Abstract
Background: Type 2 diabetes mellitus (T2DM) is characterized as a disease continuum that is marked by metabolic changes that are present for several years, sometimes well before frank diagnosis of T2DM. Genetic predisposition, ethnicity, geography, alterations in BMI, and lipid profile are considered important markers for the pathogenesis of T2DM through mechanisms that remain unresolved and controversial. The aim of this study was to investigate the relationship between triglycerides (TGs) and β-cell function, insulin resistance (IR), and insulin sensitivity (IS) in obese first-degree relatives of patients with T2DM (FDR-T2DM) among subjects from central Mexico with normal glucose tolerance (NGT).
Methods: We studied 372 FDR-T2DM subjects (ages,18–65) and determined body mass index (BMI), fasting plasma glucose (FPG), oral glucose tolerance test (OGTT), insulin, and TGs levels. Subjects were categorized based on glycemic control [NGT, prediabetes (PT2DM), or T2DM]. NGT subjects were further categorized by BMI [normal weight (Ob−) or obese (Ob+)] and TGs levels (TG−, <150 mg/dL, or TG+, ≥150 mg/dL). β-cell function, IR, and IS were determined by the homeostasis model assessment of β-cell function (HOMA2-β), homeostasis model assessment of insulin resistance (HOMA2-IR), and Quantitative Insulin Sensitivity Check Index (QUICKI) indices, respectively.
Results: The obese subjects with elevated TGs levels had 21%–60% increased β-cell function when compared to all groups (P<0.05). In addition, this group had insulin levels, IS, and IR similar to PT2DM. Furthermore, only in obese subjects did TGs correlate with β-cell function (ρ=0.502, P<0.001).
Conclusion: We characterized FDR-T2DM subjects from central Mexico with NGT and revealed a class of obese subjects with elevated TGs and β-cell function, which may precede PT2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is a severe worldwide health concern. It is predicted that over 366 million people will suffer from this disease worldwide by 2030.1 Moreover, by 2035, about 10% of the total population in Mexico and the Central American region will develop diabetes.2,3 Of these cases, it is predicted that 24%, and in certain regions as high as 50%, will go undiagnosed until a severe symptomatic state is presented.4 Consequently, there is a critical need to better understand characteristics associated with the developmental stages of the disease, particularly amongst groups that are disproportionately affected by T2DM, such as Hispanics in the United States and people of Mexico and Central America.5
Diabetes is a multifactorial disease characterized by metabolic alterations with a prevalence that is affected by ethnicity and geography.6,7 T2DM patients exhibit β-cell dysfunction, decreased sensitivity to insulin, and hyperglycemia8,9 that are associated with deficiencies in insulin secretion or resistance to insulin action or both.10 Various complex genetic determinants are considered to contribute to the development of T2DM.11 There is evidence that first-degree relatives of patients with T2DM (FDR-T2DM) show increased susceptibility to developing insulin resistance (IR) and defects in insulin sensitivity (IS).12 FDR-T2DM subjects are defined as having a parent, sibling, or child with T2DM. Indeed having one or two parents leads to a 1.6- or 3.2-fold increased risk of developing frank T2DM, respectively,13 thus further supporting the proposal of a strong genetic predisposition for developing T2DM.14–16
Dyslipidemia, presence of metabolic syndrome, and elevated triglycerides (TGs) and total cholesterol (TC) are known to affect β-cell function in diabetes.17–19 However, the mechanisms for these effects are not entirely clear. Some reports suggest that elevated TGs levels alone are associated with developing diabetes and cardiovascular disease,20 whereas others suggest that elevated TGs levels with high low-density lipoprotein (LDL) and low high-density lipoprotein (HDL) have a synergistic effect.21,22 In addition, subjects with a body mass index (BMI) greater than 25 kg/m2 have an increased risk of developing T2DM (relative risk=1.5–5.1).23,24 TGs levels and abdominal fat distribution are greater in Hispanics than Afro-Americans and Caucasians.25 Furthermore, Mexicans Americans have greater prevalence of developing T2DM.5 Thus, it is widely accepted that there is a strong link between BMI, TGs, TC, and diabetes in FDR-T2DM26 that may be affected by ethnicity.5 However, and to the best of our knowledge, the relationship between TGs levels and measures of insulin function among a uniform group of subjects with FDR-T2DM is unknown.
We characterized subjects with FDR-T2DM from the central Mexico. We found that in our cohort, obese subjects with augmented TGs levels have elevated β-cell function when compared to normal BMI subjects, obese subjects with normal TGs levels, prediabetes (PT2DM), and T2DM subjects. In addition, these subjects had similar insulin, IS, and IR values, as observed in PT2DM.
Material and Methods
Subjects and settings
We designed a cross-sectional study, which included 372 FDR-T2DM, males (n=160) and nonpregnant females (n=212), whose ages ranged from 18 to 65 years old. All subjects were from Puebla, Mexico, and met the following criteria: Otherwise healthy and FDR-T2DM. Subjects were excluded from the study, if they had an acute or chronic illness. This human study protocol was approved by the Scientific Research Committee of the Mexican Social Security Institute. All participants provided informed consent to participate in this study that was conducted in accordance with the Declaration of Helsinki.
Clinical characterization
Subjects were clinically evaluated according to a standardized protocol including personal and family clinical history. With the subjects in fasting conditions, wearing light clothing and without shoes, their height (meters) and weight (kg) were measured using the body composition analyzer (TBF-215, Tanita, Tokyo, Japan). BMI was calculated as weight/height2 (kg/m2).27 A BMI between 18.5 and 24.9 kg/m2 was normal, between 25 and 29.9 kg/m2 was overweight, and between 30 and 39.9 kg/m2 was obese. BMIs <18.4 kg/m2 and >40 kg/m2 were excluded. Blood pressure measurements [diastolic blood pressure (DBP) and systolic blood pressure (SBP)] were taken on the left arm of the seated subject with a mercury column sphygmomanometer with an appropriately sized cuff. The data are the average of two physician-obtained measurements.
Biochemical assays
Whole blood samples were collected from the antecubital vein following a 10- to 12-hr overnight fast in a Vacutainer® Plus plastic sterile tube with a BD Hemogard™ closure containing sodium fluoride and potassium oxalate (catalog no. 367925, BD Mexico City, Mexico). The samples were kept at room temperature for 2 hr to allow clotting. The serum fraction was recovered and frozen at −20°C until used. Samples were used for the following end points: Fasting plasma glucose (FPG), insulin, glycated hemoglobin (HbA1c), TC, HDL, LDL, and TGs. An additional blood sample was obtained 2 hr after oral glucose administration (75 grams) for the oral glucose tolerance test (OGTT) to determine glucose tolerance. FPG and OGTT were determined, in duplicate, using the enzymatic method/spectrophotometric glucose oxidation (Beckman Instruments, Brea, CA). Insulin levels were determined by automated immunoassay (Access, Beckman). The HbA1c levels were determined by the turbidimetric inhibition immunoassay. To determine TGs, TC, HDL, and LDL, serum samples were sent to the Central Laboratory of the Multidisciplinary Research Group. Normal values for TGs (<150 mg/dL), TC (≤200 mg/dL), HDL (≥60 mg/dL), and LDL (<100 mg/dL) were used according to the Mexican Health Ministry.28,29
Calculation of HOMA2-β, HOMA2-IR, and QUICKI
β-Cell function and IR were assessed by the homeostatic model assessment (HOMA) online calculator downloaded from www.dtu.ox.ac.uk/homacalculator/index.php on (April, 2014). The HOMA2-β is used to evaluate the β-cell function and is expressed as a percentage of normal (100%). The HOMA2-IR is used to evaluate the index of IR. The subjects were classified as IR with HOMA2-IR scores >1.8 and metabolic syndrome with HOMA2-IR scores between 1.4 and 1.8.30 IS was calculated from the Quantitative Insulin Sensitivity Check Index (QUICKI) by the following formula: QUICKI=1/(log[Insulin]+log[Glucose]). QUICKI scores <0.357 are representative of decreased IS.31
Allocation of subjects into groups and subgroups
Using the American Diabetes Association (ADA) recommendation, subjects were classified as either T2DM (FPG ≥126 mg/dL, OGTT ≥200 mg/dL, or HbA1c ≥6.5%), PT2DM (FPG, 100–125 mg/dL, OGTT 140–199 mg/dL, or HbA1c 5.7%–6.4%), or normal glucose tolerance (NGT, FPG <100 mg/dL, OGTT <140 mg/dL, or HbA1c <5.7%). The NGT group was furthered divided by BMI into normal weight (Ob−, BMI <25.0 kg/m2) and obese (Ob+, BMI ≥25.0 kg/m2). Last, the Ob− and Ob+groups were further subdivided into subgroups by TGs levels: <150 mg/dL (Ob−TG− and Ob+TG−) and ≥150 mg/dL (Ob−TG+ and Ob+TG+).
Statistical analyses
Statistical analyses were performed using Statistical Package for the Social Sciences program for Windows NT, version 11 (SPSS, Chicago, IL). Differences between groups were evaluated by one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. The results were expressed as mean±standard error (SE). Correlation analysis was done by calculating the Spearman correlation coefficient (ρ).32 P values <0.05 were considered statistically significant.
Results
Distribution of FDR-T2DM subjects by risk factors
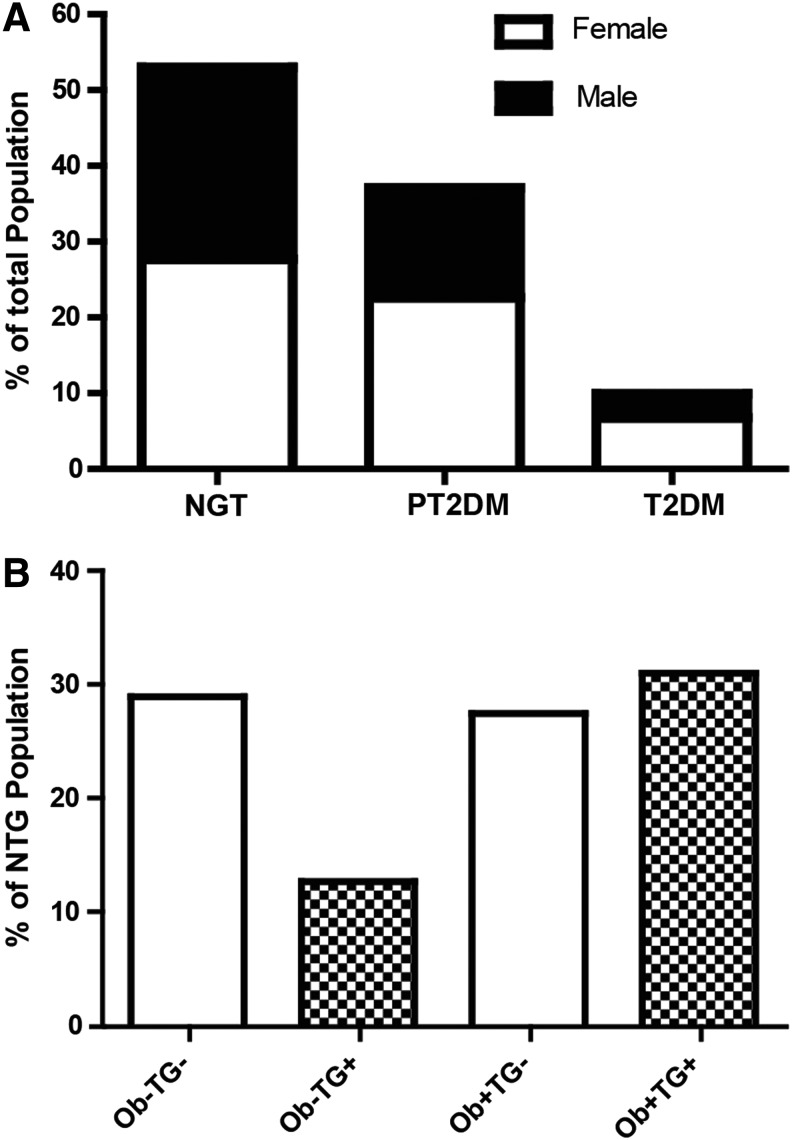
We determined the percentage of NGT, PT2DM, and T2DM for FDR-T2DM in our cohort from Central Mexico. A total of 47% were dysglycemic (PT2DM and T2DM), and 53% had NGT (Fig. 1A); however, 58% of the NGT group was obese. Because TGs levels (>150 mg/dL) have been shown to affect insulin and glucose homeostasis,33 we assessed TGs levels in our cohort. From the original sample, 6.7% of subjects had increased TGs levels with normal BMI, 14.5% of subjects were obese with normal TGs levels, and 16.4% of subjects were obese with increased TGs levels (data not shown). One can estimate from our cohort that 15.3% (or 28.9% of the NGT group) had only a genetic predisposition, whereas 37.6% (71.1% of the NGT group) have additional factors associated with T2DM, as determined by TG, BMI, FPG, OGTT, and HbA1c (Fig. 1B). The clinical and metabolic characteristics of our cohort are summarized in Table 1.
FIG. 1.
Distribution of first-degree relatives of type 2 diabetes mellitus (T2DM). (A) Subjects were categorized as either normoglycemia (NGT) or hyperglycemia [prediabetes (PT2DM) and T2DM] according to the American Diabetes Association (ADA) specifications.8 (B) Using body mass index (BMI; ≥25 kg/m2) and triglycerides (TGs; ≥150 mg/dL), NGT subjects were recategorized into normal weight (Ob−)TG−, Ob−TG+, obese (Ob+)TG−, and Ob+TG+.
Table 1.
Clinical and Metabolic Characteristics of First-Degree Relatives of Type 2 Diabetes Mellitus Selected by Body Mass Index and Triglycerides
| Group | Nonobese | Obese | Hyperglycemia | |||
|---|---|---|---|---|---|---|
| (TGs >150 mg/dL) | − | + | − | + | N/A | N/A |
| Subgroup | Ob−TG− | Ob−TG+ | Ob+TG− | Ob+TG+ | PT2DM | T2DM |
| Sample (male/female) | 56 (18/38) | 26 (15/11) | 54 (27/27) | 61 (34/27) | 138 (84/54) | 37 (25/12) |
| Age | 33.6±1.5 | 40.3±2.6 | 37.8±1.5 | 38.9±1.4 | 41.3±1.0* | 45.0±1.9* |
| Height (meters) | 1.60±0.01 | 1.64±0.02 | 1.61±0.01 | 1.64±0.01 | 1.60±0.01 | 1.60±0.01 |
| Weight (kg) | 56.9±0.9 | 62.2±1.6 | 73.5±1.7*† | 80.2±1.7*† | 71.5±1.0*†§** | 78.8±3.1*† |
| BMI (kg/m2) | 22.0±0.2 | 23.1±0.3 | 28.0±0.4*† | 29.7±0.5*† | 27.8±0.3*†§** | 30.6±1.0*†‡ |
| SBP (mmHg) | 108.7±1.3 | 108.5±2.1 | 113.5±1.6 | 116.3±1.5* | 112.6±1.2** | 120.8±2.7*† |
| DBP (mmHg) | 71.5±1.3 | 69.8±1.8 | 72.3±1.2 | 75.1±1.2 | 73.4±0.8** | 79.7±1.5*†‡ |
| Lipid profile | ||||||
| TC (mg/dL) | 176.7±5.2 | 205.9±9.6 | 172.5±5.3† | 190.2±5.7 | 197.4±4.1† | 200.6±6.6‡ |
| HDL (mg/dL) | 46.3±2.4 | 38.5±3.8 | 38.4±2.2 | 29.9±2.1* | 39.5±1.3§ | 39.0±1.7 |
| LDL (mg/dL) | 100.6±4.1 | 119.5±9.6 | 105.8±4.3 | 108.2±4.5 | 120.5±3.4* | 127.4±5.8* |
| TGs (mg/dL) | 92.6±3.9 | 209.6±8.1* | 108.1±6.5† | 289.9±22.7*†‡ | 199.8±10.3*‡§ | 205.6±17.9*‡ |
| Metabolic parameters | ||||||
| HbA1c (%) | 4.9±0.1 | 5.0±0.1 | 5.0±0.1 | 5.1±0.1 | 5.5±0.1*†‡§** | 6.9±0.3*†‡§ |
| FPG (mg/dL) | 87.6±1.1 | 90.4±1.5 | 91.2±0.8 | 88.4±1.1 | 98.5±1.1* | 132.9±9.5*†‡ |
| OGTT (mg/dL) | 92.1±2.7 | 99.6±3.8 | 98.9±2.8 | 103.2±2.7 | 126.2±2.7*§** | 232.5±19.3*†‡§¶ |
Values are mean±standard error. Significance was determined by one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test.
P<0.05 vs. Ob−TG−.
P<0.05 vs. Ob−TG+.
P<0.05 vs. Ob+TG−.
P<0.05 vs. Ob+TG+.
P<0.05 vs. PT2DM.
TGs, triglycerides; Ob−, normal weight; Ob+, obese; PT2DM, prediabetes; T2DM, type 2 diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycated hemoglobin; FPG, fasting plasma glucose; OGTT, oral glucose tolerance test.
Elevated TGs levels and obesity are associated with augmented insulin and β-cell function
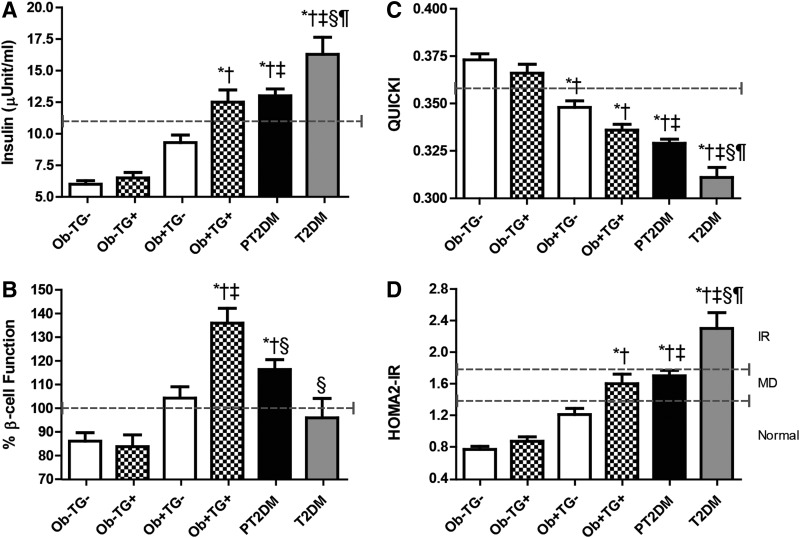
A key hallmark of the disease is deficiencies in insulin secretion or action.10 We determined the insulin levels for each subgroup of our cohort. The insulin levels were not significantly affected by increases in TGs (Ob−TG− vs. Ob−TG+ and Ob+TG− vs. Ob+TG+); however, insulin levels did increase with obesity (Fig. 2A). Even though obese subjects with normal TGs levels had elevated insulin levels compared to their nonobese counterparts, they were significantly lower than PT2DM and T2DM (P<0.05). Interestingly, only the obese subjects with elevated TGs had similar insulin levels to the PT2DM but not the T2DM group (P<0.05). These data suggest that increases in TGs are associated with insulin levels in obese subjects. To test this assumption, the TGs and insulin levels were compared using the Spearman rank correlation. When the total sample was analyzed, there was a moderate correlation (ρ=0.407, P<0.001; Table 2). However, when the two factors were compared by group, there was a very weak correlation with nonobese subjects (ρ=0.217, P<0.05) and PT2DM (ρ=0.210, P<0.05; Table 2). The obese subjects had moderate association (ρ=0.441, P<0.001).
FIG. 2.
Insulin levels and metabolic parameters in first-degree relatives of type 2 diabetes mellitus (T2DM). (A) Insulin levels (normal, <11 μU/mL). (B) β-Cell function determined by homeostasis model assessment of β-cell function (HOMA2-β) (normal, 80%–100%). (C) Insulin sensitivity (IS) determined by Quantitative Insulin Sensitivity Check Index (QUICKI) (normal, >0.357). (D) Insulin resistance (IR) determined by HOMA2-IR [normal, <1.4; degree of metabolic disorder (MD), 1.4–1.8; and IR, >1.8] for each subgroup. The dashed line corresponds to the cutoff for each parameter. Bar graphs represent the mean and standard error. (*) P<0.05 vs. obese (Ob−)TG−; (†) P<0.05 vs. Ob−TG+; (‡) P<0.05 vs. normal weight (Ob+)TG−; (§) P<0.05 vs. Ob+TG+, and (¶) P<0.05 vs. PT2DM.
Table 2.
Spearman Correlation of First-Degree Relatives of Type 2 Diabetes Mellitus
| Group | Total | Nonobese | Obese | PT2DM |
|---|---|---|---|---|
| TGs vs. | ||||
| HOMA2-β | ρ=0.271, P<0.001 | ρ=0.035, NS | ρ=0.502, P<0.001 | ρ=0.148, NS |
| HOMA2-IR | ρ=0.410, P<0.001 | ρ=0.236, P<0.05 | ρ=0.426, P<0.001 | ρ=0.212, P<0.05 |
| QUICKI | ρ=−0.400, P<0.001 | ρ=−0.254, P<0.05 | ρ=−0.387, P<0.001 | ρ=−0.194, P<0.05 |
| Insulin | ρ=0.407, P<0.001 | ρ=0.217, P<0.05 | ρ=0.441, P<0.001 | ρ=0.210, P<0.05 |
PT2DM, prediabetes; TGs, triglycerides; HOMA2-β, homeostasis model assessment for β-cell function; NS, not significant; HOMA2-IR, homeostasis model assessment for insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index.
Higher insulin levels can be a result of β-cell dysfunction; therefore, β-cell function was determined using the HOMA2-β calculator. In the nonobese subgroups, subjects with elevated TGs levels had similar β-cell function as their normal TGs levels counterparts (Ob−TG− vs. Ob−TG+; Fig. 2B). However, obese subjects with normal TGs levels had augmented β-cell function, which was within normal limits. The presence of both risk factors (obesity and elevated TGs) were associated with increased β-cell function, which was higher than any group studied (Ob+TG+ vs. all subgroups; P<0.05). Next, we determined if increases in TGs and β-cell function were associated. There was a moderate association only seen with obese subjects (ρ=0.502, P<0.001; Table 2). There was no correlation between nonobese subjects and PT2DM.
TGs levels are an indication of insulin action in subjects with FDR-T2DM
Increased insulin and β-cell function are associated with IR and IS; therefore, these parameters were examined in our group. Nonobese subjects had normal sensitivity (Fig. 2C), whereas, elevated TGs levels were associated with decreased IS but remained above the cutoff and were not significantly different. Obese subjects had decreased IS below the threshold; however, only obese subjects with elevated TGs had IS values that were similar to PT2DM. Next, we determined the relationship between TGs and IS. When examining the correlation between groups, the nonobese subjects and PT2DM had a very weak inverse correlation (ρ=−0.254, P<0.05 and ρ=−0.194, P<0.05, respectively), whereas obese subjects had a weak inverse correlation (ρ=−0.387, P<0.001). Nonobese subjects had no resistance to insulin (Fig. 2D), and increases in BMI alone did not lead to an IR state. However, obese subjects with elevated TGs levels had a HOMA2-IR score indicating some degree of IR. Interestingly, these subjects had similar HOMA2-IR scores as observed in PT2DM, but only the T2DM subjects were IR. Obese subjects showed a moderate correlation between TGs and IR (ρ=0.426, P<0.001). In contrast, the nonobese subjects and PT2DM had a weak correlation (ρ=0.236 and ρ=0.212, respectively, P<0.05).
Discussion
In this study, we determined insulin levels, β-cell function, IR, and IS in FDR-T2DM from Central Mexico that were categorized by BMI and TGs. In our cohort, we identified a group of individuals with normal glucose function that were obese and had elevated TGs levels. These subjects were identical to PT2DM with respect to insulin levels, IS, and IR; however they had increased β-cell function. Of interest, only in nondiabetic obese FDR-T2DM did serum TGs levels positively correlate with β-cell function. Thus, our study in subjects from Central Mexico supports and extends the work of others among a uniform group of FDR-T2DM individuals.
Effect of TGs on β-cell function remains inconclusive. Bardini et al. showed elevated TGs levels were associated with β-cell overstimulation in nondiabetic obese subjects from Italy.34 However, their study included subjects with and without a family history of T2DM and were grouped by a hypertriglyceridemic waist phenotype. We chose to focus our study on subjects with FDR-T2DM that were characterized by BMI and TGs levels because of the elevated risk of developing T2DM associated with their genetic predisposition and obesity. In subjects with PT2DM and T2DM, others have shown that TGs levels were negatively associated with β-cell function.33,35,36 This is in disagreement with our data. T2DM patients exhibit decreased β-cell mass and function of up to 50%, which may be present many years before clinical diagnosis.37,38 We used subjects that were not clinically diagnosed at the time of the study as having either PT2DM or T2DM. In addition, previous reports used patients that were diagnosed with T2DM or PT2DM, and as such, these subjects could have already increased β-cell damage as compared to our cohort.
TGs are present in many different tissues and have varying physiological effects.39 We used serum TGs levels to determine their relationship with β-cell function. In humans and animal models, increases in serum TGs levels were associated with increased β-cell function.40–42 Using pancreatic TGs levels, others have reported an absence of any correlation between TGs and β-cell function in NGT and PT2DM35,36,43 and no correlation35,43 or a negative correlation36 for patients with T2DM. However, in obese FDR-T2DM with high serum TGs levels, pancreatic TGs levels and their effect on β-cell function have, to the best of our knowledge, not yet been reported. Furthermore, there are reports showing that pancreatic TGs levels are affected by ethnicity.17 Consequently, there remains a need to determine the pancreatic TGs levels in FDR-T2DM among our cohort. Nonetheless, taken together, our results and those of others would suggest that serum TGs are a good indicator of β-cell function in the newly characterized obese FDR-T2DM subjects with NGT.
It has been reported that PT2DM and T2DM exhibit higher insulin levels with impaired glucose tolerance than their NGT counterparts, independent of their obesity classification.10 However, insulin levels among obese subjects with elevated TGs levels and PT2DM have not been described among the Hispanic population. In our cohort, obese FDR-T2DM with elevated TGs levels showed increased insulin levels similar to PT2DM but did not have impaired glucose tolerance. Interestingly, this group was characterized as having pronounced increases in β-cell function that were higher than those observed in PT2DM, T2DM, or their normal TGs level counterparts. In other reports, an acute exposure of a TGs supplement led to increased β-cell function (acute phase) whereas removal of the TGs supplement restored β-cell function to baseline conditions.42,44,45 However, prolonged TGs exposure led to a chronic phase, which included loss of β-cell function42,44,45 and impaired glucose tolerance.42,45 Thus, we posit that in our cohort, obese FDR-T2DM with elevated TGs levels may represent subjects in an acute phase. This group also had decreased IS with increasing degrees of IR development. In contrast, only the obese FDR-T2DM with normal TGs levels had decreased IS with normal resistance to insulin.
Because increases in TGs levels are associated with development of IR,46 various groups have proposed the notion that there is a transition between the two obese groups and would suggest that increased TGs levels may lead from a non-IR state to increased degree of IR. Moreover, prolong elevated TGs levels have been associated with β-cell loss of function and impaired glucose tolerance.42,44,45 These results suggest that in obese FDR-T2DM, TGs levels may be a factor in the development of impaired glucose tolerance. Thus, it is possible that our obese FDR-T2DM subjects with elevated TGs levels may be a group of subjects that precede the development of PT2DM in the disease continuum.
There are few limitations to this study. First, it should be noted that our sample only contained FDR-T2DM subjects, which have already been shown to have a genetic predisposition to developing T2DM. Further studies are required to determine if obese subjects with elevated TGs levels and without a FDR-T2DM are similar to PT2DM but with elevated β-cell function. Second, this is a cross-sectional study and cannot determine causal relationship between obesity and TGs levels with β-cell function. Last, this study only focused on subjects from Central Mexico. Due to the highly diverse genetic background of the Mexican population,47 confirmation of elevated β-cell function in obese subjects in other genetically distinct regions of Mexico is required.
Conclusion
In conclusion, we characterized a cohort of FDR-T2DM subjects from Central Mexico and identified a group of NGT that are obese with elevated TGs levels. This group is similar to PT2DM, in respect to insulin, IS, and IR, except they have elevated β-cell function. Understanding the pathophysiology of this specific group will aid in the development of targeted therapies for FDR-T2DM to deter the development of PT2DM.
Acknowledgment
We would like to express our gratitude to the participants of this study, Maria Carmen Sánchez-Guillén MD PhD, who contributed to the development of this project, Jesus Chacon Sanchez MD MS, who guided the statistical analysis, Alfredo Avendaño-Arenaza, and Ricardo Villegas-Tovar, from BUAP Libraries-Department.
This study was supported in part by grants from the Programa de Mejoramiento del Profesorado of the “Secretaria de Educacion Publica” and the “Vicerrrectoria de Investigación” of the Benemérita Universidad Autonoma de Puebla, Mexico (to E.T.R., M.E.G.M., and R.P.F.), the National Heart Lung and Blood Institute of the National Institutes of Health (R01 HL-096518), and a Harvard University Faculty Grant Award supported by the Banco Santander Fund of the David Rockefeller Center for Latin American Studies at Harvard University (to J.R.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Aschner P, Aguilar-Salinas C, Aguirre L, et al. Diabetes in South and Central America: An update for 2013 for the IDF Diabetes Atlas. Diabetes Res Clin Practice 2014;103:238–243 [DOI] [PubMed] [Google Scholar]
- 3.Yisahak SF, Beagley J, Hambleton IR, et al. on behalf of the IDFDA. Diabetes in North America and The Caribbean: 2013 update for the IDF Diabetes Atlas. Diabetes Res Clin Practice 2014;103:223–230 [DOI] [PubMed] [Google Scholar]
- 4.DCCT. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 5.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 6.Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab Syndr Relat Disord 2013;11:71–80 [DOI] [PubMed] [Google Scholar]
- 7.Liese AD, Lawson A, Song HR, et al. Evaluating geographic variation in type 1 and type 2 diabetes mellitus incidence in youth in four US regions. Health Place 2010;16:547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerich JE. Contributions of insulin-resistance and insulin-secretory defects to the pathogenesis of type 2 diabetes mellitus. Mayo Clin Proc 2003;78:447–456 [DOI] [PubMed] [Google Scholar]
- 9.Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med 2000;108(Suppl 6a):2S–8S [DOI] [PubMed] [Google Scholar]
- 10.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulsen P, Kyvik KO, Vaag A, et al. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 1999;42:139–145 [DOI] [PubMed] [Google Scholar]
- 12.Baez-Duarte BG, Sanchez-Guillen Mdel C, Perez-Fuentes R, et al. Beta-cell function is associated with metabolic syndrome in Mexican subjects. Diabetes Metab Syndr Obes 2010;3:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olaiz G, Rojas RSB. Encuesta Nacional de Salud 2000. La Salud de los adultos, 2003
- 14.Waki H, Yamauchi T, Kadowaki T. The epigenome and its role in diabetes. Curr Diabetes Rep 2012;12:673–685 [DOI] [PubMed] [Google Scholar]
- 15.Pimenta W, Korytkowski M, Mitrakou A, et al. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 1995;273:1855–1861 [PubMed] [Google Scholar]
- 16.Valdez R, Greenlund KJ, Khoury MJ, et al. Is family history a useful tool for detecting children at risk for diabetes and cardiovascular diseases? A public health perspective. Pediatrics 2007;120(Suppl 2):S78–S86 [DOI] [PubMed] [Google Scholar]
- 17.Lingvay IMR, Szczepaniak EW, Szczepaniak LS. Ethnic diversity in beta-cell function susceptibility to pancreatic triglyceride levels: Pilot investigation. J Diabetes Metab 2014;5:348 [Google Scholar]
- 18.Gotto AM., Jr.Triglyceride as a risk factor for coronary artery disease. Am J Cardiol 1998;82:22Q–25Q [DOI] [PubMed] [Google Scholar]
- 19.Taskinen MR. Triglyceride is the major atherogenic lipid in NIDDM. Diabetes/Metab Rev 1997;13:93–98 [DOI] [PubMed] [Google Scholar]
- 20.Chen AH, Tseng CH. The role of triglyceride in cardiovascular disease in Asian patients with type 2 diabetes—a systematic review. Rev Diabet Stud 10:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauss R.M., Triglycerides and atherogenic lipoproteins: Rationale for lipid management. Am J Med 1998;105:58S–62S [DOI] [PubMed] [Google Scholar]
- 22.Brunzell JD, Ayyobi AF. Dyslipidemia in the metabolic syndrome and type 2 diabetes mellitus. Am J Med 2003;115(Suppl 8A):24S–28S [DOI] [PubMed] [Google Scholar]
- 23.Gray B, Muhlhausler BS, Davies PS, et al. Liver enzymes but not free fatty acid levels predict markers of insulin sensitivity in overweight and obese, nondiabetic adults. Nutr Res 2013;33:781–788 [DOI] [PubMed] [Google Scholar]
- 24.Ganz ML, Wintfeld N, Li Q, et al. The association of body mass index with the risk of type 2 diabetes: A case-control study nested in an electronic health records system in the United States. Diabetol Metabolic Syndr 2014;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczepaniak LS, Victor RG, Mathur R, et al. Pancreatic steatosis and its relationship to beta-cell dysfunction in humans: Racial and ethnic variations. Diabetes Care 2012;35:2377–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardini G, Dicembrini I, Pala L, et al. Hypertriglyceridaemic waist phenotype and beta-cell function in subjects with normal and impaired glucose tolerance. Diabet Med 2011;28:1229–1233 [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health. Norma Oficial Mexicana NOM-015-SSA2-1994, “Tratamiento y control de la diabetes mellitus en la atención primaria.” Diario Oficial de la Federacion, 2010
- 28.Ministry of Health. Norma Oficial Mexicana NOM-037-SSA2-2002, “Para la prevención, tratamiento y control de las dislipidemias.” 2002
- 29.Haffner SM; American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care 2003;26(Suppl 1):S83–S86 [DOI] [PubMed] [Google Scholar]
- 30.Geloneze B, Vasques AC, Stabe CF, et al. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arquivos brasileiros de endocrinologia e metabologia 2009;53:281–287 [DOI] [PubMed] [Google Scholar]
- 31.Hrebicek J, Janout V, Malincikova J, et al. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab 2002;87:144–147 [DOI] [PubMed] [Google Scholar]
- 32.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical J 2012;24:69–71 [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Wang Y, Huang Q, et al. Impaired beta cell function in Chinese newly diagnosed type 2 diabetes mellitus with hyperlipidemia. J Diabetes Res 2014;2014:493039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardini G, Rotella CM, Giannini S. Dyslipidemia and diabetes: Reciprocal impact of impaired lipid metabolism and beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet Studies 2012;9:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007;30:2916–2921 [DOI] [PubMed] [Google Scholar]
- 36.Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: A nuclear magnetic resonance study. Diabetes/Metab Res Rev 2010;26:200–205 [DOI] [PubMed] [Google Scholar]
- 37.Levy J, Atkinson AB, Bell PM, et al. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: The 10-year follow-up of the Belfast Diet Study. Diabet Med 1998;15:290–296 [DOI] [PubMed] [Google Scholar]
- 38.Jensen CC, Cnop M, Hull RL, et al. ; American Diabetes Association. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002;51:2170–2178 [DOI] [PubMed] [Google Scholar]
- 39.Tzagournis M. Triglycerides in clinical medicine. A review. Am J Clin Nutr 1978;31:1437–1452 [DOI] [PubMed] [Google Scholar]
- 40.Magnan C, Collins S, Berthault MF, et al. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J Clin Invest 1999;103:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng T, Gao Y, Tian H. Relationship between blood lipid profiles and pancreatic islet beta cell function in Chinese men and women with normal glucose tolerance: A cross-sectional study. BMC Public Health 2012;12:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez S, Bermudez B, Pacheco YM, et al. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: Monounsaturated compared with saturated fatty acids. Am J Clin Nutr 2008;88:638–644 [DOI] [PubMed] [Google Scholar]
- 43.Saisho Y, Butler AE, Butler PC. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes: Response to Tushuizen et al. Diabetes Care 2008;31:e38; author reply e39 [DOI] [PubMed] [Google Scholar]
- 44.Carpentier A, Mittelman SD, Lamarche B, et al. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol 1999;276(6 Pt 1):E1055–E1066 [DOI] [PubMed] [Google Scholar]
- 45.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 46.Eto K, Yamashita T, Matsui J, et al. Genetic manipulations of fatty acid metabolism in beta-cells are associated with dysregulated insulin secretion. Diabetes 2002;51(Suppl 3):S414–S420 [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Estrada A, Gignoux CR, Fernandez-Lopez JC, et al. The genetics of Mexico recapitulates Native American substructure and affects biomedical traits. Science 2014;344:1280–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]