Abstract
The obese lipid profile is associated with increased free fatty acids and triacylglycerides. Currently, little is known about the plasma lipid species associated with obesity. In this study, we compared plasma lipid fatty acid (FA) profiles as a function of BMI. Profiling phospholipid (PL) FAs and their respective oxylipids could predict which obese individuals are more likely to suffer from diseases associated with chronic inflammation or oxidative stress. We investigated the relationship between BMI and plasma PL (PPL) FA composition in 126 men using a quantitative gas chromatography analysis. BMI was inversely associated with both PPL nervonic and linoleic acid (LA) but was positively associated with both dihomo-γ-linolenic and palmitoleic acid. Compared to lean individuals, obese participants were more likely to have ω-6 FAs, except arachidonic acid and LA, incorporated into PPLs. Obese participants were less likely to have EPA and DHA incorporated into PPLs compared to lean participants. Non-esterified plasma PUFA and oxylipid analysis showed ω-6 oxylipids were more abundant in the obese plasma pool. These ω-6 oxylipids are associated with increased angiogenesis (i.e. epoxyeicosatrienoates), reactive oxygen species (i.e. 9-hydroxyeicosatetraenoate), and inflammation resolution (i.e. Lipoxin A4). In summary, BMI is directly associated with specific PPL FA and increased ω-6 oxylipids.
Keywords: lipidome, omega-3, obesity, human, biomarker, inflammation, nervonic acid
1. Introduction
Obesity is associated with chronic low-grade inflammation, increased oxidative stress, insulin resistance, and metabolic dysregulation [1]. These conditions are linked to excess lipid storage in white adipose tissue (WAT). This increased lipid accumulation places demands on WAT causing macrophage polarization [2], altered adipokine secretion [3], and increased inflammation. The lipid profile (i.e. lipidome), including oxygenated FA metabolites deemed oxylipids, can influence production of inflammatory cytokines [4]. Obesity is associated with dietary shifts in FA intake [5], which alters FA composition of cellular phospholipid (PL) membranes and plasma PLs (PPLs) [6]. PUFAs within membrane PLs serve as substrates for the biosynthesis of oxylipids through either enzymatic or non-enzymatic pathways. Thus, obesity-induced changes in fatty acid concentrations or metabolism will greatly impact the character of the inflammatory response. Therefore, the plasma lipidome, which is indicative of dietary FA intake and changes in FA metabolism, may contain potential biomarkers of the subclinical chronic inflammation associated with obesity.
Although obesity is typically associated with inflammation, many lipid metabolites, including PUFA-derived resolvins and protectins, are anti-inflammatory [7]. PUFAs of the omega-6 (ω-6) and omega-3 (ω-3) families are substrates for oxylipids, which regulate cytokine production by stimulating either inflammatory or anti-inflammatory pathways [8]. Several ω-6 and ω-3 PUFAs are structurally similar and compete for elongating, desaturating, and oxygenating enzymes. An overabundance of one PUFA family (i.e. ω-3) will affect presence of the other (i.e. ω-6) and subsequently affect oxylipid production of PUFA families. For instance, elevated plasma ω-3s are associated with decreased plasma ω-6s and higher concentrations of plasma ω-3 oxylipids [9]. PUFA oxylipids are formed through oxygenation of linoleic acid (LA), alpha-linolenic acid (ALA), arachidonic acid (AA), EPA, and DHA, by cytochrome P450 enzymes [10], cyclooxygenase (COX) [11], and lipoxygenase (LOX) [12]. Through these enzymatic oxygenations, PUFA oxylipid function is enhanced or reduced; thus regulating inflammation. ω-3 PUFAs are typically considered “anti-inflammatory like” lipids, while ω-6 PUFAs are considered to be more “inflammatory like” [13]. Therefore, complex lipids such as PLs and sphingolipids affect inflammation through their regulatory functions on metabolism, depending upon the presence of specific FAs [4].
Because of its association with chronic disease, there is a need for early biomarkers of obesity-associated inflammation. Specifically, it has been suggested an increased ω-6/ω-3 ratio is associated with increased inflammation in obesity and this ratio has been proposed as a biomarker of disease [14]. Most studies evaluating the role of lipids in obesity have focused on dietary PUFA intake and supplementation, lipoprotein particle variation, serum di- and tri-gylceride composition, circulating saturated and unsaturated FFAs, or changes to ω-6/ω-3 ratio. However, PPLs, and non-esterified plasma PUFAs and oxylipids, have not been comprehensively analyzed across BMI categories. Such measurements are necessary to fully define the plasma lipidome and to elucidate its associations with metabolic disease. In the current study, we set out to characterize the PPL FA changes associated with obesity and profile obesity-associated non-esterified plasma PUFAs and oxylipids.
2. Materials and Methods
2.1. Ethics Statement
The study was approved by the Biomedical and Health Institutional Review Board of Michigan State University (IRB# 08-786). The Biomedical and Health Institutional Review Board is one of three IRB committees on the Michigan State University East Lansing campus. Michigan State University's IRBs were established to advance the goal of conducting research with diligence and integrity. The purpose of the committee is to protect the rights, welfare and privacy of human subjects who participate in research conducted by students and/or faculty affiliated with MSU. At the time of enrollment, written informed consent was obtained from each participant.
2.2. Study Population
Healthy males (n=126, > 96% Caucasian) ranging from 48–65 years of age were screened and recruited as previously reported [15]. Participants were weighed and measured by trained staff. These measurements were used to calculate BMI (kg/m2). Study participants were classified as lean (BMI <25), overweight (25≤ BMI <30), or obese (BMI ≥30).
2.3. Serum Adipokine, Inflammatory Marker, and C-peptide Analysis
In brief, at time of enrollment venous blood was collected. Serum and plasma fractions were separated from venous blood after collection, stored at −80°C. All adipokines, C-peptide, and cytokines were analyzed using ELISA or multiplex cytokine kits. A commercially available leptin ELISA kit was performed per manufacturer’s instructions (R&D Systems, DY398; Minneapolis, MN). C-Peptide concentrations were measured as directed by the manufacturer (Calbiotech, Spring Valley, CA, REF; CP1795). Multimeric adiponectin ELISAs of total, high (HMW), medium (MMW), and low (LMW) molecular weight were performed following the manufacturer’s instructions (Alpco Diagnostics, Salem, NH). Serum concentrations of TNF-α and monocyte chemotactic protein-1 (MCP-1) were determined by multiplex immunoassay per the manufacturer’s instructions (HCYTOMAG-60K, Millipore, Billerica, MA).
2.4. Extraction and Isolation of Plasma Phospholipids
PPL extraction was performed as previously described [16]. In brief, plasma samples were thawed on ice. Approximately 200 mg plasma per sample was weighed and extracted using a modified Rose and Oaklander extraction [17]. Phospholipids were isolated using Isolute-XL ® SPE aminopropyl columns (500 mg; Bioatage, Charlotte, NC) as previously described by Agren et al [18].
2.5. Fatty Acid Methyl Ester Preparation
Fatty acid methyl esters (FAMEs) were prepared from PPLs using acidified methanol as previously described [19]. Isolated FAMEs were resuspended in 5 µL n-hexane (100 µg/mL BHT) per mg plasma, and transferred to GC autosampler vials, and stored under nitrogen at - 80°C.
2.6. FAME Analysis, Identification, and Quantification
PPL FAMEs were analyzed using HS-Omega-3 Index® methodology at OmegaQuant Analytics, LLC (Sioux Falls, SD). Gas chromatography was performed using a GC2010 Gas Chromatograph (Shimadzu, Columbia, MD) equipped with a SP2560, 100-m column (Supelco, Bellefonte, PA) using hydrogen as the carrier gas. FAMEs were referenced against a standard of FAs characteristic of erythrocytes. PPL FAMEs were identified and calculated as a percentage of total identified FA after response factor correction.
2.7. Non-Esterified Plasma PUFA and Oxylipid Extraction
Non-esterified plasma PUFA and oxylipid extraction and isolation were performed as previously described [20], but modified as specified. In brief, 500 µL human plasma was thawed on ice and transferred to a 15 mL conical tube containing 1.5 mL HPLC-grade methanol. 15 µL deuterated internal standard mix and 7.4 µL LC-MS-grade formic acid were added to the plasma methanol mixture. This solution was mixed at high speed for 2 min. Protein crash and lipid extraction were performed by centrifugation at 4750 × g for 20 min at 4°C. Supernatant was collected and transferred to a solution containing 1 µL/mL LC-MS-grade formic acid in HPLC-grade water.
2.8. Non-Esterified Plasma PUFA and Oxylipid Isolation
Phenomenex Strata-X (60 mg/3 mL, Phenomenex, Torrance, CA) solid phase extraction columns were conditioned with 4 mL HPLC-grade methanol, followed by 4 mL HPLC-grade water. Extracted samples were then loaded onto columns and rinsed with 20% HPLC-grade methanol in HPLC-grade water. After rinsing, columns were dried under vacuum for 20 min to remove solvents. Non-esterified plasma PUFAs and oxylipids bound to columns were eluted into collection tubes with 4 mL acetonitrile:methanol (1:1 v/v). Collected eluents were evaporated to dryness in a SpeedVac for 4 hr at 40°C. Dried residues were re-dissolved in 100 µL HPLC-grade methanol, vortexed gently, and transferred to a microcentrifuge tube containing 50 µL HPLC-grade water. Samples were spun at 14000 × g for 15 min at 4°C. After centrifugation, 75 µL drawn from the top of the supernatant was transferred to an autosampler vial with glass insert and stored under nitrogen at −20°C for no longer than 4 days.
2.9. Non-Esterified Plasma PUFA and Oxylipid Analysis, Identification, and Quantification
A Waters Xevo TQ-S UHPLC/MS/MS was used to quantify targeted non-esterified plasma lipids, using an Ascentis Express C18 column (10cmX2.1mm; 2.7 µm particles; Sigma-Aldrich, St. Louis, MO). FFA and oxylipid standard (Cayman Chemical, Ann Arbor, MI) collision energies were optimized for precursor ion and product ion using ESI in negative-ion mode. Multiple reaction monitoring (MRM) analyses were performed in 1–3 min time segments. Analytes were quantified using response relative to internal standards. Data processing was performed using TargetLynx software version 4.1 (Waters, Milford, MA). Limit of detection (LOD) was defined as signal to noise < 3.
2.10. Statistical Analysis
Statistical differences in FA levels, serum adipokines, age, and smoking between BMI categories was determined using Kruskal-Wallis one-way analysis of variance, followed by Dunn’s test for multiple comparison. Polytomous logistic regression [21] was used to calculate Odds Ratios (ORs) and 95% confidence intervals across BMI categories. BMI was analyzed categorically as the independent variable. FAs were analyzed as continuous dependent variables, and all logistic regression models included age as a continuous dependent variable. The odds ratios of palmitoleic, docosatetraenoic acid (DTA), and docosapentaenoic acid (DPA), and delta-6-desaturase (D6D) estimated enzyme activity have been calculated on the basis that there is a unit change of 0.11 for the respective beta coefficient for each given parameter.
Forward logistic regressions [22] were performed comparing lean to overweight and lean to obese, and comparing the lowest to middle and lowest to highest tertiles of nervonic acid. BMI and nervonic acid were run as the independent variables in their respective forward logistic regression models. The dependent variables for forward logistic regressions were selected based on biological changes documented in obesity, and several fatty acids that were highly significant in our analyses. Dependent variables included in forward logistic regressions were age, palmitoleic, DGLA, LA, waist circumference (WC), nervonic acid, MCP-1, TNF-α, and C-peptide. Model diagnostics were normal, and C-statistic values >0.9 for both models. Due to statistical simultaneity between BMI and nervonic acid, forward regression results were used to determine variables for simultaneous equations. The simultaneous equations [23] were solved to determine estimates, standard errors, and P-values. Equation 1: BMI = age + palmitoleic + DGLA + LA + MCP-1 + TNF-α; Equation 2: Nervonic acid = age + BMI + WC + TNF-α + MCP-1 + palmitoleic + DGLA + C-peptide.
P-values were considered statistically significant if P < 0.05. Most statistical analyses were conducted using software SAS version 9.4 (SAS, Cary, NC), except Dunn’s nonparametric comparison for post hoc testing that was conducted using Graph Pad Prism version 4 (Graph Pad, La Jolla, CA).
3. Results
3.1. Characteristics of Study Participants
Age, smoking, anthropometric, glycemic, and inflammatory markers of 126 participants, separated into respective BMI categories, are outlined in Table 1. In brief, overweight participants were younger than lean and obese participants. Waist circumference increased with increasing BMI. Leptin was significantly increased and adiponectin significantly decreased with obesity, as previously reported [15]. Obese individuals also had the highest serum concentrations of C-peptide, MCP-1, and TNF-α.
Table 1.
| Parameter | Lean (n=28)a | Overweight (n=46)a | Obese (n=52)a | P-Valueb |
|---|---|---|---|---|
| Age (years) | 57.89 ± 4.21A | 55.00 ±4.70B | 58.13 ±4.40A | <0.005 |
| Smoker (% total) | 25 | 24 | 27 | - |
| BMI (kg/m2) | 23.33 ± 1.43A | 27.99 ± 1.30B | 34.71 ± 3.79C | <.0001 |
| WC (cm) | 87.25 ± 7.39A | 100.58 ± 7.34B | 118.85 ± 11.18C | <.0001 |
| Leptin (ng/mL) | 2.48 ± 1.72A | 5.34 ± 2.70B | 17.94 ± 12.12C | <.0001 |
| Total Adiponectin (µg/mL) | 6.12 ± 3.10A | 4.96 ± 1.76A | 3.95 ± 1.70B | <0.001 |
| HMW (µg/mL) | 2.83 ± 2.21A | 2.19 ± 1.16A | 1.57 ± 1.11B | <0.005 |
| MMW (µg/mL) | 0.75 ± 0.40 | 0.80 ± 0.43 | 0.62 ± 0.36 | - |
| LMW (µg/mL) | 2.54 ± 1.07A | 1.97 ± 0.60AB | 1.75 ± 0.57B | <0.001 |
| C-Peptide (ng/mL) | 1.86 ± 1.12A | 2.56 ± 1.56A | 3.84 ± 1.98B | <.0001 |
| TNF-α (pg/mL) | 6.95 ± 5.26A | 7.28 ± 3.73AB | 10.59 ± 9.13B | <0.0001 |
| MCP-1 (pg/mL) | 459 ±146A | 507 ± 138AB | 535 ± 166B | <0.05 |
Values expressed as Mean ± standard deviation.
Kruskal Wallis one-way ANOVA with Dunn's test for multiple comparison.
P value reported only if significant, p <0.05.
Waist circumference (WC), and high molecular weight (HMW), medium molecular weight (MMW), and low molecular weight (LMW) adiponectin.
3.2 Plasma Phospholipid Fatty Acid Analysis
Results of nonparametric ANOVAs and median values of the FAs measured are given in Table 2. Of the saturated FAs (SatFAs), stearic acid (P<0.05) was higher and palmitic acid tended to be higher in the PPLs of obese individuals compared to lean. Palmitoleic acid (P<0.001) was higher and nervonic acid (P<0.0001) was lower in obese compared to lean participants. In obesity, only the ω-3 FA EPA (P<0.005) was lower in PPLs compared to lean individuals. In addition, total PPL ω-3s (P<0.05) were also lower in obese individuals compared to lean. Most ω-6 FAs were elevated in both overweight and obese participants, most notably Dihomo-γ-linolenic acid (DGLA, P<0.0001). However, LA (P<0.005) decreased in obese individuals compared to lean. Interestingly, total ω-6 PUFA yielded no significance and the ratio of ω-6 to ω-3 was similar in the PPLs of all participants. D6D estimated enzyme activity (P<0.0001), calculated as the ratio of DGLA to LA, was greater in obese individuals than lean. Furthermore, D5D estimated enzyme activity, calculated as the ratio of AA to DGLA, tended to be lower in obese participants than lean (p=0.051).
Table 2.
| Variable (% of Total)a | Lean (n=28)b | Overweight (n=46)b | Obese (n=52)b | P-Valuec |
|---|---|---|---|---|
| C14:0 Myristic | 0.00 | 0.00 | 0.00 | - |
| C16:0 Palmitic | 28.10(25.53,30.22) | 29.42 (27.50,30.64) | 29.27 (27.80,30.63) | - |
| C18:0 Stearic | 13.56 (12.46,14.75)A | 14.00 (12.91,15.85)AB | 14.47 (13.79,15.51)B | <0.05 |
| C20:0 Arachidic | 0.52 (0.44,0.62) | 0.55 (0.44,0.62) | 0.49(0.41,0.61) | - |
| C21:0 Behenic | 1.43(1.18,1.86) | 1.48 (1.28,1.79) | 1.48(1.16,1.82) | - |
| C24:0 Lignoceric | 1.26(1.16,1.61) | 1.37(1.05,1.71) | 1.22(0.93,1.59) | - |
| Total sat | 45.60 (43.89,47.68) | 46.26 (44.95,49.90) | 47.37 (45.91,49.26) | - |
| C16:1 Palmitoleic | 0.27 (0.18,0.44)A | 0.35 (0.20,0.55)A | 0.48 (0.32,0.62)B | <0.001 |
| C18:1 Oleic | 10.46(8.81,11.80) | 9.49(8.70,11.08) | 9.73(8.79,11.43) | - |
| C20:1 Eicosenoic | 0.17(0.14,0.21) | 0.15(0.12,0.22) | 0.14(0.11,0.20) | - |
| C24:1 Nervonic | 1.90 (1.65,2.34)A | 1.54 (1.34,1.80)B | 1.41 (1.12,1.71)B | <0.0001 |
| Total MUFA | 12.79 (10.93,14.39) | 11.72(10.80,13.51) | 11.98(10.97,13.79) | - |
| C16:1 trans-Palmitoleic | 0.15(0.11,0.17) | 0.12(0.11,0.15) | 0.12(0.10,0.15) | - |
| C18:1 trans-Oieic | 0.62 (0.50,0.92) | 0.66(0.51,0.90) | 0.68 (0.52,0.88) | - |
| C18:2 trans-Linoleic | 0.24(0.17,0.32) | 0.27 (0.20,0.38) | 0.25 (0.19,0.36) | - |
| Total trans | 0.99(0.87,1.40) | 1.04(0.83,1.36) | 1.09(0.89,1.36) | - |
| C18:3n3 Alpha-Linolenic | 0.15(0.12,0.23) | 0.15(0.11,0.20) | 0.13(0.10,0.16) | - |
| C20:5n3 Eicosapentaenoic | 0.84 (0.64,1.06)A | 0.55 (0.42,1.09)AB | 0.59 (0.41,0.74)B | <0.005 |
| C20:5n3 Docosapentaenoic | 0.88 (0.73,1.05) | 0.84(0.65,1.03) | 0.80 (0.62,0.95) | - |
| C22:6n3 Docosahexaenoic | 3.18 (2.32,4.50) | 2.50(2.07,3.61) | 2.61 (2.12,3.19) | - |
| EPA+DHA | 3.84 (2.90,5.47) | 3.22 (2.56,4.53) | 3.21 (2.69,3.97) | - |
| Total ω-3 | 5.24 (3.91,7.02)A | 4.34 (3.45,5.53)AB | 4.10 (3.69,4.99)B | <0.05 |
| C18:2n6 Linoleic | 22.10 (18.13,24.19)A | 19.85 (17.39,22.55)AB | 18.68 (16.80,20.31)B | <0.005 |
| C18:3n6 Gamma-Linoleic | 0.11 (0.07,0.13)A | 0.14 (0.09,0.19)B | 0.14 (0.11,0.18)B | <0.01 |
| C20:2n6 Eicosadienoic | 0.32 (0.28,0.36) | 0.30 (0.26,0.36) | 0.30 (0.26,0.35) | - |
| C20:3n6 DGLA | 2.08 (1.91,2.53)A | 2.64 (2.26,3.38)B | 2.98 (2.46,3.56)B | <0.0001 |
| C20:4n6 Arachidonic | 9.80(7.71,11.24) | 10.38(8.48,11.17) | 10.37(9.10,12.73) | - |
| C22:4n6 Docosatetraenoic | 0.28 (0.18,0.36)A | 0.36 (0.23,0.40)AB | 0.39 (0.32,0.50)B | <0.005 |
| C22:5n6 Docosapentaenoic | 0.18 (0.12,0.29)A | 0.23 (0.14,0.32)AB | 0.27 (0.21,0.34)B | <0.01 |
| Total ω-6 | 35.41 (31.00,37.51) | 34.53 (33.10,36.55) | 33.86 (31.04,36.06) | - |
| Total ω-6/ Total ω-3 ratio | 6.63 (4.93,9.33) | 8.31(6.11,10.27) | 8.50 (6.73,9.63) | - |
| Delta-6-Desaturase | 0.10 (0.08,0.13)A | 0.14 (0.11,0.18)B | 0.15 (0.13,0.20)B | <0.0001 |
| Delta-5-Desaturase | 4.64(3.54,5.51) | 3.95 (2.76,4.80) | 3.41 (2.74,4.46) | - |
Fatty acid units expressed as percent of total.
Values expressed as Median (Q1,Q3).
Kruskal Wallis one-way ANOVA with Dunn’s test for multiple comparison.
P value reported only if significant, p <0.05.
Dihomo-γ-linolenic acid (DGLA).
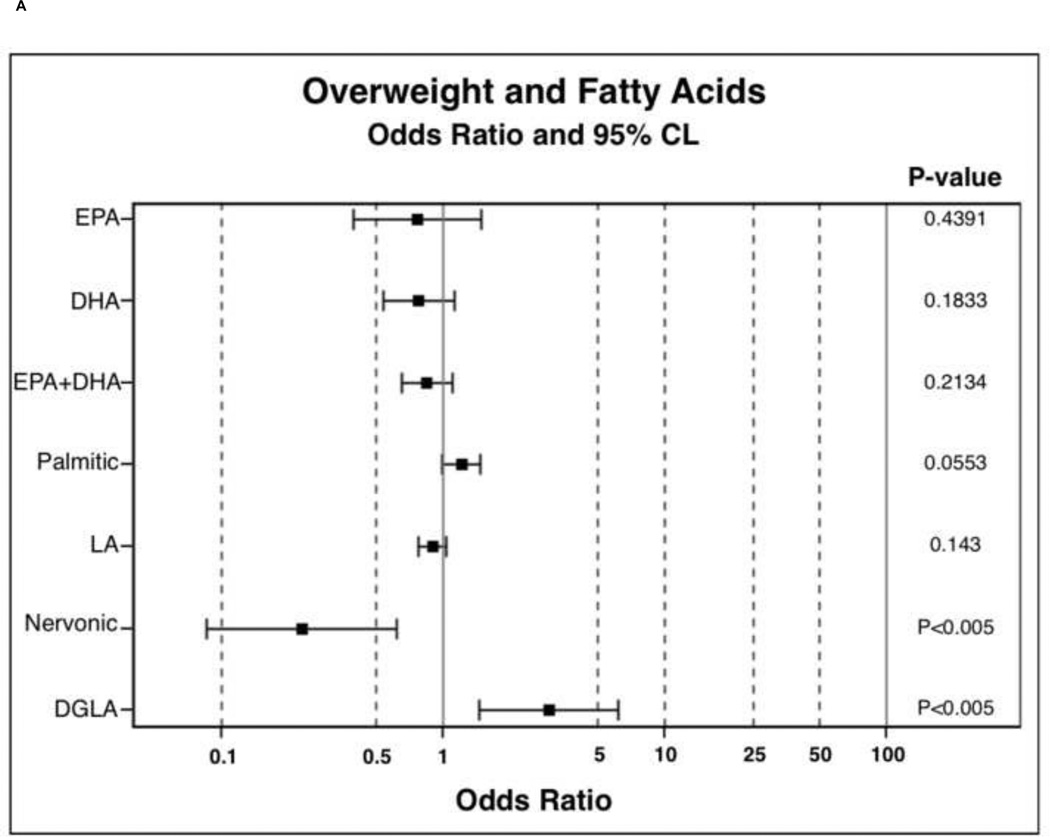
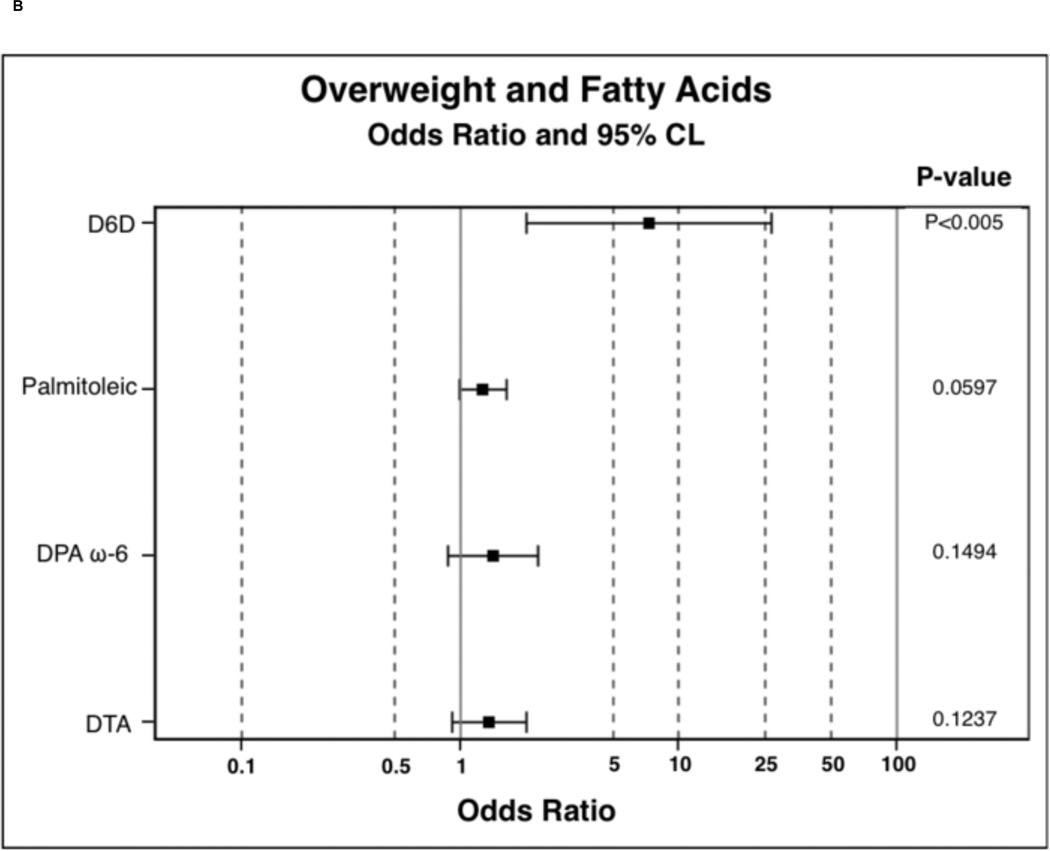
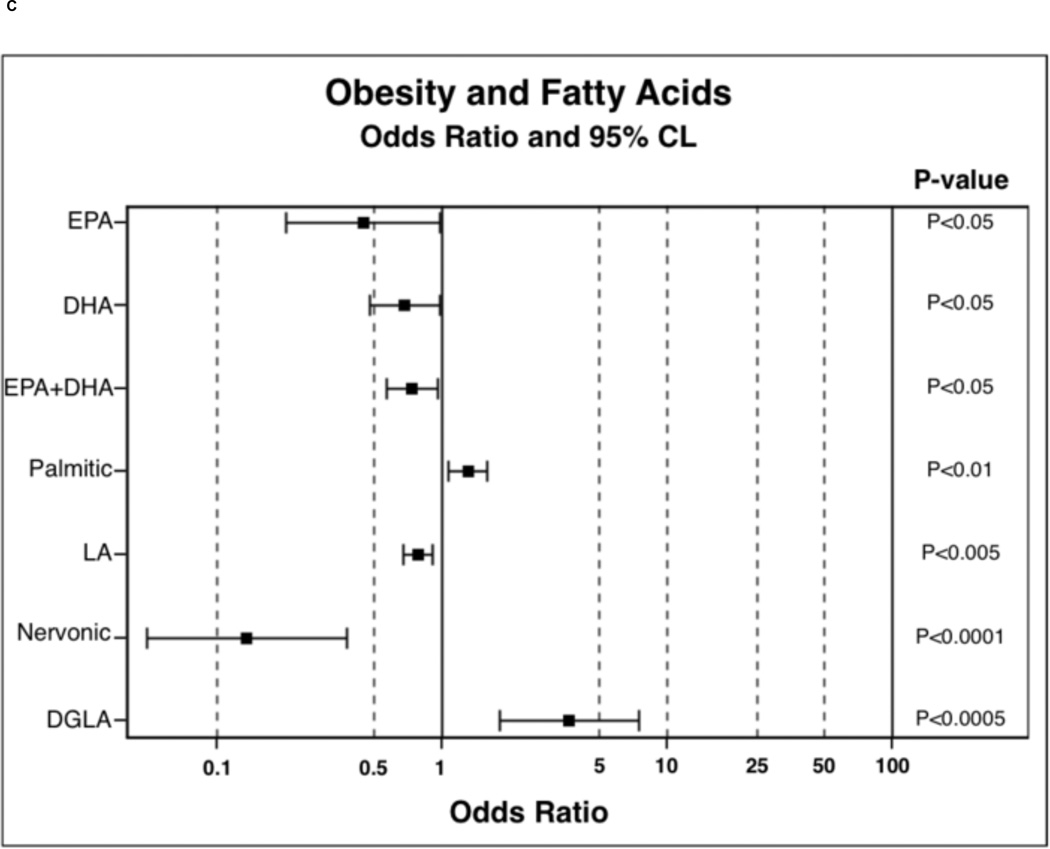
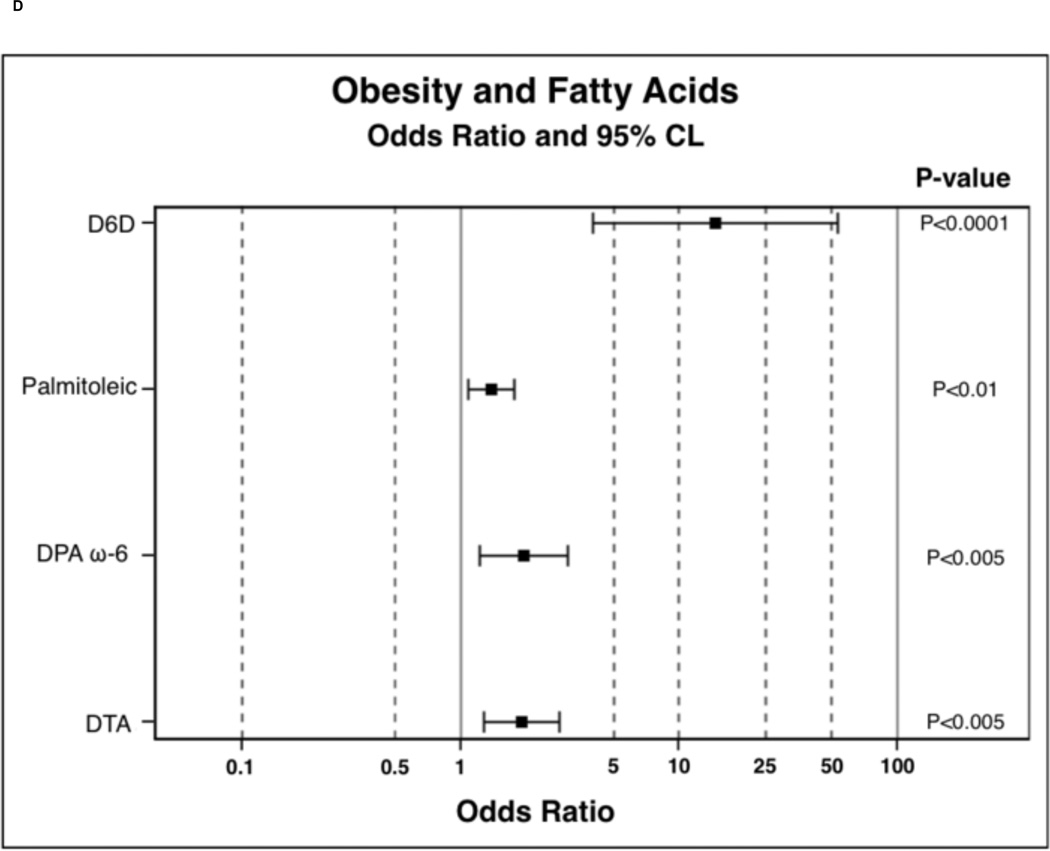
In order to determine the probability of PPL FA incorporation, in comparison to the lean BMI category, polytomous logistic regressions were run. Results of polytomous logistic regressions are displayed as OR (95% Confidence Interval) and indicate the likelihood of PPL FA incorporation in overweight and obese individuals compared to lean. FAs were selected for these analysis based on observed trends or significance in nonparametric ANOVAs. Overweight participants were 0.23 (0.09–0.62) times less likely to have nervonic acid incorporated into PPLs and were 3.01 (1.46–6.18) times more likely to have DGLA incorporated into PPLs (Figure 1A). Overweight participants were also 7.30 (2.00–26.60) times more likely to have increased D6D estimated enzyme activity (Figure 1B). Overweight individuals tended to have higher palmitic 1.21 (1.00–1.48) and palmitoleic 1.27 (0.99–1.63) acid in PPLs. Obese participants were less likely to have EPA 0.45 (0.20–0.99), DHA 0.68 (0.48–0.98), combined EPA and DHA 0.74 (0.57–0.96), LA 0.78 (0.68–0.91), and nervonic acid 0.14 (0.05–0.38) incorporation into PPLs (Figure 1C). Obese individuals were more likely to have palmitic 1.30 (1.07–1.58), DGLA 3.68 (1.81–7.46), palmitoleic 1.39 (1.09–1.77), DTA 1.90 (1.28–2.82), and DPA ω-6 1.95 (1.22–3.09) incorporation into PPLs. In obesity, individuals were 14.64 (4.01–53.40) times more likely to have elevated D6D estimated enzyme activity (Figure 1D) and tended to be 0.76 (0.57–1.01, P-value 0.06, data not shown) times less likely to have elevated D5D estimated enzyme activity.
Figure 1.
Odds ratio results from polytomous logistic regression compared to lean BMI as reference group. 1B and 1D: odds ratios have been calculated on the basis that there is a unit change of 0.11 for the respective beta coefficient for each given parameter. Dihomo-γ-linolenic acid (DGLA), delta-6-desaturase (D6D), docosatetraenoic acid (DTA), docosapentaenoic acid (DPA), and linoleic acid (LA).
Given that both BMI and nervonic acid demonstrate a high level of multicollinearity with several anthropometric measurements, serum adipokines, and proinflammatory markers (data not shown), along with the observed interactions between BMI and nervonic acid in forward logistic regressions, we hypothesized there was statistical simultaneity between BMI and nervonic acid. In order to account for this possible statistical simultaneity, we solved a system of simultaneous equations, yielding two independent statistical outputs: one for BMI and one for nervonic acid (Table 3). In equation 1, every 1-unit increase in palmitoleic (P<0.05), DGLA (P<0.0001), TNF-α (P<0.05), and LA (P<0.005) resulted in BMI (P<0.001) changing by 2.657, 1.993, 0.156, and −0.345 units respectively. In equation 2, every 1-unit increase in WC (P<0.05) and TNF-α (P<0.05) resulted in nervonic acid (P<0.005) changing by −0.043- and −0.017-units respectively.
Table 3.
| Equation 1 | Equation 2 | ||||||
|---|---|---|---|---|---|---|---|
| Model | P-value | Model | P-value | ||||
| BMI | <0.0001 | Nervonic acid | <0.005 | ||||
| Variable | Estimate | Std. Error | P-value | Variable | Estimate | Std. Error | P-value |
| Intercept | 26.529 | 5.776 | <0.0001 | Intercept | 2.566 | 0.667 | <0.0005 |
| Age | 0.032 | 0.086 | 0.7069 | Age | 0.005 | 0.010 | 0.6549 |
| Palmitoleic | 2.657 | 1.296 | <0.05 | BMI | 0.034 | 0.019 | 0.0831 |
| DGLA | 1.993 | 0.486 | <0.0001 | WC | −0.043 | 0.017 | <0.05 |
| Linoleic | −0.345 | 0.113 | <0.005 | TNF-α | −0.017 | 0.007 | <0.05 |
| Nervonic | −0.570 | 0.784 | 0.4688 | MCP-1 | 0.000 | 0.000 | 0.8383 |
| MCP-1 | 0.002 | 0.003 | 0.4903 | Palmitoleic | −0.224 | 0.149 | 0.1364 |
| TNF-α | 0.156 | 0.060 | <0.05 | DGLA | −0.067 | 0.060 | 0.2728 |
| C-peptide | −0.002 | 0.031 | 0.9540 | ||||
Independent variables are listed under respective model, with overall model significance. Respective model variables are listed under the heading variable, with respective beta-estimates and P-values.
P-value bolded if <0.05.
Dihomo-γ-linolenic acid (DGLA), waist circumference (WC), monocyte chemoattractant protein-1 (MCP-1), and Tumor necrosis factor alpha (TNF-α).
3.3 Non-Esterified Plasma PUFAs and Oxylipid Analysis
Due to changes observed in PPL PUFA composition across BMI categories, we investigated several non-esterified plasma PUFAs and their respective oxylipids in pooled plasma from obese (n=10) and lean (n=10) individuals. These results are displayed as fold changes between concentrations of non-esterified plasma PUFAs and oxylipids in the obese to lean plasma pools; visualized in tandem with results from PPL FA analysis (Figure 2 and 3). To avoid overlap in FA names between the PPL and non-esterified plasma PUFA and oxylipid analysis, we refer to all non-esterified plasma PUFAs unabbreviated and as their conjugate base ending with the suffix – ate.
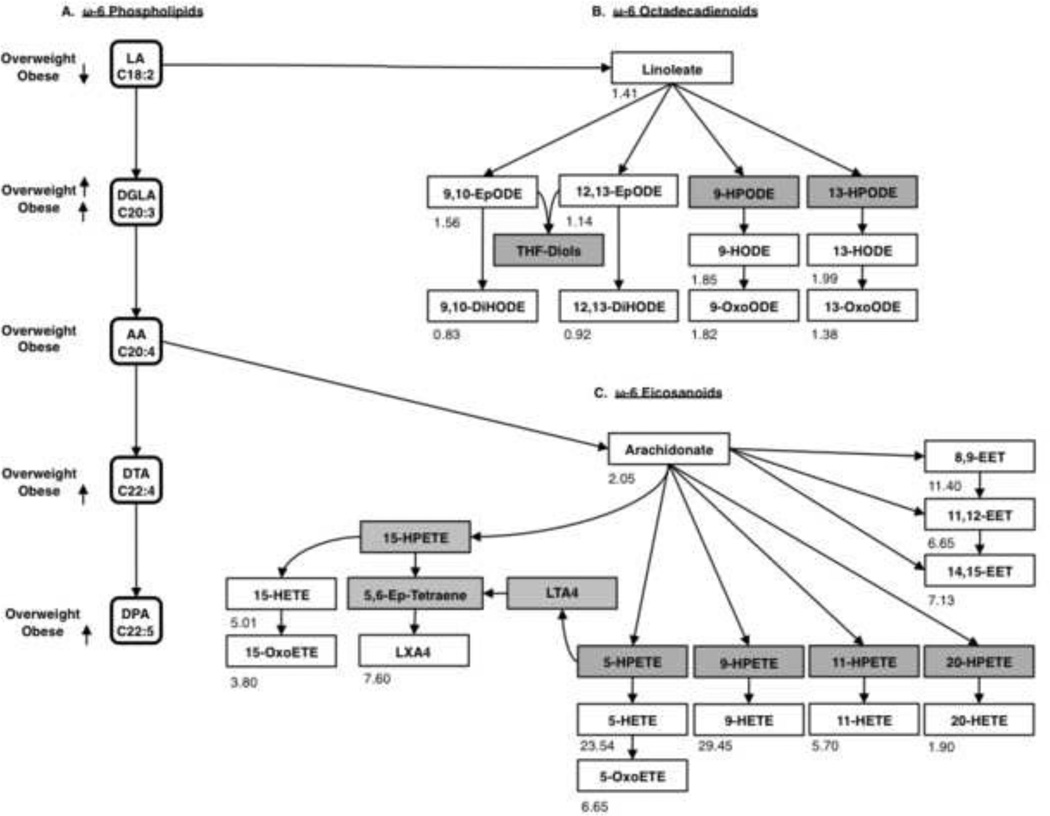
Figure 2.
(A) Visual representation of results from polytomous logistic regression ω-6 PL pathway, PPL analysis. (B) Linoleate derived ω-6 octadecadienoids. (C) Arachidonate derived ω-6 eicosanoids. (B and C) Non-esterified plasma PUFAs and oxylipids are listed in boxes: colored white if measured and grey if not measured. Number listed in the lower left-hand corner represents fold change calculated as obese to lean for each analyte, where a—represents metabolites which were below limit of detection. Epoxy eicosatrienoate (EET), lipoxin A4 (LXA4), leukotriene A4 (LTA4), hydroxy eicosatetraenoate (HETE), eicosatetraenoate (ETE), epoxy octadecadienoate (EpODE), hydroperoxy octadecadienoate (HPODE), hydroxy octadecadienoate (HODE), hydroperoxy eicosatetraenoate (HPETE), arachidonic acid (AA), Dihomo-γ-linolenic acid (DGLA), docosatetraenoic acid (DTA), Docosapentaenoic acid (DPA), and linoleic acid (LA).
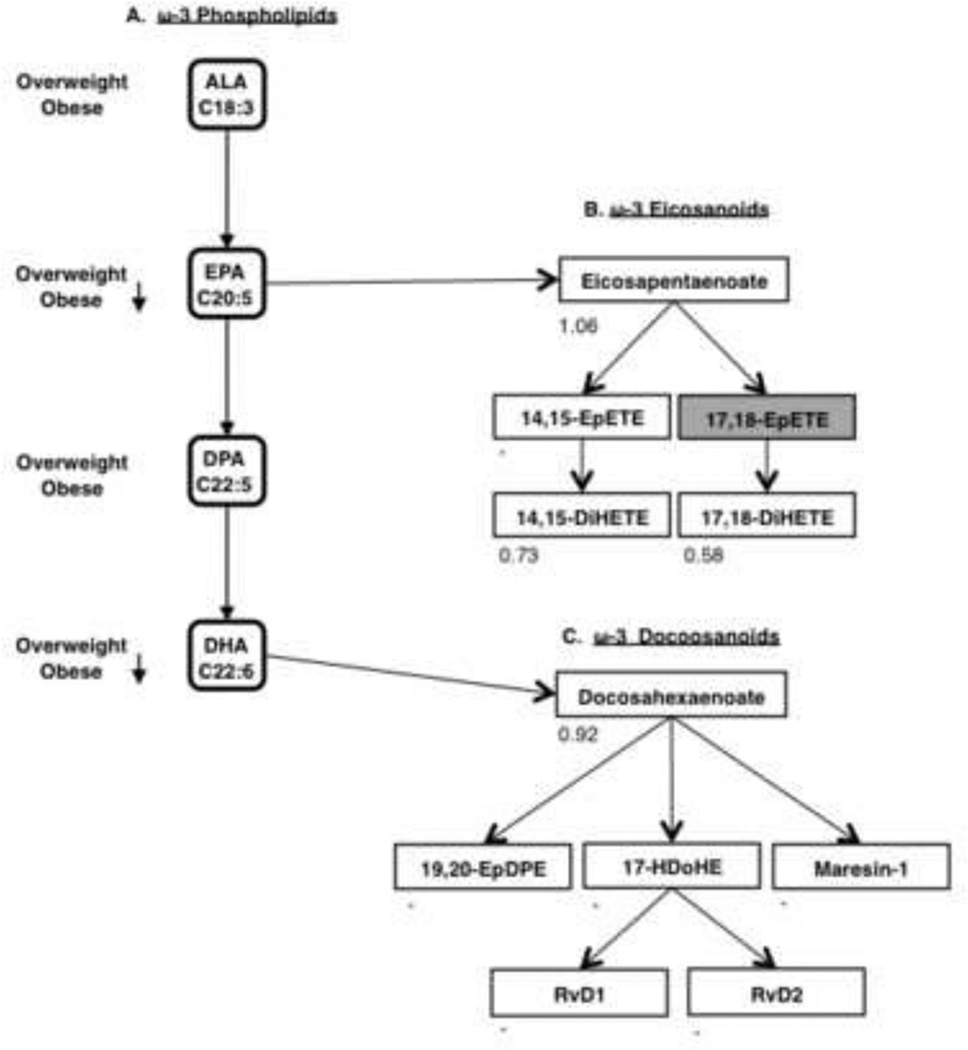
Figure 3.
(A) Visual representation of results from polytomous logistic regression ω-3 PPL pathway, PPL analysis. (B) Eicosapentaenoate derived ω-3 eicosanoids. (C) Docosahexaenoate derived ω-3 docosanoids. (B and C) Non-esterified plasma PUFAs and Oxylipids are listed in boxes: colored white if measured and grey if not measured. Number listed in the lower left-hand corner represents fold change calculated as obese to lean for each analyte, where a—represents metabolites which were below limit of detection. Maresin 1’s fold change is designated with a—since it was only above LOD in the lean pool. Alpha-linolenic acid (ALA), docosapentaenoic acid (DPA), Epoxy eicosatetraenoate (EpETE), dihydroxy eicosatetraenoate (DiHETE), Epoxy docosapentaenoate (EpDPE), Hydroxy docosahexaenoate (HDoHE), and Resolvin D-series (RvD).
Polytomous logistic regression of ω-6 FAs revealed obese participants were less likely to have LA incorporated into their PPLs and more likely to have of DGLA, DTA, and DPA incorporated into PPLs (Figure 2A). Non-esterified plasma linoleate concentration was increased in the obese pool (Figure 2B). In obesity, increased 9,10- and 12,13-epoxy octadecadienoate (EpODE) was observed along with a decrease in their downstream products 9,10- and 12,13- dihydroxy ODE (DiHODE). Also, 9- and 13- hydroxy ODE (HODE) were increased in the obese pool along with their respective ketone metabolites 9- and 13-oxoODE.
AA in PPLs did not differ across BMI categories, but there was an increase in ω-6 PPL FAs along with D6D estimated enzyme activity, warranting investigation of non-esterified plasma arachidonate and oxylipids (Figure 2C). All monohydroxy products 5-, 9-, 11-, 15-, and 20-HETE had elevated concentrations in the obese pool, with largest fold increases in 5- and 9-HETE. Respective oxidized products 5-oxo eicosatetraenoate (ETE) and 15-oxoETE were also increased in the obese pool. Arachidonate-derived epoxides 8,9-, 11,12-, and 14,15-epoxy eicosatrienoate (EET) were all increased. In the obese pool, the concentration of the arachidonate-derived resolvin LXA4 also increased.
Our results showed our obese participants had significantly decreased total ω-3 as well as EPA and DHA incorporation into PPLs, with no change in DPA ω-3 PPL incorporation (Figure 3A). In obesity, non-esterified plasma eicosapentaenoate only slightly increased, and its epoxidated product 14,15-EpETE was below LOD in both pools (Figure 3B). Both ω-3 eicosanoids 14,15-and 17,18-DiHETE decreased in obesity. Non-esterified plasma docosahexaenoate was slightly decreased in the obese pool (Figure 3C). ω-3 docosanoids 19,20-epoxy docosapentaenoate (EpDPE), 17-hydroxy docosahexaenoate (HDoHE), Resolvin D1 (RvD1), and RvD2 were below LOD in both plasma pools. Maresin 1 was detectable in the lean pool, but it was below LOD in the obese pool so no fold change could be calculated.
4. Discussion
This study characterized PPL differences associated with BMI. We show decreased nervonic acid, increased DGLA, and increased palmitoleic acid incorporation into PPLs of overweight and obese individuals. Previously, decreased nervonic acid and increased palmitoleic acid in PPLs has been documented in metabolic syndrome (MetS) [6, 24]. Compared to lean participants, obese individuals had significantly increased WC, leptin, C-peptide, MCP-1, and TNF-α, and also significantly decreased adiponectin. Obesity and MetS are both associated with altered adipokine secretion; i.e. elevated leptin and decreased adiponectin [25]. The expansion of WAT results in adipokine dysregulation, adipocyte hypoxia, and induction of the M1 macrophage [26]. M1s are primarily responsible for secretion of TNF-α and MCP-1, resulting in increased adipocyte apoptosis that is characteristic of insulin resistance [27]. Together this suggests specific PPL FA changes are associated with increasing BMI, functioning as novel biomarkers of obesity-associated inflammation and early MetS.
Early predictive biomarkers of obesity-associated inflammation and MetS are needed to reduce the risk of future chronic diseases. In this current study, we report PPL incorporation of palmitic and stearic acid was higher in obese subjects versus overweight and lean subjects. The observed SatFA increase in PPLs could result from increased dietary intake, increased hepatic SatFA synthesis, or the preferential uptake of circulating saturated FFAs (SatFFAs) by cellular phospholipid membrane leaflets to prevent toxic accumulation [28]. Perreault et al showed total serum palmitic and stearic acid increased in individuals referred to as “metabolically unhealthy obese” or obese individuals with increased inflammation [29]. Obesity is associated with higher circulating concentrations of SatFFAs, and high circulating SatFFAs can initiate an inflammatory response resulting in metabolic disruptions [30]. For instance, elevated SatFFAs influences a positive feedback loop in WAT, contributing to inflammation-associated insulin resistance and the further release of more SatFFAs from WAT [30]. Increased SatFA incorporation into PLs species is associated with higher levels of SatFFAs [31]. Therefore, a possible mechanism may exist preventing SatFFA toxicity by sequestration into PLs.
SatFAs are substrates for stearoyl-CoA desaturase (SCD) enzymes, which convert SatFAs to MUFAs. Palmitic and stearic acid are converted to the MUFAs palmitoleic and oleic acid by SCDs respectively. Increased activity of SCD enzymes is associated with metabolic syndrome (MetS) and insulin resistance [32]. We assessed PPL SCD n-7 estimated enzyme activity, calculated as the ratio of PPL palmitoleic to palmitic acid [33], through polytomous logistic regression and found obese individuals were more likely (P-value <0.05, data not shown) and overweight individuals tended to be more likely (P-value = 0.085, data not shown) to have higher SCD n-7 estimated enzyme activity compared to lean individuals. We also assessed SCD n-9 estimated enzyme activity, calculated as the ratio of oleic to stearic acid [33], and found no significant changes associated with BMI. Therefore in our obese and overweight participants, only the SCD n-7 pathway is affected, causing increased conversion of palmitoleic acid from palmitic acid.
In the current study, we report increased palmitoleic acid incorporation in PPLs with increasing BMI. Zong et al previously reported palmitoleic acid increases in RBC PLs of males with MetS and also, RBC PL palmitoleic acid is inversely correlated with serum adiponectin [6]. As mentioned, our obese group was more likely, and overweight individuals tended to be more likely, to have increased SCD n-7 estimated enzyme activity. This suggests increased SCD n-7 activity is responsible for the increased palmitoleic acid in PPLs. This increase may also result from higher cellular uptake of circulating FFA palmitoleate to be used in PL synthesis. In obesity, it is plausible decreased FFA palmitoleate and increased palmitoleic acid incorporation in PPLs occur simultaneously, since circulating non-esterified palmitoleate is thought to play a protective role against obesity [34]. Results from our system of simultaneous equations show palmitoleic acid having the highest beta coefficient, such that for every 1-unit increase in palmitoleic acid in PPLs BMI increases by 2.66-units. These findings identify increased palmitoleic acid in PPLs as a potential biomarker for metabolic dysregulation or early MetS identification.
Oleic acid had no significant correlation with inflammatory factors or odds of altered PPL incorporation across BMI categories (data not shown). This was unexpected since stearic acid increased in PPLs of obese participants, and stearic acid is a precursor in oleic acid synthesis. As mentioned, we saw no difference in SCD n-9 estimated enzyme activity across BMI categories. Previously, Stefan et al showed low SCD n-9 estimated enzyme activity is associated with insulin resistance in obesity [35] and biopsies of obese skeletal muscle reveal decreased SCD n-9 activity [36], which may suggest SCD n-9 may be involved in the progression of obesity-associated insulin resistance. In our study population, we excluded individuals with diabetes from the analysis, which may explain why SCD n-9 estimated enzyme activity was not significantly associated with obesity. Oleic acid (C18:1) is elongated to form eicosenoic acid (C20:1), and is further elongated to nervonic acid (C24:1). We did not observe significant correlations between eicosenoic acid and BMI. However, we did observe significantly decreased odds of nervonic acid incorporation in PPLs with increasing BMI.
To our knowledge, the biological role of nervonic acid in obesity and obesity-associated inflammation is undetermined. Recently Yamazaki et al reported total serum nervonic acid levels are lower in individuals with MetS compared to those without MetS [24]. In our study population, PPL nervonic acid is inversely correlated with WC, leptin, BMI, DGLA, TNF-α, and C-peptide (data not shown). Nervonic acid tertiles were significantly associated with BMI, WC, and the aforementioned metabolic and inflammatory markers when tested in forward logistic regressions. In our system of simultaneous equations, nervonic acid showed significant inverse relationships with both WC and TNF-α. Unexpectedly, BMI was not significantly associated with nervonic acid incorporation in PPLs. This suggests observed significance between nervonic acid and BMI in the polytomous logistic regression is likely due to underlying increases in WC and TNF-α. As an alternative to WC and TNF-α, nervonic acid may serve as a potential early biomarker of obesity-associated inflammation, metabolic dysregulation, or early MetS.
Given that Yamazaki saw a decrease in total serum nervonic acid in MetS and we observed nervonic acid was inversely associated with WC and TNF-a, it is likely that decreasing nervonic acid is indicative to the progression to obesity-associated inflammation and insulin resistance. As mentioned, endogenous nervonic acid synthesis is dependent on the SCD n-9 pathway, and in skeletal muscle, decreased SCD n-9 activity is associated with obesity and insulin resistance. We observed PPL SCD n-9 estimated enzyme activity was not associated with BMI. The decreased nervonic acid in PPLs observed in our study population is likely taking place in sphingomyleins, which serve as secondary messengers in the form of ceramides. Previously, Park et al showed ceramides induce apoptosis of adipocyte-derived stem cells [37]. Ceramides also function in inducing inflammation-associated pathologies such as type 2 diabetes [38]. Specifically, nervonic acid containing ceramides are increased in those with type 2 diabetes [39]. Ceramides are also glycosylated to form polar sphingolipids (i.e. cerebrosides and gangliosides), which incorporate the ceramide bound FA into the polar sphingolipid structure [40]. For instance, the ganglioside GD series (i.e. GD1, GD2, and GD3) may contain nervonic acid in their structures [41–43]. Previously, it was reported GD3 is associated with type 2 diabetes [44]. The lipid extraction method used in our and Yamazaki’s analysis would not have extracted polar sphingolipids, which elucidates that decreasing nervonic acid is a result of decreased endogenous synthesis or a preferential incorporation into polar sphingolipid species.
In obesity we observed decreased LA and increased DGLA, DTA, and DPA ω-6 in PPLs. This was expected since LA is the essential precursor for ω-6 FA synthesis and competes with ALA, the essential ω-3 FA precursor, as a substrate for D6D. We report overweight and obese individuals have increased D6D estimated enzyme activity, and in obesity, there was a decreased trend in D5D estimated enzyme activity. As previously mentioned, PPL D6D estimated enzyme activity is calculated as the ratio of DGLA to LA and D5D estimated enzyme activity is calculated as the ratio of AA to DGLA [45]. Numerous studies have shown increased D6D and decreased D5D estimated enzyme activity is linked to an increased risk of type-2 diabetes [reviewed in [46]]. Insulin resistance is associated with increased serum C-peptide, a marker of insulin production, which is elevated in obesity [47]. As mentioned, our obese participants had increased serum C-peptide. Therefore, our findings suggest D6D and D5D estimated enzyme activity may elucidate early insulin resistance in obesity.
The system of simultaneous equations revealed a significant inverse relationship between BMI and PPL LA as well as a significant direct relationship between BMI and PPL DGLA. Although dietary modification of the of ω-6/ω-3 ratio has been suggested to affect PL incorporation [48], in fact, the absolute amounts of dietary ALA and LA have greater influence of incorporation into PPL, and conversion of ALA to EPA, compared to the total dietary ratio of ALA to LA [49]. Liou et al showed higher intakes of LA decrease EPA in PPLs, but do not directly influence AA incorporation in PPLs [50]. Although we did not collect dietary information on our study participants, increased dietary intake of LA may explain the observed decrease in EPA and no change in AA with obesity. Interestingly, a dietary reduction of LA has shown to significantly reduce ODEs [5]. The observed increase in ω-6 PPLs and an increase in non-esterified plasma linoleate and several ω-6 ODEs in obesity is consistent with documented effects of increased dietary LA intake. Together these data suggests, in our population, increased dietary intake of LA may be responsible for the observed changes.
We report obesity is associated with increased EpODEs but decreased DiHODEs, signifying pathway shunting to THF-diols is likely occurring. Linoleate derived HODEs and oxo-ODEs are potent PPAR-γ ligands and were increased in our obese plasma pool. PPAR-γ ligands increase mRNA expression and circulating concentrations of adiponectin, which is an anti-inflammatory adipokine inversely associated with BMI [51]. Recently, Mirzaei et al reported that obese individuals with increased concentrations of serum PPAR-γ ligands are at a higher risk for MetS [52]. It has also been suggested certain tissues may exhibit higher expressions of HODE and ODE converting enzymes, to aid in controlling local tissue inflammation [53]. Therefore, in obesity, the increased production of ω-6 ODE PPAR-γ ligands may function in regulating inflammation in WAT, as a possible mechanism of pro-resolution to the underlying obesity-associated inflammation.
In our obese pool, arachidonate and ω-6 eicosanoids were increased, with 9-HETE having highest observed fold change. 9-HETE is an auto-oxidative product of arachidonate, indicative of oxidative stress [54]. In obesity both lipid peroxidation and ROS are increased [55]. Elevated 9-HETE indicates increased ROS in our obese plasma pool, likely due to obesity-associated inflammation. We also observed an increased concentration of 20-HETE in obesity. Previously, Tsai et al reported plasma 20-HETE was significantly increased in MetS [56]. Together these HETE findings suggest increased 9-HETE in obesity as a marker of obesity-associated inflammation or early MetS.
We report an increase in LXA4 in our obese pool, which is a potent pro-resolving oxylipid referred to as a “stop” signal in inflammatory responses [57]. LXA4 is also considered an endogenous anti-diabetic molecule able to reduce TNF-α and other inflammatory markers associated with insulin resistance [58]. The observed increase in 5- and 15-HETE implicates increased 5- and 15-LOX activity, demonstrating a possible mechanism of LXA4 production through leukotriene A4 (LTA4) conversion. As mentioned, expanded WAT is associated with increased inflammation and adipocyte hypoxia. 15-HETE stimulates angiogenesis in adipose tissue [59], suggesting a countermeasure to the hypoxic-like features obese adipocytes often exhibit [60]. Interestingly 8,9-, 11,12-, and 14,15-EET were also elevated in obesity, and all three EETs are implicated in cellular hypoxic responses [61]. Results of the ω-6 non-esterified plasma PUFA and oxylipid analysis indicate increases in metabolites linked to metabolic syndrome, insulin resistance, inflammation resolution, and potential responses to the obesity-associated pathology observed in WAT.
PPL EPA decreased significantly in obese participants and non-esterified plasma eicosapentaenoate increased only slightly in the obese pool. Both 14,15- and 17,18-DiHETE decreased in obesity. This may be due to competition between ω-6 epoxides and ω-3 epoxides, since both depend on soluble epoxide hydrolases to form their respective DiHETEs. As mentioned, we observed increases in all ω-6 epoxides measured. Non-esterified plasma docosahexaenoate decreased in the obese pool. The only docosanoid above the LOD was maresin-1, in the lean pool. Maresin-1 is considered a pro-resolving oxylipid produced by the macrophage phenotype M2 [62]. As mentioned, the M1 phenotype is responsible for TNF-α secretion and is associated with macrophage infiltration of excess WAT. Each excess kilogram of human fat results in the accumulation of an estimated 20–30 million macrophages [63]. Therefore, this observation may suggest the increased M1 phenotype in obesity may result in decreased maresin-1 production.
Our cross-sectional study was conducted on a population of males (n=126, > 96% Caucasian, ages 48–65) to indentify associations between PPL, oxylipids, and BMI. We recognize that the generalizability of these observations is limited and should be verified in larger, more diverse populations. In addition, we report FA-based estimated enzyme activities of D5D, D6D, and SCD n-7 and n-9 are associated with BMI. These estimated enzyme activity estimates have not been extensively validated and may not be representative of enzyme kinetics in tissues. Thus, reported altered estimated enzyme activities could be related to other factors. Although we indicate our observed FA differences are associated with altered dietary intake, we did not directly collect or assess dietary intake in this small study. We acknowledge the limitations of not assessing diet, % energy from fat and supplement use in this study. Future studies should investigate the relationship between dietary intake, FA changes in the lipidome, and subsequent effects on oxylipid production in obesity.
5. Conclusion
In conclusion, we report BMI is associated with PPL FA differences potentially due to altered lipid metabolism and FA intake. Furthermore, obesity is associated with increased ω-6 non-esterified plasma PUFAs and oxylipids, suggestive of increased ROS, production of PPAR-γ ligands, and cellular hypoxia. Together these data suggest an inability to resolve obesity-associated inflammation is related to a decreased presence of EPA and DHA in PPLs, decreased non-esterified plasma docosahexaenoate, and decreased non-esterified plasma eicosapentaenoate-derived oxylipids, as well as an imbalance in the enzymatic competition between the ω-3 and ω-6 substrates of fatty acid modifying enzymes.
Acknowledgments
We would like to thank Wenjuan Ma from MSU Center for Statistical Training and Consulting for statistical help and input, MSU Mass Spectrometry and Metabolomics Core Facility, Daniel Jones for metabolomic consultation and project design, and Ami Lane-Elliot and Catherine Belcher for helping with sample processing. Research supported in part by the National Cancer Institute 1R03CA142000 and the Clinical and Translational Sciences Institute at MSU.
Abbreviations used
- AA
Arachidonic acid
- ALA
Alpha-linolenic acid
- BHT
Butylated hydroxytoluene
- COX
Cyclooxygenase
- D5D
Delta-5-desaturase
- D6D
Delta-6-desaturase
- DGLA
Dihomo-γ-linolenic acid
- DiHETE
Dihydroxy eicosatetraenoate
- DPA
Docosapentaenoic acid
- DTA
Docosatetraenoic acid
- EET
Epoxy eicosatrienoate
- EpDPE
Epoxy docosapentaenoate
- EpODE
Epoxy octadecadienoate
- ETE
Epoxy eicosatetraenoate
- FAMEs
Fatty acid methyl esters
- HDoHE
Hydroxy DHA
- HETE
Hydroxy eicosatetraenoate
- HMW
High molecular weight adiponectin
- HODE
Hydroxy octadecadienoate
- IL-6
Interleukin-6
- LA
Linoleic Acid
- LMW
Low molecular weight adiponectin
- LOD
Limit of detection
- LTA4
Leukotriene A4
- LOX
Lipoxygenase
- LXA4
Lipoxin A4
- MCP-1
Monocyte chemoattractant protein-1
- MetS
Metabolic syndrome
- MMW
Medium molecular weight adiponectin
- MRM
Multiple reaction monitoring
- NF-κB
Nuclear factor kappa of activated B cells
- ODE
octadecadienoate
- OR
odds ratio
- PGE
Prostaglandin E-series
- PL
Phospholipid
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- PPL
Plasma phospholipid
- RvD
Resolvin D-series
- SatFAs
Saturated fatty acids; SatFFAs
- TNF-α
Tumor necrosis factor alpha
- WAT
White adipose tissue
- ω-3
Omega-3
- ω-6
Omega-6;
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emanuela F, Grazia M, Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a Link between Obesity and Metabolic Syndrome. J Nutr Metab. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu M, Zhou H, Zhao J, Xiao N, Roychowdhury S, Schmitt D, Hu B, Harding CV, Hise AG, Hazen SL, DeFranco AL, Fox PL, Morton RE, Dicorleto PE, Febbraio M, Nagy LE, Smith JD, Wang JA, Li X. MyD88-dependent interplay between myeloid and endothelial cells in the initiation and progression of obesity-associated inflammatory diseases. J Exp Med. 2014;211:887–907. doi: 10.1084/jem.20131314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo HJ, Choi KM. Adipokines as a novel link between obesity and atherosclerosis. World J Diabetes. 2014;5:357–363. doi: 10.4239/wjd.v5.i3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramsden CE, Ringel A, Feldstein AE, Taha AY, Macintosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong G, Ye X, Sun L, Li H, Yu Z, Hu FB, Sun Q, Lin X. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged and older Chinese. Am J Clin Nutr. 2012;96:970–976. doi: 10.3945/ajcn.112.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu Q, Xuan W, Fan G. Roles of resolvins in the resolution of acute inflammation. Cell biology international. 2014 doi: 10.1002/cbin.10345. [DOI] [PubMed] [Google Scholar]
- 8.Volpe CM, Nogueira-Machado JA. The dual role of free fatty acid signaling in inflammation and therapeutics. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:189–197. doi: 10.2174/18715303113139990041. [DOI] [PubMed] [Google Scholar]
- 9.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. Journal of lipid research. 2012;53:1662–1669. doi: 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Mabalirajan U, Agrawal A, Ghosh B. 15-Lipoxygenase eicosanoids are the putative ligands for vanilloid receptors and peroxisome proliferator-activated receptors (PPARs) Proceedings of the National Academy of Sciences of the United States of America. 2012;109 doi: 10.1073/pnas.1118477109. E1; author reply E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 14.Raphael W, Sordillo LM. Dietary polyunsaturated fatty acids and inflammation: the role of phospholipid biosynthesis. Int J Mol Sci. 2013;14:21167–21188. doi: 10.3390/ijms141021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comstock SS, Hortos K, Kovan B, McCaskey S, Pathak DR, Fenton JI. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study. PLoS One. 2014;9:e85939. doi: 10.1371/journal.pone.0085939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurzell EA, Wiesinger JA, Morkam C, Hemmrich S, Harris WS, Fenton JI. Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA+DHA content? Prostaglandins Leukot Essent Fatty Acids. 2014 doi: 10.1016/j.plefa.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose HG, Oklander M. Improved Procedure for the Extraction of Lipids from Human Erythrocytes. Journal of lipid research. 1965;6:428–431. [PubMed] [Google Scholar]
- 18.Agren JJ, Julkunen A, Penttila I. Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. Journal of lipid research. 1992;33:1871–1876. [PubMed] [Google Scholar]
- 19.Burdge GC, Wright P, Jones AE, Wootton SA. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br J Nutr. 2000;84:781–787. [PubMed] [Google Scholar]
- 20.Mattmiller SA, Carlson BA, Gandy JC, Sordillo LM. Reduced macrophage selenoprotein expression alters oxidized lipid metabolite biosynthesis from arachidonic and linoleic acid. J Nutr Biochem. 2014;25:647–654. doi: 10.1016/j.jnutbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 21.y Y. Multiple Imputation Using SAS Software. Journal of Statistical Software. 2011;45 doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source code for biology and medicine. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King TM. Using simultaneous equation modeling for defining complex phenotypes. BMC genetics. 2003;4(Suppl 1):S10. doi: 10.1186/1471-2156-4-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki Y, Kondo K, Maeba R, Nishimukai M, Nezu T, Hara H. The proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci. 2014;63:527–537. doi: 10.5650/jos.ess13226. [DOI] [PubMed] [Google Scholar]
- 25.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Annals of the New York Academy of Sciences. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 29.Perreault M, Zulyniak MA, Badoud F, Stephenson S, Badawi A, Buchholz A, Mutch DM. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One. 2014;9:e88539. doi: 10.1371/journal.pone.0088539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci. 1998;3:d169–d175. doi: 10.2741/a272. [DOI] [PubMed] [Google Scholar]
- 31.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Progress in lipid research. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjermo H, Riserus U. Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care. 2010;13:703–708. doi: 10.1097/MCO.0b013e32833ec41b. [DOI] [PubMed] [Google Scholar]
- 33.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Progress in lipid research. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 34.Sauma L, Stenkula KG, Kjolhede P, Stralfors P, Soderstrom M, Nystrom FH. PPAR-gamma response element activity in intact primary human adipocytes: effects of fatty acids. Nutrition. 2006;22:60–68. doi: 10.1016/j.nut.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Haring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51:648–656. doi: 10.1007/s00125-008-0938-7. [DOI] [PubMed] [Google Scholar]
- 36.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JY, Kim MJ, Kim YK, Woo JS. Ceramide induces apoptosis via caspase-dependent and caspase-independent pathways in mesenchymal stem cells derived from human adipose tissue. Arch Toxicol. 2011;85:1057–1065. doi: 10.1007/s00204-011-0645-x. [DOI] [PubMed] [Google Scholar]
- 38.Yuyama K, Mitsutake S, Igarashi Y. Pathological roles of ceramide and its metabolites in metabolic syndrome and Alzheimer's disease. Biochim Biophys Acta. 2014;1841:793–798. doi: 10.1016/j.bbalip.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 41.Muthing J, Peter-Katalinic J, Hanisch FG, Unland F, Lehmann J. The ganglioside GDI alpha' IV3Neu5Ac, III6Neu5Ac-GgOse4Cer, is a major disialoganglioside in the highly metastatic murine lymphoreticular tumour cell line MDAY-D2. Glycoconjugate journal. 1994;11:153–162. doi: 10.1007/BF00731155. [DOI] [PubMed] [Google Scholar]
- 42.Ladisch S, Li R, Olson E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1974–1978. doi: 10.1073/pnas.91.5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popa I, Pons A, Mariller C, Tai T, Zanetta JP, Thomas L, Portoukalian J. Purification and structural characterization of de-N-acetylated form of GD3 ganglioside present in human melanoma tumors. Glycobiology. 2007;17:367–373. doi: 10.1093/glycob/cwm006. [DOI] [PubMed] [Google Scholar]
- 44.Tiberti C, Dotta F, Anastasi E, Torresi P, Multari G, Vecci E, Andreani D, Di Mario U. Anti-ganglioside antibodies in new onset type 1 diabetic patients and high risk subjects. Autoimmunity. 1995;22:43–48. doi: 10.3109/08916939508995298. [DOI] [PubMed] [Google Scholar]
- 45.Hodge AM, English DR, O'Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 46.Kroger J, Schulze MB. Recent insights into the relation of Delta5 desaturase and Delta6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol. 2012;23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Webb P, Zimmet P, Bonser A, King H, Bottomley S. Factors affecting fasting serum C-peptide levels in Micronesians: comparison with a Caucasoid population. Diabetologia. 1984;27:23–26. doi: 10.1007/BF00253496. [DOI] [PubMed] [Google Scholar]
- 48.Harnack K, Andersen G, Somoza V. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr Metab (Lond) 2009;6:8. doi: 10.1186/1743-7075-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006;84:44–53. doi: 10.1093/ajcn/84.1.44. [DOI] [PubMed] [Google Scholar]
- 50.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 51.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 52.Mirzaei K, Hossein-Nezhad A, Keshavarz SA, Koohdani F, Saboor-Yaraghi AA, Hosseini S, Eshraghian MR, Djalali M. Crosstalk between circulating peroxisome proliferator-activated receptor gamma, adipokines and metabolic syndrome in obese subjects. Diabetol Metab Syndr. 2013;5:79. doi: 10.1186/1758-5996-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, Rogler G. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol. 2007;74:612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Shishehbor MH, Zhang R, Medina H, Brennan ML, Brennan DM, Ellis SG, Topol EJ, Hazen SL. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. 2006;41:1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai IJ, Croft KD, Mori TA, Falck JR, Beilin LJ, Puddey LB, Barden AE. 20-HETE and F2-isoprostanes in the metabolic syndrome: the effect of weight reduction. Free Radic Biol Med. 2009;46:263–270. doi: 10.1016/j.freeradbiomed.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 57.Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, Serhan CN. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 58.Das UN. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot Essent Fatty Acids. 2013;88:201–210. doi: 10.1016/j.plefa.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Soumya SJ, Binu S, Helen A, Reddanna P, Sudhakaran PR. 15(S)-HETE-induced angiogenesis in adipose tissue is mediated through activation of PI3K/Akt/mTOR signaling pathway. Biochem Cell Biol. 2013;91:498–505. doi: 10.1139/bcb-2013-0037. [DOI] [PubMed] [Google Scholar]
- 60.Trayhurn P. Hypoxia and Adipocyte Physiology: Implications for Adipose Tissue Dysfunction in Obesity. Annu Rev Nutr. 2014 doi: 10.1146/annurev-nutr-071812-161156. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki S, Oguro A, Osada-Oka M, Funae Y, Imaoka S. Epoxyeicosatrienoic acids and/or their metabolites promote hypoxic response of cells. J Pharmacol Sci. 2008;108:79–88. doi: 10.1254/jphs.08122fp. [DOI] [PubMed] [Google Scholar]
- 62.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]