Abstract
Leaky expression of adenovirus (Ad) genes occurs following transduction with a conventional replication-incompetent Ad vector, leading to an induction of cellular immunity against Ad proteins and Ad protein-induced toxicity, especially in the late phase following administration. To suppress the leaky expression of Ad genes, we developed novel Ad vectors by incorporating four tandem copies of sequences with perfect complementarity to miR-122a or miR-142-3p into the 3′-untranslated region (UTR) of the E2A, E4, or pIX gene, which were mainly expressed from the Ad vector genome after transduction. These Ad vectors easily grew to high titers comparable to those of a conventional Ad vector in conventional 293 cells. The leaky expression of these Ad genes in mouse organs was significantly suppressed by 2- to 100-fold, compared with a conventional Ad vector, by insertion of the miRNA-targeted sequences. Notably, the Ad vector carrying the miR-122a–targeted sequences into the 3′-UTR of the E4 gene expressed higher and longer-term transgene expression and more than 20-fold lower levels of all the Ad early and late genes examined in the liver than a conventional Ad vector. miR-122a–mediated suppression of the E4 gene expression in the liver significantly reduced the hepatotoxicity which an Ad vector causes via both adaptive and non-adaptive immune responses.
Introduction
Replication-incompetent adenovirus (Ad) vectors are widely used in not only clinical gene therapy but also basic researches. Theoretically, Ad genes should not be expressed following transduction with a replication-incompetent Ad vector because the E1A gene, which is crucial for the transcription of other Ad genes, is deleted from the Ad genome. However, Ad genes are indeed expressed from the vector genome, resulting in an induction of cellular immunity against Ad proteins as well as Ad protein-induced toxicity. Such Ad protein-induced cellular immunity and toxicity frequently cause tissue damages and/or an elimination of Ad vector-transduced cells, leading to short-lived transgene expression.1,2 The Leaky expression of Ad genes have been observed for E2A,1,3–5 E4,5 pIX,5,6 hexon,3 and fiber.3,4
In order to suppress the leaky expression of Ad genes, various types of replication-incompetent Ad vectors have been developed. Ad vectors in which not only E1 genes but also E2A and/or E4 genes were deleted2–4,7–10 have also been developed. The E2A- and/or E4-deleted Ad vectors showed significant reduction in the leaky expression of viral proteins,2–4 resulting in a decreased cytotoxic T lymphocyte (CTL) response,2 diminished hepatotoxicity,2,9,10 and increased in vivo transgene expression persistence.2 pIX gene-deleted Ad vectors have also been developed.11 The E2A-, E4-, and/or pIX-deleted Ad vectors are highly valuable and promising; however, special packaging cell lines complementing not only E1 gene products but also the E2A, E4, and/or pIX gene products are necessary for the production of these Ad vectors. It is relatively difficult to generate special packaging cells expressing these Ad genes at levels high enough for high titer production of E2A-, E4-, and/or pIX-deleted Ad vectors. The titers of these Ad vectors using these packaging cells were often lower than that of a conventional Ad vector.4,7,10 A helper-dependent Ad (HD-Ad) vector that lacks all viral coding regions has also been developed. The HD-Ad vector shows reduced inflammation in the organs, and persistent transgene expression following intravenous administration.12,13 However, the production systems of HD-Ad vectors are constrained by technical complexity and are limited to the production of low titers of HD-Ad vectors. In addition, the complete removal of helper virus contamination is a difficult and complicated procedure. The development of safe and efficient Ad vectors that can be easily produced at high titers by a conventional method using normal 293 cells is necessary for gene therapy and, even more, for basic researches.
In order to develop a replication-incompetent Ad vector of the type described above, a microRNA (miRNA)-regulated gene expression system was utilized to suppress the leaky expression of Ad genes in this study. Several groups, including our own, have demonstrated that insertion of miRNA-targeted sequences into the 3′-untranslated region (UTR) of a transgene reduced the expression levels of the transgene in the cells, with the extent of reduction being dependent on the cellular expression levels of the corresponding miRNA.14–18 Thus we hypothesized that incorporation of the complementary sequences for miR-122a or miR-142-3p, which respectively exhibit liver- or spleen-specific expression,14,19,20 into the 3′-UTR of the E2A, E4, or pIX genes suppressed the leaky expression of Ad genes in an miRNA-dependent manner and that high titer production of an Ad vector was achieved using conventional 293 cells.
Results
Construction of a replication-incompetent Ad vector carrying the miRNA-targeted sequences for suppression of the leaky expression of Ad genes
In order to suppress the leaky expression of Ad genes, four tandem copies of sequences with perfect complementarity to miR-122a or miR-142-3p, which respectively exhibit liver- or spleen-specific expression but almost undetectable levels of expression in 293 cells, were inserted into the 3′-UTR of the E2A, E4, or pIX gene of the replication-incompetent Ad vector genome (Figure 1). The liver is the main organ where intravenously administered Ad vectors accumulate. The spleen is largely involved in the innate and acquired immune responses following Ad vector administration. The insertion sites of miRNA-targeted sequences were behind the stop codon in the 3′-UTR of each gene; at bp 4032 in the pIX gene, bp 22442 in the E2A gene, and bp 32913 in the E4 gene. The E4 gene is composed of at least six open reading frames (ORFs), all of which share the common 3′-terminal sequences.21 The miRNA-targeted sequences were inserted into the common 3′-terminal sequences of the E4 gene and upstream of the poly A signal sequences in order that expression of all the E4 ORFs would be suppressed by miRNA. The Ad vectors carrying the miRNA-targeted sequences were normally propagated in conventional 293 cells and exhibited titer productions comparable to those of a conventional Ad vector, Ad-L2,22 which is a conventional E1-deleted Ad vector (Table 1). Real-time RT-PCR analysis demonstrated that the Ct values for miR-122a and miR-142-3p in 293 cells were above 34 (data not shown). Rapid amplification of 3′-cDNA ends (3′-RACE) analysis confirmed that four tandem copies of sequences with perfect complementarity to miR-122a or miR-142-3p were inserted into the 3′-UTR of the E2A, E4 (including all the E4 ORFs) or pIX genes (data not shown). No mutations were found in the miRNA-targeted sequences.
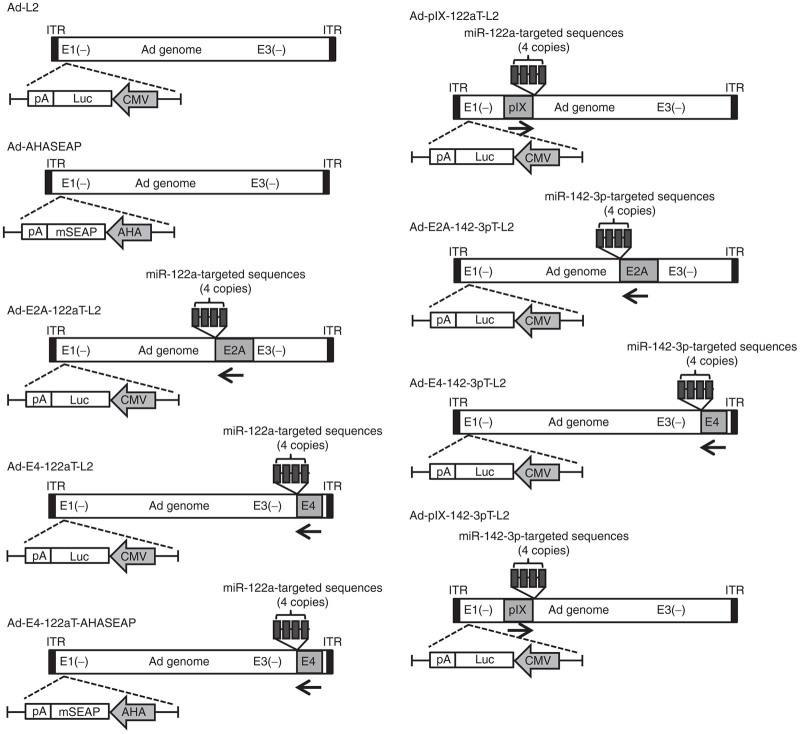
Figure 1.
Schematic diagrams of replication-incompetent Ad vectors used in this study. A luciferase expression cassette was inserted into the E1-deleted region in Ad-L2, Ad-E2A-122aT-L2, Ad-E4-122aT-L2, Ad-pIX-122aT-L2, Ad-E2A-142-3pT-L2, Ad-E4-142-3pT-L2, and Ad-pIX-142-3pT-L2. A murine secreted embryo alkaline phosphatase (mSEAP) expression cassette was inserted into the E1-deleted region in Ad-AHASEAP and Ad-E4-122aT-AHASEAP. AHA, a synthetic promoter composed of apolipoprotein E enhancer, the hepatocyte control region, and human α1-antitrypsin promoter; CMV, cytomegalovirus promoter; ITR, inverted terminal repeat; 122aT, miR-122a–targeted sequences; 142-3pT, miR-142-3p–targeted sequences.
Table 1. Ad vectors used in this study.
| Vector name | Promoter | Transgene | miRNA-targeted sequences | Virus particle | Infectious unit |
|---|---|---|---|---|---|
| Ad-L2 | CMV | Luciferase | – | 2.2 × 1012 | 3.2 × 1011 |
| Ad-AHASEAP | AHA | mSEAP | – | 2.2 × 1012 | 3.4 × 1011 |
| Ad-E2A-122aT-L2 | CMV | Luciferase | miR-122a | 2.7 × 1012 | 4.0 × 1011 |
| Ad-E4-122aT-L2 | CMV | Luciferase | miR-122a | 2.8 × 1012 | 4.2 × 1011 |
| Ad-E4-122aT-AHASEAP | AHA | mSEAP | miR-122a | 1.7 × 1012 | 2.6 × 1011 |
| Ad-pIX-122aT-L2 | CMV | Luciferase | miR-122a | 2.9 × 1012 | 4.3 × 1011 |
| Ad-E2A-142-3pT-L2 | CMV | Luciferase | miR-142-3p | 4.3 × 1012 | 5.8 × 1011 |
| Ad-E4-142-3pT-L2 | CMV | Luciferase | miR-142-3p | 3.8 × 1012 | 5.5 × 1011 |
| Ad-pIX-142-3pT-L2 | CMV | Luciferase | miR-142-3p | 3.7 × 1012 | 5.3 × 1011 |
Total amount of titers recovered from 20 plates of 150-mm dishes.
AHA, a synthetic promoter composed of apolipoprotein E enhancer, the hepatocyte control region, and human α1-antitrypsin promoter; CMV, cytomegalovirus promoter; mSEAP, murine secreted embryonic alkaline phosphatase; –, Ad vector does not contain the corresponding sequences.
miRNA-mediated suppression of the leaky expression of the Ad genes in vitro
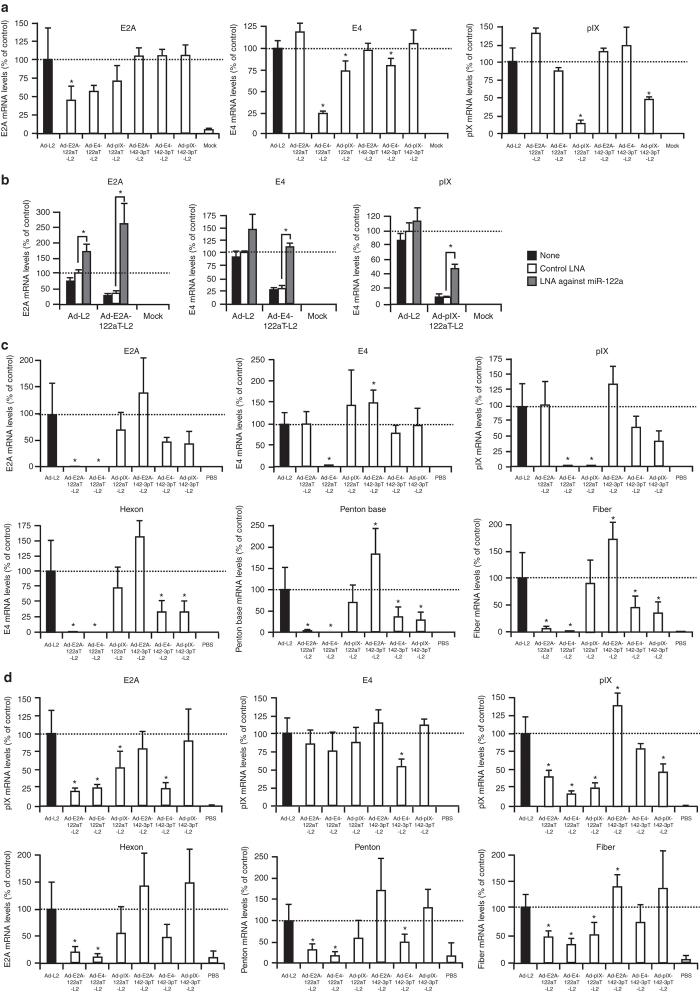
In order to examine whether leaky expression of the Ad genes was suppressed by insertion of the miRNA-targeted sequences in an miRNA-dependent manner, HuH-7 cells, which highly express miR-122a,23 were transduced with the luciferase-expressing Ad vectors, and the Ad gene expression levels were determined 12 hours after transduction. All the Ad vectors examined exhibited similar levels of luciferase production in HuH-7 cells (data not shown). Ad-E2A-122aT-L2 mediated 2.2-fold lower E2A gene expression than did Ad-L2 (Figure 2a). Ad-E4-122aT-L2 and Ad-pIX-122aT-L2 exhibited 4- and 6.5-fold lower E4 and pIX gene expression levels, respectively, than did Ad-L2. Insertion of the miR-142-3p–targeted sequences in the 3′-UTR also reduced the expression levels of the E4 and pIX genes in spite of the undetectable levels of miR-142-3p expression in HuH-7 cells, probably due to non-specific suppression via the insertion of miRNA-targeted sequences; however, the levels of suppression of the E4 and pIX genes realized by insertion of the miR-142-3p–targeted sequences were significantly lower than those by insertion of the miR-122a–targeted sequences. A reduction in E4 gene expression was also found for Ad-pIX-122aT-L2. It was unclear why the E4 gene expression was reduced for Ad-pIX-122aT-L2. The expression levels of Ad genes other than those described above were not significantly reduced in HuH-7 cells. The E2A, E4, and pIX gene expressions were also significantly suppressed in K562 cells, which is a human chronic myelogenous leukemia cell line highly expressing miR-142-3p,24 by insertion of the miR-142-3p–targeted sequences in the 3′-UTR of these Ad genes (Supplementary Figure S1). Note that all the E4 ORFs can be detected by the primers for the E4 gene used in this study. The E4 mRNA levels in the graph represent the sum of each E4 ORF mRNA level.
Figure 2.
Suppression of the leaky expression of Ad genes in culture cells and mouse organs by insertion of the miRNA-targeted sequences. (a) HuH-7 cells were transduced with Ad vectors at an MOI of 10 for 1 hour and harvested at 12 hours after transduction. (b) Restoration of the leaky expression of Ad genes in HuH-7 cells by LNA-modified ASO complementary to miR-122a. HuH-7 cells were transfection with LNA-modified ASO complementary to miR-122a or an LNA control at 10 nmol/l. Twenty-four hours after transduction, HuH-7 cells were transduced with Ad vectors at an MOI of 10 for 1 hour, and harvested at 12 hours after transduction. The Ad gene expression levels in the cells transduced with Ad vectors were determined by real-time RT-PCR. (c,d) C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Two days after administration, (c) the livers and (d) the spleens were harvested. The Ad gene expression levels in the cells and mice transduced with Ad vectors were determined by real-time RT-PCR. The data are expressed as the mean values ± SD (a,b: n = 4; c,d: n = 5–6). *P < 0.05 in comparison with (a,c,d) Ad-L2 or the (b) LNA control.
In order to examine whether the suppression of the leaky expression of Ad genes by insertion of the miRNA-targeted sequences was miRNA-dependent, miR-122a was inhibited by pre-treatment with a locked nucleic acid (LNA)-modified antisense oligonucleotides (ASO) against miR-122a. The average expression levels of the E2A, E4, and pIX genes by Ad-L2 were elevated by transfection with the LNA-modified ASO against miR-122a via an unknown mechanism (Figure 2b). By contrast, more than threefold elevation in the expression of the E2A, E4, and pIX genes was found by the LNA-modified ASO against miR-122a in the cells treated with the Ad vectors containing the miR-122a–targeted sequences. The control LNA-modified ASO failed to restore the miR-122a–mediated suppression of these Ad genes. These results indicate that the reduction in the leaky expression levels of these Ad genes by insertion of the miR-122a–targeted sequences was miRNA-dependent.
Suppression of the leaky expression of Ad genes in mouse organs following intravenous administration
Next, to examine whether in vivo leaky expression of Ad genes in the organs was suppressed by incorporation of the miRNA-targeted sequences, Ad gene expression levels in the liver and spleen were determined by real-time RT-PCR following intravenous administration of Ad vectors. Among the Ad genes examined (E2A, E4, pIX, hexon, penton base, and fiber genes), the pIX gene exhibited the highest level of expression in the liver following intravenous administration of Ad-L2 (Supplementary Figure S2a). The pIX gene expression levels were approximately 40-fold lower than the expression level of a cytomegalovirus (CMV) promoter-driven luciferase gene in the E1-deleted region of the Ad vector genome. The expression levels of the other Ad genes were similar.
Insertion of the miR-122a–targeted sequences into the 3′-UTR of the Ad genes reduced the leaky expression of the corresponding Ad genes in the liver (Figure 2c). The expressions of the E2A, E4, and pIX genes by Ad-E2A-122aT-L2, Ad-E4-122aT-L2, and Ad-pIX-122aT-L2, respectively, in the liver were significantly suppressed by 20- to 30-fold, compared with the corresponding expressions by Ad-L2. The expression of the Ad late genes (hexon, penton base, and fiber genes), which did not possess miR-122a–targeted sequences in the 3′-UTR, was also decreased in the livers of mice treated with Ad-E2A-122aT-L2 and Ad-E4-122aT-L2. In particular, the livers of mice treated with Ad-E4-122aT-L2 exhibited larger reductions—more than 20-fold in the expression of not only the E4 gene but also all the other Ad genes examined compared with livers treated with the other Ad vectors (Figure 2c). It is well known that the E4 gene products are required to express the E2A and late genes.3,21,25 The E2A gene product also regulates the expression of the viral late genes.4 We consider that miRNA-mediated suppression of the E2A or E4 gene expression resulted in a reduction of the Ad late gene expression in the liver. The livers treated with Ad-E4-142-3pT-L2 and Ad-pIX-142-3pT-L2 showed a reduction in the expression of the Ad late genes in spite of the almost undetectable levels of miR-142-3p expression in the liver. Ad-E4-142-3pT-L2 and Ad-pIX-142-3pT-L2 induced a reduction in the average E2A mRNA levels in the liver, although the reductions in the E2A mRNA levels were not statistically significant. A non-specific reduction in the E2A mRNA expression by Ad-E4-142-3pT-L2 or Ad-pIX-142-3pT-L2 could have caused the reduction in the Ad late gene mRNA levels in the liver.
The leaky expression levels of the Ad genes in the spleen were 50- to 5,000-fold lower than those in the liver (Supplementary Figure S2b). The highest level of leaky expression in the spleen was found for the E4 gene, followed by the E2A, pIX, and fiber genes, following intravenous administration of Ad-L2. Ad-E4-142-3pT-L2 and Ad-pIX-142-3pT-L2 mediated approximately twofold lower levels of the E4 gene and pIX gene, respectively, than did Ad-L2 in the spleen (Figure 2d). Ad-E2A-142-3pT-L2 administration failed to suppress the E2A gene expression in the spleen. It is unclear why the E2A gene expression was not reduced in the spleens of mice treated with Ad-E2A-142-3pT-L2. Overall, the levels of miR-142-3p–mediated suppression of the leaky expression of Ad genes in the spleen were lower than the corresponding levels of miR-122a–mediated suppression in the liver, probably due to the lower levels of miR-142-3p expression in the spleen, compared with miR-122a expression in the liver. Alternatively, the Ad vectors might transduce not only blood cells but also non-blood cells, which have negligible levels of miR-142-3p expression, in the spleen. The expressions of most of the Ad genes examined were also reduced in the spleen when miR-122a–targeted sequences were inserted in the 3′-UTR, although the spleen exhibited undetectable level of miR-122a expression. We confirmed that miR-142-3p did not suppress the expression of renilla luciferase gene possessing the miR-122a–targeted sequences in the 3′-UTR in the in vitro reporter gene assay (data not shown). It remains unclear why insertion of the miR-122a–targeted sequences in the 3′-UTR led to the reduction in the expressions of the Ad genes in the spleen. The 3′-UTR of an mRNA plays an important role in the stability of mRNA.26 Insertion of the miR-122a–targeted sequences might affect the stability of mRNA for Ad genes in the spleen in an miRNA-independent manner.
Reduction in Ad vector-induced hepatotoxicity by incorporation of the miR-122a–targeted sequences into the 3′-UTR of the E4 gene
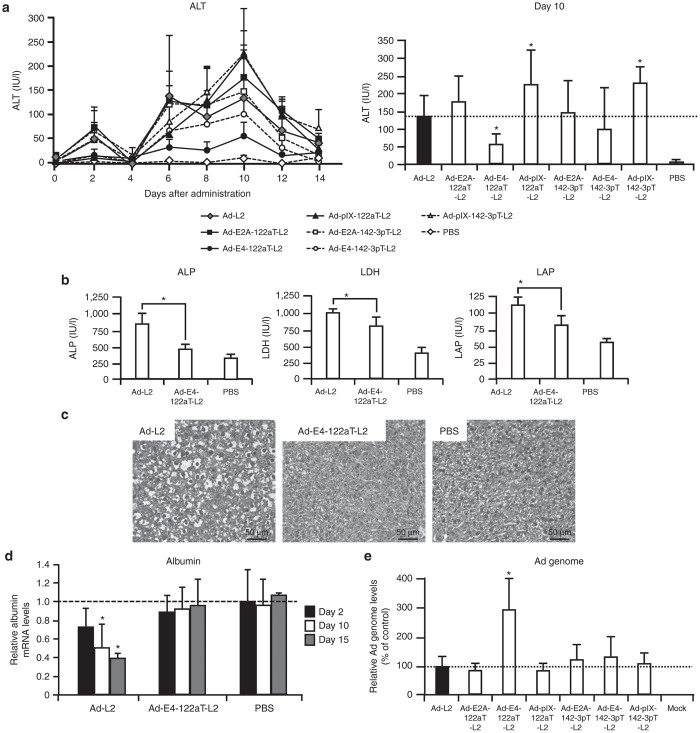
In order to examine whether a reduction in the leaky expression of Ad genes by incorporation of miRNA-targeted sequences leads to the suppression of hepatotoxicity associated with replication-incompetent Ad vectors after intravenous administration, serum alanine aminotransferase (ALT) levels, enzymatic biomarkers of hepatotoxicity, were measured after intravenous administration of Ad vectors (Figure 3a). The profiles of serum ALT levels contained two peaks, as previously reported.13,27 The first peak was found on day 2. Serum ALT levels were significantly elevated on day 2 in the mice treated with Ad-L2, Ad-E2A-122aT-L2, Ad-E2A-142-3pT-L2, and Ad-pIX-142-3pT-L2. On the other hand, Ad-E4-122aT-L2, Ad-E4-142-3pT-L2, and Ad-pIX-122aT-L2 exhibited significantly lower ALT levels than Ad-L2 on day 2. The serum ALT levels reached the second peak on day 10. The serum ALT levels were elevated by all the Ad vectors examined, but among the Ad vectors tested, Ad-E4-122aT-L2 induced the lowest levels of serum ALT; the levels were approximately twofold lower than those by Ad-L2. On the other hand, Ad-pIX-122aT-L2 and Ad-pIX-142-3pT-L2 induced statistically significant elevations in the serum ALT levels 10 days after administration, compared with Ad-L2. The reason for the elevation of serum ALT levels by Ad-pIX-122aT-L2 and Ad-pIX-142-3pT-L2 remains unclear. Previous studies have demonstrated that pIX has various other functions in addition to its role as a capsid cement.28 For example, pIX acts as a transcriptional activator in the nucleus, and pIX might be involved in Ad vector-induced hepatotoxicity. Lower ALT levels were induced by Ad-E4-122aT-L2 than by Ad-L2 at the dose of 2 × 109 infectious unit (IFU)/mouse (data not shown). Other hepatotoxicity parameters, i.e., serum alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and leucine aminopeptidase (LAP) levels, were also significantly higher in mice treated with Ad-L2, compared with the mice receiving Ad-E4-122aT-L2 (Figure 3b).
Figure 3.
Suppression of Ad vector-mediated hepatotoxicity by incorporation of miR-122a–targeted sequences into the 3′-UTR of the E4 gene. The serum (a) ALT, (b) ALP, LDH, LAP levels in mice after intravenous injection of Ad vectors. C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Blood samples were collected via retro-orbital bleeding at the indicated number of days after administration. The bar graph shows the serum ALT levels 10 days after administration. The data are expressed as the mean values ± SD (n = 6). *P < 0.05 in comparison with Ad-L2. Statistically significant differences in the serum ALT levels relative to Ad-L2 were found at 2, 6, 8, 10, 12 days after Ad-E4-122aT-L2 administration. (c) Liver sections of mice following intravenous administration of Ad-L2 (left), Ad-E4-122aT-L2 (middle), or PBS (right). C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Ten days after administration, the livers were isolated, and histological analysis was performed using hematoxylin and eosin staining. The scale bar = 50 µm. (d) The albumin mRNA levels in mice after intravenous injection of Ad vectors. C57BL/6 mice were treated with Ad vectors at 1 × 1010 IFU/mouse. The livers were harvested from the mice 2, 10, and 15 days after administration. The data are expressed as the mean values ± SD (n = 4–6). *P < 0.05 in comparison with PBS. (e) Ad genome copy numbers in the liver. C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Fifteen days after administration, the Ad genome copy numbers in the mouse liver were determined by real-time PCR. The data are expressed as the mean values ± SD (n = 5–6). *P < 0.05 in comparison with Ad-L2.
Next, in order to compare the hepatotoxicity profiles of Ad-L2 and Ad-E4-122aT-L2, histopathological examination of the liver sections was performed 10 days after Ad vector administration (Figure 3c). Many vacuolated cells were observed in the livers of mice treated with Ad-L2. Moreover, there were several necrotic areas in the sections. On the other hand, the livers of Ad-E4-122aT-L2-treated mice exhibited a much lower level of vacuolation than those of Ad-L2-treated mice.
To examine the influence of Ad vector-mediated hepatotoxicity on the expression of liver-specific genes, albumin mRNA levels in the liver were quantified by real-time RT-PCR (Figure 3d). Albumin, which plays an important role in the maintenance of oncotic pressure and transport of small molecules such as calcium, unconjugated bilirubin, free fatty acids, and cortisol, is abundantly expressed in hepatocytes. The mice treated with Ad-L2 showed 53% and 37% reductions in albumin mRNA in the liver on days 10 and 15, respectively, compared with those of PBS-treated mice. On the other hand, the albumin mRNA expression levels in the livers of mice treated with Ad-E4-122aT-L2 were comparable to those of PBS-treated mice. These results indicate that administration of a replication-incompetent Ad vector possessing the miR-122a–targeted sequences into the 3′-UTR of the E4 gene resulted in significantly lower hepatotoxicity than treatment with a conventional Ad vector. A reduction in the hepatotoxicity induced by Ad-E4-122aT-L2 would lead to the higher copy numbers of Ad vector genome remaining in the liver. We found that the genome copy number of Ad-E4-122aT-L2 was approximately threefold higher than those of the other Ad vectors 15 days following administration (Figure 3e).
Immune responses against Ad proteins following Ad vector administration
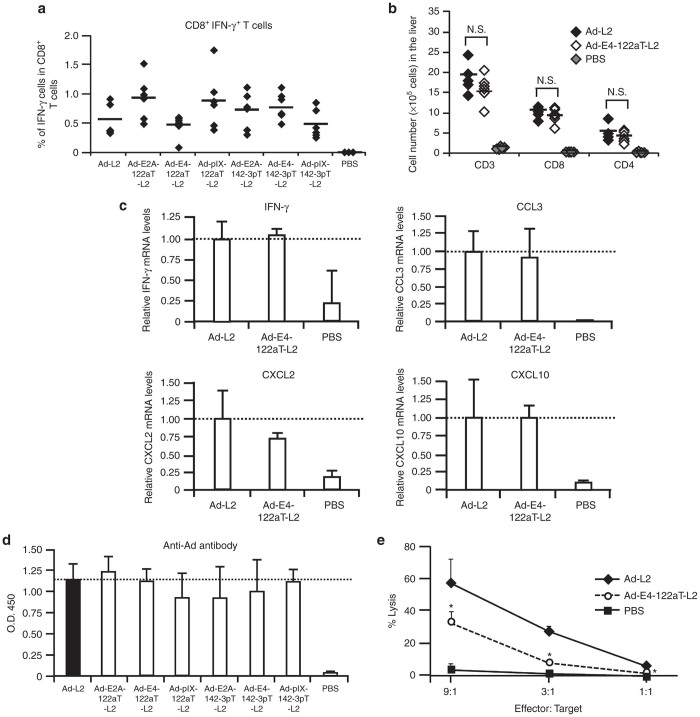
In order to determine whether suppression of the E4 gene expression by incorporation of the miR-122a–targeted sequences in the 3′-UTR of the E4 gene would lead to a low level of cellular immune responses against Ad protein, the numbers of CTLs against the hexon, which is one of the major capsid proteins and the dominant epitope of Ad vector,29 in the splenocytes were determined 15 days following administration by an intracellular cytokine staining assay. All the Ad vectors induced elevation in the numbers of hexon-specific CD8+ T cells producing IFN-γ, but these cell numbers were not significantly different between the Ad vectors examined (Figure 4a).
Figure 4.
Ad vector-induced immune responses following intravenous administration of Ad vectors into mice. (a) Hexon-specific IFN-γ+ CD8+ T cells in the splenocytes. C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Fifteen days after the injection, the splenocytes were harvested. The splenocytes were incubated with hexon peptide for 6 hours. (b) Infiltration of lymphocytes into the liver following Ad vector administration. C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Ten days after administration, liver mononuclear cells were isolated and analyzed for cells expressing CD3, CD4, and CD8 by fluorocytometry. The data are expressed as the mean values ± SD (n = 6). N. S., not significant. (c) IFN-γ and chemokine mRNA levels in the liver after administration of Ad-L2 and Ad-E4-122aT-L2. C57BL/6 mice were treated with Ad vectors at 1 × 1010 IFU/mouse. Ten days after administration, IFN-γ and chemokine mRNA levels in the liver were determined by real-time RT-PCR. The data are expressed as the mean values ± SD (n = 3–6). (d) Anti-Ad antibody levels in the serum following intravenous administration of Ad vectors. C57BL/6 mice were intravenously administered as described above. Anti-Ad antibody levels in the serum were determined by ELISA 14 days after administration. The data are expressed as the mean values ± SD (n = 6). (e) Ad-specific CTL-mediated lysis of the hepatocytes transduced with Ad vectors. C57BL/6 mice were intravenously administered Ad-null at a dose of 1 × 1010 IFU/mouse. Ten days after the injection, the splenocytes were harvested and incubated with Ad-null at an MOI of 10 for 4 days. Primary mouse hepatocytes were transduced with Ad-L2 or Ad-E4-122aT-L2 at an MOI of 10 for 24 hours and were incubated with the splenocytes at 37 °C. LDH levels in the medium were measured 4 hours after incubation. The data are expressed as the mean values ± SD (n = 4). *P < 0.05 in comparison with Ad-L2.
We next examined the infiltration of lymphocytes into the liver 10 days following administration of Ad-L2 and Ad-E4-122aT-L2 (Figure 4b). Dramatic increases in the numbers of CD4+ and CD8+ T cells in the liver were found following administration of Ad-L2 and Ad-E4-122aT-L2. However, the numbers of CD4+ and CD8+ cells infiltrated into the liver were comparable between the mice treated with Ad-L2 and those treated with Ad-E4-122aT-L2. Furthermore, there were no significant differences in the mRNA levels of IFN-γ or chemokines, including CCL3, CXCL2, and CXCL10, in the livers of mice injected with Ad-L2 and Ad-E4-122aT-L2 on day 10, although administration of both Ad vectors significantly elevated the expression of IFN-γ and all the chemokines examined in the liver (Figure 4c). The liver mRNA levels of other chemokines, including CCL2, CCL4, CCL5, and CX3CL were also comparable between mice receiving Ad-L2 and Ad-E4-122aT-L2 (Supplementary Figure S3). There were no significant differences in anti-Ad antibody levels in the serum for all the Ad vectors tested on day 14 (Figure 4d) and day 28 (data not shown).
Next, we hypothesized that the liver hepatocytes transduced with Ad-E4-122aT-L2 might be less susceptible to Ad-specific CTL attack, due to the lower levels of Ad antigen presentation, compared with the hepatocytes transduced with Ad-L2, even though the levels of Ad-specific CTL induction in the spleen and infiltration of Ad-specific CTL in the liver were comparable between the mice receiving Ad-L2 and those receiving Ad-E4-122aT-L2. In order to examine this hypothesis, primary mouse hepatocytes transduced with Ad-L2 or Ad-E4-122aT were incubated with splenocytes isolated from the mice receiving a conventional Ad vector (Ad-null). Significant LDH releases were found in both the hepatocytes transduced with Ad-L2 and the hepatocytes transduced with Ad-E4-122aT; however, the hepatocytes transduced with Ad-L2 showed a 1.8- to 3.2-fold higher LDH release than the hepatocytes transduced with Ad-E4-122aT (Figure 4e). These results indicate that the hepatocytes transduced with Ad-L2 were more susceptible to Ad-specific CTL attack than the hepatocytes transduced with Ad-E4-122aT.
Hepatotoxicity profile of Ad-E4-122aT-L2 in immune-incompetent mice
In order to examine whether suppression of the E4 gene expression in the liver by insertion of the miR-122a–targeted sequences would result in a reduction in immune-independent hepatotoxicity induced by replication-incompetent Ad vectors, serum ALT levels were measured following intravenous administration into Rag2/Il2rγ double-knockout mice, which have global defects in both cellular and humoral immunity due to the lack of T, B, and natural killer (NK) cells.30,31 Rag2/Il2rγ double-knockout mice exhibited significant elevation in serum ALT levels following intravenous administration of Ad-L2 (Figure 5). On the other hand, no increases in the serum ALT levels were found in Ad-E4-122aT-L2-treated mice. These results indicate that the Ad vector-induced hepatotoxicity via a non-adaptive immune response was almost completely eradicated by miR-122a–mediated suppression of the E4 gene expression, and further that E4 gene expression in the liver is one of the main causes of replication-incompetent Ad vector-mediated hepatotoxicity.
Figure 5.
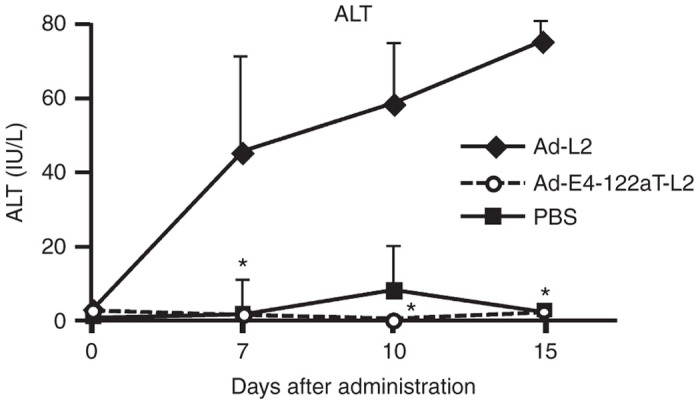
The serum ALT levels in the serum in Rag2/Il2rγ double-knockout mice after intravenous administration of Ad vectors. Rag2/Il2rγ double-knockout mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Blood samples were collected via retro-orbital bleeding on the indicated number of days after administration. The data are expressed as the mean values ± SD (n = 3). *P < 0.05 in comparison with Ad-L2.
In vivo transgene expression by the Ad vectors containing miRNA-targeted sequences in the 3′-UTR of Ad genes
In order to evaluate the in vivo transgene expression levels induced by the Ad vectors carrying miRNA-targeted sequences, luciferase expression in the liver was examined 2 days following administration (Figure 6a). The luciferase expression levels in the liver induced by the Ad vectors carrying miRNA-targeted sequences, with the exceptions of Ad-E2A-122aT-L2 and Ad-E4-122aT-L2, were comparable to those by Ad-L2. Ad-E4-122aT-L2–mediated luciferase expression in the liver was 15-fold lower than that mediated by Ad-L2. The lower luciferase expression levels of Ad-E4-122aT-L2 in the liver were probably due to the significant suppression of E4 gene expression. Previous studies demonstrated that the E4 gene products, especially the E4 ORF3 gene product, enhanced the transcriptional activity of a CMV promoter.9,10,32
Figure 6.
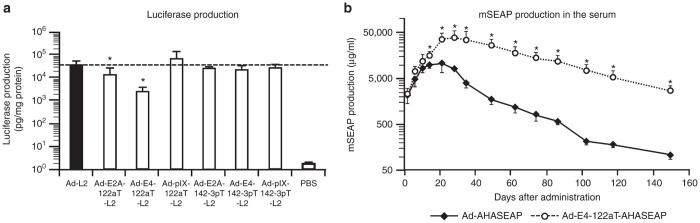
Ad vector-mediated transgene expression in mice. (a) Luciferase production in the liver following Ad vector administration. C57BL/6 mice were intravenously administered Ad vectors expressing the luciferase gene under the control of a CMV promoter at 1 × 1010 IFU/mouse, and the livers were harvested and subjected to luciferase expression analysis 2 days after administration. Luciferase production in the liver was determined by luminescence assay. The data are expressed as the mean values ± SD (n = 5–6). *P < 0.05 in comparison with Ad-L2. (b) mSEAP production in the serum following Ad vector administration. C57BL/6 mice were intravenously administered Ad vectors expressing the mSEAP gene under the control of an AHA promoter at 1 × 1010 IFU/mouse. Blood samples were collected via retro-orbital bleeding on the indicated days after injection. mSEAP production in the serum was determined by SEAP chemiluminescence assay. The data are expressed as the mean values ± SD (n = 4). *P < 0.05 in comparison with Ad-AHASEAP. mSEAP expression in the PBS-treated mice was below the detectable level.
To circumvent the influence of suppressing the E4 gene expression on the CMV promoter activity, the liver-specific synthetic promoter composed of apolipoprotein E enhancer, the hepatocyte control region, and human α1-antitrypsin (AHA) promoter was used for transgene expression. The transcriptional silencing was not observed in the AHA promoter, which made it possible to properly evaluate the influence of Ad vector-mediated hepatotoxicity on the transgene expression profile. In order to exclude the influence of immune responses to transgene products on the transgene expression profile and to examine whether the suppression of hepatotoxicity by insertion of the miR-122a–targeted sequences in the 3′-UTR of the E4 gene improves the transgene expression profiles, the murine secreted embryonic alkaline phosphatase (mSEAP) gene was inserted into the Ad vector genome as a reporter gene. mSEAP is a secreted form of murine embryonic alkaline phosphatase, which is an endogenous protein in mice. Ad-AHASEAP and Ad-E4-122aT-AHASEAP exhibited the comparable mSEAP expression on day 2, indicating that the E4 gene expression did not affect the AHA promoter activity (Figure 6b). Ad-AHASEAP–mediated mSEAP expression levels were gradually increased, and they reached a plateau at 10 days after administration. Subsequently, the mSEAP expression levels induced by Ad-AHASEAP gradually declined. On the other hand, the serum mSEAP levels induced by Ad-E4-122aT-AHASEAP were maintained for at least 149 days. In addition, the mSEAP expression levels by Ad-E4-122aT-AHASEAP were 1.5- to 34.1-fold higher than those by Ad-AHASEAP. These results indicate that suppression of the E4 gene expression in the liver led to the higher and longer-term transgene expression.
Discussion
The aim of this study was to develop a replication-incompetent Ad vector that exhibits an improved safety profile by suppressing the leaky expression of Ad genes, but that can be easily produced at high titers using conventional 293 cells. For this purpose, four tandem copies of sequences with perfect complementarity to miR-122a or miR-142-3p were incorporated into the 3′-UTR of the E2A, E4, or pIX genes. All Ad vectors developed in this study were efficiently produced at high titers comparable to a conventional Ad vector using normal 293 cells. Among the Ad vectors developed, an Ad vector containing the miR-122a–targeted sequences in the 3′-UTR of the E4 gene exhibited lower levels of hepatotoxicity and higher and longer-term transgene expression than a conventional Ad vector. This study also indicates that expression of the E4 gene in the liver is one of the main causes of replication-incompetent Ad vector-induced hepatotoxicity.
Previous studies have developed several types of replication-incompetent Ad vectors lacking the E2A, E4, and/or pIX genes to eliminate the leaky expression of Ad genes and to increase the transgene insertion capacity.2,10,11 However, the yields of these Ad vectors were frequently much lower than that of a conventional Ad vector. The preparation of HD-Ad vectors also suffers from low yield and a contamination of helper virus.33 In contrast, the Ad vectors carrying the miRNA-targeted sequences in the 3′-UTR of Ad genes easily grew to high titers comparable to those of a conventional Ad vector via a conventional Ad vector preparation method using normal 293 cells without any trouble. High titers of these Ad vectors can be prepared by anyone without much experience in Ad vector preparation. This property is highly crucial not only for studies on potential gene therapies but also for gene function analyses. In particular, an Ad vector with miR-122a–targeted sequences in the 3′-UTR of the E4 gene, which exhibits lower hepatotoxicity and higher and longer-term transgene expression than a conventional Ad vector, would be suitable for gene function analysis in the liver, especially when Ad vector-mediated hepatotoxicity or Ad gene products affect the function of genes of interest. We are currently performing an analysis of gene function in the liver using this Ad vector.
Another advantage of an Ad vector containing the miRNA-targeted sequences in the 3′-UTR of the E4 gene is that the expression of all E4 ORFs is suppressed by miRNA. The miRNA-targeted sequences were inserted into the common terminal sequence, which is shared by all E4 ORFs, in the 3′-UTR.21 We confirmed that all E4 ORF mRNAs possessed the miRNA-targeted sequences in the 3′-UTR without mutations. In addition, all of the RT-PCR products of E4 gene transcripts by 4 different pairs of primers, each of which can detect several different E4 ORFs, were reduced, indicating that all of the E4 ORF mRNA levels were reduced by insertion of the miRNA-targeted sequences in the common terminal sequence in the 3′-UTR (data not shown). It is highly difficult to produce high titers of an Ad vector in which the E4 ORFs are completely deleted. There have been only two studies demonstrating the in vivo transduction properties of Ad vectors with deletion of all E4 ORFs,8,34 although various types of Ad vectors containing mutations in the E4 gene have been developed.2,3,10 As described below, the E4 gene products have inhibitory effects on various cellular functions, suggesting that the suppression of all E4 ORF expression is crucial in order to reduce Ad vector-mediated hepatotoxicity.
In addition to the E4 gene-deleted Ad vectors, the E2A gene-deleted Ad vectors were developed and exhibited the reduction in expression of not only the E2A gene but also Ad late genes in the cultured cells.4 In this study, insertion of the miR-122a–targeted sequences in the 3′-UTR of the E2A gene resulted in a 2-log order reduction in the expression of the E2A gene in the liver, compared with Ad-L2. Ad-E2A-122aT-L2 also exhibited a 2-log order reduction in the expression of the Ad late genes in the liver because the E2A gene product is essential for viral genome replication;4 however, serum ALT levels following administration of Ad-E2A-122aT-L2 were comparable to those by Ad-L2 and higher than those by Ad-E4-122aT-L2. These results suggest that the E4 gene expression in the liver plays a crucial role in Ad vector-induced hepatotoxicity. Christ et al. also demonstrated that deletion of the E4 gene resulted in much greater suppression of the hepatotoxicity than deletion of the E2A gene.4
miR-122a, which is a liver-specific miRNA, is a very effective choice of miRNA for the regulation of the Ad vector-mediated expression of both the transgene and Ad genes, because Ad vectors have strong hepatotropism. In addition, a very high copy number of miR-122a is expressed in the liver hepatocytes. miR-122a accounts for approximately 70% of the miRNAs expressed in the hepatocytes.20 We previously reported that Ad vector-mediated transgene expression in the liver and replication of oncolytic adenoviruses containing a tumor-specific promoter in the hepatocytes were efficiently suppressed by insertion of miR-122a–targeted sequences into the 3′-UTR of transgene.15,16 Ylösmaki et al. reported that the insertion of miR-122a–targeted sequences into the E1A gene in replication-competent Ad reduced hepatotoxicity.35
At the beginning of this study, we hypothesized that leaky expression in the spleen, which is considered to play a crucial role in induction of CTLs, should be suppressed in order to suppress the induction of Ad protein-specific CTLs, and that the insertion of miR-142-3p–targeted sequences into the 3′-UTR of Ad genes would reduce the leaky expression of Ad genes in the spleen. However, the leaky expression levels of Ad genes in the spleen were 50- to 5,000-fold lower than those in the liver following intravenous administration of Ad-L2. In particular, the expression levels of the Ad late genes by all Ad vectors in the spleen were extremely low and slightly above the detection limit of real-time RT-PCR analysis. Hexon-specific CTL induction was not suppressed by miRNA-mediated inhibition of Ad gene expression in the spleen, although all the Ad vectors except for Ad-E2A-142-3pT-L2 and Ad-pIX-142-3pT-L2 exhibited significantly lower leaky expression levels of Ad genes in the spleen, compared with Ad-L2. In addition, the numbers of lymphocytes infused into the liver and IFN-γ mRNA levels in the liver were comparable between mice receiving Ad-L2 and those treated with Ad-E4-122aT-L2. These results suggest that Ad protein-specific CTLs might be mainly induced by Ad input proteins taken up by spleen antigen-presenting cells. Several studies have demonstrated the contribution of Ad input proteins to Ad protein-specific CTL responses.36,37 Significant levels of CTLs were detected by HD-Ad vectors and UV-inactivated Ad vectors following intravenous administration into mice.36,38 A previous study reported a significant reduction in the CTL levels following administration of an Ad vector with deletion of the E2A or E4 gene;2 however, a CTL against the transgene product might have been measured in that study. Although Ad vector-mediated CTL induction in the spleen and infiltration of lymphocytes in the liver were comparable between Ad-L2 and Ad-E4-122aT-L2, the CTL-mediated damages in the hepatocytes transduced with Ad-E4-122aT-L2 were significantly lower than those in the cells treated with Ad-L2. This is probably because the leaky expression of Ad genes in the liver was greatly reduced in the case of Ad-E4-122aT-L2 transduction, leading to a reduction in Ad antigen presentation on the hepatocytes.
This study demonstrated that miR-122a–mediated suppression of the E4 gene expression in the liver significantly reduced the hepatotoxicity that was caused by a replication-incompetent Ad vector via not only an adaptive immune response but also a non-adaptive immune response, indicating that the E4 gene products expressed in the liver directly induced hepatotoxicity via a non-adaptive immune response. Several studies have also reported that E4 gene products caused hepatotoxicity even in immune-incompetent mice following systemic administration.2,39 The E4 gene products have various functions. For example, the E4 ORF3 and E4 ORF6 augment viral DNA replication, late viral protein synthesis, and shut-off of host protein synthesis, and the E4 ORF4 has been demonstrated to be involved in apoptosis.21,25 However, the detailed mechanism of the E4 gene product-mediated hepatotoxicity following Ad vectors administration remains to be elucidated. Christ et al. prepared a series of Ad vectors containing various combinations of the E4 ORFs, and examined the hepatotoxicity profiles of these Ad vectors. They demonstrated that liver injury was markedly reduced with vectors containing either the E4 ORF3 alone or the ORF3+ ORF4, while vectors containing the ORF4 alone, the ORF6+ ORF6/7, or ORF3+ ORF6+ ORF6/7 still displayed elevated hepatotoxicity.9 Other groups also reported the hepatotoxicity profiles of the Ad vectors containing various patterns of deletion in the E4 genes.2,10 Taken together, these results indicate that the mechanism of the E4 ORF-induced hepatotoxicity is highly complex, and it has remained unclear which E4 ORF is crucial for Ad vector-mediated hepatotoxicity. Therefore, suppression of the expression of all E4 gene products from the Ad vector genome is crucial to suppress the Ad vector-mediated hepatotoxicity, as described above.
In summary, replication-incompetent Ad vectors exhibiting miRNA-mediated suppression of the leaky expression of Ad genes were developed in this study. All Ad vectors developed were easily produced at high titers comparable to a conventional Ad vector using normal 293 cells. Among the Ad vectors developed, an Ad vector containing miR-122a–targeted sequences into the 3′-UTR of the E4 gene exhibited lower levels of liver damage and higher transgene expression profiles, compared with a conventional Ad vector. Furthermore, this study indicates that expression of the E4 gene in the liver is one of the main causes of Ad vector-induced hepatotoxicity. An Ad vector with miR-122a–targeted sequences in the 3′-UTR of the E4 genes would be a promising framework for safe and effective gene therapy, basic research including gene function analysis, and elucidation of the mechanisms underlying Ad vector-mediated toxicity.
Materials and Methods
Mice and cells
Female C57BL/6 mice aged 5–7 weeks were obtained from Nippon SLC (Hamamatsu, Japan). Rag2/Il2rγ double-knockout mice of a C57BL/6 background, also aged 5–7 weeks, were obtained from Taconic Farms (Hudson, NY).31 All animal experimental procedures used in this study were performed in accordance with the institutional guidelines for animal experiments at Osaka University. HuH-7 cells (a human well-differentiated hepatocellular carcinoma cell line) were cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St Louis, MO) supplemented with 10% fetal calf serum (FCS) and antibiotics. A total of 293 cells (a human embryonic kidney cell line) were cultured in Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FCS, 2 mmol/l glutamine (Wako Pure Chemical Industries), and antibiotics. Primary mouse hepatocytes were cultured in Williams’ Medium E (Life Technologies, Carlsbad, CA) supplemented with 10% FCS, 10 µg/ml insulin (Sigma-Aldrich), 4 µg/ml dexamethasone (Wako Pure Chemical Industries), and antibiotics.
Plasmid and replication-incompetent Ad vectors
Ad vector plasmids containing miRNA-targeted sequences were constructed as follows. Briefly, for construction of the plasmid for an Ad vector incorporating miRNA-targeted sequences into the 3′-UTR of the E2A gene, the Ad genome fragment (bp 21562-25204) was cloned into the BamHI/SacI sites in pHM5.40 Subsequently, the BstXI site was introduced into the DraI site (bp 22444) in the 3′-UTR of the E2A gene by ligation with the oligonucleotides encoding the BstXI site (E2A-3′-UTR-F and E2A-3′-UTR-R; Supplementary Table S1). Following the introduction of oligonucleotides encoding two copies of miR-122a complementary sequences and PacI/SpeI sites (E2A-miR-122aT-BstXI-S3 and E2A-miR-122aT-BstXI-AS3) into the BstXI site, oligonucleotides encoding two copies of miR-122a complementary sequences (E2A-miR-122aT-BstXI-S4 and E2A-miR-122aT-BstXI-AS4) were introduced into the PacI/SpeI sites. The fragment containing the miRNA-targeted sequences was replaced with the corresponding sequences in the conventional Ad vector genome in pAdHM440 by homologous recombination, resulting in pAdHM4-E2A-122aT. For construction of the plasmid for an Ad vector incorporating miRNA-targeted sequences into the 3′-UTR of the E4 gene, the Ad genome fragment (bp 31993-33283) was cloned into pHM3.3, which was constructed based on pHM3.40 Subsequently, the KpnI site was introduced into the ApoI/BspMI sites (bp 32835-35943), which were located in the 3′-UTR of the E4 gene, by ligation with oligonucleotides encoding the KpnI site (E4-3′-UTR-F1 and E4-3′-UTR-R1). Thereafter, the NotI site was introduced into the KpnI/BspMI sites (bp 32943), which were located in the 3′-UTR of the E4 gene, by ligation with oligonucleotides encoding the NotI site (E4-3′-UTR-F2 and E4-3′-UTR-R2). Following the introduction of oligonucleotides encoding two copies of miR-122a complementary sequences and the PacI site (E4-miR-122aT-S1 and E4-miR-122aT-AS1) into the KpnI/NotI sites, oligonucleotides encoding two copies of miR-122a complementary sequences (E4-miR-122aT-S2 and E4-miR-122aT-AS2) were introduced into the KpnI/PacI sites. The fragment containing the miRNA-targeted sequences was replaced with the conventional Ad vector genome in pAdHM4 by homologous recombination, resulting in pAdHM4-E2A-122aT. For construction of a plasmid for an Ad vector incorporating miRNA-targeted sequences into the 3′-UTR of the pIX gene, the KpnI and EcoRI sites were introduced into the XbaI site (bp 4030), which is located in the 3′-UTR of the pIX gene, in pAd3′-IX5, which was constructed based on pAdHM41.41 Oligonucleotides encoding the miR-122a–targeted sequences (pIX-miR-122aT-S1, pIX-miR-122aT-AS1, pIX-miR-122aT-S2, and pIX-miR-122aT-AS2) were inserted into the KpnI and EcoRI sites. The resulting plasmid was then digested with PI-SceI/BstZ17I, and ligated with PI-SceI/BstZ171-digested pAdHM4, resulting in pAdHM4-pIX-122aT. The sequences of the oligonucleotides are shown in Supplementary Table S1. Ad vector plasmids incorporating four tandem copies that were perfectly complementary to miR-142-3p were similarly constructed using the oligonucleotides encoding miR-142-3p–targeted sequences (Supplementary Table S1). The sequences of the miRNA-targeted sequences in the Ad vector plasmids were verified by sequence analysis.
Ad vectors were prepared by an improved in vitro ligation method.40,42 An AHA-driven mSEAP-expressing plasmid, pAHA-mSEAP, was constructed using pHMRSV6,42 pBS-ApoEHCR-hAATp-hFIX-Int-bpA,43 and pCpG-mSEAP (InvivoGen, SanDiego, CA). pCMVL1,22 which has a CMV promoter-driven luciferase expression cassette, and pAHA-mSEAP were digested with I-CeuI/PI-SceI, and subsequently ligated with I-CeuI/PI-SceI–digested Ad vector plasmids. Further details on the construction method of Ad vector plasmids are available upon request. Each Ad vector plasmid was digested with PacI to release the recombinant viral genome, and was transfected into conventional 293 cells plated on 60-mm dishes. All Ad vectors were propagated in 293 cells, purified by two rounds of cesium chloride-gradient ultracentrifugation, dialyzed, and stored at −80 °C. The virus particles (VPs) were determined using a spectrophotometric method,44 and biological titers were measured using an Adeno-X-rapid titer kit (Clontech, Mountain View, CA). The ratio of the particle-to-biological titer was between 6.5 and 8 for each Ad vector used in this study. We confirmed by PCR analysis that none of the viral stocks used in this study contained detectable replication-competent virus.45 The Ad vectors used in this study are listed in Table 1.
In vitro Ad gene expression analysis
HuH-7 cells were seeded into 12-well plates at 1 × 105 cells/well. On the following day, cells were transduced with Ad vectors at a multiplicity of infection (MOI) of 10 for 1 hour. The medium containing Ad vectors was replaced with fresh medium after a 1-hour incubation. HuH-7 cells were harvested 12 hours after transduction, and total RNA was extracted from the cells. The mRNA levels of Ad genes and the glyceroaldehyde-3-phosphate-dehydrogenase (GAPDH) gene were evaluated by real-time PCR analysis as previously described.46 In the inhibition experiments using LNA-modified ASO complementary to miR-122a (miRCURY LNA Power Inhibitor; Exiqon, Vedbaek, Denmark) or an LNA control,47 HuH-7 cells were transfected with the ASO at 10 nmol/l using Lipofectamine2000 (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, HuH-7 cells were transduced with Ad vectors at MOIs of 10 for 1 hour, and harvested 12 hours after transduction. After total RNA isolation, mRNA levels of Ad genes were determined by real-time RT-PCR as described above.
In vivo Ad gene expression analysis
Ad vectors were intravenously administered into C57BL/6 mice at a dose of 1 × 1010 IFU/mouse via the tail vein. Total RNA was extracted from the livers and spleens at the indicated number of days following administration. The Ad gene mRNA levels in the organs were determined as previously described.46 The sequences of the primers and probe of mouse GAPDH were as follows: forward, 5′-CAA TGT GTC CGT CGT GGA TCT-3′; reverse, 5′-GTC CTC AGT GTA GCC CAA GAT G-3′; probe, FAM-CGT GCC GCC TGG AGA AAC CTG CC-TAMRA.
Analysis of Ad vector-mediated hepatotoxicity following intravenous administration
Ad vectors were intravenously administered into C57BL/6 and Rag2/Il2rγ double-knockout mice at a dose of 1 × 1010 IFU/mouse. The blood samples were collected via retro-orbital bleeding at the indicated days, and the serum samples were obtained by centrifugation. The serum ALT levels were determined using a transaminase-CII kit (Wako Pure Chemical Industries). The serum levels of ALP, LDH, and LAP were analyzed at the Oriental Yeast Corporation (Tokyo, Japan). For the histopathological examination of liver sections, the livers were recovered from C57BL/6 mice 10 days following Ad vector administration. The livers were washed, fixed in 10% buffered formalin (Wako Pure Chemical Industries), embedded in paraffin, and processed for histology.
Albumin mRNA levels in the liver following Ad vector administration were determined by real-time RT-PCR using THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan). The protocol for thermal cycling consisted of 60 seconds at 95 °C, followed by 40 cycles of 15 seconds at 95 °C and 60 seconds at 60 °C. The sequences of the primers were as follows: forward, 5′-TCC AAA CCT CCG TGA AAA CTA TG-3′; reverse, 5′-TGT GTT GCA GGA AAC ATT CGT-3′.
Analysis of Ad vector genome copy numbers in the liver
Ad vectors were intravenously administered into C57BL/6 mice at a dose of 1 × 1010 IFU/mouse, and liver homogenates were prepared as described above at 15 days after administration. Total DNA, including Ad vector genome, was extracted from the liver homogenates. Ad genome copy numbers in the liver were examined similarly as Ad gene expression analysis.
Analysis of Ad hexon-specific CTLs by intracellular cytokine staining assay
Levels of Ad hexon-specific CTLs in the spleen were evaluated 15 days after administration of Ad vectors at 1 × 1010 IFU/mouse using a Cytofix/CytoPerm Plus kit (BD Biosciences, San Diego, CA) as previously described with slight modification.48,49 Briefly, the spleen was harvested from the mice 15 days after administration, and 2 × 106 splenocytes were incubated for 6 hours with Ad hexon peptide (Milteny Biotec, Bergisch Gladbach, Germany), costimulatory antibodies (Ab) (CD28 and CD49d, 37.51 and R1-2,1 µg/ml; eBioscience, San Diego, CA), and 1 µg/ml of GolgiStop (BD Biosciences) in the RPMI1640 (Sigma-Aldrich) medium at 37 °C. After the incubation, the cells were washed with PBS and stained for viability (Live/Dead Fixable Dead Cell Stain Kits; Invitrogen) for 30 minutes at room temperature. The cells were then washed and stained with phycoerythrin (PE)-Cy7-conjugated anti-mouse CD3ϵ Ab (145-2C; eBioscience) and allophycocyanin (APC)-Cy7-conjugated anti-mouse CD8 Ab (53-6.7; BioLegend, San Diego, CA) for 30 minutes at 4 °C. Following incubation and washing with PBS, the cells were incubated with Cytofix/Cytoperm solution for 25 minutes at 4 °C for permeabilization, and stained with PE-conjugated anti-mouse interferon (IFN)-γ Ab (XMG1.2; eBioscience) after washing with Perm/Wash solution (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Evaluation of intrahepatic lymphocytes following Ad vector administration
Intrahepatic lymphocytes were recovered from the livers of Ad vector-treated C57BL/6 mice 10 days after administration as follows. The livers were perfused via the portal vein with PBS, cut, and homogenized using a plunger. The liver cell suspension was passed through a mesh in PBS containing 5 mg/ml collagenase and 100 U/ml DNaseI (Roche, Basel, Switzerland). The cell suspension was incubated for 45 minutes at 37 °C and centrifuged at 300g for 6 minutes at 4 °C. The cell pellet was then resuspended in serum-free RPMI1640 medium containing 44% Percoll, added to PBS containing 55% Percoll, and centrifuged at 300g for 20 minutes at room temperature. The cell fraction was harvested, added to 2% FCS-PBS, centrifuged at 300g, washed with 2% FCS-PBS, and resuspended in 1 ml of red blood cell lysis solution (1×Ack buffer) for 1 minutes at 4 °C. Cells were then washed twice and resuspended in 2% FCS-PBS. For fluorescence activated cell sorting (FACS) analysis, total mononuclear cells were stained for viability with fluorescein isotiocyanate (FITC)-conjugated anti-mouse CD4 Ab (GK1.5, eBioscience), anti-mouse CD3ϵ Ab, and anti-mouse CD8 Ab, and subsequently subjected to FACS analysis. Data were analyzed using FlowJo software (TreeStar).
Expression of IFN-γ and chemokines in the liver after Ad vector administration
The Ad vectors were intravenously administered to C57BL/6 mice at a dose of 1 × 1010 IFU/mouse. Ten days after administration, total RNA was extracted from the livers. The IFN-γ and chemokine production levels were determined by real-time RT-PCR using THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan) as described above. The sequences of the primers used in this study are listed in Supplementary Table S2.
CTL-mediated lysis of Ad vector-transduced primary mouse hepatocytes
A C57BL/6 mouse was intravenously administered Ad-null,40 which does not possess a transgene expression cassette, at 1 × 1010 IFU/mouse to induce Ad-specific CTL. The spleen was harvested from the mouse 10 days after administration, and splenocytes were restimulated with Ad-null at an MOI of 10 for 4 days. Primary hepatocytes were isolated from a naive mouse using the hepatic portal perfusion technique via a conventional method.50 Primary hepatocytes were seeded into a 96-well round bottom plate at 1 × 104 cells/well. One day after isolation, primary hepatocytes were transduced with Ad-L2 or Ad-E4-122aT-L2 at an MOI of 10. The medium containing Ad vectors was replaced with fresh medium after a 24-hour incubation. Subsequently, the primary hepatocytes were incubated with the splenocytes isolated as described above for 4 hours at 37 °C. The CTL-mediated lysis was measured by an LDH assay using a CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) as previously described.38,51
Anti-Ad antibody levels in the serum following intravenous administration of Ad vectors
C57BL/6 mice were intravenously administered Ad vectors at 1 × 1010 IFU/mouse. Blood samples were collected via retro-orbital bleeding fourteen days after administration. Anti-Ad antibody levels in the serum were determined by enzyme-linked immunosorbent assay (ELISA). For the ELISA, a 96-well plate was coated with Ad-null (5 × 105 IFU/well) overnight at 4 °C, washed with PBS-0.05% Tween (PBST), and blocked in ImmunoBlock (DS Pharma Biomedical, Osaka, Japan) for 1 hour at room temperature. The serum samples (diluted 1:500) were added to the antigen-coated plate and incubated for 2 hours at 37 °C. The plate was washed with PBST and incubated with biotin-conjugated goat anti-mouse IgG (H+L) (SouthernBiotech, Birmingham, AL) for 2 hours at 37 °C. The plates was then washed with PBST and incubated with streptavidin-HRP (SouthernBiotech) for 1 hour at room temperature. Finally, the plate was washed with PBST and TMB ELISA Peroxidase Substrate (Rockland Immunochemicals, Gilbertsville, PA) was added. The reaction was stopped by the addition of 0.5 mol/l HCl, and absorbance was read at 450 nm on a TriStar LB941 (Berthold Technologies, Bad Wildbad, Germany).
In vivo transgene expression analysis
Ad vectors were intravenously administered into C57BL/6 mice at a dose of 1 × 1010 IFU/mouse. Two days after administration, the livers were recovered from the mice and homogenized. The luciferase production in the homogenates was measured as previously described.52 For determination of mSEAP levels in the serum, C57BL/6 mice were administered the Ad vectors expressing mSEAP at a dose of 1 × 1010 IFU/mouse. The blood samples were collected on the indicated days via retro-orbital bleeding. mSEAP expression levels were determined using Great EscAPe SEAP Chemiluminescence Kit, version 2.0 (Clontech).
Statistical analysis
Statistical significance (P < 0.05) was determined using Student’s t-test. Data are presented as means ± SD.
Acknowledgments
The authors thank Kazuo Ohashi (Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan), Takako Ichinose (National Institute of Biomedical Innovation, Osaka, Japan), and Sayuri Okamoto (Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan) for their help, and David R Corey (University of Texas, Southwestern Medical Center, Dallas, TX) for advice. The AHA promoter was kindly provided by Mark A Kay (Stanford University, CA). This work was supported by a grant-in-aid for Young Scientists (A) and a Grant-in-Aid for Scientific Research (A) and (B) from the Ministry of Education, Culture, Sports, Sciences, and Technology (MEXT) of Japan, by a grant from the Ministry of Health, Labour and Welfare of Japan, and a grant from the Japan Research Foundation for Clinical Pharmacology. K.S., K.T., and Y.N. are research fellows of the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.
References
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Yang Y, Wilson JM. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedieu JF, Vigne E, Torrent C, Jullien C, Mahfouz I, Caillaud JM. Long-term gene delivery into the livers of immunocompetent mice with E1/E4-defective adenoviruses. J Virol. 1997;71:4626–4637. doi: 10.1128/jvi.71.6.4626-4637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaack J, Qiao L, Walkiewicz MP, Stonehouse M, Engel DA, Vazquez-Torres A. Insertion of CTCF-binding sites into a first-generation adenovirus vector reduces the innate inflammatory response and prolongs transgene expression. Virology. 2011;412:136–145. doi: 10.1016/j.virol.2010.12.053. [DOI] [PubMed] [Google Scholar]
- Nakai M, Komiya K, Murata M, Kimura T, Kanaoka M, Kanegae Y. Expression of pIX gene induced by transgene promoter: possible cause of host immune response in first-generation adenoviral vectors. Hum Gene Ther. 2007;18:925–936. doi: 10.1089/hum.2007.085. [DOI] [PubMed] [Google Scholar]
- Zhou H, O’Neal W, Morral N, Beaudet AL. Development of a complementing cell line and a system for construction of adenovirus vectors with E1 and E2a deleted. J Virol. 1996;70:7030–7038. doi: 10.1128/jvi.70.10.7030-7038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough DE, Hsu C, Kulesa VA, Lee GM, Cantolupo LJ, Lizonova A. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ M, Louis B, Stoeckel F, Dieterle A, Grave L, Dreyer D. Modulation of the inflammatory properties and hepatotoxicity of recombinant adenovirus vectors by the viral E4 gene products. Hum Gene Ther. 2000;11:415–427. doi: 10.1089/10430340050015888. [DOI] [PubMed] [Google Scholar]
- Andrews JL, Kadan MJ, Gorziglia MI, Kaleko M, Connelly S. Generation and characterization of E1/E2a/E3/E4-deficient adenoviral vectors encoding human factor VIII. Mol Ther. 2001;3:329–336. doi: 10.1006/mthe.2001.0264. [DOI] [PubMed] [Google Scholar]
- Krougliak V, Graham FL. Development of cell lines capable of complementing E1, E4, and protein IX defective adenovirus type 5 mutants. Hum Gene Ther. 1995;6:1575–1586. doi: 10.1089/hum.1995.6.12-1575. [DOI] [PubMed] [Google Scholar]
- Mian A, Guenther M, Finegold M, Ng P, Rodgers J, Lee B. Toxicity and adaptive immune response to intracellular transgenes delivered by helper-dependent vs. first generation adenoviral vectors. Mol Genet Metab. 2005;84:278–288. doi: 10.1016/j.ymgme.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, Brann T. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol Ther. 2002;5:63–73. doi: 10.1006/mthe.2001.0510. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sakurai F, Nakamura S, Kouyama E, Kawabata K, Kondoh M. miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy. Mol Ther. 2008;16:1719–1726. doi: 10.1038/mt.2008.159. [DOI] [PubMed] [Google Scholar]
- Bennett D, Sakurai F, Shimizu K, Matsui H, Tomita K, Suzuki T. Further reduction in adenovirus vector-mediated liver transduction without largely affecting transgene expression in target organ by exploiting microrna-mediated regulation and the Cre-loxP recombination system. Mol Pharm. 2012;9:3452–3463. doi: 10.1021/mp300248u. [DOI] [PubMed] [Google Scholar]
- Kron MW, Espenlaub S, Engler T, Schirmbeck R, Kochanek S, Kreppel F. miRNA-mediated silencing in hepatocytes can increase adaptive immune responses to adenovirus vector-delivered transgenic antigens. Mol Ther. 2011;19:1547–1557. doi: 10.1038/mt.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio K, Sakurai F, Katayama K, Tashiro K, Matsui H, Kawabata K. Enhanced safety profiles of the telomerase-specific replication-competent adenovirus by incorporation of normal cell-specific microRNA-targeted sequences. Clin Cancer Res. 2011;17:2807–2818. doi: 10.1158/1078-0432.CCR-10-2008. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Russell SJ. MicroRNAs and the regulation of vector tropism. Mol Ther. 2009;17:409–416. doi: 10.1038/mt.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Täuber B, Dobner T. Molecular regulation and biological function of adenovirus early genes: the E4 ORFs. Gene. 2001;278:1–23. doi: 10.1016/s0378-1119(01)00722-3. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Koizumi N, Hosono T, Utoguchi N, Watanabe Y, Kay MA. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001;8:730–735. doi: 10.1038/sj.gt.3301453. [DOI] [PubMed] [Google Scholar]
- Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5:e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkerova M, Belickova M, Bruchova H. Differential expression of microRNAs in hematopoietic cell lineages. Eur J Haematol. 2008;81:304–310. doi: 10.1111/j.1600-0609.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- Weitzman MD. Functions of the adenovirus E4 proteins and their impact on viral vectors. Front Biosci. 2005;10:1106–1117. doi: 10.2741/1604. [DOI] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- Gallo-Penn AM, Shirley PS, Andrews JL, Tinlin S, Webster S, Cameron C. Systemic delivery of an adenoviral vector encoding canine factor VIII results in short-term phenotypic correction, inhibitor development, and biphasic liver toxicity in hemophilia A dogs. Blood. 2001;97:107–113. doi: 10.1182/blood.v97.1.107. [DOI] [PubMed] [Google Scholar]
- Parks RJ. Adenovirus protein IX: a new look at an old protein. Mol Ther. 2005;11:19–25. doi: 10.1016/j.ymthe.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Ségard H. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Armentano D, Smith MP, Sookdeo CC, Zabner J, Perricone MA, St George JA. E4ORF3 requirement for achieving long-term transgene expression from the cytomegalovirus promoter in adenovirus vectors. J Virol. 1999;73:7031–7034. doi: 10.1128/jvi.73.8.7031-7034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormond E, Chahal P, Bernier A, Tran R, Perrier M, Kamen A. An efficient process for the purification of helper-dependent adenoviral vector and removal of helper virus by iodixanol ultracentrifugation. J Virol Methods. 2010;165:83–89. doi: 10.1016/j.jviromet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Armentano D, Zabner J, Sacks C, Sookdeo CC, Smith MP, St George JA. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylösmäki E, Martikainen M, Hinkkanen A, Saksela K. Attenuation of Semliki Forest virus neurovirulence by microRNA-mediated detargeting. J Virol. 2013;87:335–344. doi: 10.1128/JVI.01940-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Cheng Q, Harui A, Basak SK, Mitani K, Low TA. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J Immunol. 2002;169:4651–4656. doi: 10.4049/jimmunol.169.8.4651. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Linthout S, Lusky M, Collen D, De Geest B. Persistent hepatic expression of human apo A-I after transfer with a helper-virus independent adenoviral vector. Gene Ther. 2002;9:1520–1528. doi: 10.1038/sj.gt.3301824. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther. 1998;9:2577–2583. doi: 10.1089/hum.1998.9.17-2577. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Mizuguchi H, Utoguchi N, Watanabe Y, Hayakawa T. Generation of fiber-modified adenovirus vectors containing heterologous peptides in both the HI loop and C terminus of the fiber knob. J Gene Med. 2003;5:267–276. doi: 10.1002/jgm.348. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Kay MA. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum Gene Ther. 1999;10:2013–2017. doi: 10.1089/10430349950017374. [DOI] [PubMed] [Google Scholar]
- Miao CH, Ohashi K, Patijn GA, Meuse L, Ye X, Thompson AR. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Murata T, Watanabe S, Kujime Y, Hirose M, Pan J. A simple method for the simultaneous detection of E1A and E1B in adenovirus stocks. Oncol Rep. 2004;11:173–178. [PubMed] [Google Scholar]
- Shimizu K, Sakurai F, Machitani M, Katayama K, Mizuguchi H. Quantitative analysis of the leaky expression of adenovirus genes in cells transduced with a replication-incompetent adenovirus vector. Mol Pharm. 2011;8:1430–1435. doi: 10.1021/mp200121z. [DOI] [PubMed] [Google Scholar]
- Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- Shoji M, Yoshizaki S, Mizuguchi H, Okuda K, Shimada M. Immunogenic comparison of chimeric adenovirus 5/35 vector carrying optimized human immunodeficiency virus clade C genes and various promoters. PLoS ONE. 2012;7:e30302. doi: 10.1371/journal.pone.0030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine. 2010;28:1932–1941. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gao X, Fukumoto S, Tademoto S, Sato K, Hirai K. Post-isolation inducible nitric oxide synthase gene expression due to collagenase buffer perfusion and characterization of the gene regulation in primary cultured murine hepatocytes. J Biochem. 1998;124:892–899. doi: 10.1093/oxfordjournals.jbchem.a022204. [DOI] [PubMed] [Google Scholar]
- Kano A, Watanabe Y, Takeda N, Aizawa S, Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J Biochem. 1997;121:677–683. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272:149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.