Abstract
Objectives
To examine changes between 2006 and 2011 in the proportion of HIV-positive patients newly-enrolled in HIV care with advanced disease and the median CD4+ cell count at enrollment; and identify patient-, facility-, and contextual-level factors associated with late enrollment in care in 2011.
Design
Cross sectional over time.
Methods
For time trends analyses, routinely-collected patient-level data (307,110 adults newly-enrolled in 138 HIV clinical care facilities) in Kenya, Mozambique, Rwanda and Tanzania; and for analyses of correlates, patient-level data (46,201 in 195 facilities), and facility- and population-level survey data were used. Late enrollment was defined as CD4+ count ≤350 cells/μl and/or WHO clinical stage 3/4.
Results
Late enrollment declined from 69.9% to 57.2%, (p<0.0001); median CD4+ count increased from 242 to 292 cells/μL (ptrend<0.0001). In 2011, risk of late enrollment was significantly higher for men and non-pregnant women vs. pregnant women; patients aged >25 vs. 15-25 years; non-married vs. married; and those entering from sites other than prevention of mother to child transmission (PMTCT). More extensive HIV testing coverage in the region of a facility was significantly associated with lower risk of late enrollment.
Conclusions
Despite improvement, in 2011, 57% of patients entered HIV care already ART-eligible. The lower risk of late enrollment among those referred from PMTCT and in regions where HIV testing coverage was higher suggests that innovative approaches to rapidly increase testing uptake among people living with HIV prior to the development of symptoms have the potential to reduce late enrollment in care.
Introduction
Enrollment in HIV clinical care at advanced stage of disease (late enrollment in care) denotes critical missed opportunities to improve the health and well-being of HIV-positive people[1-3], and limits the potential of treatment at earlier stages of HIV disease to reduce HIV transmission[4] and potentially attenuate incidence[5-7]. Most studies of the prevalence, correlates, and outcomes of late enrollment in HIV care have been conducted in Europe and North America[1, 2, 8-18]. In sub-Saharan Africa, studies have largely focused on advanced disease at ART initiation[19, 20, 21]. Far less is known about advanced disease at enrollment in HIV care, a critical precursor to late ART initiation in this region[22]. In particular, whether the prevalence of late enrollment has changed over time with scale-up of care and treatment in sub-Saharan Africa is unknown. Additionally, multiple barriers to early diagnosis and prompt linkage to care likely exist in sub-Saharan Africa, but with few exceptions[23-25] only individual-level correlates have been examined. Characteristics of the HIV care facility and features of the broader social environment in which a facility operates[21, 26] may also influence timing of enrollment in HIV care.
Advanced HIV disease has frequently been defined as a CD4+ count ≤200 cells/μL, or even lower (e.g., [15, 17, 25]. In 2010, the World Health Organization (WHO) released expanded criteria for ART eligibility in low and middle income countries, recommending ART initiation for persons with CD4+ count <350 cells/μL or WHO stage 3 or 4[27]. In this study we defined late enrollment in care based on the WHO 2010 treatment guidelines, in order to describe progress toward meeting the goal of universal access to treatment. Specifically, we (1) examined changes between 2006 and 2011 in the proportion of HIV+ patients who enrolled late in HIV care and in the median CD4+ cell count at enrollment; and (2) identified individual-, program-, and contextual-level factors associated with late enrollment in care in 2011.
Methods
Study population
The study population included HIV-positive adults aged 15 to 85 years newly enrolling in HIV care between January 1, 2006 and December 31, 2011 at 138 HIV care facilities that provided continuous services during this time period in four sub-Saharan African countries: Kenya (64 facilities), Mozambique (29), Rwanda (24), and Tanzania (21). An additional 57 facilities providing services in 2011 were included in the analysis of correlates of late enrollment in 2011 (N=195). All programs were receiving technical support from ICAP at Columbia University through funding from the United States President's Emergency Plan for AIDS Relief (PEPFAR). Provision of services at each facility was conducted according to national guidelines with all providing both pre-ART and ART services. These facilities had implemented electronic databases to manage patient information routinely collected during each visit; when compared to all facilities supported by ICAP in those same countries during the study period, the facilities included in this analysis had higher patient volume, and were more likely to be located in urban areas and to be higher-level facilities (data not shown).
For this study, we included patients if CD4+ cell count and/or WHO clinical staging information were available up to three months after enrollment in HIV care, given that CD4+ counts are not always performed immediately at enrollment. Additionally, patients who were missing both CD4+ count and clinical stage were included if they initiated ART within one month of enrollment, as these patients enrolled late and likely were initiated on ART without documentation of CD4+ count and/or clinical stage.
Data sources
Patient information routinely collected during each clinic visit was documented by clinicians on national patient forms. This information was regularly entered into on-site electronic databases by data clerks. Data quality assessments were conducted at least annually at each facility using a standardized protocol. This entailed drawing a random sample of approximately 5-20% (depending on total patient volume) and assessing completeness and accuracy of key data elements. Remediation plans were developed for sites with poor data quality (<80% completeness and accuracy) and data quality was re-assessed after their implementation. Each quarter, data were de-identified at the site-level, encrypted, and imported into a common-format database. Use of these de-identified patient-level data for research purposes was approved by National Ethics Committees in each country, the Columbia University Medical Center Institutional Review Board, the United States Centers for Disease Control and Prevention, and the PEPFAR Office of the Global AIDS Coordinator (OGAC).
Data on facility characteristics were derived from structured assessments completed by ICAP staff in-country in August 2011. The assessment comprised 85 closed-ended questions about the types of HIV-related services provided at the facility and the characteristics of the HIV program (e.g., staffing configurations, availability of supportive services). Administration of this assessment was considered non-human subjects research because data referred to facilities, not individuals.
Contextual-level data were obtained from national Census and from country-specific, population-based surveys, namely the Demographic and Health Surveys and AIDS Indicator Surveys, using the most recently completed survey–2008 for Kenya, 2009 for Rwanda, 2011 for Tanzania and Mozambique. For Kenya and Rwanda, 2011 estimates for contextual-level variables were imputed by extending the slope between the most recent available data and that from the previous time point.
Measures
The main study outcome, derived from the routinely collected patient data, was enrollment in HIV care with advanced HIV disease, or late enrollment in care. Late enrollment in care was defined as having CD4+ count ≤350 cells/μl and/or WHO clinical stage 3/4 documented within three months of enrollment. In addition, those who were missing these parameters were considered to have enrolled in care late if they initiated ART within one month of enrollment in care.
Predictors were selected based on our conceptual framework, including factors that might influence late testing or delayed enrollment after diagnosis(22). Individual-level variables examined included age, sex, pregnancy status, marital status, and point of entry into care. The latter was categorized as voluntary counseling and testing [VCT] service, prevention of mother-to-child transmission [PMTCT] program, or provider-initiated testing and counseling [PITC] (including HIV testing among inpatients or outpatients in the general outpatient department, family planning clinic, TB clinic, or sexually transmitted infections [STI] clinic, depending on the country).
Facility-level variables examined included type of setting (urban, rural), type of facility (primary, secondary, tertiary, private), program size (i.e., number of patients active in HIV care, both in HIV care and on ART in the quarter prior to each individual's date of enrollment), number of days/week of facility operation, and availability of VCT and PMTCT on site.
Contextual measures were created for each of the 15 sub-national regions where one of the study facilities was located; they included HIV prevalence, HIV knowledge (mean score on five items assessing comprehensive HIV knowledge), testing coverage (percent of population tested and received results within the past 12 months), stigma (mean score on four items indicating acceptance of HIV+ people, reverse coded), education (percent of population having completed primary school), employment (percent of population employed), income distribution (percent of households in the first quintile of the national wealth index).
Statistical Analyses
We conducted two statistical analyses. The first assessed change over time in late enrollment in care and median CD4+ cell count at enrollment, and included the 138 facilities that provided services from 2006 to 2011. For late enrollment, statistical significance tests were performed using a Poisson regression model with generalized estimating equations (GEE) to account for clustering within facilities, treating calendar time as a continuous time measure. The models produced risk ratios for late enrollment per half year increase in calendar time. For median CD4+ count at enrollment we used the Kruskal Wallis test for comparisons over time. To assess the effect of missing outcome data on estimates of change over time, we multiply imputed outcomes for those missing both CD4+ count and WHO clinical stage at enrollment and compared the findings to those observed with complete data.
The second analysis examined correlates of late enrollment in care in 2011 and included the 195 facilities providing services at that time. Relative risks of late enrollment in care were estimated using generalized linear models with a binomial distribution and a log link with GEE. Modeling was done in several steps. First, individual-level variables that were significantly associated with late enrollment in bivariate analyses were included in a multivariable model and retained if they were significant at p<0.05. Second, the same was done for facility-level variables. Next, variables that were significant in each of these two multivariable models were combined into a two-level model and retained if they were significant. Fourth, as we did not have sufficient regions to assess the independent associations of contextual-level variables with late enrollment, each contextual-level variable was added to the two-level model and retained if statistically significant. Finally, non-significant correlates were dropped unless they altered other estimates by 10% or more. We conducted sensitivity analyses to determine if findings were altered if (1) the definition of late enrollment was based on the 2006 WHO treatment guidelines (CD4+≤200 cells/μL or WHO Stage 4 or; if missing CD4+ count, WHO Stage 3 or; if pregnant or active TB, CD4+≤350 cells/μL)[28]; (2) data for each country were removed; (3) the analysis was restricted to the 138 facilities used in the time trend analysis; and (4) patients defined as enrolling late only on the basis of having initiated ART within one month of enrollment were removed.
Results
Study population and missing data
In the 138 facilities that reported data from 2006 to 2011, 307,100 HIV-positive newly-enrolled adult patients had data on CD4+ count and/or WHO stage. In 2006, 44,804 patients (83.5% of all newly enrolled adults) met these criteria; in 2011, the respective number was 39,725 (91.4%) (Table 1). Considering the proportion missing both CD4+ count and WHO stage in 2006 and 2011, a consistent decrease was observed across all patient characteristics (Supplementary Table 1a). Additionally, among those missing CD4+ count but not WHO stage, the distribution of stage did not differ substantially between the two years (Supplementary Table 1b).
Table 1. Percent distribution of newly-enrolled patients and percent enrolled late by patient charateristics in 138 ICAP facilities in 4 sub-Saharan countries, 2006 to 20111.
| All patients enrolled between 2006 and 2011 | 2006 | 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Percent of patients | Percent late | N | Percent of patients2 | Percent late3 | N | Percent of patients | Percent late3 | |
| All patients | 3,07,100 | 100.0 | 63.8 | 44,804 | 100 | 69.9 | 39,725 | 100.0 | 57.2 |
| Sex and pregnancy status | |||||||||
| Women (not pregnant) | 1,83,565 | 59.8 | 62.4 | 28,111 | 62.7 | 67.6 | 22,412 | 56.4 | 57.1 |
| Women (pregnant) | 21,194 | 6.9 | 36.4 | 1,215 | 2.7 | 46.3 | 4,708 | 11.9 | 29.1 |
| Men | 1,02,341 | 33.3 | 72.1 | 15,478 | 34.6 | 76.0 | 12,605 | 31.7 | 67.9 |
| Age at enrollment | |||||||||
| 15 – 25 years | 62,371 | 20.3 | 50.5 | 7,557 | 16.9 | 58.0 | 9,449 | 23.8 | 44.2 |
| 26 – 35 years | 1,18,032 | 38.4 | 63.5 | 17,130 | 38.2 | 69.7 | 15,120 | 38.1 | 56.9 |
| 36 – 45 years | 77,849 | 25.4 | 70.0 | 12,563 | 28 | 73.9 | 9,150 | 23.0 | 65.0 |
| ≥46 years | 48,848 | 15.9 | 71.8 | 7,554 | 16.9 | 75.8 | 6,006 | 15.1 | 66.7 |
| Marital status | |||||||||
| Married/living with partner | 1,45,568 | 47.4 | 60.4 | 17,330 | 38.7 | 67.9 | 20,454 | 51.5 | 53.2 |
| Single | 69,216 | 22.5 | 65.8 | 9,110 | 20.3 | 72.1 | 9,651 | 24.3 | 59.3 |
| Divorced/widowed | 42,655 | 13.9 | 68.3 | 6,140 | 13.7 | 71.8 | 5,017 | 12.6 | 63.7 |
| Missing | 49,661 | 16.2 | 67.4 | 12,224 | 27.3 | 70.2 | 4,603 | 11.6 | 63.8 |
| Enrollment point of entry | |||||||||
| VCT | 1,19,910 | 39.1 | 63.0 | 20,723 | 46.3 | 68.6 | 14,137 | 35.6 | 57.4 |
| PMTCT | 30,301 | 9.9 | 35.3 | 2,975 | 6.6 | 41.3 | 5,466 | 13.8 | 31.0 |
| PITC (TB/HIV, in/outpatient, other) | 1,37,328 | 44.7 | 70.5 | 17,154 | 38.3 | 75.3 | 18,100 | 45.6 | 64.7 |
| Unknown | 19,561 | 6.4 | 66.9 | 3,952 | 8.8 | 74.9 | 2,022 | 5.1 | 59.8 |
| Country | |||||||||
| Kenya - 64 facilities | 76,617 | 25.0 | 63.9 | 10,691 | 23.9 | 74.3 | 8,282 | 20.9 | 58.1 |
| Mozambique - 29 facilities | 1,68,199 | 54.8 | 66.3 | 23,829 | 53.2 | 72.1 | 24,049 | 60.5 | 58.8 |
| Rwanda - 24 facilities | 23,638 | 7.7 | 48.9 | 6,331 | 14.1 | 53.0 | 1,816 | 4.6 | 43.3 |
| Tanzania - 21 facilities | 38,646 | 12.6 | 62.0 | 3,953 | 8.8 | 72.3 | 5,578 | 14.0 | 53.6 |
138 facilities are those reporting in all 6 years.
All comparisons of proportions of population characteristics, 2006 vs, 2011, were significant at p<.0001, with the exception of age group 26-35 years, for which p=0.61.
All comparisons of proportions enrolled late, 2006 vs. 2011, were significant at p<.0001.
As shown in Table 1, across all study years, most patients were non-pregnant women (59.8%); aged 26 and 35 years (38.4%); and married or living with a partner (47.4%). Patients were most likely to have entered care via a PITC site (44.7%) or VCT (39.1%), with only 9.9% enrolled through PMTCT services. The largest proportion of patients was enrolled in Mozambique (54.8%). Between 2006 and 2011 there were statistically significant changes in the distribution of patients across all characteristics (p<.0001) except for those aged 25-36 years.
Change in late enrollment in HIV care and CD4+ cell count at enrollment, 2006-2011
Between 2006 and 2011 a significant decrease in late enrollment in HIV care was observed; the percent of patients that enrolled late declined from 69.9% in 2006 to 57.2% in 2011, corresponding to a 1.5% decline per half year change in calendar time (p<0.0001) (Table 1 and Figure 1a). When we multiply imputed the outcome for those missing both CD4+ and stage, the estimate for change over time was only slightly attenuated; therefore, all reported results are those with complete data.
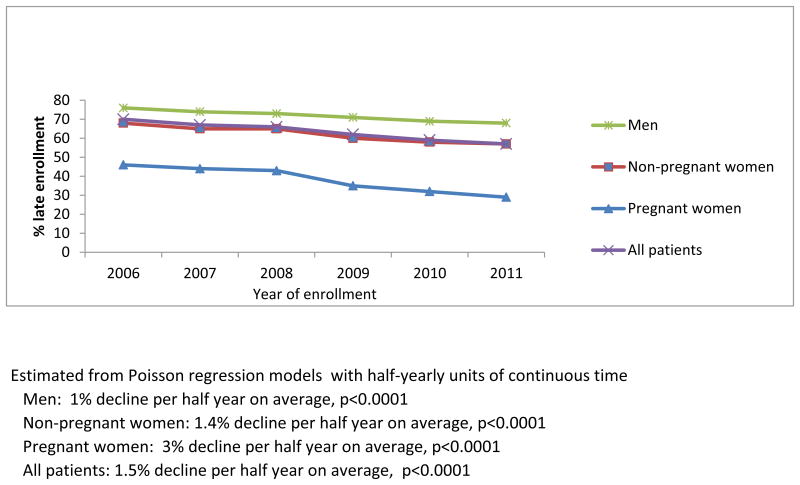
Figure 1a. Percent late enrollment by sex and pregnancy status among newly-enrolled patients in 138 facilities in 4 sub-Saharan African countries, 2006-2011.
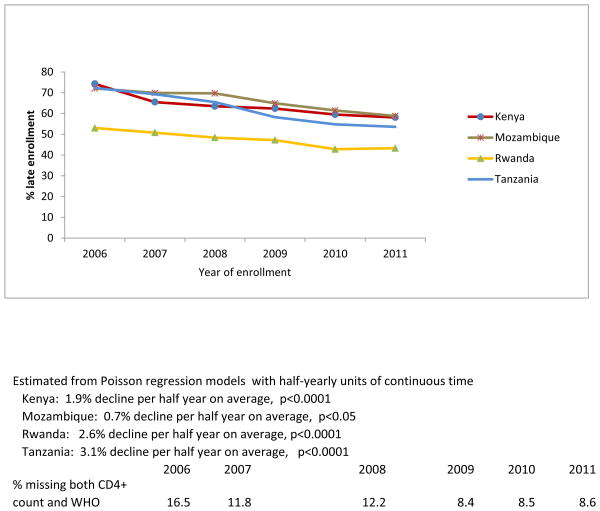
A statistically significant decline was observed among women, both non-pregnant (1.4%/half-year, p<0.0001) and pregnant (3%/half-year, p<0.001), as well as among men (1%/half-year, p<0.0001), and across all four countries (Kenya 1.9%/half-year, p<0.0001, Mozambique 0.7%/half-year, p=0.05, Rwanda 2.6%/half-year, p<0.0001, Tanzania 3.1%/half-year, p<0.0001) (Figure 1b). Additionally, the risk of late enrollment was significantly lower in 2011 compared with 2006 across subgroups defined by age, marital status, and enrollment point of entry (p<.0001 for all pairwise comparisons) (Table 1).
Figure 1b. Percent late enrollment by country among newly-enrolled patients in 138 facilities in 4 sub-Saharan African countries, 2006-2011.
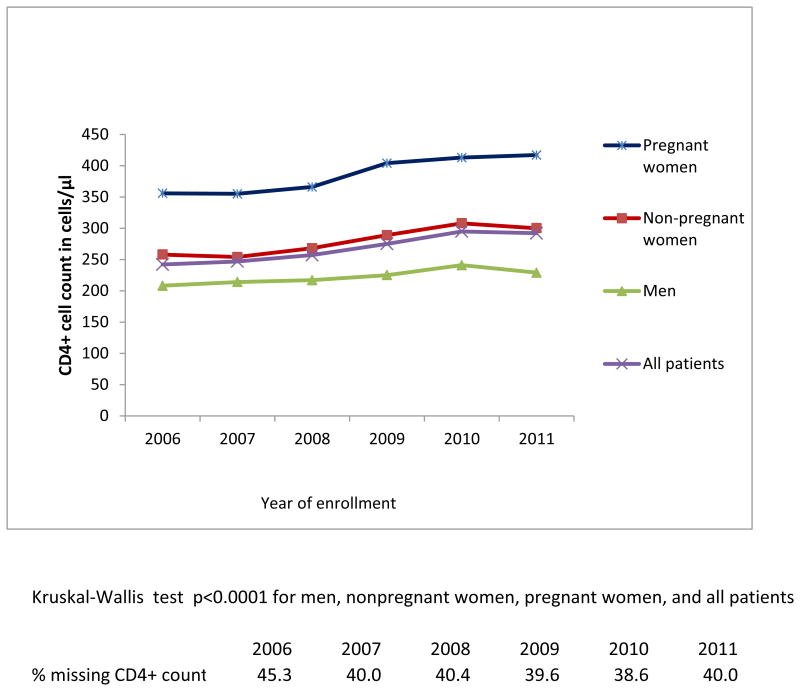
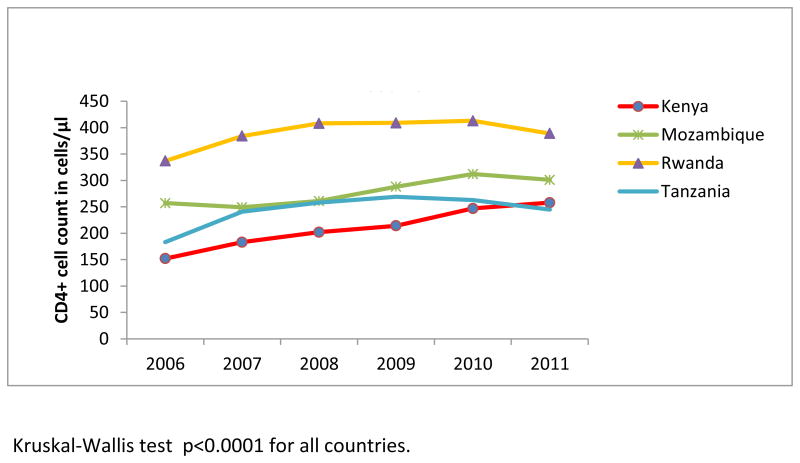
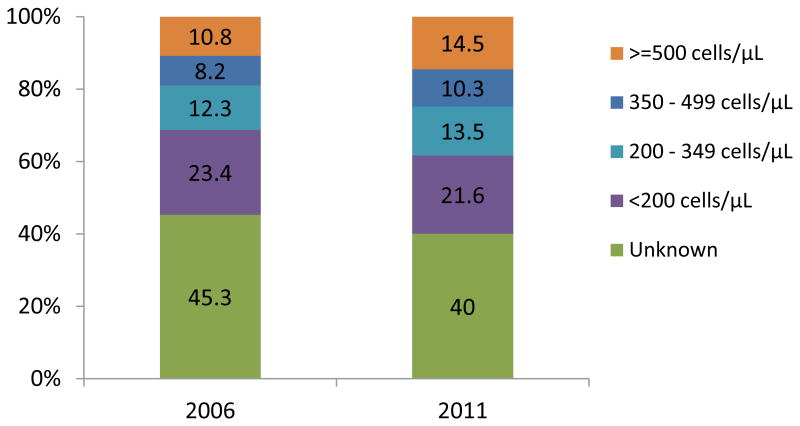
Among patients who had CD4+ count at enrollment (54.7% in 2006 and 60% in 2011), the median CD4+ count increased significantly from 242 cells/μL in 2006 to 292 cells/μL in 2011 (ptrend<0.0001), or 50 cells/μL in 6 years (Figure 2a). A significant increase in median CD4+ count was noted over the study years among non-pregnant and pregnant women, and men (ptrend<0.0001) (Figure 2a), and in all countries (ptrend<0.0001) (Figure 2b). Considering the full distribution of CD4+ count at enrollment including those missing (Figure 3), the proportion with CD4+ ≥350 cells/μL increased from 19% to 24.8% of patients (with an increase from 10.8% to 14.5% among those with CD4+ ≥500 cells/μL), and the proportion with CD4+ <200 cells/μL decreased from 23.4% to 21.6% (overall χ2 p<.0001).
Figure 2a. Median CD4+ count at enrollment by sex and pregnancy status among newly-enrolled patients in 138 facilities in 4 sub-Saharan African countries, 2006-2011.
Figure 2b. Median CD4 count at enrollment by country among newly-enrolled patients in 138 facilities in 4 sub-Saharan African countries, 2006-2011.
Figure 3. Distribution of CD4 count in 2006 and 2011 among newly-enrolled patients in 138 facilities in 4 sub-Saharan countries.
Chi-squared test p<0.0001
Total n=53,641 for 2006 and 43,466 for 2011.
Multi-level correlates of late enrollment in HIV care, 2011
In the 195 facilities that reported data in 2011, 46,201 newly-enrolled adult patients had sufficient information to define whether they enrolled late in care (91% of all newly-enrolled adults); of these, 55.6% enrolled late (Table 2). In unadjusted analyses, all patient-level variables examined were significantly associated with late enrollment. At the facility level, risk of late enrollment was lower in primary and private sites relative to tertiary sites, and it was higher in facilities with larger patient loads. At the contextual level, higher HIV prevalence, HIV stigma, and coverage of primary education were significantly associated with higher risk of late enrollment in care, whereas higher population testing coverage, HIV knowledge, and employment were associated with lower risk of late enrollment.
Table 2. Individual-, facility-, and contextual-level correlates of late enrollment in 2011, 195 ICAP supported HIV care clinics in Kenya, Mozambique, Rwanda, and Tanzania.
| N | Percent of patients | Percent Late | Crude RR (95% CI)1 | ARR (95% CI)1,2 | |
|---|---|---|---|---|---|
| All | 46201 | 100.0 | 55.6 | ||
| INDIVIDUAL-LEVEL FACTORS | |||||
| Sex and pregnancy status | |||||
| Women (pregnant) | 5,678 | 12.3 | 28.9 | 1 | 1 |
| Women (not pregnant) | 25,762 | 55.8 | 55.9 | 2.11 (1.81, 2.46) | 1.52 (1.34, 1.72) |
| Men | 14,761 | 32.0 | 65.7 | 2.51 (2.15, 2.94) | 1.74 (1.54, 1.98) |
| Age at enrollment | |||||
| 15 – 25 years | 10,689 | 23.1 | 42.8 | 1 | 1 |
| 26 – 45 years | 28,560 | 61.8 | 58.1 | 1.46 (1.39, 1.54) | 1.28 (1.22, 1.34) |
| 46 – 55 years | 6,952 | 15.1 | 65.4 | 1.67 (1.58, 1.78) | 1.35 (1.28, 1.42) |
| Marital status | |||||
| Married/living with partner | 23,691 | 51.3 | 51.4 | 1 | 1 |
| Widowed/Divorced | 5,972 | 12.9 | 61.5 | 1.25 (1.21, 1.29) | 1.14 (1.11, 1.17) |
| Never married | 11,219 | 24.3 | 58.2 | 1.13 (1.08, 1.17) | 1.12 (1.10, 1.15) |
| Unknown | 5,319 | 11.5 | 62.7 | 1.16 (1.10, 1.21) | 1.1 (1.06, 1.15) |
| Point of entry | |||||
| PMTCT | 6,552 | 14.2 | 30.0 | 1 | 1 |
| VCT | 16,546 | 35.8 | 54.9 | 1.93 (1.70, 2.22) | 1.34 (1.23, 1.47) |
| PITC (TB/HIV, in/outpatient, other) | 20,604 | 44.6 | 63.8 | 2.21 (1.94, 2.53) | 1.54 (1.41, 1.69) |
| Unknown | 2,499 | 5.4 | 60.9 | 2.10 (1.83, 2.40) | 1.46 (1.33, 1.6) |
| Country | |||||
| Kenya - 69 facilities | 8,397 | 18.2 | 58.0 | 1 | 1 |
| Mozambique - 31 facilities | 26,260 | 56.8 | 59.1 | 1.07 (0.98, 1.18) | 0.79 (0.63, 1.00) |
| Rwanda - 44 facilities | 2,678 | 5.8 | 41.0 | 0.68 (0.62, 0.75) | 0.84 (0.69, 1.03) |
| Tanzania - 51 facilities | 8,866 | 19.2 | 47.7 | 0.80 (0.72, 0.89) | 0.93 (0.83, 1.03) |
| FACILITY-LEVEL FACTORS Location | |||||
| Rural | 12,549 | 27.2 | 55.1 | 1 | |
| Urban | 33,652 | 72.8 | 55.9 | 1.06 (0.97, 1.15) | |
| Type of site | |||||
| Tertiary | 1,328 | 2.9 | 63.9 | 1 | |
| Secondary | 18,705 | 40.5 | 59.4 | 0.87 (0.70, 1.1) | |
| Primary | 22,547 | 48.8 | 53.5 | 0.74 (0.60, 0.94) | |
| Private | 3,621 | 7.8 | 47.3 | 0.70 (0.54, 0.90) | |
| Number of patients visited the facility during quarter prior to enrollment | |||||
| <300 | 3,170 | 6.9 | 46.5 | 1 | 1 |
| 300-699 | 6,778 | 14.7 | 47.0 | 1.05 (0.95, 1.18) | 1.03 (0.93 1.14) |
| >=700 | 36,253 | 78.5 | 58.1 | 1.12 (1.01, 1.25) | 1.09 (1.00, 1.21) |
| VCT service | |||||
| No | 3,314 | 7.2 | 60.5 | 1 | |
| Yes | 42,887 | 92.8 | 55.3 | 0.88 (0.76, 1.02) | |
| PMTCT services | |||||
| No | 282 | 0.6 | 54.6 | 1 | |
| Yes | 45,919 | 99.4 | 55.7 | 1.09 (0.63, 1.87) | |
| Days open at facility | |||||
| <2.5 days | 1,579 | 3.4 | 43.8 | 1 | |
| 2.5-4.9 days | 6,118 | 13.2 | 53.9 | 1.14 (0.95, 1.39) | |
| >=5 days | 38,504 | 83.3 | 56.5 | 1.13 (0.96, 1.34) | |
| CONTEXTUAL-LEVEL FACTORS | |||||
| Population tested for HIV and received results last 12 months, % | |||||
| <=20 | 11,889 | 25.7 | 63.3 | 1 | 1 |
| 21 - 39 | 27,736 | 60.0 | 54.9 | 0.89 (0.78, 1.02) | 0.86 (0.67, 1.11) |
| >=40 | 6,576 | 14.2 | 45.0 | 0.68 (0.59, 0.78) | 0.76 (0.58, 0.97) |
| HIV prevalence, % | |||||
| <=3.5 | 4,820 | 10.4 | 51.1 | 1 | 1 |
| >3.5 to <=10.0 | 21,799 | 47.2 | 53.2 | 1.12 (1.01, 1.24) | 1.03(0.78, 1.35) |
| >10.0 | 19,582 | 42.4 | 59.6 | 1.30 (1.14, 1.47) | 1.04 (0.94, 1.15) |
| Population median comprehensive HIV knowedge score | |||||
| Low | 23,978 | 51.9 | 58.3 | 1 | |
| High | 22,223 | 48.1 | 52.9 | 0.85 (0.78, 0.94) | |
| Population median HIV stigma score | |||||
| Low | 19,882 | 43.0 | 52.7 | 1 | |
| High | 26,319 | 57.0 | 57.9 | 1.17 (1.07, 1.27) | |
| Population completed primary education,% | |||||
| <=30 | 25,174 | 54.5 | 57.1 | 1 | |
| 31 – 74 | 12,630 | 27.3 | 51.3 | 1.0 (0.89, 1.12) | |
| >=75 | 8,397 | 18.2 | 58.0 | 1.19 (1.08, 1.32) | |
| Population employed,% | |||||
| <=60 | 20,996 | 45.4 | 59.5 | 1 | |
| 61 – 74 | 17,858 | 38.7 | 55.2 | 0.90 (0.82, 0.99) | |
| >=75 | 7,347 | 15.9 | 45.9 | 0.69 (0.62, 0.78) | |
| Households in the lowest quintile of national wealth index, % | |||||
| <6 | 20,643 | 44.7 | 56.4 | 1 | |
| >=6.0 to <=20.0 | 12,390 | 26.8 | 49.3 | 0.83 (0.76, 0.90) | |
| >20.0 to <=30.0 | 4,105 | 8.9 | 55.4 | 0.72 (0.63, 0.83) | |
| >30.0 | 9,063 | 19.6 | 62.9 | 1.06 (0.93, 1.21) |
Relative risks were estimated using generalized linear models with a binomial distribution and a log link, with GEE to account for clustering within clinics. Each variable represents a separate model.
Model is adjusted for all variables shown.
In multivariable analyses, relative to pregnant women, non-pregnant women had a significantly higher risk of late enrollment (ARR=1.52; 95%CI: 1.34, 1.72), as did men (ARR=1.74; 95% [CI: 1.54, 1.98). Other individual-level factors associated with higher risk of late enrollment were older age relative to age 15-25 years (ARR:26-45 years=1.28; 95%CI:1.22, 1.34; ARR:46-55 years=1.35; 95%CI:1.28, 1.42); and, relative to being married or living with a partner, being widowed or divorced (ARR=1.14;95%CI:1.11, 1.71), never married (ARR=1.12; 95%CI:1.10, 1.15), or of unknown marital status (ARR=1.10; 95%CI:1.06, 1.15). Compared with those enrolled through PMTCT services, risk of late enrollment in care was significantly higher for patients enrolled through VCT (ARR=1.34; 95%CI:1.23, 1.47), PITC (ARR=1.54; 95%CI:1.41, 1.69), or an unknown point of entry (ARR=1.46; 95%CI:1.33, 1.60).
Of facility-level factors, patients enrolled in care at larger vs. smaller programs–measured by number of active patients–had a slightly higher risk of late enrollment (ARR=1.10; 95%CI:1.00, 1.21 for programs with ≥700 vs. <300 active patients). With respect to contextual-level factors, risk for late enrollment in care was significantly lower in facilities located in regions with higher HIV testing coverage. Relative to regions where ≤20% of the population tested and received HIV results in the past year, in regions where ≥40% of the population did so the ARR=0.76 (95%CI:0.58, 0.97). No other contextual-level variable retained significance in the final model.
In a sensitivity analysis using the WHO 2006 criteria for ART initiation to define late enrollment in care, non-pregnant vs. pregnant women did not have higher risk of late enrollment. The results of all other sensitivity analyses were consistent with the final model, although some estimates were slightly attenuated (data not shown).
Discussion
This study, using data from a large population of patients in diverse settings across four sub-Saharan countries, is one of the few analyses, and the largest to date, of late enrollment in HIV care in this region. It demonstrated that enrollment in HIV care with advanced HIV disease declined over time concurrent with efforts to scale up HIV treatment in the same countries. (In 2006, 23% of those needing ART in sub-Saharan Africa were receiving it[29], and this percentage increased to 57% by 2011[30]). In our study, between 2006 and 2011, the prevalence of late enrollment in HIV care decreased from 69.9% to 57.2%, and the median CD4+ count at enrollment in care increased from 242 cells/μL to 292 cells/μL. A decline in late enrollment was observed across all categories of patients and in all four countries, strengthening the conclusion that these results reflect actual changes and are not merely a consequence of shifts in the characteristics of the patient populations enrolling in these programs. The possibility remains that some portion of the decline in late enrollment into care that we observed is due to a relative increase in the number of healthier individuals who had outcome data in the latter years of this study. However, based on analyses examining characteristics of patients missing outcome data in 2006 and 2011 and on reviewing multiply imputed outcome data, we believe this attenuation, if present, is small.
Despite these encouraging findings, our analysis also showed that in 2011, nearly a decade after HIV service scale-up began in sub-Saharan Africa, more than half of all individuals enrolled in care when they already were eligible for ART by 2010 WHO guidelines. Without a significant change in the observed rate of decrease in late enrollment in care—3% per year—more than another decade will pass before fewer than one-quarter of patients will enroll late according to those guidelines. Given that the median time from HIV infection to reaching a CD4+ count <350 cells/μL is approximately four years[31], our study suggests that opportunities are missed to diagnose individuals earlier in disease, and underscores that greater efforts are needed to identify and link to care those with HIV infection prior to development of symptoms.
To further understand the factors associated with late enrollment in HIV care, we examined its correlates in 2011. More extensive HIV testing coverage in the areas surrounding the care facility was associated with a lower risk of late enrollment, even controlling for HIV prevalence, suggesting that expanded testing could lead to earlier enrollment in care. Community-based testing campaigns[7] and home-based counseling and testing[32] are important to pursue, although linkage to care after a positive HIV test may be a challenge with these approaches[32-34]. Research urgently needs to continue to explore these and other approaches to increase early diagnosis and prompt linkage to care.
Of the facility characteristics examined, only larger program size, in terms of number of active patients, was associated with a small increase in risk of late enrollment. This could reflect greater travel distance from outlying areas to a large central clinic or scheduling constraints at large facilities. Individual characteristics were strong predictors of late enrollment, even accounting for facility and contextual factors. As observed in two other studies in sub-Saharan Africa[23, 35], being male, unmarried, and of older age were characteristics significantly associated with late enrollment in HIV care in our study. Women typically have more contact with the health care system[36], thus likely facilitating access to earlier HIV testing and engagement in HIV care, and this is especially true for pregnant women. Older people may be less likely to believe they are at risk for HIV, potentially resulting in delayed testing[23]. Being married or living with a partner may facilitate earlier enrollment, as having a partner known or suspected to be HIV-positive is a common motivation for HIV testing[37] and partner support may encourage prompt enrollment in HIV care[38].
A patient's point of entry into HIV care also was an important correlate of late enrollment. Individuals who were diagnosed through VCT or in a PITC setting were significantly more likely to enroll late, whereas individuals who entered care through PMTCT sites were less likely to do so, even accounting for sex and pregnancy status, probably reflecting the fact that all PMTCT clients are routinely offered HIV testing. Therefore, although costly, a policy of screening rather than testing based on symptoms or the presence of risk factors might reduce the proportion of individuals who enroll late in care.
This study had a number of strengths including the large number of patients from diverse sites across sub-Saharan Africa, use of data from routine clinical care, six years of data from the same facilities, which permitted analysis of trends over time unconfounded by changes in the number of facilities included each year, and the inclusion of facility and contextual correlates of late enrollment in addition to individual characteristics. A limitation was that CD4+ cell count data at enrollment were missing for 40-45% of individuals and, other than excluding those who transferred from another site, we were unable to exclude those who may have “newly-enrolled” more than once. Furthermore, we had limited power to detect associations with contextual-level factors with only 15 sub-national regions.
In conclusion, we observed a substantial decrease in the proportion of patients enrolled in HIV care at advanced stages of HIV disease between 2006 and 2011 in 138 facilities across four sub-Saharan African countries. Despite this progress, the decline in late enrollment was slow, and 57% of patients in 2011 enrolled in care at thresholds below WHO 2010 ART treatment guidelines. Although the expansion of testing, care, and treatment has resulted in significant improvements in outcomes for HIV-positive individuals, substantial efforts are needed to ensure that further gains are achieved. Encouragingly, we found that several of the correlates of late enrollment in 2011 reflect modifiable factors. That people entering care via PMTCT and those living in regions with higher HIV testing coverage have lower risk of late enrollment underscores the importance of continuing to scale up testing in order to identify individuals earlier in disease. Innovative approaches to increase testing uptake prior to symptom development may most rapidly reduce late enrollment in care.
Supplementary Material
Acknowledgments
Drs. Hoffman, Wu, Nash, and Elul conceptualized the study; they, along with Drs. Lahuerta and El-Sadr, Ms. Gorrell Kulkarni, and Drs. Mugisha, Hawken, Nuwagaba-Biribonwoha, Remien, Chuva implemented the study; Dr. Wu conducted the statistical analysis; Drs. Hoffman and Elul wrote the manuscript. All authors critically reviewed and edited the manuscript.
We would like to express our gratitude to all patients at the health facilities included in this analysis. We want to acknowledge the efforts of the ICAP staff in-country and health facility staff for their commitment to confronting the HIV epidemic. We also want to thank the members of the Optimal Model Steering Committees in Kenya, Mozambique, Rwanda and Tanzania.
This work was supported by the President's Emergency Plan for AIDS Relief and by the US Centers for Disease Control and Prevention through the Optimal Models Collaboration (Grant number: 5U2GPS001537-03), and by a research grant from the National Institute of Mental Health (Grant Number R01MH089831). All the clinics included in this analysis received support from ICAP at Columbia University through funding from the President's Emergency Plan for AIDS Relief. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: The authors declare no competing interests.
References
- 1.Lanoy E, Mary-Krause M, Tattevin P, Perbost I, Poizot-Martin I, Dupont C, et al. Frequency, determinants and consequences of delayed access to care for HIV infection in France. Antiviral Therapy. 2007;12:89–96. doi: 10.1177/135965350701200111. [DOI] [PubMed] [Google Scholar]
- 2.Smit C, Hallett TB, Lange J, Garnett G, de Wolf F. Late entry to HIV care limits the impact of anti-retroviral therapy in The Netherlands. PLoS One. 2008;3:e1949. doi: 10.1371/journal.pone.0001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metsch LR, Pereyra M, Messinger S, del Rio C, Strathdee SA, Anderson-Mahoney P, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clinical Infectious Diseases. 2008;47:577–584. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M, Chen Y, McCauley M, Gamble T, Hosseinipour M, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clinical Infectious Diseases. 2010;50:1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghi V, Girardi E, Bellelli S, Angeletti C, Mussini C, Porter K, et al. Late presenters in an HIV surveillance system in Italy during the period 1992-2006. J Acquir Immune Defic Syndr. 2008;49:282–286. doi: 10.1097/QAI.0b013e318186eabc. [DOI] [PubMed] [Google Scholar]
- 10.Begovac J, Gedike K, Lukas D, Lepej SZ. Late presentation to care for HIV infection in Croatia and the effect of interventions during the Croatian Global Fund Project. AIDS Behav. 2008;12:S48–53. doi: 10.1007/s10461-008-9398-9. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans: a problem of access or screening? Med Care. 2007;45:1105–1109. doi: 10.1097/MLR.0b013e3181271476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. AIDS. 2006;20:775–778. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- 13.Girardi E, Aloisi MS, Arici C, Pezzotti P, Serraino D, Balzano R, et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. Journal of Acquired Immune Deficiency Syndromes. 2004;36:951–959. doi: 10.1097/00126334-200408010-00009. [DOI] [PubMed] [Google Scholar]
- 14.Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006;99:472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndiaye B, Salleron J, Vincent A, Bataille P, Bonnevie F, Choisy P, et al. Factors associated with presentation to care with advanced HIV disease in Brussels and Northern France: 1997-2007. BMC Infectious Diseases. 2011;11:11. doi: 10.1186/1471-2334-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohl M, Tate J, Duggal M, Skanderson M, Scotch M, Kaboli P, et al. Rural residence is associated with delayed care entry and increased mortality among veterans with human immunodeficiency virus infection. Medical Care. 2010;48:1064–1070. doi: 10.1097/MLR.0b013e3181ef60c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabin CA, Smith CJ, Gumley H, Murphy G, Lampe FC, Phillips AN, et al. Late presenters in the era of highly active antiretroviral therapy: uptake of and responses to antiretroviral therapy. AIDS. 2004;18:2145–2151. doi: 10.1097/00002030-200411050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Zoufaly A, an der Heiden M, Marcus U, Hoffmann C, Stellbrink H, Voss L, et al. Late presentation for HIV diagnosis and care in Germany. HIV Medicine. 2012;13:172–181. doi: 10.1111/j.1468-1293.2011.00958.x. [DOI] [PubMed] [Google Scholar]
- 19.Egger M. Immunodeficiency at the start of combination antiretroviral therapy in low, middle and high-income countries. Journal of Acquired Immune Deficiency Syndromes. 2013 doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahuerta MLJ, Nuwagaba-Biribonwoha H, Okamura M, Alvim MF, Fernandes R, Assan M, Hoos D, Elul B, El-Sadr WM, Nash D. Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PLoS One. 2012;7:e37125. doi: 10.31371/journal.pone.0037125. 371. PLoS ONE 2012,7:e37125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24:359–383. doi: 10.1353/hpu.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni SG, Remien RH, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006-2011: Findings from four sub-Saharan African countries. Clin Infect Dis. 2014;58:432–441. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52:280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash D, Wu Y, Elul B, Hoos D, El-Sadr W, for the International Center for AIDS Care and Treatment Programs Program-level and contextual-level determinants of low-median CD4R cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25:1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drain PK, Losina E, Parker G, Giddy J, Ross D, Katz JN, et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS One. 2013;8:e55305. doi: 10.1371/journal.pone.0055305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Trop Med Int Health. 2008;13:904–913. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 28.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach (2006 revision) Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 29.UNAIDS. UNAIDS AIDS Epidemic Update. Geneva, Switzerland: UNAIDS; 2006. [Google Scholar]
- 30.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2012. [Google Scholar]
- 31.Kiwanuka N, Robb M, Laeyendecker O, Kigozi G, Wabwire-Mangen F, Makumbi FE, et al. HIV-1 viral subtype differences in the rate of CD4+ T-cell decline among HIV seroincident antiretroviral naive persons in Rakai district, Uganda. Journal of Acquired Immune Deficiency Syndromes. 2010;54:180–184. doi: 10.1097/QAI.0b013e3181c98fc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. Journal of Acquired Immune Deficiency Syndromes. 2013 doi: 10.1097/QAI.0b013e31829b567d. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcellin F, Abe C, Loubiere S, Boyer S, Blanche J, Koulla-Shiro S, et al. Delayed first consultation after diagnosis of HIV infection in Cameroon. AIDS. 2009;23:1015–1019. doi: 10.1097/QAD.0b013e32832a5996. [DOI] [PubMed] [Google Scholar]
- 34.Hatcher AM, Turan JM, Leslie HH, Kanya LW, Kwena Z, Johnson MO, et al. Predictors of linkage to care following community-based HIV counseling and testing in rural Kenya. AIDS and Behavior. 2012;16:1295–1307. doi: 10.1007/s10461-011-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS One. 2010;5:e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–623. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 37.Musheke M, Ntalasha H, Gari S, Mckenzie O, Bond V, Martin-Hilber A, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC Public Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. http://www.biomedcentral.com/1471-2458/1413/1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawad S, Hoffman S, De Menezes ID, Mngomezulu N, Blanchard K, Stiefvater E, et al. XIX International AIDS Conference (AIDS 2012) Washington, D.C.: Jul 22-27, 2012. Linkage to care: Barriers and facilitators from the perspectives of counselors and clients. Poster presentation, A-452-0310-17640. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.