Abstract
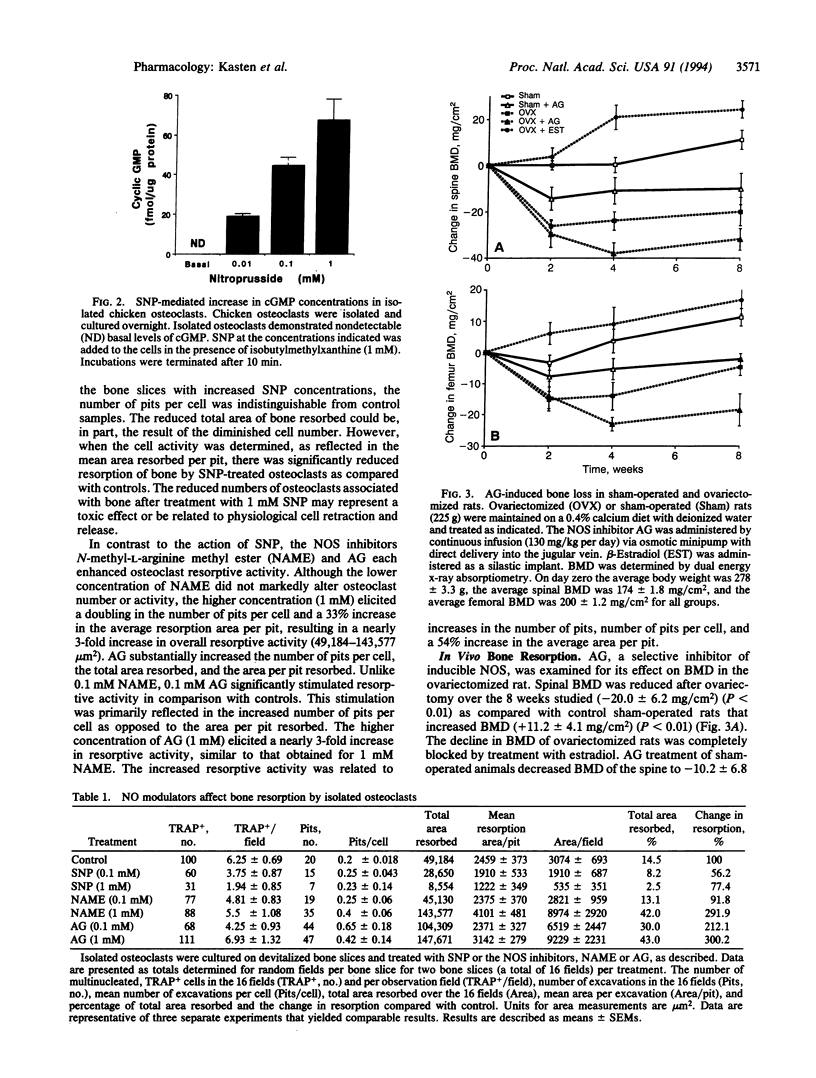
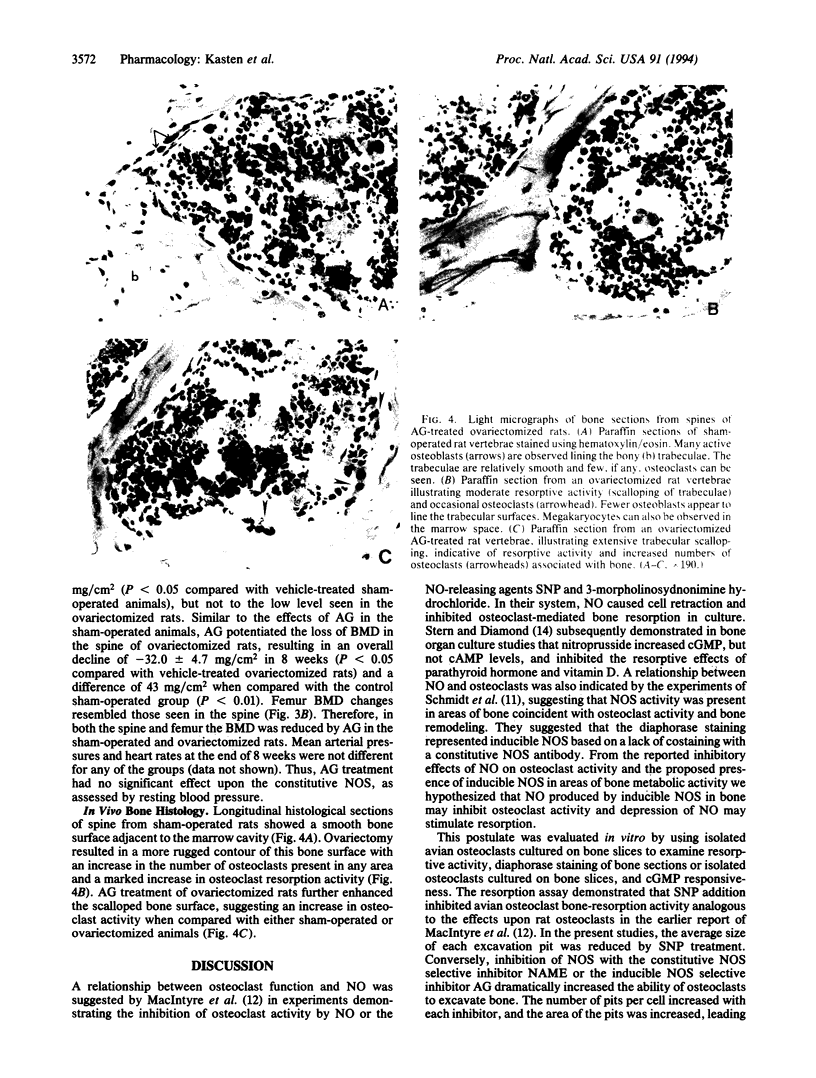
We have examined the effects of modulating nitric oxide (NO) levels on osteoclast-mediated bone resorption in vitro and the effects of nitric oxide synthase (NOS) inhibitors on bone mineral density in vivo. Diaphorase-based histochemical staining for NOS activity of bone sections or highly enriched osteoclast cultures suggested that osteoclasts exhibit substantial NOS activity that may account for basal NO production. Chicken osteoclasts were cultured for 36 hr on bovine bone slices in the presence or absence of the NO-generating agent sodium nitroprusside or the NOS inhibitors N-nitro-L-arginine methyl ester and aminoguanidine. Nitroprusside markedly decreased the number of bone pits and the average pit area in comparison with control cultures. On the other hand, NOS inhibition by N-nitro-L-arginine methyl ester or aminoguanidine dramatically increased the number of bone pits and the average resorption area per pit. In a model of osteoporosis, aminoguanidine potentiated the loss of bone mineral density in ovariectomized rats. Aminoguanidine also caused a loss of bone mineral density in the sham-operated rats. Inhibition of NOS activity in vitro and in vivo resulted in an apparent potentiation of osteoclast activity. These findings suggest that endogenous NO production in osteoclast cultures may regulate resorption activity. The modulation of NOS and NO levels by cells within the bone microenvironment may be a sensitive mechanism for local control of osteoclast bone resorption.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges R. S. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984 Mar;114(3):930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- MacIntyre I., Zaidi M., Alam A. S., Datta H. K., Moonga B. S., Lidbury P. S., Hecker M., Vane J. R. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2936–2940. doi: 10.1073/pnas.88.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Oursler M. J., Collin-Osdoby P., Anderson F., Li L., Webber D., Osdoby P. Isolation of avian osteoclasts: improved techniques to preferentially purify viable cells. J Bone Miner Res. 1991 Apr;6(4):375–385. doi: 10.1002/jbmr.5650060409. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Osdoby P., Pyfferoen J., Riggs B. L., Spelsberg T. C. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler M. J., Pederson L., Pyfferoen J., Osdoby P., Fitzpatrick L., Spelsberg T. C. Estrogen modulation of avian osteoclast lysosomal gene expression. Endocrinology. 1993 Mar;132(3):1373–1380. doi: 10.1210/endo.132.3.8440193. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., Teitelbaum S., Slatopolsky E., McCracken R., Bergfeld M., Lee W., Avioli L. V., Peck W. A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Stern P. H., Diamond J. Sodium nitroprusside increases cyclic GMP in fetal rat bone cells and inhibits resorption of fetal rat limb bones. Res Commun Chem Pathol Pharmacol. 1992 Jan;75(1):19–28. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Wilkins M. R., Settle S. L., Needleman P. Augmentation of the natriuretic activity of exogenous and endogenous atriopeptin in rats by inhibition of guanosine 3',5'-cyclic monophosphate degradation. J Clin Invest. 1990 Apr;85(4):1274–1279. doi: 10.1172/JCI114564. [DOI] [PMC free article] [PubMed] [Google Scholar]




