Abstract
As a consequence of intracerebral hemorrhage (ICH), blood components enter brain parenchyma causing progressive damage to the surrounding brain. Unless hematoma is cleared, the reservoirs of blood continue to inflict injury to neurovascular structures and blunt the brain repair processes. Microglia/macrophages (MM Φ) represent the primary phagocytic system that mediates the cleanup of hematoma. Thus the efficacy of phagocytic function by MM Φ is an essential step in limiting ICH-mediated damage. By using primary microglia to model red blood cell (main component of hematoma) clearance, we studied the role of transcription factor Nrf2, a master-regulator of anti-oxidative defense, in the hematoma clearance process. We showed that in cultured microglia, activators of Nrf2 1) induce anti-oxidative defense components, 2) reduce peroxide formation, 3) upregulate phagocytosis-mediating scavenger receptor CD36, and 4) enhance RBC phagocytosis. Through inhibiting Nrf2 or CD36 in microglia, by DNA-decoy or neutralizing antibody, we documented the important role of Nrf2 and CD36 in RBC phagocytosis. Using autologous blood injection ICH model to measure hematoma resolution, we showed that Nrf2 activator, sulforaphane, injected to animals after the onset of ICH, induced CD36 expression in ICH-affected brain and improved hematoma clearance in rats and wild-type mice, but expectedly not in Nrf2-knockout-(KO) mice. Normal hematoma clearance was impaired in Nrf2-KO mice. Our experiments suggest that Nrf2 in microglia play an important role in augmenting the anti-oxidative capacity, phagocytosis and hematoma clearance after ICH.
Keywords: intracerebral hemorrhage, Nrf2, sulforaphane, microglia, phagocytosis, hematoma
INTRODUCTION
Intracerebral hemorrhage (ICH) has the highest mortality rate of all stroke subtypes; about 40,000 Americans die annually from the disease (Broderick et al. 1993a). ICH can injure the brain in two ways: initially via mass effect (primary injury) and then secondarily via toxic blood (e.g. hemolysis) products and pro-inflammatory and -oxidative responses (Aronowski & Hall 2005, Xi et al. 2006, Wagner et al. 2003, Hanley 2009, Wang et al. 2002). One of the key predictors of poor outcome after ICH is hematoma volume (Broderick et al. 1993b). A larger hematoma may cause greater injury to the brain not only because of mass effect, but also because it results in a larger reservoir of potentially neurotoxic iron-rich blood. One could certainly hypothesize that faster and more efficient clearance of toxic blood products may be essential in limiting ICH-mediated secondary injury. In agreement with this notion, we have shown that targeting hematoma cleanup via the transcription factor PPARγ may represent a therapeutic target in ICH (Zhao et al. 2007b, Gonzales et al. 2012).
Following ICH, cleanup of the hematoma is accomplished by microglia – the resident macrophages of the brain – along with hematogenous macrophages that enter the site of injury. To achieve cleanup, microglia/macrophages (MMΦ) engulf the hematoma components (Zhao et al. 2007b, Woo et al. 2012); however, they generate large quantities of oxidant by-products in doing so (Splettstoesser & Schuff-Werner 2002). Thus, microglia involved in cleaning up ICH need to be able to withstand both the oxidative stress generated from the initial injury along with the oxidant byproducts generated by the microglia themselves. In other words, to retain their functionality, microglia must possess some unique ability to adapt to the pro-oxidative environment. The Keap1-Nrf2 stress-response pathway is activated by electrophiles and pro-oxidants and it provides the key stress-sensing system that allows cells under oxidative stress to combat oxidative insults by inducing genes with anti-oxidative functions (Brigelius-Flohe & Flohe 2011). While Nrf2 is fairly ubiquitous, it is likely that its anti-oxidative role may be uniquely instrumental for cells with scavenging functions, such as microglia.
The objective of this study was to establish whether microglia involved in phagocytosis-mediated cleanup of ICH do so with the assistance of Nrf2 pathways. We hypothesized that Nrf2 is important for effective hematoma cleanup and that activation of Nrf2 would enhance hematoma resolution, whereas inhibition of Nrf2 would compromise hematoma resolution after ICH.
MATERIAL AND METHODS
All animal studies followed the guidelines outlined in Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Animal Welfare Committee of University of Texas Health Science Center at Houston.
Microglia culture
We isolated microglia using p1–p2 mouse pups, as previously described (Zhao et al. 2007b). Briefly, the cells from brain tissue were seeded in 75 cm2 TC flasks and cultured for 14d. The loosely adherent microglia were harvested, centrifuged and re-plated onto poly-L-lysine coated TC plates, with or without 12-mm diameter German-glass, at a density of 2~5×105 cells/ml.
Immunohistochemistry
To characterize the expression of Nrf2 in microglia, microglia in culture were fixed in cold methanol or 2% formalin and labeled with rabbit anti-Nrf2 (Santa Cruz) according to the protocol as we described (Zhao et al. 2007b). To demonstrate the ability of microglia to internalize RBC and to determine the spatial relationship between hematoma and microglia/macrophages in the ICH-affected animal brains, we performed double immunofluorescence using rabbit anti-rat RBC antibody (Fitzgerald) and mouse anti-rat CD68 antibody (Serotec) to label phagocytic cells. The RBC and the CD68 were visualized with goat anti-rabbit IgG-Alexa Fluor 546 and goat anti-mouse IgG-Alexa Fluor 488, respectively. The nuclei of the cells were stained with Hoechst 33258.
Image capture and cell counting
A Zeiss Axioskop 2 microscope equipped with a CCD camera and operated with MetaMorph 7.5 software was used for image acquisition. The fluorescence-labeled cells were visualized using Ex/Em of 490/520 nm for Alexa Fluor 488, 550/575 nm for Alexa Fluor 546, and 365/480 nm for Hoechst 33258.
RBC isolation and labeling for phagocytosis assay
The red blood cells (RBC) were purified using density gradient centrifugation (BD Vacutainer® CPT™). After washing with PBS, the purified RBC were labeled with the fluorescent dye 5 (6)-carboxyfluorescein diacetate (CFDA; Molecular Probes). The labeled RBCs were diluted to109 cells/ml and used as an indicator/target of phagocytosis, as we reported (Zhao et al. 2007b).
Phagocytosis in vitro
The phagocytosis assay was performed as we described earlier (Zhao et al. 2007b). At indicated time points after adding the CFDA-labeled RBC to the cultured microglia (10:1 ratio), we separated the unphagocytosed free floating RBC from phagocytes that are attached to the plastic by aspiration. Microglia containing engulfed RBC were lysed in distilled water and fluorescence intensity in the supernatant from the cell lysate was measured using a fluorometer with a 490/520nm filter set. The fluorescence intensity (OD) was referred as phagocytosis index.
To study the role of CD36 during phagocytosis by rat microglia, we added 10 μg/ml of CD36 neutralizing antibody (clone FA6-152, Beckman Coulter) or mouse anti-FITC antibody (control antibody, Gene Therapy) at 15min prior to adding CFDA-labeled RBCs, the targets of phagocytosis.
In all instances SF (2 μM) was added 16h prior to initiation of the phagocytosis assay.
Hydrogen peroxide (H2O2) assay
To ensure the accuracy of our conclusions, we measured H2O2 concentrations in microglia culture media using OxiSelect™ ADHP/Resorufin fluorescence assay (Cell Biolabs, STA-344). We removed the phenol red from DMEM culture medium as its presence interfered with the OD reading.
Sulforaphane and tert-butylhydroquinone (tBHQ) treatment
For the animal experiments, 5 mg/kg sulforaphane (SF, LKT Laboratories, S8044) dissolved in 10% corn oil was injected, i.p., at 30 min and 24h after ICH. A solution of 10% corn oil in PBS was used as the vehicle control. For the cell culture experiments, 1~10 μM SF or 5 μM tBHQ in DMSO was directly applied into the culture medium. The vehicle (0.1~0.5% DMSO in culture media) was used as the control for SF and tBHQ.
Nuclear extraction and Nrf2 EMSA
After exposing microglial cells to SF, we fractionated the cells into cytosol and nuclear fractions as we described (Zhao et al. 2007a, Zhao et al. 2007c). Using nuclear fractions, the Nrf2 activity was measured with an Nrf2 EMSA kit (Active Motif) according to the manufacturer’s instructions. Using 96-well plates containing oligonucleotide representing the ARE consensus binding site (5′-GTCACAGTGACTCAGCAGAATCTG), we added 2 μg of nuclear extract protein per well. The DNA bounded Nrf2 was probed with an anti-Nrf2 antibody. After incubation with HRP-conjugated secondary antibody and developing color, the optical density (OD) was measured at 450nm. The nuclear extract from Nrf2 transfected COS-7 cells was used as the positive control. After subtracting the baseline (mutated oligonucleotide) value, the average OD of two independent set of samples was calculated.
Western blot
The Western blot was performed as we described (Zhao et al. 2007a). Rabbit anti-Nrf2 (Santa Cruz), rabbit anti-CD36 (Cayman), mouse anti-heme oxygenase 1 (HO-1, Stressgen) and mouse anti-GAPDH (INEUROMICS) were used as primary antibodies. After incubation with appropriate secondary antibody conjugated with HRP (1:5000, Zymed) the immunopositive bands were visualized with ECL (Pierce, Rockford, IL). Semi-quantification of luminescence signal intensity was determined by analyses of optical density on X-ray film using a Computer-Assisted Kodak Analysis (EDAS) 290 system.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
The primary microglia in culture were washed with RNase/DNase-free PBS. Trizol-Reagent was used for RNA isolation. RT-PCR analyses were done as described previously (Zhao et al. 2009c, Zhao et al. 2007b, Zhao et al. 2006). We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal standard. The sequences of primers are listed in Tab 1.
ARE decoy
The double stranded oligonucleotide ARE decoy (ctaatggtgacaaagcaacttt; containing Nrf2 core binding sequence) or mutant-decoy (cgactgccttcaaaataacttt) in JetPEI-Macrophage transfection reagent (Polyplus Transfection Inc.) was added to microglia in culture for 24h at 2.5 μM. After 24h incubation, the microglial cells were used for phagocytosis assay.
Intracerebral hemorrhage
We induced intracerebral hemorrhage in rats and mice via intra-striatal injection of autologous blood, as described previously (Felberg et al. 2002, Hickenbottom et al. 1999, Zhao et al. 2009a, Zhao et al. 2006, Zhao et al. 2007b). Briefly, male Sprague-Dawley rats (250 ~ 350g) along with Nrf2+/+ and Nrf2−/− mice (Chan et al. 1996, Zhao et al. 2007a) (both C57BL/6 background; 25 ~ 30g, of both sexes equally distributed between the groups), were immobilized onto a stereotaxic frame under chloral hydrate anesthesia (0.35g/kg; i.p.). A 1 mm diameter burr hole was drilled into the skull and a 26-gauge stainless steel cannula was inserted for blood infusion (collected from the femoral artery; 15 μl/5 min for mice, or 35 μl/5 min for rats) into the left corpus striatum. Core body temperature was maintained at 37±0.5°C during the entire surgery and for 2h afterwards.
Tissue harvesting
Animals were deeply anesthetized with chloral hydrate (0.5 g/kg; i.p.) and intracardially perfused with ice-cold PBS. For histology and biochemical analyses, the whole brains or the subdissected tissues representing hematoma-affected striatum were snap frozen by submersion in −80°C 2-methylbutane and stored in the −80°C freezer prior to cryosectioning, RNA isolation, or protein analyses.
Hematoma size measurement
Hematoma resolution was assessed by measuring the amount of hemoglobin (Hb) remaining in the hematoma–affected brain at 7 days after ICH, as we detailed previously (Zhao et al. 2009a, Zhao et al. 2007b). Briefly, the mice under chloral hydrate anesthesia were perfused with ice-cold PBS to remove intravascular blood. Hemoglobin content in the ipsilateral striatum was measured using Drabkin’s reagent (Asahi et al. 2000, Zhao et al. 2007b). Peripheral blood (0, 2, 4, 8, 16, or 20 μl) was added to the homogenate of naïve perfused brain and used to prepare a standard calibration curve as described (Zhao et al. 2007b). The data were expressed as blood volume per brain homogenate.
Nrf2 knockout mice
Nrf2 knockout mice were constructed and characterized in Dr. Yuet Wai Kan’s laboratory (Department of Laboratory Medicine, and Howard Hughes Medical Institute and Cardiovascular Research Institute, UCSF) (Chan et al. 1996) and used in our earlier studies (Zhao et al. 2007a). All the experimental groups included both male and females mice that were equally distributed among all groups.
Statistical Analyses
All data is expressed as a mean ± SEM. For the in vitro experiments, we pooled the samples from three culture wells and repeated the experiments three times. We performed statistical analyses using the GraphPad and InStat programs. One-way analysis of variance (ANOVA) followed by Newman-Keuls post-test was used for multiple comparisons. Non-paired t-test was used when two groups were compared.
RESULTS
Nrf2 promotes phagocytosis of RBCs by microglia/macrophages
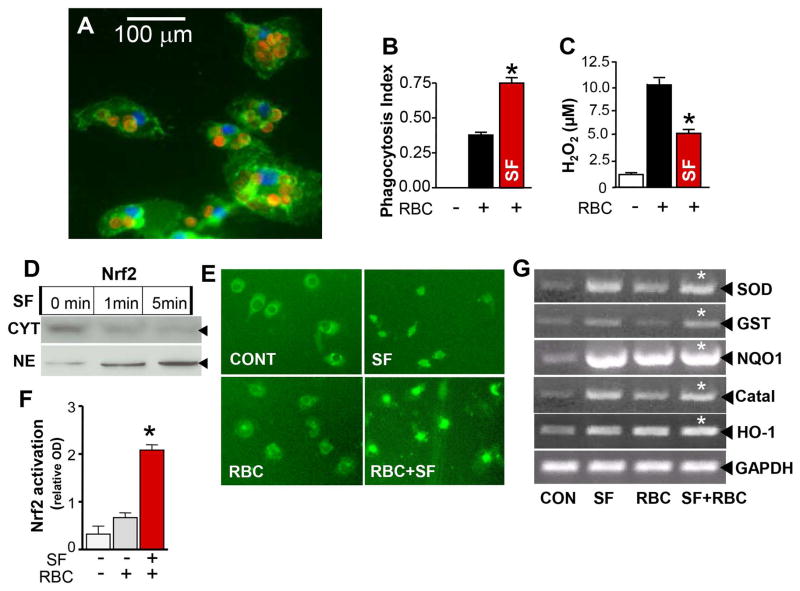
With our first set of in vitro experiments, we assesses the of RBC phagocytosis efficacy by cultured microglia (Fig 1A) and also characterized the oxidative environment generated during this process. As displayed in Fig 1C, RBC cleanup by microglia was associated with robust oxidative stress as evidenced by accumulation of H2O2 in the culture media. Phagocytosis of RBC was significantly enhanced by treatment of the microglia with the prototypic Nrf2 activator, sulforaphane (SF) (Fig 1B; red bar). Interestingly, this enhanced phagocytosis was accompanied by a decrease in the levels of H2O2 generated. Similar results were obtained when microglia were treated with a different Nrf2 activator, tBHQ. tBHQ increased phagocytosis of RBC by 36.7% and reduced H2O2 production by 31.5%.
Fig 1.
(A) Phagocytosis of RBC by microglia. Upon exposure to RBC the rat microglia (green; CD11b staining) became much bigger in size and filled out with RBC, as shown at 1h after starting phagocytosis. The RBC and the nuclei were labeled with a rabbit anti-rat RBC antibody (red) and DAPI (blue). Note that RBC are engulfed by microglia. Phagocytosis of CFDA-RBC by microglia in culture (B) was associated with the increased H2O2 in the culture media (C), as determined at 2h after initiation of phagocytosis. Pre-treatment of microglia with SF (2μM) increased engulfment of RBC (B; red bar) and reduced accumulation of H2O2 in the culture media (C; red bar). The data are expressed as mean ± SEM (n=3 independent experiments). *p ≤ 0.05, vs. RBC alone. (D) Nrf2 Western blot showing Nrf2 protein in the cytoplasm (CYT) and nuclear fractions (NE) at 1min and 5min after adding 2μM SF to rat primary microglia. Note very fast nuclear translocation/activation of Nrf2 with SF. (E) Representative photograph of Nrf2 immunofluorescence in rat microglia at the baseline (CONT) and at 2 min after exposure to 2μM SF, RBC or SF+RBC. Note increased nuclear presence of Nrf2 upon activation with SF. (F) DNA binding activity of nuclear Nrf2 in microglia upon adding RBC or RBC plus SF, checked by Nrf2 EMSA. (G) Photograph of representative gels showing RT-PCR products to demonstrate the gene expression profile - indicatives of Nrf2 activation in rat microglia at 6h after exposure to 2μM SF, RBC or SF+RBC.
To determine whether this enhanced phagocytic effect was indeed mediated by SF-mediated activation of Nrf2, we tested the translocation of Nrf2 in treated microglia. Indeed; SF treatment resulted in rapid Nrf2 nuclear translocation (Fig 1D – Western blot, and 1E - immunohistochemistry) along with elevated Nrf2 DNA binding (Fig 1F). In addition, the expression of prototypic Nrf2 gene targets including superoxide dismutase-1 (SOD1), glutathione S-transferase (GST), catalase, NAD(P)H dehydrogenase, quinone 1 (NQO1), and heme oxygenase-1 (HO-1) was increased by SF treatment (Fig 1G).
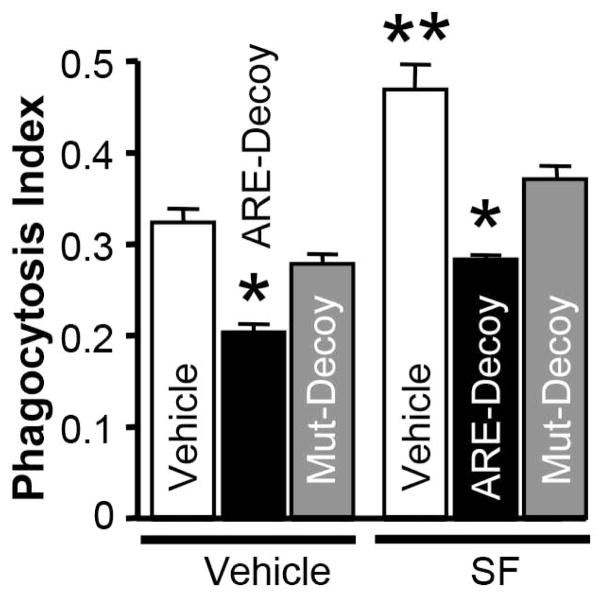
Lastly, we tested the effects of Nrf2 sequestration by treating microglial cell cells with a fragment of DNA containing a Nrf2 binding site (ARE decoy). The resultant inactivation of Nrf2 was associated with a reduction in the phagocytosis efficacy of the microglia, as compared to microglia treated with a mutated DNA decoy (control) (Fig 2). Moreover, the ARE-DNA decoy attenuated the ability of SF treatment to enhance phagocytosis, providing additional evidence that Nrf2 is likely the primary target of SF in this setting.
Fig 2.
Phagocytosis index indicating efficacy of RBC engulfment by rat brain microglia at 2h after adding CFDA-RBC with Nrf2-decoy (ARE-Decoy) or mutant decoy (Mut-Decoy), and absence or presence of 2μM SF. Microglia were pre-treated with the Nrf-2 decoy or mutant-decoy for 24h before the assessment of phagocytosis, while SF was added 16h before the exposure to CFDA-RBC. Results are expressed as mean ± SEM (n=3). *p<0.05 and **p 0.01, compared with the vehicle control (JetPEI+RBC).
Nrf2 induces CD36 expression, a potential target for enhancement of microglial phagocytosis
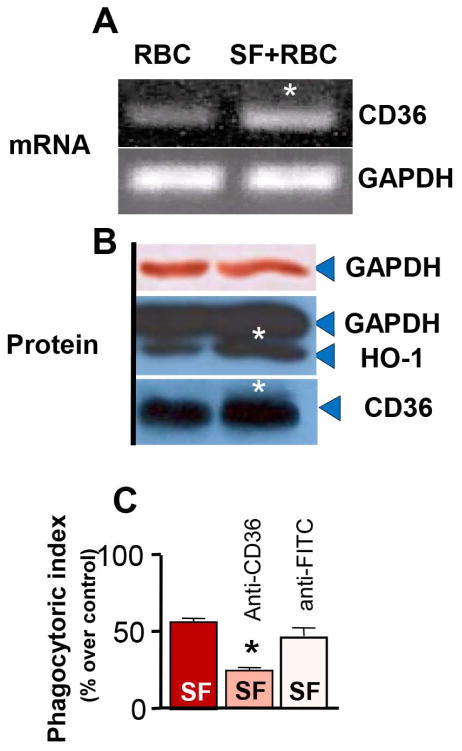
Nrf2 is a transcriptional regulator of CD36 (Ishii et al. 2004, Olagnier et al. 2011), a key scavenger receptor on MMΦ whose important role in the process of phagocytosis is documented (Ren et al. 1995, Zhao et al. 2007b, Woo et al. 2012). However, the relevance of microglial Nrf2 to CD36 expression and phagocytotic function is unclear. Now, we report that treatment with SF in addition to activating Nrf2 and enhancing RBC phagocytosis (Fig 1B and 1D–F), resulted in the increased expression of CD36 mRNA in microglia (Fig 3A). As anticipated, we further determined that SF could effectively induce CD36 and HO-1 protein expression within 6h, less than the window whereby phagocytosis efficacy was measured (16h after adding SF)(Fig 3B). In addition, a CD36 neutralizing antibody, but not an isotype-control antibody, inhibited SF-induced phagocytosis of RBC (Fig 3C); suggesting coupling between Nrf2 and CD36 in the RBC engulfment process.
Fig 3.
(A) Photograph of RT-PCR gels showing CD36 and GAPDH gene expression profile and (B) CD36, HO-1 and GAPDH protein expression levels in rat microglia upon exposure to RBC or RBC+2 μM SF for 6h. GAPDH is used as an internal control – the same GAPDH band was visualized using alkaline phosphatase (brows; upper band) and ECL (lower band). (C) Phagocytosis Index of rat microglia at 2h after exposure to CFDA-RBC in presence of 2μM SF in microglia treated with or without 10 μg/ml of anti-CD36 or anti-FITC antibodies (isotope control). The data are expressed as mean±SEM (n=3 independent experiments). *p ≤0.05, compared with all other groups.
Finally, using the rat model of intracerebral hemorrhage, we observed that administering SF to our rats (2.5 mg/kg; i.p. at 30 min and 2h after ICH) resulted in an approximate 2.7-fold increase of CD36 mRNA expression in ICH-affected brain, as measured at 48h after the onset of ICH, suggesting that CD36 may play a role in Nrf2-mediated phagocytosis/cleanup in ICH-affected brain.
Nrf2 promotes hematoma clearance after ICH in animals
The logical extension of our in vitro investigations was to examine whether Nrf2 was involved in hematoma resolution after experimental ICH in animals. We and others have previously demonstrated that Nrf2 contributes to the induction of anti-oxidant enzymes within the brain and improves neurological deficits in an animal model of ICH (Zhao et al. 2007a, Wang et al. 2007). We therefore hypothesized that SF therapy would result in more effective hematoma clearance by MMΦ via attenuating oxidative stress and augmenting expression of the scavenger receptor, CD36.
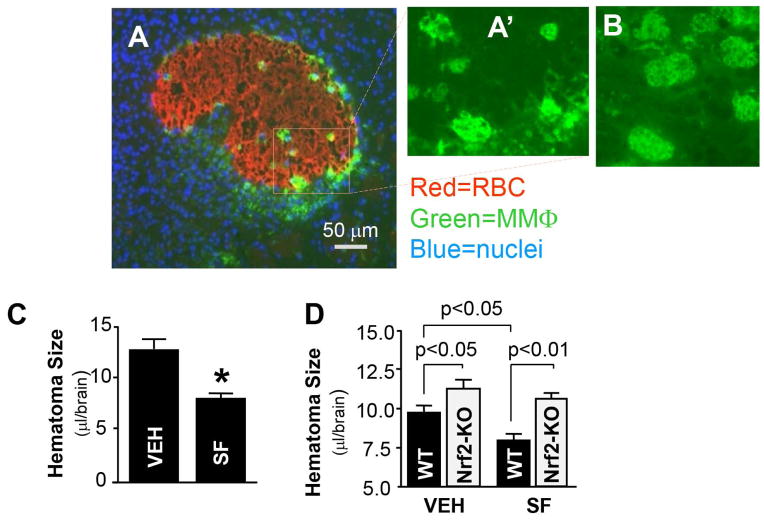
In this study, we used a clinically relevant animal model of ICH that is based on intracerebral injection of a constant volume of autologous blood. Following blood injection, vehicle versus SF was administered (5 mg/kg for mouse and 2.5 mg/kg for rat, i.p.) at 0.5h and 24h. Hematoma volume was subsequently measured at day 7 post-injection in mice and at day 10 post-injection in rats using the amount of hemoglobin remaining in the brain as an indicator of hematoma size. First, we showed that phagocytic microglia were primarily localized to the periphery of the hematoma, in the perihematomal tissue (Fig 4A, 4A″ and 4B). Second, we observed that SF treatment resulted in a significantly greater degree of hematoma resolution in both rat (Fig 4C) and mouse (Fig 4D). We repeated these experiments in Nrf2-knockout (KO) mice (Fig 4D). Hematoma resolution was significantly impaired in the Nrf2-KO mice as compared to wild type mice. Furthermore, treatment with SF did not result in enhanced hematoma cleanup in the Nrf2-KO mice, suggesting that Nrf2 is indeed necessary for SF-mediated hematoma clearance.
Fig 4.
(A) Photomicrograph of hematoma in rat brain on day 7 after ICH, double-stained with anti-RBC (red) and anti-CD68 (green) antibodies. The panel A″ and B shows the close-up morphology of CD68+-microglia/macrophages at the hematoma-affected brain at 7 and 3 days after ICH, respectively. (C) Hematoma size on day 10 after ICH in the SD rats treated with vehicle (10% corn oil in PBS) or SF (2.5 mg/kg at 30min and 24h after ICH). The data (mean±SEM, n=7) are expressed as the blood volume in the ICH-affected brain tissue. *p ≤0.05, compared with the vehicle control. (D) Hematoma size on day 7 after ICH in WT and Nrf2-KO mice treated with vehicle (10% corn oil in PBS) or SF (5 mg/kg at 30min and 24h after ICH). The data (mean±SEM, n=5–7) are expressed as the blood volume in the ICH-affected brain tissue.
DISCUSSION
We have demonstrated here that Nrf2 in microglia/macrophages plays a pivotal role in regulating the phagocytic functions of these cells and that in an experimental model of ICH, Nrf2 appears to be essential to hematoma clearance. In the initial set of experiments, we used an in vitro system to demonstrate that microglia are capable of phagocytosing RBC and that activators of Nrf2 such as sulforaphane enhance this process whereas inactivation of Nrf2 inhibits cleanup. We also demonstrated that the expression of CD36 – a member of scavenger receptor class B and a target gene of Nrf2 – was indeed upregulated in response to sulforaphane and likely participates in modulation of phagocytic function by Nrf2. Finally, we demonstrated that sulforaphane treatment of mice and rats with experimentally-induced ICH significantly increased hematoma resolution and that this effect was dependent upon Nrf2.
Efficient removal of hematoma components after ICH is necessary for achieving inflammation resolution and for allowing new homeostatic environment and functional recovery to be achieved (Zhao et al. 2007b, Zhao et al. 2009a, Manoonkitiwongsa et al. 2001). In general, the majority of post-injury cleanup functions are conducted by MMΦ that either infiltrate the damaged tissue or proliferate locally at the injury site (Murray & Wynn 2011, Ransohoff & Perry 2009). In this study, we also noted an abundance of phagocytic (CD68+) cells surrounding the hematoma (e.g. in the perihematomal tissue) as early as 24 to 72 hours post-ictus. This location of phagocytic cells appears to be highly strategic to conduct an effective cleanup. In fact, the close-up images of peri-ICH zone phagocytes indicate that they are indeed enlarged and contain many vesicular structures, suggesting that they are actively involved in the engulfment process. Normally after ICH, phagocytic microglia/macrophages progressively increase in the peri-hemorrhagic areas for approximately the first 7 days, and then slowly subside over 1 to 2 weeks (Gong et al. 2000) (JA, unpublished results). This time-profile corresponds to the time profile of hematoma resolution, suggesting that these cells indeed play an important role in the hematoma resolution process. In our in vitro experiments with cultured microglia, individual microglia were capable of engulfing many RBC, supporting the notion that microglia are important players in the cleanup process after ICH.
The Keap1-Nrf2 system is a vital sensing system, enabling cells to adapt to and to combat oxidative stress elaborated during tissue injury and in response to xenobiotics (van Muiswinkel & Kuiperij 2005, Zhang et al. 2014, Zhao et al. 2007a, Moi et al. 1994). Nrf2 itself is a ubiquitous pleiotropic transcription factor and a key genomic homeostatic regulator of intracellular stress (Moi et al. 1994). Upon activation by oxidative and electrophilic stress, Nrf2 transactivates the antioxidant response element (ARE) (Itoh et al. 1999) within the regulated region of many cytoprotective target genes which encode for critical mediators of cellular defense functions. Among many, these genes include: SOD (Park & Rho 2002), catalase, GST, HO-1 (Alam et al. 1999), haptoglobin (Hp) (Zhao et al. 2009b, Alam et al. 1999, Kwak et al. 2001). In addition, Nrf2, similar to transcription factor PPARγ, controls the expression of CD36 (Ishii et al. 2004, Maruyama et al. 2008), an essential scavenger receptor that in phagocytic cells assists in engulfment of various phagocytic targets (Ren et al. 1995) (including extravascular/displaced RBC) which may represent a key component in achieving brain cleanup after stroke or intracerebral hemorrhage (Zhao et al. 2009a, Zhao et al. 2007b). Removal of RBC by microglia may have a profound indirect effect on oxidative stress, as it could reduce the accumulation of free iron (from lysed unphagocytosed RBC) and consequently the formation of iron-catalyzed free radicals.
To activate Nrf2 in our animal studies, we utilized a prototypic agonist, sulforaphane. Sulforaphane is a sulfur-containing isothiocyanate derivative found in cruciferous vegetables that was demonstrated to selectively activate Nrf2 (Thimmulappa et al. 2002, Zhao et al. 2007a). In order to ensure penetration into the central nervous system, in our in vivo experiments we adopted an approximately 10-fold higher (weight/volume) dose than previously identified as effective in reducing oxidative damage to microglia from H2O2 (200 mM). An identical dose of sulforaphane was used in prior studies of Nrf2 after ICH and was effective in activating Nrf2 in the brain tissue and inducing Nrf2-target gene expression (Zhao et al. 2007a).
While phagocytosis-mediated cleanup is essential for resolution of inflammation and achieving the repair of injured brain, phagocytosis/phagosome-mediated catabolism of the engulfed dead cells and cellular debris is known to generate highly pro-oxidative responses that could adversely affect neighboring cells and hamper the benefits of the cleanup process (Splettstoesser & Schuff-Werner 2002, Yenari et al. 2006, Banati et al. 1993, Cox et al. 1995, Maderna & Godson 2003, Taylor & Sansing 2013). Thus, activation of Nrf2 by improving the anti-oxidative capacity of cells, could protect not only all neurovascular components of the affected brain, but could also prevent the generation and spilling of free radicals by phagocytes themselves, protecting them from oxidative self-injury. It is furthermore intriguing to note that under conditions where Nrf2 through ARE transcriptionally augments production of both CD36 and anti-oxidants, the increased oxidative stress resulting from the augmented CD36-assisted phagocytosis could be buffered by the improved anti-oxidative defense system. This functional CD36-antioxidative defense coupling could in fact have key roles in allowing the cleanup process to be more efficient. For instance, in the context of ischemic stroke, it appears that CD36 may act as a source of oxidative damage and pro-inflammatory responses (mediated by NF-κB activation in microglia) (Cho et al. 2005, Abe et al. 2010, Cho & Kim 2009), as CD36-deficient mice subjected to focal ischemia experienced less damage than CD36-proficient mice (Cho et al. 2005). In this respect, we believe that Nrf2-mediated anti-oxidative function in microglia is essential for preventing CD36-associated oxidative stress and NF-κB activation (Kunz et al. 2008), a process that requires the presence of oxidants for its induction (Bowie & O’Neill 2000). CD36 may also control the pro-oxidative environment directly by mediating removal of oxidatively damaged cells (including oxidized low density lipoprotein; OxLDL) (Sambrano et al. 1994) and cell fragments accumulated at the site of injury. In fact, in studies with neonatal stroke, deficient phagocytosis caused by CD36 deletion was postulated to contribute to augmented damage after experimental ischemic stroke (Woo et al. 2012).
It is well-documented that after ICH, hematoma is gradually cleared out over time from the brain (Zhao et al. 2007b). We have demonstrated with our work here that the residual hematoma is significantly larger in animals deficient in Nrf2 as compared to control animals, suggesting impaired cleanup processes in Nrf2 knockout animals. We also demonstrated that Nrf2 activation via sulforaphane was capable of improving hematoma resolution in control mice, but not in the Nrf2-KO mice. We therefore propose that Nrf2 plays an essential role in the effective cleanup process after ICH, perhaps via coordinated efforts to enhance phagocytosis while concomitantly limiting oxidative stress.
Table 1.
The sequences of primers
| Gene Name | Sequence | product Size (bp) | Gene ID | location of Primer | |
|---|---|---|---|---|---|
| GAPDH | F | AGACAGCCGCATCTTCTTGT | 323 | X 02231 | 24- |
| R | TACTCAGCACCAGCATCACC | -346 | |||
| CD36 | F | GCAAAGAAGGAAAGCCTGTG | 329 | NM_031561 | 1231- |
| B | GCCCAGGAGCTTTATTTTCC | -1559 | |||
| Nrf2 | F | CAGTCTTCACCACCCCTGAT | 451 | AF037350 | 509- |
| B | CTAATGGCAGCAGAGGAAGG | -959 | |||
| Catalase | F | ATGCAAAGGGAGCAGGTG | 576 | M11670 | 307–324 |
| B | AATGGGAAGGTTTCTGCC | 947–964 | |||
| GST | F | GCTGGAGAAGGTGTCCAGAG | 340 | NM_181371 | 373- |
| R | AAGTCTGGCATTCAGGGTTG | -712 | |||
| NQO1 | F | ACCTTGCTTTCCATCACCA | 476 | BC083542 | 516 |
| R | CAAAGGCGAAAACTGAAAGC | -991 | |||
| SOD1 | F | CGTCATTCACTTCGAGCAGA | 341 | X05634 | 43- |
| R | CACCTTTGCCCAAGTCATCT | -380 | |||
| HO-1 | F | CACCAGCCACACAGCACTAC | 599 | NM_012580 | 22–42 |
| R | AAGGCGGTCTTAGCCTCTTC | 600–620 | |||
Acknowledgments
This work was supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grants RO1NS060768 and R01NS064109 of NIH/NINDS.
Abbreviations
- Nrf2
Nuclear factor-erythroid 2 p45-related factor 2
- NF-κB
nuclear factor κB
- ICH
intracerebral hemorrhage
- RT-PCR
Reverse Transcription-Polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- EGTA
ethylenebis(oxyethylenenitrilo) tetra acetic acid
- PBS
Phosphate buffered saline
- DTT
dithiothreitol
- PMSF
phenylmethylsulfonyl fluoride
- TEMED
N,N,N′,N′-tetramethyletheylenediamine
- PPARγ
Peroxisome proliferator-activated receptor gamma
- SF
Sulforaphane
- tBHQ
tert-butylhydroquinone
- RBC
red blood cells
- CFDA
fluorescent dye 5 (6)-carboxyfluorescein diacetate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
Footnotes
The authors have no conflicts of interest to declare.
References
- Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, Iadecola C. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke; a journal of cerebral circulation. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. The Journal of biological chemistry. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurological research. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants & redox signaling. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. Journal of Neurosurgery. 1993a;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke; a journal of cerebral circulation. 1993b;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. Journal of neurochemistry. 2009;109(Suppl 1):126–132. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: The “black hole” model of hemorrhagic damage. Annals of neurology. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain research. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- Gonzales NR, Shah J, Sangha N, et al. Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC) International journal of stroke : official journal of the International Stroke Society. 2012 doi: 10.1111/j.1747-4949.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- Hanley DF. Intraventricular Hemorrhage. Severity Factor and Treatment Target in Spontaneous Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2009 doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke; a journal of cerebral circulation. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. discussion 2477–2478. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz A, Abe T, Hochrainer K, Shimamura M, Anrather J, Racchumi G, Zhou P, Iadecola C. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Maruyama A, Tsukamoto S, Nishikawa K, et al. Nrf2 regulates the alternative first exons of CD36 in macrophages through specific antioxidant response elements. Archives of biochemistry and biophysics. 2008;477:139–145. doi: 10.1016/j.abb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier D, Lavergne RA, Meunier E, et al. Nrf2, a PPARgamma alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog. 2011;7:e1002254. doi: 10.1371/journal.ppat.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EY, Rho HM. The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Mol Cell Biochem. 2002;240:47–55. doi: 10.1023/a:1020600509965. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrano GR, Parthasarathy S, Steinberg D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3265–3269. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splettstoesser WD, Schuff-Werner P. “Oxidative stress in phagocytes--”the enemy within. Microsc Res Tech. 2002;57:441–455. doi: 10.1002/jemt.10098. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clinical & developmental immunology. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4:267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free radical biology & medicine. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke; a journal of cerebral circulation. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- Woo MS, Wang X, Faustino JV, Derugin N, Wendland MF, Zhou P, Iadecola C, Vexler ZS. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Annals of neurology. 2012;72:961–970. doi: 10.1002/ana.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke; a journal of cerebral circulation. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang S, Mao L, et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke; a journal of cerebral circulation. 2009a;40:S92–94. doi: 10.1161/STROKEAHA.108.533158. [DOI] [PubMed] [Google Scholar]
- Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, Aronowski J. Neuroprotective role of haptoglobin after intracerebral hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:15819–15827. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009c;29:6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2007a;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Annals of neurology. 2007b;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Zhang J, Grotta JC, Aronowski J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. Journal of neurochemistry. 2007c;101:652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]