Abstract
Mephedrone (4-methymethcathinone) is a synthetic cathinone designer drug that disrupts central nervous system (CNS) dopamine (DA) signaling. Numerous central neuropeptide systems reciprocally interact with dopaminergic neurons to provide regulatory counterbalance, and are altered by aberrant DA activity associated with stimulant exposure. Endogenous opioid neuropeptides are highly concentrated within dopaminergic CNS regions and facilitate many rewarding and aversive properties associated with drug use. Dynorphin, an opioid neuropeptide and kappa receptor agonist, causes dysphoria and aversion to drug consumption through signaling within the basal ganglia and limbic systems, which is affected by stimulants. This study evaluated how mephedrone alters basal ganglia and limbic system dynorphin content, and the role of DA signaling in these changes. Repeated mephedrone administrations (4 × 25 mg/kg/injection, 2-h intervals) selectively increased dynorphin content throughout the dorsal striatum and globus pallidus, decreased dynorphin content within the frontal cortex, and did not alter dynorphin content within most limbic system structures. Pre-treatment with D1-like (SCH-23380) or D2-like (eticlopride) antagonists blocked mephedrone-induced changes in dynorphin content in most regions examined, indicating altered dynorphin activity is a consequence of excessive DA signaling.
Keywords: Mephedrone, Dynorphin, Basal Ganglia, Limbic System, Dopamine, Stimulant
INTRODUCTION
4-Methylmethcathinone (mephedrone) is an emerging synthetic cathinone designer drug distributed as a primary constituent of 'bath salts' mixtures worldwide and as a substitute for methylenedioxymethamphetamine (MDMA) within ecstasy tablets throughout Europe (for review, see (German et al., 2014a)). Abused for its euphoric and stimulant effects in humans (Winstock et al., 2011), mephedrone acutely disrupts central dopamine (DA) and serotonin (5-HT) systems and demonstrates a high abuse liability within animal pre-clinical models (Aarde et al., 2013; Bonano et al., 2014; Hadlock et al., 2011). The direct action of stimulants to increase extracellular DA by blocking reuptake or driving release within the basal ganglia and limbic systems is closely tied to many of the stimulant and rewarding properties of these drugs (for review, see Fleckenstein et al., 2007; Nestler, 2005), which likely occurs with mephedrone. Mephedrone is a DA transporter (DAT) substrate, reducing DA uptake and releasing DA through transporter-mediated efflux by presumably increasing intracellular DAT substrate concentration (Baumann et al., 2012; Hadlock et al., 2011).
Neuropeptide systems provide a regulatory counterbalance to endogenous dopaminergic neuron activity by altering DA receptor sensitivity, neurotransmitter release, and electrical activity (for review, see Angulo and McEwen, 1994). The interaction between the dopaminergic and neuropeptide systems is often reciprocal in nature – DA initiates neuropeptide activity, and neuropeptides feedback to regulate DA signaling. Excess DA signaling, as occurs during stimulant exposure, dysregulates and decouples these feedback interactions. For example, repeated high-doses of cocaine, methamphetamine (METH), and mephedrone all disrupt basal ganglia and limbic system neurotensin, a neuropeptide that inhibits dopaminergic activity within these brain regions and is involved in the escalation of drug consumption over time (German et al., 2014b; Gygi et al., 1994).
Endogenous opioid neuropeptides - endorphins, enkephalins and dynorphins - and their receptors - delta, mu, kappa, and opiate receptor-like - are highly concentrated within the basal ganglia and limbic system, and reciprocally interact with dopaminergic neurons (for review see Trigo et al., 2010). Activation of central kappa opioid receptors by dynorphin inhibits dopaminergic firing and neurotransmitter release (Broderick, 1987; Reid et al., 1988). Kappa receptor activation simultaneously exerts analgesic and dysphoric/aversive effects (for review see Wee and Koob, 2010), leading to the hypothesis that dynorphin functions as an endogenous anti-addiction system. Indeed, kappa receptor antagonists increase cocaine self-administration (Wee et al., 2009) and dynorphin itself dose-dependently blocks cocaine-induced rises in extracellular DA (Zhang et al., 2004). The dynorphin system, however, is sensitive to DA signaling: dramatic increases in basal ganglia and limbic system DA transmission lead to increased dynorphin release, increased dynorphin tissue content, and increased feedback to dopaminergic neurons (Hanson et al., 1987; Li et al., 1988; Quirion et al., 1985). Consequently, understanding how the dynorphin system is altered by stimulants provides insight into the loss of endogenous dopaminergic regulation.
The goal of this study was to determine whether or not mephedrone alters dynorphin within the basal ganglia and limbic systems, the role of DA signaling in any mephedrone-induced changes, and to compare any changes to those caused by other stimulants. To do this, rats were given repeated high-dose mephedrone injections and dynorphin tissue content was measured by assessing dynorphin-like immunoreactivity (DLI) within basal ganglia and limbic structures. The involvement of DA signaling in the responses of dynorphin to mephedrone was assessed by pre-treatment with D1-like (SCH-23380) or D2-like (eticlopride) receptor antagonists. This study establishes the first profile of the impact of mephedrone upon the dynorphin system, providing groundwork for subsequent studies to investigate causal links between altered dynorphin within the basal ganglia and limbic structures and mephedrone-related behaviors.
MATERIALS AND METHODS
Animals, Reagents and Drug Treatment
Male Sprague-Dawley rats (250–325 g; Charles River Laboratories; Raleigh, NC) were housed with food and water ad libitum in a temperature and light-controlled environment. All experiments were approved by the University of Utah Institutional Animal Care and Use committee. 4-Methylmethcathinone (mephedrone) was synthesized by the Research Triangle Institute (Research Triangle Park, NC) and was a generous gift from the National Institute on Drug Abuse. S-(−)-eticlopride hydrochloride and R-(+)-SCH-23390 hydrochloride were purchased from Sigma Aldrich (St. Louis, MO). Drug dosages were calculated as free base and prepared in sterile saline solution (0.9% wt/vol NaCl, pH 7.4). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
The dynorphin A antiserum was raised in New Zealand White rabbits as previously described (Hanson et al., 1987). This antiserum recognizes the dynorphin carboxy terminus and is highly selective, expressing no cross-reactivity with 1000-fold excess concentrations of other endogenous neuropeptides such as neurotensin, metenkephalin, cholecystokinin, substance P or substance K.
Rats were administered four subcutaneous injections of saline (1.0 ml/kg/injection) or mephedrone (25 mg/kg/injection) at 2-h intervals and sacrificed 18 h after the last injection by decapitation. This dosage of mephedrone was chosen because it has been demonstrated to acutely disrupt the DA and 5-HT systems within the striatum and hippocampus, respectively (Hadlock et al., 2011), and to alter DA-related NT systems (German et al., 2014b). Animal groups were pre-treated with a D1-like (SCH-23390; 0.5 mg/kg/injection) antagonist, D2-like (eticlopride; 0.5 mg/kg/injection) antagonist, or saline 15 min prior to each mephedrone injection. Dosages were based on previous studies demonstrating their effectiveness within the basal ganglia and limbic systems (Alburges et al., 2011; Wang and McGinty, 1996). Immediately following sacrifice brains were removed, flash-frozen on dry ice, and stored at −80 °C until dissection. The striatum, divided into four regions according to the anterior-posterior and medial-lateral locations (Gygi et al., 1994), globus pallidus, lateral habenula, nucleus accumbens, substantia nigra, ventral tegmental area, and frontal cortex were dissected according to locations defined in The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 1986). Specifically, the frontal cortex in this study was the dorsolateral cortical regions between Bregma 2.2 and 3.2. All dissected areas were stored at −80 °C until radioimmunoassays were performed.
Dynorphin Radioimmunoassay
Dynorphin-like immunoreactivity (DLI) within the defined brain regions was determined by solid-phase radioimmunoassay (RIA) as previously described (Alburges et al., 2009). Briefly, tissue was homogenized in 300 µL 10 mM HCl, boiled for 10 min, then centrifuged at 17000 g for 30 min. Protein content was assessed by Bradford assay of the supernatant, the remainder of which was lyophilized and stored at −80 °C until further use. Lyophilized samples were reconstituted in assay buffer consisting of 300 µL phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, and 1.8 mM KH2PO4 in dH20; pH 7.4), 0.1% (wt/vol) gelatin and 0.1% (wt/vol) Triton X-100. Nunc immunoplates (ISI Bio-Express; Kaysville, UT) were prepared for the assay by incubating 50 µL protein G solution (50 ng/100 ml in 0.1 mol/l NaHCO3; Invitrogen; Carlsbad, CA) per well overnight at 4 °C followed by three washes with wash buffer (150 mM K2HPO4, 20 mM Na2HPO4, 0.2 mM ascorbic acid, 0.2% (vol/vol) Tween-20 and 0.1% (wt/vol) sodium azide in dH20; pH 7.4). Dynorphin (25 µL; 1:10,000 dilution) antiserum was diluted in assay buffer, incubated in plate wells for 2 h at 25 °C to attach antibody to protein G surface, and then washed three times with wash buffer. Samples or standards (25 µL) were added to wells and incubated for 3 h at 25 °C. Radiolabeled dynorphin ([125I]dynorphin; 6500 dpm per 25 µL diluted in assay buffer) was then added to each well and incubated for 2 h at 25 °C. Following incubation wells were washed with wash buffer and protein G was removed from wells, placed in polypropylene tubes, and radioactivity was counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co.; Meriden, CT). Dynorphin concentration was determined by comparing bound to free [125I]dynorphin in each sample to a standard curve ranging from 1 to 125 pg protein per assay tube.
Data Analysis
Results are graphed as percentage differences from saline controls (mean ± standard error of the mean) with saline control absolute values reported in the figure legends. Data were analyzed using both one-way ANOVA comparisons between all groups and two-way ANOVA comparisons of pre-treatment (saline, eticlopride, or SCH-23390) and drug-treatment (saline or mephedrone). Bonferroni post-hoc tests were then performed using significance set at p < 0.05 in GraphPad Prism 5.01 (GraphPad Software; La Jolla, CA).
RESULTS
The impact of mephedrone exposure upon dynorphin A tissue content within basal ganglia and limbic system structures was evaluated by radioimmunoassay (RIA) of dynorphin-like immunoreactivity (DLI) in the brains of animals receiving repeated mephedrone or saline (see Materials and Methods). This treatment paradigm was chosen based on human user reports of repeated mephedrone binging over an 8 – 12 h period with approximate 2-h intervals (Addiction, 2011; Winstock et al., 2011) and the dosage was based on studies demonstrating 25 mg/kg/injection of mephedrone causes acute disruption of central DA, 5-HT (Hadlock et al., 2011), and neurotensin (German et al., 2014b) systems in rats. The involvement of DA signaling in mephedrone-induced DLI changes was assessed by pre-treating animals with a D1-like receptor antagonist (SCH-23390), D2-like receptor antagonist (eticlopride), or saline 15 min prior to each mephedrone injection.
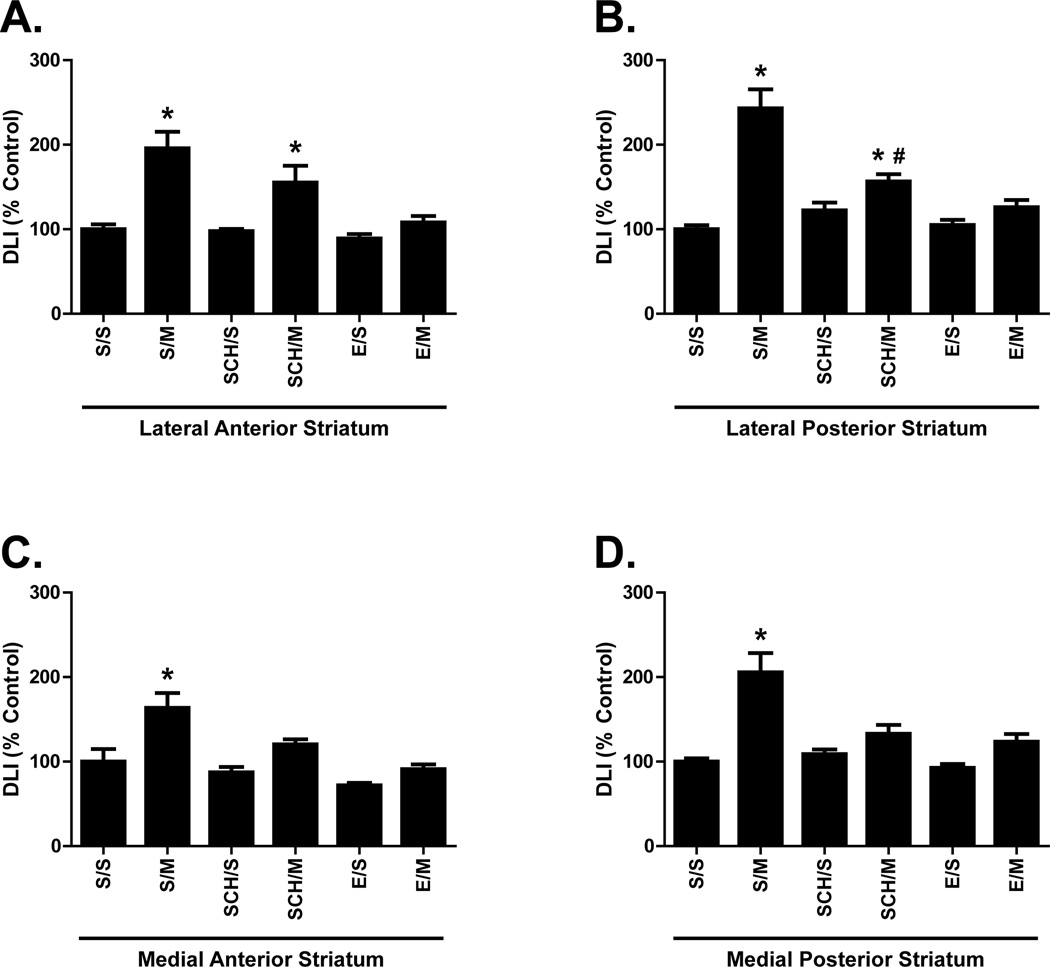
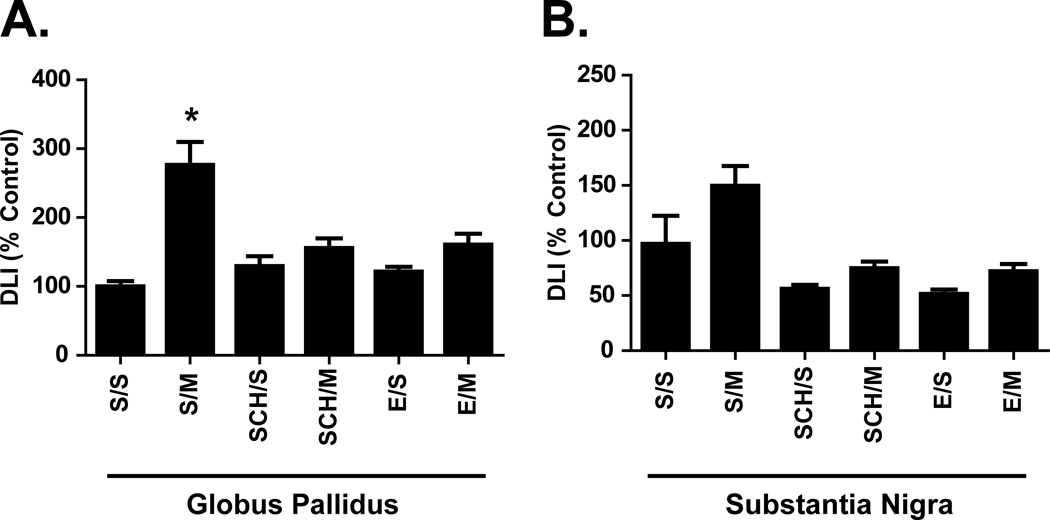
Within the basal ganglia, repeated mephedrone treatments increased DLI in all dorsal striatum regions (S/M; Fig. 1A–D). DLI differed significantly as a function of drug treatment (saline or mephedrone) and pre-treatment (saline, eticlopride, or SCH-23390) within the lateral anterior striatum (drug treatment: p < 0.0001, F(1,42) = 32.69; pre-treatment: p = 0.0011, F(2, 42) = 8.084), lateral posterior striatum (drug treatment: p < 0.0001, F(1,42) = 49.37; pre-treatment: p < 0.0001, F(2,42) = 11.85), medial anterior striatum (drug treatment: p < 0.0001, F(1,42) = 21.08; pre-treatment: p < 0.0001, F(2,42) = 12.00), and medial posterior striatum (drug treatment: p < 0.0001, F(1,42) = 33.55; pre-treatment: p = 0.0009; F(2,42) = 8.323). Pre-treatment with either SCH-23390 or eticlopride blocked mephedrone-induced increases in DLI within the medial anterior and medial posterior dorsal striatum (Fig. 1C, D). Only eticlopride significantly blocked mephedrone-induced increased DLI within the lateral anterior dorsal striatum (Fig. 1A). Within the lateral posterior striatum, mephedrone-induced DLI increases were blocked by eticlopride and partially blocked by SCH-23390 (Fig. 1B). DLI was significantly increased in lateral posterior striatum in SCH-23390 plus mephedrone-treated animals when compared to saline controls (Fig. 1B; SCH/M vs. S/S), but DLI was also significantly reduced in SCH-23390 pre-treated animals when compared to those receiving mephedrone alone (Fig. 1B; SCH/M vs. S/M). DLI differed significantly as a function of drug treatment and pre-treatment within both the globus pallidus (drug treatment: p < 0.0001, F(1,42) = 31.05; pre-treatment: p = 0.0162, F(2,42) = 4.556) and substantia nigra (drug treatment: p = 0.0074, F(1,42) = 7.895; pre-treatment: p < 0.0001, F(2,42) = 13.37). Compared to saline controls (S/S), mephedrone alone (S/M) significantly increased DLI within the globus pallidus (Fig. 2A) but not the substantia nigra, despite a trend towards an increase (Fig. 2B). Similar to regions of the dorsal striatum, increased globus pallidus DLI after repeated mephedrone administrations was blocked by pre-treatment with either SCH-23390 or eticlopride (Fig. 2A).
Figure 1.
Effects of repeated mephedrone administrations and the role of D1-like and D2-like receptors on dynorphin-like immunoreactivity (DLI) within the dorsal striatum. Rats received either saline (S; 1 ml/kg/injection, s.c.) four injections (2-h intervals) of either the D2-like antagonist eticlopride (E; 0.5 mg/kg/injection, i.p.) or the D1-like antagonist SCH-23390 (SCH; 0.5 mg/kg/injection, i.p.) 15 min prior to each of 4 injections (2-h intervals) of either saline (S; 1 ml/kg/injection, s.c.) or mephedrone (M; 25 mg/kg/injection, s.c.) and were sacrificed 18 h following the last injection. DLI was assessed within the lateral anterior striatum (A; S/S = 139.6 ± 8.9 pg/mg protein), medial anterior striatum (B; S/S = 231.7 ± 36.6 pg/mg protein), lateral posterior striatum (C; S/S = 118.5 ± 6.0 pg/mg protein), and medial posterior striatum (D; 208.7 ± 8.6 pg/mg protein). Results are expressed as percentages of the saline control group (S/S) and represent the mean ± SEM. S/S = saline/saline, S/M = saline/mephedrone, E/S = eticlopride/saline, E/M = eticlopride/mephedrone, SCH/S = SCH-23390/saline, SCH/M = SCH-23390/mephedrone. * p < 0.05 vs. S/S within each group. N = 8 rats per treatment group. # = p < 0.05 vs S/M.
Figure 2.
Effects of repeated mephedrone administrations and the role of D1-like and D2-like receptors on basal ganglia dynorphin-like immunoreactivity (DLI). Rats were treated as described in Fig. 1 and DLI was assessed in the globus pallidus (A; S/S = 438.3 ± 36.4 pg/mg protein) and substantia nigra (B; S/S = 2739.0 ± 741.6 pg/mg protein). * p < 0.05 vs. S/S within each group. N = 8 rats per treatment group.
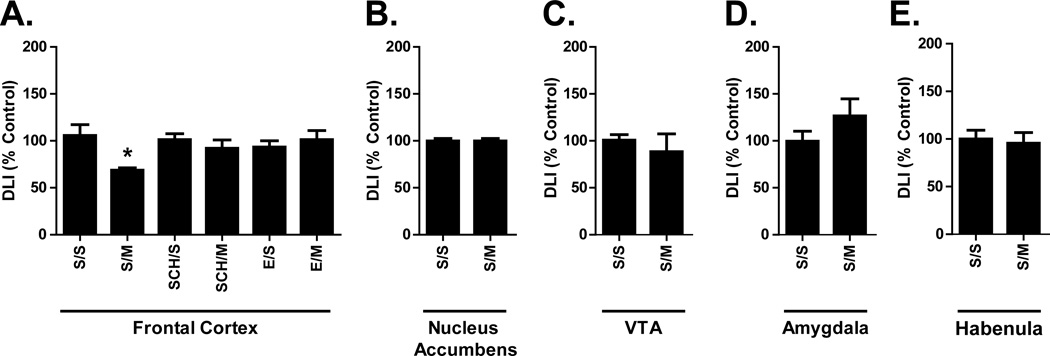
Repeated mephedrone treatment alone had limited impact on the limbic system, leaving DLI in the nucleus accumbens (Fig. 3B), ventral tegmental area (Fig. 3C), amygdala (Fig. 3D), and habenula (Fig. 3E) unaffected. Mephedrone alone (S/M) significantly decreased DLI within frontal cortex DLI (Fig. 3A), which was prevented by pre-treatment with D1-like (SCH/M) or D2-like (E/M) antagonists (Fig. 3A). DLI did not differ significantly as a function of either drug treatment or pre-treatment when assessed by two-way ANOVA.
Figure 3.
Effects of repeated mephedrone administration and the role of D1-like and D2-like receptors on limbic system DLI. Animals were treated as described in Fig. 1 and DLI was assessed within the frontal cortex (A; S/S = 138.2 ± 17.1 pg/mg protein), nucleus accumbens (B; S/S = 1819.0 ± 56.8 pg/mg protein), ventral tegmental area (C; VTA; S/S = 796.0 ± 49.5 pg/mg protein), amygdala (D; S/S = 1452.0 ± 165.3 pg/mg protein), and habenula (E; S/S = 434.9 ± 43.2 pg/mg protein). Results are expressed as percentages of the saline control group (S/S) and represent the mean ± SEM. * p < 0.05 vs. S/S within each group. N = 8 rats per treatment group.
DISCUSSION
This study demonstrates that repeated mephedrone administrations selectively increased dynorphin-A content within the basal ganglia in a selective D1-like and D2-like receptor-dependent fashion. In all brain areas studied, DA signaling mediated repeated mephedrone-induced changes in dynorphin content. Increased DLI within the medial dorsal striatum (Fig. 1C, D) and globus pallidus (Fig. 2A) as well as decreased DLI within the frontal cortex (Fig. 2A) were all blocked by D1-like (SCH-23380) and/or D2-like (eticlopride) antagonist treatment. Increased DLI within the lateral anterior dorsal striatum (Fig. 1A, B) was blocked by a D2-like antagonist alone and increased DLI within the lateral posterior dorsal striatum was partially blocked by a D1-like antagonist and completely blocked by a D2-like antagonist. DLI changes in basal ganglia and frontal cortex are likely a consequence of mephedrone acutely increasing extracellular DA through DAT blockade and DA release (Hadlock et al., 2011), and subsequent elevated D1-like and D2-like receptor signaling.
The main objective of this study was to compare the profile of mephedrone-induced changes in dynorphin content throughout the basal ganglia and limbic systems to those observed following exposure to other stimulants such as cocaine, METH, and methylphenidate (MPD). Mephedrone significantly increased DLI within all regions of the striatum and the globus pallidus in a manner similar to repeated cocaine, METH, and MPD administrations (Figs. 1 & 2) (Alburges et al., 2011; Hanson et al., 1987; Hanson et al., 1988; Smiley et al., 1990). Mephedrone caused a trend towards increased nigral DLI, although this did not reach significance likely due to variability in the saline control group (Fig 2B). Even though dynorphin is localized to D1-like receptor-expressing efferent neurons in the striatum, increased D2-like receptor activity is necessary for mephedrone to increase striatal dynorphin content, suggesting that indirect dopaminergic signaling influences striatal dynorphin activity. D2-like receptors are prominent on striatal GABAergic interneurons that provide regulatory feedback to D1 receptor-containing efferent striatal neurons. Thus, D2-mediated GABAergic receptor activation by excess DA following mephedrone administration would reduce inhibitory GABAergic feedback onto D1 receptor-containing striatal neurons, increase activity within D1 receptor-containing striatal neurons, and presumably increase dynorphin content. Such indirect signaling has been observed in other neuropeptide systems. Neurotensin, for example, inhibits striatal dopaminergic function through the modulation of striatal GABAergic activity (for review, see (Binder et al., 2001)). Dynorphin regulates basal ganglia DA signaling and many of the subsequent stimulant behaviors of these drugs, such as increased stereotypy and locomotion (Trigo et al., 2010). Increased basal ganglia dynorphin content is likely involved in the locomotor, stereotypy and drug consumption behaviors observed with mephedrone administration.
A major difference between the effects of mephedrone and other stimulants exists within the limbic system. Repeated cocaine, METH, and MPD treatments all increase dynorphin content within the nucleus accumbens (Alburges et al., 2011; Singh et al., 1991; Smiley et al., 1990). In contrast, mephedrone did not change dynorphin content within most limbic structures examined, including the nucleus accumbens, ventral tegmental area, and lateral habenula (Fig. 3B–E). Increased limbic system dynorphin signaling following acute and chronic stimulant use has been tied to dysphoria, aversion, and anhedonia in both humans and animal models (Butelman et al., 2012). Consequently, the absence of altered limbic system dynorphin content suggests mephedrone may not cause the same dysphoric effects associated with the acute use of other stimulants.
Unlike other limbic regions, mephedrone decreased frontal cortex DLI, which was blocked by both D1-like and D2-like receptor antagonists, indicating excess DA signaling is a primary driver of this response (Fig. 3A). Since kappa opioid receptor activation inhibits DA release within the frontal cortex (Tejeda et al., 2013), reduced dynorphin content (Fig. 3A) suggests kappa receptor oversight of the frontal cortex DA system is likely impaired by mephedrone. Because the frontal cortex is responsible for oversight of executive function - selecting behaviors, assigning value, responding to reward-related cues - and working memory, changes in frontal cortex dynorphin content are likely related to mephedrone-linked alterations in cognitive functions such as reduced working memory and enhanced impulsivity, schizotypy, and depression (den Hollander et al., 2013; Freeman et al., 2012).
Mephedrone-induced changes in basal ganglia and frontal cortex DLI paralleled other DA-linked neuropeptide systems, such as neurotensin. Neurotensin provides inhibitory feedback to basal ganglia and limbic DA systems - mitigating DA receptor signaling, reducing DA release, and limiting drug consumption behavior. Mephedrone treatment similar to that used within this study substantially increases striatal, nigral and pallidal neurotensin levels in a D1-like and D2-like receptor-dependent fashion (German et al., 2014b). The relationship between behavioral consequences of changes in the dynorphin and neurotensin systems by mephedrone is currently unknown.
This study demonstrates mephedrone selectively alters dynorphin content through increased DA signaling and activation of D1-like and D2-like receptors within most basal ganglia structures and the frontal cortex, but not in other limbic system structures, in a pattern distinct from that caused by cocaine, METH, and MPD. Though only the dynorphin responses to investigator-administered mephedrone were evaluated, work with other mechanistically similar stimulants suggests a similar dynorphin response profile would occur in animals self-administering mephedrone. Similar dynorphin responses in basal ganglia and limbic systems were observed after both investigator-administered and self-administered METH, a stimulant similar to mephedrone (Frankel et al., 2011). Additional studies are necessary in order to determine why basal ganglia and limbic regions respond differently to mephedrone when compared to the other stimulants, and whether causal links exist between dynorphin changes in different brain regions. Reward and consumptive behaviors of stimulants are closely linked to DA signaling within the basal ganglia and limbic systems, and dynorphin feedback on related dopaminergic pathways shapes many of these behaviors. Consequently, differences in reward and behavior between stimulants are likely linked to drug-related shifts in basal ganglia and limbic dynorphin activity. These possibilities merit further study.
Acknowledgements
This work was supported by grants DA031883, DA019447, and DA11389.
Footnotes
Conflicts of Interest
All authors have no conflicts of interest to declare.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addiction biology. 2013;18(5):786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addiction EMCfDaD. Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances. Luxembourg: The Publications Office of the European Union; 2011. p. 193. [Google Scholar]
- Alburges ME, Frankel PS, Hoonakker AJ, Hanson GR. Responses of limbic and extrapyramidal substance P systems to nicotine treatment. Psychopharmacology. 2009;201(4):517–527. doi: 10.1007/s00213-008-1316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Horner KA, Fleckenstein AE, Hanson GR. Methylphenidate alters basal ganglia neurotensin systems through dopaminergic mechanisms: a comparison with cocaine treatment. Journal of neurochemistry. 2011;117(3):470–478. doi: 10.1111/j.1471-4159.2011.07215.x. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain research Brain research reviews. 1994;19(1):1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacological reviews. 2001;53(4):453–486. [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic "bath salts" cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231(1):199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick PA. Striatal neurochemistry of dynorphin-(1–13): in vivo electrochemical semidifferential analyses. Neuropeptides. 1987;10(4):369–386. doi: 10.1016/s0143-4179(87)90128-4. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends in neurosciences. 2012;35(10):587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of "bath salt" designer drugs methylone and mephedrone. Pharmacology, biochemistry, and behavior. 2013;103(3):501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. The Journal of pharmacology and experimental therapeutics. 2011;336(3):809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a 'new legal high'. Addiction. 2012;107(4):792–800. doi: 10.1111/j.1360-0443.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life sciences. 2014a;97(1):2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Hoonakker AH, Fleckenstein AE, Hanson GR. Mephedrone alters basal ganglia and limbic neurotensin systems. Journal of neurochemistry. 2014b doi: 10.1111/jnc.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Gibb JW, Hanson GR. Differential effects of antipsychotic and psychotomimetic drugs on neurotensin systems of discrete extrapyramidal and limbic regions. The Journal of pharmacology and experimental therapeutics. 1994;270(1):192–197. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339(2):530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Methamphetamine-induced changes in the striatal-nigral dynorphin system: role of D-1 and D-2 receptors. European journal of pharmacology. 1987;144(2):245–246. doi: 10.1016/0014-2999(87)90527-9. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of methamphetamine effects on the striatal-nigral dynorphin system. European journal of pharmacology. 1988;155(1–2):11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Li SJ, Sivam SP, McGinty JF, Jiang HK, Douglass J, Calavetta L, Hong JS. Regulation of the metabolism of striatal dynorphin by the dopaminergic system. The Journal of pharmacology and experimental therapeutics. 1988;246(1):403–408. [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; 1986. p. 166. [Google Scholar]
- Quirion R, Gaudreau P, Martel JC, St-Pierre S, Zamir N. Possible interactions between dynorphin and dopaminergic systems in rat basal ganglia and substantia nigra. Brain research. 1985;331(2):358–362. doi: 10.1016/0006-8993(85)91563-x. [DOI] [PubMed] [Google Scholar]
- Reid M, Herrera-Marschitz M, Hokfelt T, Terenius L, Ungerstedt U. Differential modulation of striatal dopamine release by intranigral injection of gamma-aminobutyric acid (GABA), dynorphin A and substance P. European journal of pharmacology. 1988;147(3):411–420. doi: 10.1016/0014-2999(88)90176-8. [DOI] [PubMed] [Google Scholar]
- Singh NA, Midgley LP, Bush LG, Gibb JW, Hanson GR. N-Methyl-D-aspartate receptors mediate dopamine-induced changes in extrapyramidal and limbic dynorphin systems. Brain research. 1991;555(2):233–238. doi: 10.1016/0006-8993(91)90346-w. [DOI] [PubMed] [Google Scholar]
- Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. The Journal of pharmacology and experimental therapeutics. 1990;253(3):938–943. [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Backman CM, Chefer V, O'Donnell P, Shippenberg TS. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(9):1770–1779. doi: 10.1038/npp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug and alcohol dependence. 2010;108(3):183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. D1 and D2 receptor regulation of preproenkephalin and preprodynorphin mRNA in rat striatum following acute injection of amphetamine or methamphetamine. Synapse. 1996;22(2):114–122. doi: 10.1002/(SICI)1098-2396(199602)22:2<114::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205(4):565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106(11):1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology. 2004;172(4):422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]