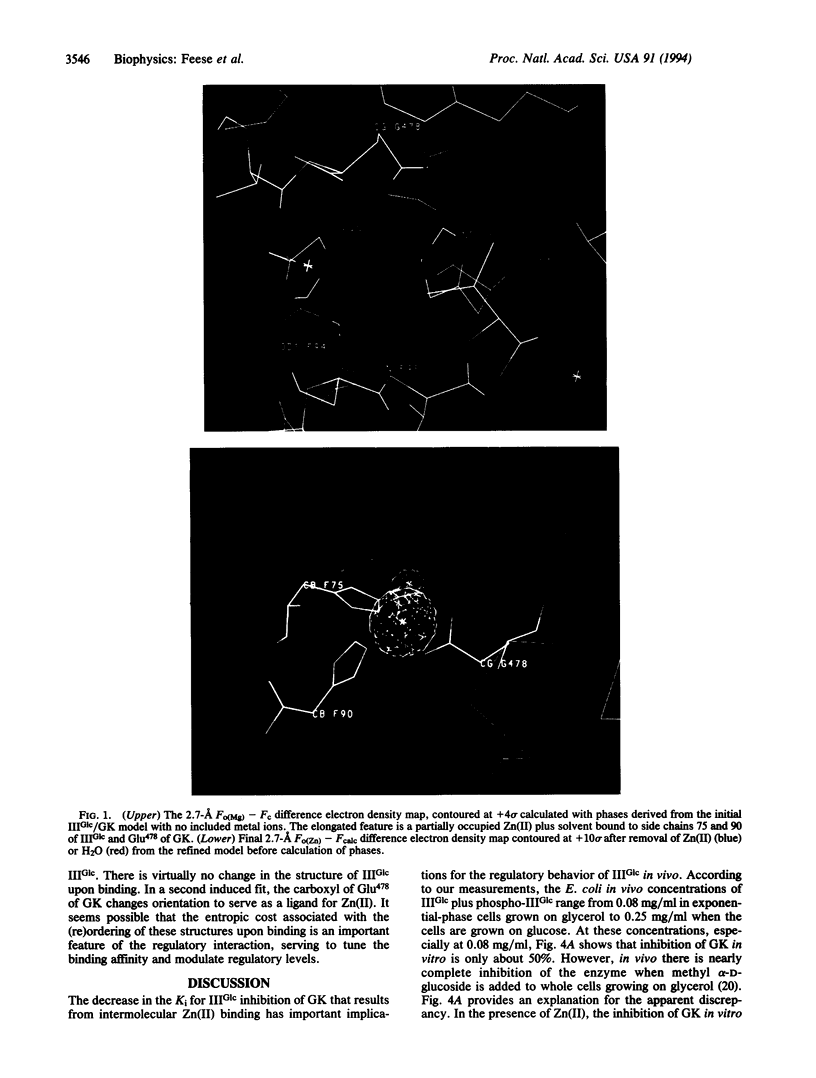
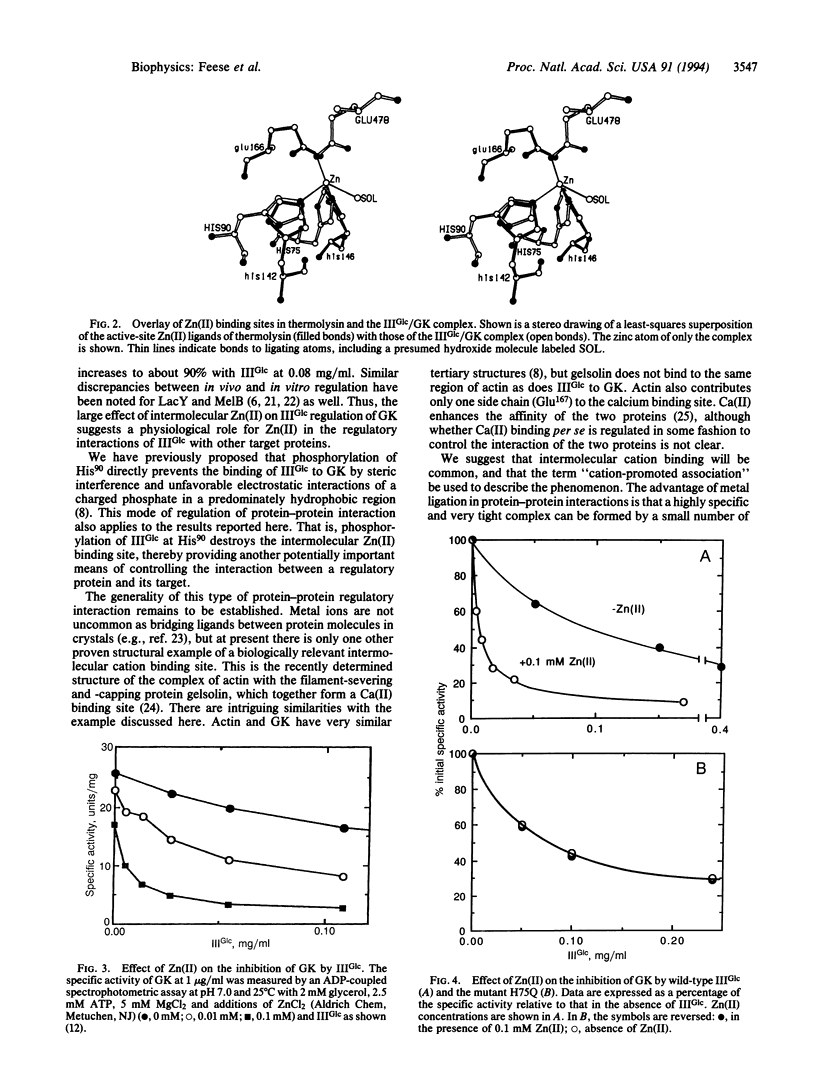
Abstract
A central question in molecular biology concerns the means by which a regulatory protein recognizes different targets. IIIGlc, the glucose-specific phosphocarrier protein of the bacterial phosphotransferase system, is also the central regulatory element of the PTS. Binding of unphosphorylated IIIGlc inhibits several non-PTS proteins, but there is little or no sequence similarity between IIIGlc binding sites on different target proteins. The crystal structure of Escherichia coli IIIGlc bound to one of its regulatory targets, glycerol kinase, has been refined at 2.6-A resolution in the presence of products, adenosine diphosphate and glycerol 3-phosphate. Structural and kinetic analyses show that the complex of IIIGlc with glycerol kinase creates an intermolecular Zn(II) binding site with ligation identical to that of the zinc peptidase thermolysin. The zinc is coordinated by the two active-site histidines of IIIGlc, a glutamate of glycerol kinase, and a water molecule. Zn(II) at 0.01 and 0.1 mM decreases the Ki of IIIGlc for glycerol kinase by factors of about 15 and 60, respectively. The phosphorylation of one of the histidines of IIIGlc, in its alternative role as phosphocarrier, provides an elegant means of controlling the cation-enhanced protein-protein regulatory interaction. The need for the target protein to supply only one metal ligand may account for the lack of sequence similarity among the regulatory targets of IIIGlc.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell T. L., Pitts J. E., Tickle I. J., Wood S. P., Wu C. W. X-ray analysis (1. 4-A resolution) of avian pancreatic polypeptide: Small globular protein hormone. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4175–4179. doi: 10.1073/pnas.78.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A., Reizer J., Nikaido H., Saier M. H., Jr Regulation of the maltose transport system of Escherichia coli by the glucose-specific enzyme III of the phosphoenolpyruvate-sugar phosphotransferase system. Characterization of inducer exclusion-resistant mutants and reconstitution of inducer exclusion in proteoliposomes. J Biol Chem. 1990 Dec 5;265(34):21005–21010. [PubMed] [Google Scholar]
- Dills S. S., Schmidt M. R., Saier M. H., Jr Regulation of lactose transport by the phosphoenolpyruvate-sugar phosphotransferase system in membrane vesicles of Escherichia coli. J Cell Biochem. 1982;18(2):239–244. doi: 10.1002/jcb.1982.240180211. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Matthews B. W. Structure of thermolysin refined at 1.6 A resolution. J Mol Biol. 1982 Oct 5;160(4):623–639. doi: 10.1016/0022-2836(82)90319-9. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Faber H. R., Worthylake D., Meadow N. D., Roseman S., Pettigrew D. W., Remington S. J. Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase. Science. 1993 Jan 29;259(5095):673–677. [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Comparison of the structures of carboxypeptidase A and thermolysin. J Biol Chem. 1977 Nov 10;252(21):7704–7710. [PubMed] [Google Scholar]
- Kuroda M., de Waard S., Mizushima K., Tsuda M., Postma P., Tsuchiya T. Resistance of the melibiose carrier to inhibition by the phosphotransferase system due to substitutions of amino acid residues in the carrier of Salmonella typhimurium. J Biol Chem. 1992 Sep 15;267(26):18336–18341. [PubMed] [Google Scholar]
- Kühnau S., Reyes M., Sievertsen A., Shuman H. A., Boos W. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J Bacteriol. 1991 Apr;173(7):2180–2186. doi: 10.1128/jb.173.7.2180-2186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Mitchell W. J., Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J Biol Chem. 1987 Nov 25;262(33):16261–16266. [PubMed] [Google Scholar]
- Novotny M. J., Frederickson W. L., Waygood E. B., Saier M. H., Jr Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1985 May;162(2):810–816. doi: 10.1128/jb.162.2.810-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D. W., Yu G. J., Liu Y. Nucleotide regulation of Escherichia coli glycerol kinase: initial-velocity and substrate binding studies. Biochemistry. 1990 Sep 18;29(37):8620–8627. doi: 10.1021/bi00489a018. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presper K. A., Wong C. Y., Liu L., Meadow N. D., Roseman S. Site-directed mutagenesis of the phosphocarrier protein. IIIGlc, a major signal-transducing protein in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4052–4055. doi: 10.1073/pnas.86.11.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S., Meadow N. D. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J Biol Chem. 1990 Feb 25;265(6):2993–2996. [PubMed] [Google Scholar]
- Ross J. B., Senear D. F., Waxman E., Kombo B. B., Rusinova E., Huang Y. T., Laws W. R., Hasselbacher C. A. Spectral enhancement of proteins: biological incorporation and fluorescence characterization of 5-hydroxytryptophan in bacteriophage lambda cI repressor. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12023–12027. doi: 10.1073/pnas.89.24.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Vallee B. L., Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Enzyme action: views derived from metalloenzyme studies. Chem Br. 1968 Sep;4(9):397–402. [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]