevich VV. Arrestin-1 expression in rods: balancing functional performance and photoreceptor health. Neuroscience. 2011;174:37–49. doi: 10.1016/j.neuroscience.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Zhan X, Chen Q, Iverson TI, Gurevich VV. Arrestin Expression in E. coli and Purification. Curr Protocols Pharmacol. 2014 doi: 10.1002/0471141755.ph0211s67. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Willoughby EA, Perkins GR, Collins MK, Whitmarsh AJ. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J Biol Chem. 2003;278:10731–10736. doi: 10.1074/jbc.M207324200. [DOI] [PubMed] [Google Scholar]
- Yan C, Kaoud T, Lee S, Dalby KN, Ren P. Understanding the specificity of a docking interaction between JNK1 and the scaffolding protein JIP1. The journal of physical chemistry. B. 2011;115:1491–1502. doi: 10.1021/jp1073522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual arrestins. J Mol Biol. 2011a;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Kaoud TS, Dalby KN, Gurevich VV. Non-visual arrestins function as simple scaffolds assembling MKK4-JNK3α2 signaling complex. Biochemistry. 2011b;50:10520–10529. doi: 10.1021/bi201506g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Kaoud TS, Dalby KN, Gurevich VV. Nonvisual arrestins function as simple scaffolds assembling the MKK4-JNK3alpha2 signaling complex. Biochemistry. 2011c;50:10520–10529. doi: 10.1021/bi201506g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Kaoud TS, Kook S, Dalby KN, Gurevich VV. JNK3 binding to arrestin-3 differentially affects the recruitment of upstream MAP kinase kinases. J Biol Chem. 2013a;288:28535–28547. doi: 10.1074/jbc.M113.508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Kaoud TS, Kook S, Dalby KN, Gurevich VV. JNK3 enzyme binding to arrestin-3 differentially affects the recruitment of upstream mitogen-activated protein (MAP) kinase kinases. J Biol Chem. 2013b;288:28535–28547. doi: 10.1074/jbc.M113.508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Perez A, Gimenez LE, Vishnivetskiy SA, Gurevich VV. Arrestin-3 binds the MAP kinase JNK3α2 via multiple sites on both domains. Cell Signal. 2014;26:766–776. doi: 10.1016/j.cellsig.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]