Abstract
Excess weight gain, especially when associated with increased visceral adiposity, is a major cause of hypertension, accounting for 65–75% of the risk for human primary (essential) hypertension. Increased renal tubular sodium reabsorption impairs pressure natriuresis and plays an important role in initiating obesity hypertension. The mediators of abnormal kidney function and increased blood pressure during development of obesity hypertension include 1) physical compression of the kidneys by fat in and around the kidneys, 2) activation of the renin-angiotensin-aldosterone system (RAAS), and 3) increased sympathetic nervous system (SNS) activity. Activation of the RAAS system is likely due, in part, to renal compression as well as SNS activation. However, obesity also causes mineralocorticoid receptor activation independent of aldosterone or angiotensin II. The mechanisms for SNS activation in obesity have not been fully elucidated but appear to require leptin and activation of the brain melanocortin system. With prolonged obesity and development of target organ injury, especially renal injury, obesity-associated hypertension becomes more difficult to control, often requiring multiple antihypertensive drugs and treatment of other risk factors, including dyslipidemia, insulin resistance and diabetes, and inflammation. Unless effective anti-obesity drugs are developed, the impact of obesity on hypertension and related cardiovascular, renal and metabolic disorders is likely to become even more important in the future as the prevalence of obesity continues to increase.
Keywords: Blood pressure, kidney, sympathetic activity, renin-angiotensin system, mineralocorticoids, leptin, melanocortins, chronic kidney disease
INTRODUCTION
Obesity and its associated cardiovascular, metabolic and renal disorders have rapidly become a major threat to global health. Worldwide obesity has nearly doubled since 1980 and current estimates indicate that more than 1.4 billion adults are overweight or obese1. In the United States, more than 65% of adults are overweight and 36% are obese with a body mass index (BMI) greater than 30 kg/m2 2,3. Several other countries report even higher rates of obesity. For example, the estimated prevalence of obesity in adults exceeded 50% for men in Tonga and for women in Kuwait, Kiribati, Federated States of Micronesia, Libya, Qatar, Tonga, and Samoa4.
Major consequences of being overweight or obese include higher prevalence of hypertension and a cascade of associated cardiorenal and metabolic disorders. Studies in diverse populations throughout the world have shown that the relationship between BMI and systolic and diastolic blood pressure (BP) is nearly linear5,6. Risk estimates from the Framingham Heart Study, for example, suggest that 78 percent of primary (essential) hypertension in men and 65% in women can be ascribed to excess weight gain7. Clinical studies indicate that maintenance of a BMI < 25 kg/m2 is effective in primary prevention of hypertension and that weight loss reduces BP in most hypertensive subjects8,9.
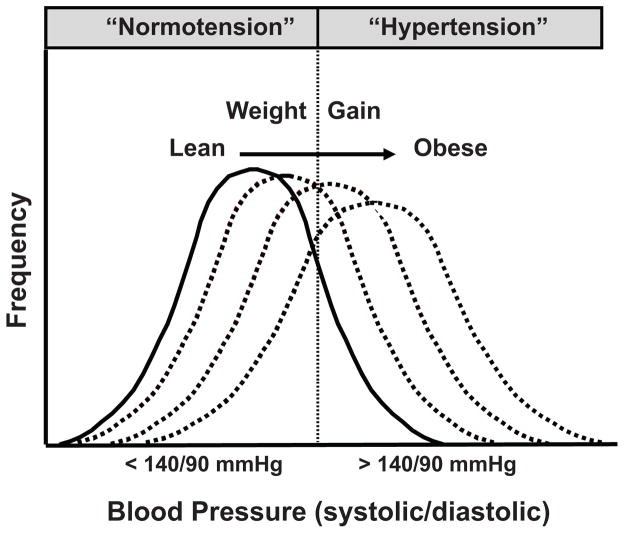
Despite impressive evidence indicating a major role for excessive weight gain in raising BP, not all obese persons are hypertensive. It is clear, however, that excess weight gain shifts the BP frequency distribution toward higher levels increasing the probability that a person’s BP will register in the hypertensive range (Figure 1). Thus, some obese people will have BPs lower than 140/90 mmHg, the level usually used to indicate “hypertension”. However, even those who are classified as obese “normotensives” have higher BP than they would at a lower body weight and weight loss lowers BP in “normotensive” and “hypertensive” obese subjects10. As discussed later, the impact of obesity on BP also depends on how long a person has been overweight, worsening as excess adiposity is maintained over several years.
Figure 1.
Effect of weight gain to shift the frequency distribution of blood pressure toward higher levels.
The distribution of fat is another important consideration. Most population studies that have investigated the relationship between obesity and BP have measured BMI rather than visceral or retroperitoneal fat which appear to be better predictors of increased BP than subcutaneous fat11.
Although the importance of obesity, especially when associated with increased visceral or retroperitoneal fat, is well established as a cause of hypertension, the pathophysiological mechanisms involved are complex and have not been fully elucidated. In this review we focus mainly on those factors that initiate obesity-induced increases in BP by impairing renal-pressure natriuresis since previous studies suggest that all forms of hypertension, including obesity hypertension, are associated with impaired renal-pressure natriuresis5.
There are additional factors that contribute to the pathophysiology of obesity-associated hypertension as metabolic disorders and increased BP are sustained over many years, leading to development of diabetes, dyslipidemia, and target organ injury, including chronic kidney disease (CKD). Some of these issues have been considered in previous reviews5,10,12; therefore, our discussion of obesity’s impact on CKD is brief and intended mainly to illustrate how this may gradually exacerbate hypertension and make it more difficult to control. In fact, BP appears to be more difficult to control in obese patients and obesity is recognized to be a common cause of treatment resistant hypertension.
HEMODYNAMIC AND RENAL CHANGES IN OBESITY-INDUCED HYPERTENSION
Studies in experimental animals have provided mechanistic insights into the cardiovascular and renal changes associated with obesity. A highly reproducible rise in BP is observed with excess weight gain induced by a high fat diet in dogs and rabbits. The metabolic, endocrine, cardiovascular, and renal changes caused by dietary-induced obesity in these experimental animals closely mimic the changes observed in obese humans5,13–16 (Table 1). Some of these changes occur rapidly during overfeeding and later become obscured by pathological changes. For example, glomerular hyperfiltration early in obesity may be replaced by a gradual decline in glomerular filtration rate (GFR) as renal injury and nephron loss occur in association with prolonged hypertension, diabetes and dyslipidemia12. Some of the same changes occur in obese rodents, although systemic hemodynamics and renal changes are not as well documented in many cases. Also, many commonly used rodent models of genetic obesity have disruption of central nervous system (CNS) signaling pathways that link obesity with sympathetic nervous system (SNS) activation and hypertension. Therefore, some rodent models of obesity, such as those with melanocortin 4 receptor (MC4R) or leptin gene mutations, have normal or reduced SNS activity and decreased BP despite inflammation, insulin resistance, dyslipidemia, and other metabolic changes associated with obesity17.
Table 1.
Hemodynamic, neurohumoral, and renal changes in obese humans, compared to lean subjects, and in experimental animal models of obesity caused by a high fat diet
| Parameter | Humans | Dogs | Rabbits | Rats | Mice |
|---|---|---|---|---|---|
| Arterial pressure | ↑ | ↑ | ↑ | ↑ | ↑ |
| Heart rate | ↑ | ↑ | ↑ | ↑ | ↑ |
| Baroreflex sensitivity | ↓ | ↓ | ↓ | ↓ | ↓ |
| Cardiac output | ↑ | ↑ | ↑ | ↑ | ? |
| VO2 (ml/min) | ↑ | ↑ | ↑ | ↑ | ↑ |
| VO2 (ml/min/kg body weight) | ↓ | ↓ | ↓ | ↓ | ↓ |
| Cardiac hypertrophy | |||||
| Eccentric | ↑ | ↑ | ↑ | ↑ | ↑ |
| Concentric | ↑ | ↑ | ↑ | ↑ | ↑ |
| Cardiac diastolic function | ↓ | ↓ | ↓ | ↓ | ↓ |
| Muscle blood flow (resting) | ↑ | ↑ | ↑ | ? | ? |
| Muscle blood flow “reserve” | ↓ | ↓ | ? | ? | ? |
| GFR* | ↑ | ↑ | ↑ | ↑ | ↑ |
| Renal blood flow* | ↑ | ↑ | ↑ | ↑ | ? |
| Renal Na+ reabsorption | ↑ | ↑ | ↑ | ↑ | ↑ |
| Sympathetic “activity”# | |||||
| Renal | ↑ | ↑ | ↑ | ? | ? |
| Cardiac | ↔↓ | ? | ? | ? | ? |
| Muscle | ↑ | ? | ? | ? | ? |
| Plasma insulin | ↑ | ↑ | ↑ | ↑ | ↑ |
| Insulin sensitivity | ↓ | ↓ | ↓ | ↓ | ↓ |
| Plasma leptin | ↑ | ↑ | ↑ | ↑ | ↑ |
VO2, total body oxygen consumption; GFR, glomerular filtration rate;
the GFR and renal blood flow refer to the early phases of obesity before major loss of nephron function has occurred;
In some instance, sympathetic “activity” is inferred from indirect measurements such as tissue norepinephrine spillover or from denervation studies; plasma insulin and leptin concentrations refer to fasting levels.
Obesity Increases Tissue Blood Flow and Cardiac Output, and Decreases Blood Flow “Reserve”
Obesity is associated with extracellular fluid volume expansion and increased blood flow in many tissues which, in turn, increases venous return and cardiac output15. Cardiac output increases in parallel with increased body weight and part of this increase is due to blood flow that supplies the extra adipose tissue. However, blood flow is increased in other tissues, including the heart, kidneys, gastrointestinal tract, and skeletal muscles10,13,16. Some of the extra blood flow is due to growth of tissues and organs in response to increased workload and metabolic demands associated with obesity. However, blood flows of tissues such as the kidneys, skeletal muscle, and heart are increased in obese subjects even when flow is expressed per gram tissue weight13. Thus, obesity is associated with functional vasodilation that is likely due to increased metabolic rate and higher tissue oxygen consumption.
Despite higher resting tissue blood flow, there is reduced blood flow “reserve” which limits the rise in blood flow that normally occurs in exercise. This decreased blood flow reserve may be due, in part, to endothelial dysfunction and weight reduction often improves flow-mediated vasodilation in obese individuals18. Accelerated arterial stiffening occurs in elderly, middle-aged, young adults (20–40 years of age) and even in children who are obese18,19. Moreover, higher aortic pulse-wave velocity, a measure of aortic stiffness, strongly correlates with increases in BMI, waist circumference and waist-hip ratio independent of systolic blood pressure, race, and sex19,20.
The mechanisms responsible for the deleterious effects of obesity on the vasculature have not been fully elucidated, but are likely due to interactions of multiple disorders, including increased BP, inflammation, hyperglycemia, “lipotoxicity” caused by excessive non-β-oxidative metabolism of fatty acids, oxidative stress, and activation of multiple neurohumoral systems. There is evidence that excess visceral fat is an important source of cytokines and other factors that create a milieu of oxidative stress and inflammation that contributes to endothelial dysfunction, vascular stiffening, and eventually atherosclerosis20.
Obesity Impairs Renal-Pressure Natriuresis
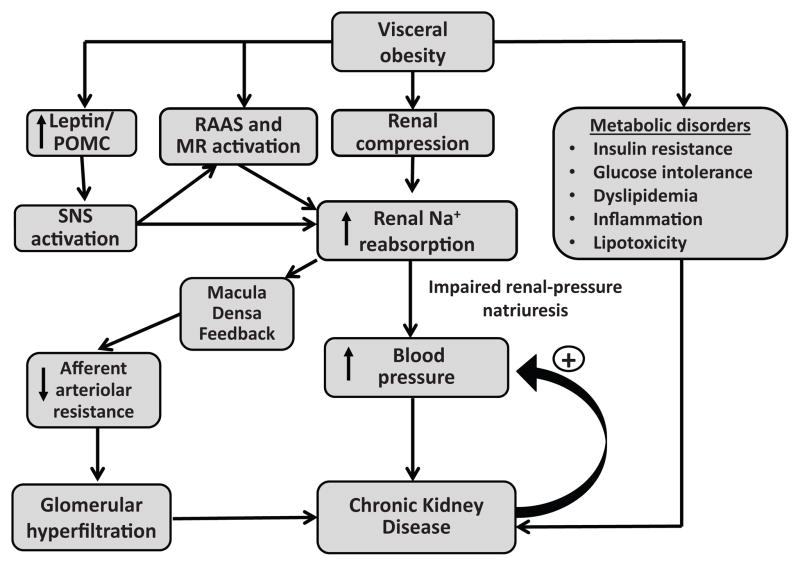
Increased renal sodium reabsorption plays a major role in initiating the rise in BP associated with excess weight gain and obese subjects require higher than normal BP to maintain sodium balance, indicating impaired renal-pressure natriuresis21. At least three major factors appear to impair renal-pressure natriuresis and increase BP during rapid, excessive weight gain (Figure 2): 1) physical compression of the kidneys due to increased visceral, retroperitoneal and renal sinus fat; 2) renin-angiotensin-aldosterone system (RAAS) activation, including activation of mineralocorticoid receptors (MR) independent of aldosterone; and 3) SNS activation, especially increased renal sympathetic nerve activity (RSNA). Also, CKD may over a much longer time, amplify the BP effects of these mechanisms, making obesity-associated hypertension more difficult to control and less easily reversed by weight loss.
Figure 2.
Summary of mechanisms by which obesity initiates development of hypertension and renal injury. Sympathetic nervous system (SNS); Renin–angiotensin–aldosterone system, RAAS; Mineralocorticoid receptor, MR; Proopiomelanocortin, POMC, neurons.
Possible role of natriuretic peptide deficiency in obesity hypertension
Although this review focuses mainly on the role of physical compression of the kidneys and activation of the RAAS and SNS in obesity hypertension, a relative deficiency of natriuretic peptides has also been suggested to contribute to impaired salt and water excretion and hypertension in obese subjects. For example, obese hypertensive men have lower levels of proatrial natriuretic peptide despite higher sodium intake compared to lean normotensive men22. Not only are levels of natriuretic peptides inappropriately low in obese subjects23, but their release in response to volume loading is impaired24. Therefore, dysfunction of the natriuretic peptide system may also contribute to impaired salt and water homoeostasis in obesity hypertension.
Cardiac-derived natriuretic peptides may also play a role in metabolic regulation. Genetically engineered mice that lack receptors (NPR-C) to promote clearance of atrial natriuretic peptide and B-type natriuretic peptide have increased circulating levels of these peptides, “browning” of white adipocytes, and increased thermogenesis compared to wild-type mice25. Thus, high levels of natriuretic peptides may protect against development of obesity and associated metabolic disorders whereas deficiency of natriuretic peptides could exacerbate obesity-induced metabolic abnormalities. However, the importance of abnormal secretion and clearance of natriuretic peptides in contributing to obesity hypertension and associated metabolic abnormalities is still unclear and has been considered more extensively in other reviews24,26.
RENAL COMPRESSION CAUSED BY VISCERAL, RETROPERITONEAL AND RENAL SINUS FAT
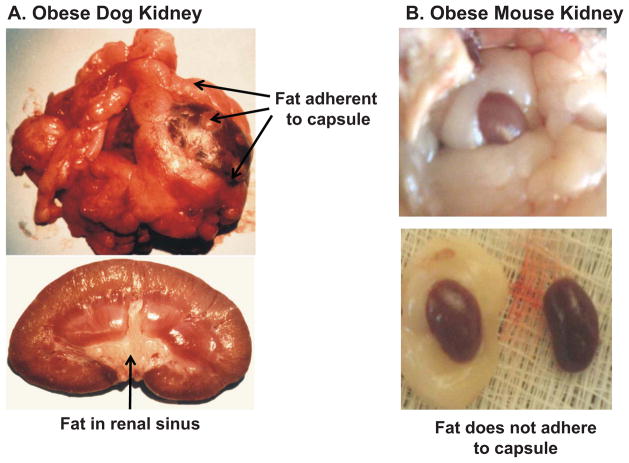
Increased visceral and retroperitoneal fat may increase BP by physically compressing the kidneys. Excess fat accumulation in and around the kidneys is associated with increased intrarenal pressures, impaired pressure natriuresis, and hypertension12. In patients with visceral obesity, intra-abdominal pressure rises in proportion to sagittal abdominal diameter, reaching levels as high as 35–40 mmHg27. These high pressures compress the renal veins, lymph vessels, ureters and renal parenchyma. Also, in obese dogs, rabbits and humans, retroperitoneal fat often encapsulates the kidney, adheres tightly to the renal capsule, and invades the renal sinuses, causing additional compression and increased intrarenal pressures (Figure 3)10,12. In obese rabbits fed a high fat diet, increased renal sinus fat was associated with distortion and prolapse of the renal medullary ducts of Bellini which would tend to restrict urinary outflow28,29.
Figure 3.
Kidneys from an obese dog fed a high fat diet for 8 weeks (A) and a severely obese (ob/ob) mouse with leptin deficiency (B). In obese dogs, the fat adheres tightly to the renal capsule, penetrates the renal capsule, and invades the renal sinuses. In obese mice the kidney is surrounded by fat but the fat does not adhere to the kidneys.
In obese humans increases in retroperitoneal and renal sinus fat are associated with hypertension. Chandra et al30 found in a large cohort of the Dallas Heart Study that visceral obesity and especially retroperitoneal fat were uniquely correlated with incident hypertension. Chughtai et al31 reported that renal sinus fat was associated with stage II hypertension and the number of antihypertensive medications required to control BP. In the Framingham Heart Study, individuals with “fatty kidneys” (high perinephric fat levels) had more than a 2-fold higher risk for hypertension which persisted after adjustment for BMI and visceral fat32. Fatty kidneys were also associated with increased risk for CKD after adjustment for BMI and visceral adiposity.
In addition to compressing the kidneys, retroperitoneal and renal sinus fat may cause inflammation and expansion of renal medullary extracellular matrix that could further impair renal function. Glycosaminoglycan content and tissue concentration of hyaluronan, important components of renal medullary extracellular matrix, are markedly elevated in the renal medulla of obese dogs and rabbits compared to lean controls29,33. Although the stimulus for increased hyaluronan in renal medulla is uncertain, its accumulation is associated with increased interstitial fluid pressure, tissue edema and inflammation in other conditions besides obesity.
Supporting the concept that visceral obesity compresses the kidneys is the observation that renal interstitial fluid hydrostatic pressure was elevated to 19 mmHg in obese dogs compared to only 9–10 mmHg in lean dogs34. Although small increases in renal interstitial fluid pressure inhibit renal tubular reabsorption, large increases of the magnitude found in obese animals would compress the thin loops of Henle and vasa recta, reducing renal tubule flow and medullary blood flow, and increasing fractional NaCl reabsorption in the loops of Henle34.
Increased sodium reabsorption caused by renal compression could indirectly contribute to renal vasodilation, glomerular hyperfiltration, and increased renin secretion in obese subjects12. Increased loop of Henle reabsorption would reduce macula densa NaCl delivery and cause, via tubuloglomerular feedback, reductions in afferent arteriolar resistance and increases in renal blood flow, GFR, and renin secretion. Glomerular hyperfiltration and elevated BP would tend to return macula densa NaCl delivery toward normal despite increased loop of Henle reabsorption, helping to restore sodium balance. Although renal compression cannot account for the initial rise in BP during rapid weight gain, they may partly explain why visceral obesity and increased renal fat are more closely associated with hypertension than subcutaneous fat.
Accumulation of fat in and around the kidneys may have additional “lipotoxic” effects on the kidneys. Lipid accumulation in key organs (ectopic fat storage) appears to impair organ function due to increased oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress35.
In contrast to multiple studies in dogs, rabbits, and humans suggesting that kidney compression contributes to obesity hypertension, this does not appear to be a major factor in rodents. Even in morbidly obese rodents (e.g. ob/ob mice), there is little adherence of fat to the kidney capsule or evidence of renal compression. In fact, kidneys from obese rats and mice appear to be “floating” in the surrounding fat with minimal invasion of the fat into the capsule or renal sinuses (Figure 3). However, the mechanisms responsible for the relative protection from adherence and invasion of fat into the kidneys of rodents, compared to humans and dogs, are unknown and a potentially interesting area for further research.
Thus far, the only proven strategies to reduce visceral, retroperitoneal and renal sinus fat and their adverse effects on cardiovascular, metabolic and renal function are bariatric surgery for severely obese patients and lifestyle modification with dietary changes and increased physical activity. A small, randomized controlled trial in which a small amount of visceral fat (0.8% of total body fat) was removed by omentectomy in patients who underwent gastric banding demonstrated improvements in glucose regulation, compared to those who only received gastric banding treatment36. Improved glucose tolerance, increased insulin sensitivity, and decreased fasting glucose occurred in the absence of significant effects of omentectomy on body weight, although effects on BP and kidney function were not reported; further longitudinal studies are needed to determine the impact on ectopic fat depots in and around the kidneys on blood pressure and renal function.
RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM (RAAS) ACTIVATION IN OBESITY
The importance of the RAAS in obesity hypertension has been previously reviewed10,37–39 and will therefore be considered briefly. Obese subjects, especially those with visceral obesity, often have mild to moderate increases in plasma renin activity (PRA), angiotensinogen, ACE activity, AngII, and aldosterone40. RAAS activation occurs despite NaCl retention, volume expansion, and hypertension, which typically suppress renin secretion and AngII formation.
Multiple mechanisms activate the RAAS activation in obesity including compression of the kidneys and increased SNS activation. Some studies also suggest a role for a local RAAS in adipose tissue41. Although angiotensinogen is produced in adipocytes, the importance of adipose tissue as a source of AngII formation is still unclear. There have been no studies, to our knowledge, directly demonstrating that adipocyte-specific derived angiotensinogen or AngII have a major influence on BP regulation in obesity. AngII has also been suggested to play a role in adipocyte growth and differentiation in rodents42 but there is little evidence in humans that inhibitors of the RAAS have major effects on adiposity or body weight.
Role of AngII in Obesity Hypertension
An important role for AngII in stimulating renal NaCl reabsorption and in mediating obesity hypertension is supported by studies in experimental animals demonstrating that AngII receptor blockade or ACE inhibition attenuates sodium retention, volume expansion, and increased BP in obesity43,44. In obese Zucker rats there is increased sensitivity to the BP effects of AngII and RAAS blockade lowers BP to a greater extent than in lean rats despite lower PRA45.
Although smaller clinical trials have clearly shown that angiotensin receptor blockers (ARBs), renin inhibitors, or angiotensin converting enzyme (ACE) inhibitors are effective in lowering BP in obese hypertensive patients46–48, there have been no large-scale clinical studies comparing the effectiveness of RAAS blockers in obese and lean hypertensive patients.
Activation of the RAAS may contribute to glomerular injury and nephron loss associated with obesity not only by increasing BP but also through intrarenal effects. For example, constriction of efferent arterioles by AngII exacerbates the rise in glomerular hydrostatic pressure caused by arterial hypertension. Studies in obese type II diabetic patients indicate that ACE inhibitors or ARBs slow progression of CKD49,50. However, further studies are needed in non-diabetic, obese subjects to determine the efficacy of RAAS blockers compared to other antihypertensive agents, in treating hypertension and reducing the risk of renal injury.
Role of MR Activation in Obesity Hypertension
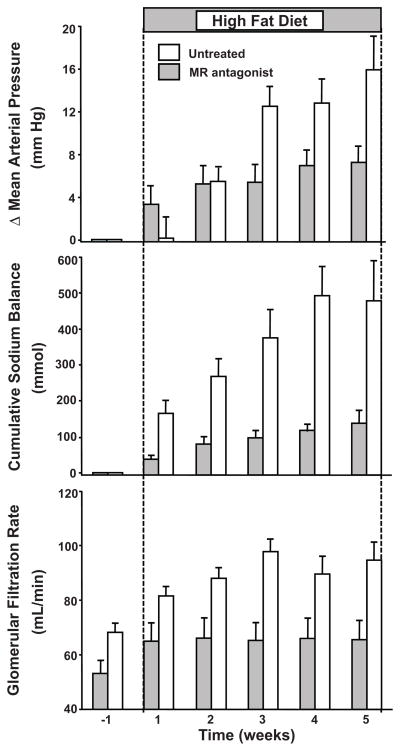
Antagonism of MR also provides an important therapeutic tool for lowering BP and attenuating target organ injury in obesity hypertension. In obese dogs, for example, MR antagonism markedly attenuated sodium retention, hypertension, and glomerular hyperfiltration51 (Figure 4). The observation that MR antagonism attenuated glomerular hyperfiltration may also have important implications for renal protection, although there are no studies, to our knowledge, that have directly tested this in obese humans.
Figure 4.
Changes (Δ) in mean arterial pressure (mmHg), cumulative sodium balance (mmol), and glomerular filtration rate (ml/min) in control, untreated dogs and mineralocorticoid receptor antagonist (eplerenone) treated dogs fed a high fat diet for 5 weeks to develop obesity. (Redrawn from data in reference 46).
Administration of MR antagonists provides significant antihypertensive benefit in treatment resistant obese patients, although there was no correlation between plasma aldosterone levels and BP responses to MR blockade52,53. BP reductions after MR antagonism in obese patients with resistant hypertension occurred despite concurrent therapy with ACE inhibitors or ARBs, suggesting that MR activation in obesity can occur independently of AngII-mediated stimulation of aldosterone secretion54.
It is not clear, however, why MR blockade is so effective in lowering BP and altering renal function in obesity despite only mild increases, or even slight decreases in plasma aldosterone, especially in those treated with ACE inhibitors or ARBs. One explanation is that obesity enhances sensitivity to aldosterone-mediated MR activation. Although obese Zucker rats have increased abundance of the α subunit of epithelial sodium channel (ENaC) in the renal cortex55, it is unclear whether there is increased expression of the other subunits necessary to form functional ENaCs or whether these changes play a major role in obesity hypertension.
Obesity also increases renal tubular epithelial cell expression of Rac1, a small guanosine triphosphate (GTP)-binding protein member of the Rho family of GTPases that activates MR signal transduction54. Several metabolic factors associated with obesity, including hyperglycemia, appear to induce renal Rac1 activation and Rac1 inhibitors attenuate proteinuria and renal injury in obese hypertensive animals54.
Glucocorticoids may also contribute to MR activation in obesity. Although cortisol can bind to MR with high affinity, renal epithelial cells are normally “protected” by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which converts cortisol to cortisone, a glucocorticoid that does not avidly bind MR. We found that 11β-HSD2 is markedly down-regulated in kidneys of obese dogs, providing a potential mechanism by which cortisol might activate the MR. Also, the ability of cortisol to activate MR may be influenced by the intracellular redox state, with increased oxidative stress resulting in increased MR activation by cortisol56. However, the importance of these mechanisms is still unclear and further studies are needed to determine why MR blockade is so effective in lowering BP in obesity hypertension.
AUTONOMIC NERVOUS SYSTEM AND RENAL NERVES IN OBESITY
Decreased Cardiac Parasympathetic Activity in Obesity
Obesity generally decreases parasympathetic tone and increases sympathetic activity. These changes in autonomic activity are associated with increased heart rate (HR), decreased HR variability, and reduced baroreflex sensitivity as well as hypertension17,57,58. The elevated HR is mainly due to decreased parasympathetic tone rather than increased sympathetic activity57,59. Conversely, weight reduction increases parasympathetic tone and HR variability while decreasing HR60. Although obesity increases sympathetic activity in many tissues, including the kidneys and skeletal muscles, cardiac sympathetic activity, as assessed by the norepinephrine spillover method, may be normal or reduced because of baroreflex inhibition of cardiac SNS activity61.
Sympathetic Nervous System Activation in Obesity
Multiple lines of evidence indicate that increased SNS activity contributes to obesity hypertension5,12: 1) SNS activity, assessed by direct recordings of muscle sympathetic nerve activity (MSNA) or norepinephrine spillover, is increased in some, but not all, tissues of obese hypertensive subjects; 2) administration of α/β-adrenergic blockers or clonidine, which stimulates central α-2 adrenergic receptors and reduces SNS activity, prevents most of the obesity-induced rise in BP in obese animals10,62 and α/β-adrenergic blockade reduces ambulatory BP significantly more in obese than in lean hypertensive patients63; 3) renal denervation (RDN) markedly attenuates sodium retention and hypertension in obese animals and obese patients with resistant hypertension. Although excessive caloric intake and weight gain clearly increases SNS activity and BP, SNS overactivity, via ensuing downregulation of β-adrenergic responsiveness, has also been suggested to exacerbate weight gain in some hypertensive patients64.
Obesity causes mild SNS activation that is differentially controlled in various tissues and depends on body fat distribution
Although cardiac SNS activity may not be elevated, RSNA and MSNA are generally increased in obese compared to lean subjects65,66. In rabbits, for example, RSNA increases within one week after starting a high fat diet while baroreflex control of RSNA is attenuated14.
Obesity-induced increases in SNS activity are usually mild and not sufficient to reduce tissue blood flow. In fact, obesity is often associated with increased blood flow in the kidneys and many other tissues. However, increased RSNA stimulates renin secretion and renal sodium reabsorption which, in turn, contribute to development and maintenance of obesity hypertension5,12.
Ethnicity and other factors such as fat distribution influence SNS responses to obesity10. For reasons that are still unclear, visceral obesity elicits greater SNS activation than does subcutaneous obesity65. In most cases MSNA has been measured rather than RSNA, the primary pathway by which the SNS causes chronic obesity hypertension. Because there is heterogeneity of autonomic outflow to different organs, measurements of MSNA activity may not always reflect RSNA. Studies by Esler and colleagues61,66 indicate that obese hypertensive subjects generally have increased RSNA, as assessed by the norepinephrine spillover method, although studies comparing different ethnic groups are limited. Thus, additional studies are needed to assess factors that influence the relationships among visceral obesity, RSNA, and hypertension in different populations.
Role of efferent and afferent renal nerves in obesity hypertension
Considerable evidence suggests that the chronic BP effects of SNS activation in obesity are mediated mainly by renal nerves. Bilateral RDN greatly attenuated sodium retention and development of hypertension in obese dogs fed a high fat diet67. RDN also returned BP to nearly normal levels in established obesity hypertension in dogs68 (Figure 5). Even partial RDN (42% reduction in renal norepinephrine) using catheter-based radiofrequency ablation substantially reduced BP in obese dogs69. Similar results have been found in obese humans with resistant hypertension in which catheter-based radiofrequency RDN consistently lowered office BP by 25–30 mmHg systolic BP and 10–12 mmHg diastolic BP for up to 24 months70,71. When 24-hour ambulatory BP was measured in a subgroup of patients, the reductions in BP after RDN averaged −11/−7 mmHg systolic/diastolic BP70,71, although, a recent trial failed to find a major effect of RDN on BP, compared to sham controls72. However, these patients were already on at least 3 antihypertensive medications, including blockers of the RAAS which may mediate at least part of the effect of the renal nerves on BP, and the extent of RDN was not verified; it appears that even under optimal conditions the radiofrequency method causes only 40–50% ablation of the renal nerves69,73. Overall, experimental and clinical studies in which RDN has been verified indicate that the renal nerves mediate most of the chronic effects of SNS activation on BP in obesity.
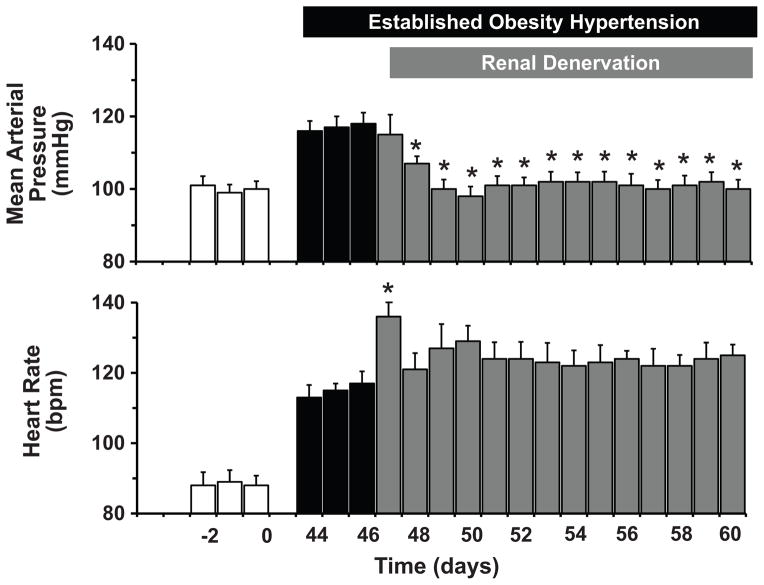
Figure 5.
Mean arterial pressure and heart rate responses to bilateral renal denervation in dogs with established obesity hypertension. Obesity was induced by feeding a high fat diet for 46 days prior to surgical denervation of both kidneys. Lean control values prior to beginning the high fat diet are shown by the open bars. (Data are from reference 62).
RDN, in addition to removing sympathetic renal efferent nerves, also eliminates renal afferent nerve fibers. These afferents carry information from mechanoreceptors and chemoreceptors to the central nervous system (CNS) and have been suggested to contribute to various forms of experimental hypertension, including renovascular hypertension70. However, the role of renal afferents in hypertension has been controversial. Early investigators, including Harry Goldblatt who first developed reproducible models of renovascular hypertension, found no evidence that renal or splanchnic nerves are necessary for development or maintenance of renovascular hypertension74,75.
The concept that renal afferents contribute to the BP lowering effects of RDN initially derived from a report that whole-body norepinephrine spillover and MSNA were reduced one year after RDN in a single patient76. Further support for this concept came from the observation that in obese patients with resistant hypertension, radiofrequency RDN decreased MSNA (by only 12–14%) as well as BP for up to one year71,77. These findings were interpreted as evidence that interrupting afferent renal nerve pathways to the brain may contribute to generalized sympathoinhibition and BP reduction after RDN70,71. However, reductions in BP following RDN do not appear to be correlated with reductions in MSNA and some investigators have failed to observe long-term reductions in MSNA after RDN78. Thus, it is still uncertain whether RDN causes significant decreases in SNA to other organs besides the kidneys. Also, measurements made for a few minutes under resting conditions may not adequately reflect SNA when a person is undergoing normal daily activities.
We assessed the role of renal sensory afferents in obesity hypertension by surgical removal of renal afferent traffic with dorsal root ganglionectomies from T10 to L2. Excision of renal afferents did not attenuate development of obesity hypertension in dogs fed a high fat diet79. These findings suggest that most of the BP effect of RDN in obese dogs can be attributed to removal of renal sympathetic efferent fibers rather than afferents. Whether this is true in obese humans has not been directly tested.
Several mediators of SNS activation in obesity have been suggested, including: 1) impaired baroreceptor reflexes; 2) activation of chemoreceptor-mediated reflexes associated with sleep apnea and intermittent hypoxia; 3) hyperinsulinemia; 4) AngII; 5) cytokines released from adipocytes such as leptin, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6); 6) the CNS CNS proopiomelanocortin (POMC) pathway. Although the role of many of these factors is still uncertain, leptin and the CNS POMC pathway appear to be important in obesity-induced SNS activation and hypertension17,80–82.
Role of Leptin in Obesity-Induced SNS Activation and Hypertension
Acute and chronic effects of leptin on RSNA and BP
Shortly after leptin’s discovery in 1994, investigators found a positive association between plasma leptin concentration and MSNA activity83 and that acute leptin administration in rodents increased SNS activity in brown adipose tissue (BAT), the adrenal gland, and the kidneys84. More recent studies indicate that acute hyperleptinemia increases MSNA in humans85. Although these findings suggest a possible role for leptin in controlling renal function and BP, acute injections of leptin have little effect on BP despite increasing SNS activity, perhaps due, in part, to counterbalancing vasodilator effects of nitric oxide (NO) which is also stimulated by leptin80,86.
We demonstrated, however, that chronic increases in plasma leptin levels, comparable to those found in severe obesity, caused sustained increases in BP and HR in rodents87. Aizawa-Abe et al.88 subsequently reported increased BP in transgenic mice with ectopic leptin overexpression in the liver. Leptin-mediated increases in BP occur gradually over several days, consistent with modest increases in SNS activity that are not sufficient to directly cause vasoconstriction but sufficient to increase renal sodium reabsorption. The BP effects of leptin were completely abolished by combined α/β-adrenergic receptor blockade; in fact, after adrenergic blockade leptin infusion lowered BP and HR, perhaps due to weight loss or stimulation of NO62.
The chronic hypertensive effect of leptin in lean rodents is modest and some investigators have reported no changes in BP during leptin infusions89. However, chronic hyperleptinemia in lean animals also markedly reduces food intake and body weight which would tend to lower SNS activity and BP. Moreover, leptin stimulates NO production which opposes increases in BP. After blocking NO synthesis the chronic effects of leptin to cause hypertension and tachycardia were markedly amplified despite reduced food intake and weight loss86. To the extent that obesity causes endothelial dysfunction and impaired NO release as well as resistance to the anorexic and weight loss effects of leptin, one might predict enhanced BP effects of leptin in obese subjects, if the effects on SNS activity are maintained.
Most of the available data on BP effects of leptin are from rodents and leptin’s effects on SNA and BP in humans have not been extensively studied. In a randomized, double-blind, placebo controlled study in overweight or obese humans, Zelissen et al.90 reported that administration of recombinant leptin for 12 weeks did not raise BP, although they also observed no significant changes in body weight compared to control subjects. Brooke et al.91 observed that acute leptin administration in lean humans did not raise BP, a finding that is not surprising in view of previous studies showing that leptin’s effects on BP require several days to manifest87.
A role for endogenous leptin in obesity hypertension is supported by the finding that administration of a leptin receptor antagonist reduced BP and renal SNS activity in obese rabbits fed a high fat diet92. Similar studies have not, to our knowledge, been conducted in humans. However, the limited available information suggests that acute leptin administration stimulates SNS activity in humans85, similar to findings in experimental animals17. Overall, the current data suggest that leptin, at levels comparable to those found in obesity, raises BP and RSNA in experimental animals and increases SNS activity in humans, although there are no studies directly demonstrating that leptin administration raises BP in humans.
CNS sites of leptin action
Leptin receptors (LepR) are expressed in many areas of the brain, including the ventromedial hypothalamus, arcuate nucleus (ARC) and dorsomedial areas of the hypothalamus, as well as in vasomotor centers of the brainstem and spinal cord intermediolateral nucleus (IML)93. Although the CNS centers that mediate leptin’s action on SNS activity and cardiovascular function have not been precisely mapped, hypothalamic centers and extra-hypothalamic regions such as the brainstem and subfornical organ (SFO) have been implicated93,94. Microinjections of leptin into the ARC increased RSNA and BAT SNS activity (BATSNA)95 and ARC lesions prevented increased BATSNA when leptin was infused93. LepR deletion in the ARC attenuated increases in RSNA and BATSNA evoked by leptin96. These observations are consistent with studies showing that ARC neurons send projections to sympathetic preganglionic neurons in the IML and suggest that the ARC is an important site for leptin’s effects on SNS activity. In fact, deletion of LepR only in POMC neurons, a major type of neuron in the ARC, prevents increased BP during chronic hyperleptinemia97.
Other hypothalamic nuclei have been implicated in leptin’s effects on SNS activity. The ventromedial and dorsomedial hypothalamus, for example, may contribute to the acute effects of leptin on RSNA, MSNA and BATSNA93. Extra-hypothalamic centers may also mediate leptin’s effect on SNS activity. Microinjections of leptin into the nucleus tractus solitarius (NTS) of the brainstem increased RSNA and acutely raised BP while BATSNA remained unchanged98. In contrast, leptin microinjections into the forebrain SFO decreased BP in lean rats, an effect that was absent in diet-induced obese rats99. Young and colleagues100, however, showed that mice with specific deletion of LepR in SFO neurons had normal BATSNA responses to leptin administration but did not exhibit the expected increase in RSNA. Taken together, these studies suggest that several brain regions may coordinate SNS responses to leptin and that leptin’s effects on SNS activity in various organs and tissues can be differentially controlled by activation of LepR in multiple brain regions.
Does obesity cause “selective” leptin resistance?
Leptin is less effective in suppressing appetite in obese compared to lean experimental animals93. However, if leptin is administered ICV, bypassing the blood brain barrier, food intake is substantially decreased in obese rodents fed a high fat diet101. This suggests that the attenuated response to leptin in obesity is partly due to defective transport, or saturation of transport, of leptin across the blood brain barrier102. However, obese subjects have higher cerebrospinal fluid (CSF) leptin levels compared to lean subjects102 suggesting that obesity also causes resistance to leptin signaling, likely related to a post-receptor defect103.
To the extent that obesity induces global leptin resistance, one might expect attenuation of leptin’s effects on SNS activity and BP, as well as attenuation of its anorexic effects. However, obesity has been suggested to induce “selective” leptin resistance, whereby leptin’s effects to increase RSNA are preserved while appetite suppression is attenuated93. In obese mice, leptin’s anorexic effects were blunted whereas the acute effects to increase RSNA were preserved104. Unfortunately, few studies have tested whether there is resistance to the chronic effects of hyperleptinemia on BP and SNS activity in obese compared to lean subjects. Also, the CNS pathways and cell signaling mechanisms that underlie selective leptin resistance in obesity are poorly understood and represent an important area for further investigation.
Molecular mechanisms for leptin’s differential effects on BP and metabolic function
The LepR is a cytokine receptor that activates janus tyrosine kinase 2 (JAK2). After binding to its CNS receptors, leptin increases activity of JAK2 leading to subsequent activation of three major intracellular pathways (Figure 6): 1) phosphorylation of tyrosine (Tyr) 1138 to recruit latent signal transducers and activators of transcription 3 (STAT3) to the LepR-JAK2 complex, causing phosphorylation and nuclear translocation of STAT3 to regulate transcription; 2) insulin receptor substrate 2 (IRS2) phosphorylation activates phosphatidylinositol 3-kinase (PI3K) which may elicit rapid non-genomic effects on neuronal activity and neuropeptide release; and 3) Tyr985 phosphorylation which recruits the tyrosine phosphatase (SHP2) to activate mitogen-activated protein kinase (MAPK).
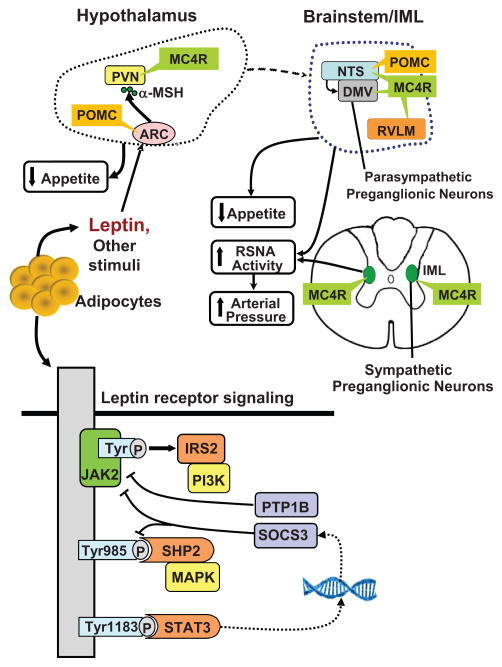
Figure 6.
Leptin-melanocortin activation in distinct areas of the brain and through multiple intracellular signaling pathways may differentially regulate appetite, energy expenditure, and arterial pressure. Leptin binding to the leptin receptor (LepR) activates its associated JAK2 tyrosine kinase (Tyr), leading to the autophosphorylation of tyrosine residues on JAK2 and phosphorylation of Tyr985 and Tyr1138. Phosphorylation of Tyr985 activates SHP2/MAPK and phosphorylation of Tyr1183 activates STAT3, a transcription factor. STAT3 activation, in addition to mediating multiple effects of leptin, also induces transcription of SOCS3 which binds to phosphor-Tyr985 and to the LepR-JAK2 complex and has a feedback effect to attenuate LepR-mediated signaling. PTP1B attenuates leptin signaling by dephosphorylation of JAK2. LepR activation of proopiomelanocortin (POMC) neurons causes release α-melanocyte stimulating hormone (α-MSH) which stimulates melanocortin 4 receptors (MC4R) in second-order neurons of the hypothalamus, brainstem, and spinal cord intermediolateral nucleus (IML).
Deletion of each of these CNS signaling pathways causes increased adiposity, but only neuron-specific STAT3 deletion recapitulates the obese phenotype found in leptin-deficient mice105. In addition to its importance in regulating body weight, STAT3 signaling also contributes to leptin’s chronic effects on BP. Deletion of STAT3 specifically in POMC neurons attenuated leptin’s effect to raise BP but had only minor effects on food intake and energy expenditure106. These observations suggest that leptin-mediated activation of STAT3 in POMC neurons is important for BP regulation whereas STAT3 activation in other neuronal types is more important in mediating leptin’s effects on appetite and energy expenditure.
The IRS2-PI3K pathway may also mediate leptin’s effect on SNS activity and BP. Pharmacological blockade of PI3K abolished the acute effects of leptin on RSNA107. CNS deletion of IRS2 signaling also attenuated the chronic BP effects of leptin but caused only moderate obesity and did not alter the anorexic responses to leptin108. These observations suggest that IRS2-PI3K signaling contributes modestly to body weight regulation but mediates at least part of leptin’s effects on SNS activity and BP.
Deletion of SHP2 in the entire CNS or specifically in the forebrain causes early-onset obesity associated with hyperphagia and impaired glucose regulation109. Also, the chronic BP effects of leptin were attenuated in mice with forebrain SHP2 deletion110 suggesting that SHP2 signaling may contribute to the effects of leptin on SNS activity and BP.
These observations suggest that each of these three intracellular signaling pathways contributes to BP regulation but may have variable effects on metabolic function. To the extent that these pathways are differentially regulated in obesity, this may help explain development of selective leptin resistance. LepR activation in different regions of the CNS may also contribute to differential control of cardiovascular and metabolic function in obesity. As discussed later, POMC neurons appear to be key sites for leptin’s effects on RSNA, BP and glucose but mediate only a small fraction of leptin’s anorexic effects. Further studies are needed, however, to ascertain how these pathways are regulated in obesity and if their differential activation contributes to selective leptin resistance.
Role of protein tyrosine phosphatase 1B (PTP1B) and suppressor of cytokine signaling 3 (SOCS3) as negative regulators of leptin’s effects on SNS activity and BP
As discussed previously, LepR activation causes phosphorylation of tyrosine residues on the LepR, including Tyr1138 which facilitates the binding of STAT proteins. One of the regulators of this signaling pathway is PTP1B which dephosphorylates JAK2 (Figure 6). Genetic deletion of PTP1B enhances leptin sensitivity and protects against obesity but exacerbates leptin’s BP effects89. Mice with whole body PTP1B deletion had reduced body fat compared to control mice, but higher BP and greater BP responses to leptin. Moreover, ganglionic blockade caused a larger fall in BP in PTP1B deficient mice compared to control mice, suggesting a greater contribution of SNS to BP in PTP1B deficient mice89. The finding that PTP1B deletion raised BP by SNS activation, even in the absence of high levels of leptin, suggests that this pathway may also modulate SNA through other mechanisms besides leptin signaling.
SOCS3 expression is regulated by the STAT3 pathway and, like PTP1B, is a negative regulator of LepR signaling that may contribute to leptin resistance in obesity (Figure 6). SOCS3 deficiency increases leptin sensitivity and attenuates obesity development in mice fed a high fat diet111. The importance of SOCS3 signaling in regulating SNS activity and BP, however, is unclear. Together, PTP1B and SOCS3 could play an important role in modulating the appetite, metabolic and cardiovascular actions of leptin and contribute to the development of selective leptin resistance in obesity. However, additional studies are needed to determine how obesity alters PTP1B and SOCS3 expression and the role of these pathways in cardiovascular regulation.
Other factors associated with obesity, such as endothelial dysfunction and impaired NO release, also selectively amplify the leptin’s effects on SNS activity and BP. Thus, obesity induces complex pathophysiological events that not only modulate LepR signaling but also elicit changes in renal, vascular and SNS function that contribute to unaltered, or even augmented, effects of leptin on SNS activity and BP (Figure 7) whereas leptin’s action on appetite, energy expenditure, and glucose homeostasis are attenuated.
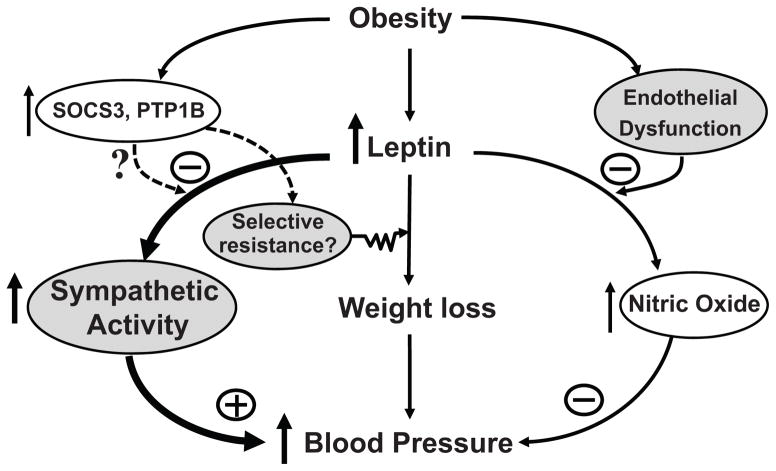
Figure 7.
Possible interactions among leptin, sympathetic activity, endothelial dysfunction and nitric oxide (NO) synthesis, and negative regulators of leptin signaling, cytokine signaling 3 (SOCS3) and protein tyrosine phosphatase 1B (PTP1B), in obesity hypertension. The net effect of leptin on blood pressure depends on the degree of endothelial dysfunction and whether there is selective resistance to the weight loss effects of leptin with preservation of the sympathetic effects of leptin in obesity.
Sympathetic activity and BP regulation in obese, leptin deficient subjects
Mice with leptin deficiency (ob/ob) mice have severe obesity, insulin resistance, hyperinsulinemia, and dyslipidemia but lower BP and reduced SNS activity compared to lean mice112. Moreover, leptin infusion in ob/ob mice increased BP despite reducing body weight113.
Humans with leptin deficiency also exhibit early-onset morbid obesity and many features of the metabolic syndrome114. Although BP and SNS activity have been assessed in only a few leptin-deficient humans, they generally are not hypertensive and do not have increased SNS activity. Of 4 individuals with leptin gene mutations who were studied by Ozata et al115, 3 had normal BP despite severe obesity. One patient had elevated BP associated with high levels of adrenocorticotrophic hormone that could have contributed to the hypertension. However, all of these leptin-deficient individuals had sympathetic hypofunction as evidenced by postural hypotension, attenuated RAAS responses to upright posture, and reduced BP responses to cold pressor tests.
We interpret these observations to be mainly consistent with observations in ob/ob mice which have normal or reduced BP despite severe obesity, insulin resistance and dyslipidemia. Collectively, clinical and experimental observations support a role for leptin as a link between obesity, increased SNS activity and elevated BP although data on leptin’s chronic BP effects in humans are still limited.
Role of CNS Proopiomelanocortin Pathway in Obesity-Induced SNS Activation and Hypertension
The CNS POMC pathway is a major regulator of appetite, energy expenditure and body weight. POMC-expressing neurons are located in the ARC and send projections to neurons in the paraventricular nucleus (PVN) and lateral hypothalamus where they release α-melanocyte stimulating hormone (α-MSH), an agonist for melanocortin 3/4 receptors (MC3/4R)116 (Figure 6). The brainstem NTS also contains POMC neurons and MC3/4R that may be important in regulating energy balance117.
Increased food intake and weight gain stimulate POMC neurons and MC3/4R signaling whereas negative energy balance reduces activity of this pathway. The importance of melanocortins in regulating energy balance is demonstrated by the finding that defects of POMC neuronal function or MC4R signaling cause severe obesity118. Although MC3R regulates energy expenditure, MC4R is much more important in regulating appetite and body weight. MC4R deficiency, for example, causes severe obesity whereas MC3R deficiency causes only mild increases in body weight119. Mutations of MC4R cause 5–6% of early onset, morbid obesity in children, illustrating the powerful role of this pathway in body weight regulation120.
Activation of CNS POMC-MC4R pathway increases SNS activity and BP
In addition to regulating energy balance, the CNS POMC-MC4R system may contribute to obesity-induced SNS activation and hypertension. Chronic pharmacological activation of CNS MC4R in rats increases BP while reducing appetite and body weight121. The rise in BP after chronic activation of MC4R is abolished after α/β-adrenergic blockade indicating that it is due to increased adrenergic activity122. Conversely, blockade of CNS MC4R in rodents causes voracious feeding, rapid weight gain, and reduces rather than increases BP123. MC4R deficient mice are also hyperphagic, obese, and have many features of the metabolic syndrome but are not hypertensive on normal or high salt diets124.
The BP-lowering effects of MC4R antagonism are especially pronounced in spontaneously hypertensive rats (SHR), a genetic model of hypertension characterized by increased SNS activity123. CNS antagonism of MC4R for 12 days caused a much greater reduction in BP in SHR than in normotensive Sprague-Dawley or Wistar-Kyoto rats despite marked increases in food intake, weight gain and insulin resistance; the fall in BP in SHR after MC4R blockade was similar to that achieved with α/β-adrenergic blockade. These observations suggest that MC4R activation contributes to tonic SNS activation and may be necessary for rapid weight gain to increase BP.
Studies in humans also suggest that MC4R activation contributes to obesity-induced hypertension. Hypertension prevalence is lower in MC4R deficient humans compared to control subjects, despite severe obesity and associated metabolic disorders125,126. Even after exclusion of patients taking antihypertensive medications, BP, heart rate, and 24-hour norepinephrine excretion were significantly lower in MC4R-deficient subjects than in control subjects. Moreover, administration of a synthetic MC4R agonist increased BP, similar to the responses observed in rodents. Thus, in humans and rodents, chronic activation of MC4R raises BP and a functional POMC-MC4R system appears to be necessary for obesity to increase SNS activity and BP.
POMC-MC4R regulates BP in non-obese subjects, independent of leptin
The CNS POMC-MC4R pathway may increase SNS activity in response to additional stimuli besides leptin and obesity. MC4R blockade markedly reduced BP in SHR, a non-obese model of hypertension associated with increased SNS activity123. MC4R antagonism also reduced BP in obese Zucker rats with defective LepR signaling127 and attenuated BP effects of other peptides besides leptin, including nesfatin-1 and neuronostatin128,129. Also, MC4R blockade attenuated hypertension associated with inhibition of NO synthesis but did not lower BP in AngII hypertension which is associated with decreased RSNA130. Studies in humans also show that increased MSNA during hypoxic stress is attenuated in MC4R deficient subjects131. These observations support the concept the POMC-MC4R pathway may contribute to SNS activation during acute stress as well as during chronic increases in SNS activity, and that this system plays a more fundamental role controlling SNS activity and BP than previously appreciated.
CNS centers for BP and SNS regulation by POMC-MC4R
The CNS regions with the greatest abundance of MC4R are the PVN, lateral hypothalamus (LH), the amygdala, the dorsal motor complex which includes the NTS and the dorsal motor nucleus of the vagus (DMV)116, and preganglionic sympathetic neurons of the IML132 which are all important sites for autonomic regulation (Figure 6). However, the specific brain regions where MC4R are most important in regulating SNS activity and BP are still unclear. Much of what is known comes from acute studies where MC4R agonists and antagonists have been injected into discrete CNS nuclei of anesthetized animals. Microinjection of an MC4R agonist into the PVN, for example, increased RSNA and BP133 and the effect of hyperinsulinemia to acutely raise lumbar SNS activity was prevented by blockade of MC4R in the PVN134.
The few studies that have examined chronic cardiovascular actions of MC4R in specific neuronal populations suggest a role for MC4R on cholinergic preganglionic parasympathetic and sympathetic neurons in contributing to obesity hypertension135. MC4R on POMC neurons may also play a role in modulating POMC activity and autonomic function136. Rescue of MC4R function specifically in POMC neurons of mice with whole-body MC4R deficiency partially restored the BP response to acute stress, suggesting that MC4R may serve to autopotentiate POMC neuronal activity136. However, the specific neurons that mediate the effects of MC4R on SNS activity and BP in obesity are still largely unknown.
Signaling pathways that mediate effects of MC4R activation on BP and metabolic functions
The MC4R is a G protein-coupled receptor that increases cAMP phosphorylation and activates protein kinase A (PKA); blockade of these intracellular pathways attenuates or abolishes MC4R actions137. Although alternative cAMP-independent mediators of MC4R have been proposed138 their physiological importance is unknown.
Previous studies of potential downstream mediators have generally been acute, lasting for only minutes to a few hours. Pharmacological activation of MC4R increased brain-derived neurotrophic factor (BDNF) protein in the DMV of rats, and the acute effect of an MC4R antagonist injected in the 4th ventricle to increase food intake was blocked by co-administration of BDNF139. Also, MC4R-mediated reductions in food intake and increases in BP were attenuated by prior CNS injection of an anti-BDNF antibody138. Other candidates, such as corticotrophin-releasing hormone (CRH), melanin-concentrating hormone (MCH), and oxytocin have also been suggested to contribute to MC4R’s actions on appetite137. However, it is still unclear if they mediate the BP effects of MC4R activation.
Thus, the CNS POMC-MC4R pathway regulates appetite, energy expenditure, autonomic nervous system activity and cardiovascular responses to stress, among many other important functions. However, the brain regions and downstream pathways that mediate MC4R actions are only beginning to be elucidated.
CKD MAY AMPLIFY THE IMPACT OF OBESITY ON HYPERTENSION
In addition to increasing BP by renal compression and activation of the RAAS and SNS, obesity may eventually cause CKD that amplifies the hypertension and makes it more difficult to control. The impact of obesity on CKD is obvious when one considers that type II diabetes and hypertension, both of which are closely associated with obesity, account for more than 70% of end stage renal disease (ESRD). Also, the rapid rise in CKD in the past three decades has paralleled increasing obesity and there is evidence that obesity may be an independent risk factor for CKD, beyond its effects to cause hypertension and diabetes12.
In a study of almost 6,500 non-diabetic participants, increasing BMI and waist circumference were associated with reduced estimated glomerular filtration rate (eGFR) and CKD140. Abdominal obesity was associated with higher risk of renal insufficiency even after adjustment for dyslipidemia, hyperglycemia, BP and BMI in patients with essential hypertension. In a retrospective analysis of 320,252 adults followed for 15–35 years, the rate of ESRD increased stepwise as BMI increased and this relationship remained after adjustment for BP, diabetes, smoking, age, and several other variables141.
Early in development of obesity and even in obese children, there is often interstitial fibrosis, microalbuminuria or proteinuria, expansion of mesangial matrix, glomerulomegaly, focal segmental glomerular sclerosis, and podocyte disorder associated with glomerular hyperfiltration142–144. As obesity and its associated hypertension and metabolic abnormalities are sustained, glomerular hyperfiltration subsides and may be replaced by declining GFR associated with nephron loss12,142,143. With nephron loss there is increasing salt-sensitivity of BP143. Obesity also aggravates the deleterious effects of other primary kidney insults, including unilateral nephrectomy, kidney transplantation, unilateral renal agenesis, and immunoglobulin A (IgA) nephropathy142,143.
Although the mechanisms by which obesity causes renal injury, in addition to hypertension and diabetes, are still unclear and beyond the scope of this review, multiple factors have been proposed including inflammation, mitochondrial dysfunction, oxidative stress, dyslipidemia and “lipotoxicity” caused by fat infiltration into and around the kidneys143. Regardless of the precise causes of obesity-induced CKD, it is likely that the gradual decline in kidney function helps to explain why most patients with treatment resistant hypertension are also overweight or obese145. With declining renal function adequate BP control becomes increasingly challenging.
SUMMARY AND PERSPECTIVES
There is overwhelming evidence that excess weight gain and visceral obesity are major causes of hypertension, perhaps accounting for as much as 65–75% of the risk for human essential hypertension. Although the mechanisms of obesity-induced hypertension are still being intensively studied, research in experimental animals and humans suggest important roles for impaired renal-pressure natriuresis due to physical compression of the kidneys and activation of the RAAS and SNS. As obesity and its metabolic and hemodynamic consequences are sustained over many years, renal injury gradually makes the hypertension more severe and more resistance to therapy.
Although weight loss is helpful in managing hypertension, many obese patients are unable to sustain adequate weight loss through lifestyle modifications and there are few available drugs that safely and effectively produce adequate long-term weight loss. Current therapeutic approaches are therefore aimed mainly at treating the hypertension and metabolic consequences of obesity, including diabetes, dyslipidemia, and inflammation. Specific guidelines for treating obesity-associated hypertension, in addition to the recommendation of reducing weight, are needed.
The most important therapeutic goal for obese hypertensive patients should be to treat their underlying causes of obesity. However, the physiological and behavioral factors that regulate energy balance are still not well understood despite an explosion of obesity-related research in the past two decades and the discovery of many adipokines, gastrointestinal hormones, and CNS pathways that influence food intake and energy expenditure. Unfortunately, the most powerful known mechanisms that control energy balance and adiposity, such as leptin and the CNS melanocortin system, also tend to increase BP and HR. Although further research is needed to better understand the molecular pathways that link metabolic and cardiovascular regulation, the rapid advances that are occurring in this field provide reasons to be optimistic that more effective therapies for obesity will be developed in the near future.
Acknowledgments
We thank Stephanie Lucas and Gerry McAlpin for their expert assistance in preparation of this manuscript.
SOURCES OF FUNDING
The authors’ research was supported by grants from the National Heart, Lung, and Blood Institute (P01 HL51971), the National Institute of General Medical Sciences (P20 GM104357), and the American Heart Association.
Nonstandard Abbreviations and Acronyms
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- α-MSH
α-melanocyte stimulating hormone
- ACE
angiotensin converting enzyme
- AngII
angiotensin II
- ARC
arcuate nucleus
- BATSNA
brown adipose tissue sympathetic nerve activity
- BDNF
brain-derived neurotrophic factor
- BMI
body mass index
- BP
blood pressure
- CKD
chronic kidney disease
- CNS
central nervous system
- CRH
corticotrophin-releasing hormone
- CSF
cerebrospinal fluid
- DMV
dorsal motor nucleus of the vagus
- ENaC
epithelial sodium channel
- ESRD
end stage renal disease
- GFR
glomerular filtration rate
- GTP
guanosine triphosphate
- HR
heart rate
- IL-6
interleukin-6
- IML
spinal cord intermediolateral nucleus
- IRS2
insulin receptor substrate 2
- JAK2
janus tyrosine kinase 2
- LepR
leptin receptor
- MAPK
Mitogen-activated protein kinase
- MC4R
melanocortin 4 receptor
- MCH
melanin-concentrating hormone
- MR
mineralocorticoid receptor
- MSNA
muscle sympathetic nerve activity
- NO
nitric oxide
- NTS
nucleus tractus solitarius
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- POMC
proopiomelanocortin
- PRA
plasma renin activity
- PVN
paraventricular nucleus
- PTP1B
protein tyrosine phosphatase 1B
- RAAS
renin-angiotensin-aldosterone system
- RDN
renal denervation
- RSNA
renal sympathetic nerve activity
- SFO
subfornical organ
- SHP2
Src homology-2 tyrosine phosphatase
- SHR
spontaneously hypertensive rats
- SNA
sympathetic nerve activity
- SNS
sympathetic nervous system
- SOCS3
suppressor of cytokine signaling 3
- STAT3
signal transducers and activators of transcription 3
- TNF-α
tumor necrosis factor-α
- TYR
tyrosine
Footnotes
DISCLOSURES
J.E. Hall is the principal investigator of research grants from St. Jude Medical, Inc. and from Palatin Technologies, Inc. to the University of Mississippi Medical Center.
Reference List
- 1.Obesity and Overweight Fact Sheet N°311. 2014 http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.U.S.Department of Health and Human Services - Center for Disease Control and Prevention. 2014 http://www.cdc.gov/obesity/data/index.html.
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 6.Jones DW, Kim JS, Andrew ME, Kim SJ, Hong YP. Body mass index and blood pressure in Korean men and women: the Korean National Blood Pressure Survey. J Hypertens. 1994;12:1433–1437. doi: 10.1097/00004872-199412000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Garrison RJ, Kannel WB, Stokes J, III, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 8.Jones DW, Miller ME, Wofford MR, Anderson DC, Jr, Cameron ME, Willoughby DL, Adair CT, King NS. The effect of weight loss intervention on antihypertensive medication requirements in the hypertension Optimal Treatment (HOT) study. Am J Hypertens. 1999;12:1175–1180. doi: 10.1016/s0895-7061(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 9.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 12.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll JF, Huang M, Hester RL, Cockrell K, Mizelle HL. Hemodynamic alterations in hypertensive obese rabbits. Hypertension. 1995;26:465–470. doi: 10.1161/01.hyp.26.3.465. [DOI] [PubMed] [Google Scholar]
- 14.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60:163–171. doi: 10.1161/HYPERTENSIONAHA.111.190413. [DOI] [PubMed] [Google Scholar]
- 15.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 16.Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, Frohlich ED. Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981;141:81–85. doi: 10.1001/archinte.141.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 19.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 20.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 21.Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S–55S. [PubMed] [Google Scholar]
- 22.Asferg CL, Nielsen SJ, Andersen UB, Linneberg A, Moller DV, Hedley PL, Christiansen M, Goetze JP, Esler M, Jeppesen JL. Relative atrial natriuretic peptide deficiency and inadequate renin and angiotensin II suppression in obese hypertensive men. Hypertension. 2013;62:147–153. doi: 10.1161/HYPERTENSIONAHA.111.00791. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 24.Savoia C, Volpe M, Alonzo A, Rossi C, Rubattu S. Natriuretic peptides and cardiovascular damage in the metabolic syndrome: molecular mechanisms and clinical implications. Clin Sci (Lond) 2010;118:231–240. doi: 10.1042/CS20090204. [DOI] [PubMed] [Google Scholar]
- 25.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11:403–412. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]
- 27.Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71–79. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer TM, Bigler SA, Moore NA, Carroll JF, Hall JE. The altered structure of renal papillary outflow tracts in obesity. Ultrastruct Pathol. 2000;24:251–257. doi: 10.1080/01913120050176707. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer TM, Banks SA, Alonso-Galicia M, Cockrell K, Carroll JF, Bigler SA, Hall JE. Distribution of renal medullary hyaluronan in lean and obese rabbits. Kidney Int. 2000;58:721–729. doi: 10.1046/j.1523-1755.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the dallas heart study. J Am Coll Cardiol. 2014;64:997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 31.Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, Nicklas B, Hamilton C, Hundley WG. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso-Galicia M, Dwyer TM, Herrera GA, Hall JE. Increased hyaluronic acid in the inner renal medulla of obese dogs. Hypertension. 1995;25:888–892. doi: 10.1161/01.hyp.25.4.888. [DOI] [PubMed] [Google Scholar]
- 34.Hall JE, Brands MW, Henegar JR. Mechanisms of hypertension and kidney disease in obesity. Ann N Y Acad Sci. 1999;892:91–107. doi: 10.1111/j.1749-6632.1999.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH, Scherer PE, Holland WL. Dichotomous roles of leptin and adiponectin as enforcers against lipotoxicity during feast and famine. Mol Biol Cell. 2013;24:3011–3015. doi: 10.1091/mbc.E12-10-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 37.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–H1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 40.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001;79:21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 41.Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol. 2013;378:1–14. doi: 10.1016/j.mce.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R181–R186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- 44.Robles RG, Villa E, Santirso R, Martinez J, Ruilope LM, Cuesta C, Sancho JM. Effects of captopril on sympathetic activity, lipid and carbohydrate metabolism in a model of obesity-induced hypertension in dogs. Am J Hypertens. 1993;6:1009–1015. doi: 10.1093/ajh/6.12.1009. [DOI] [PubMed] [Google Scholar]
- 45.Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- 46.Grassi G, Seravalle G, Dell’Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens. 2003;21:1761–1769. doi: 10.1097/00004872-200309000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Reisin E, Weir MR, Falkner B, Hutchinson HG, Anzalone DA, Tuck ML. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo-controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension. 1997;30:140–145. doi: 10.1161/01.hyp.30.1.140. [DOI] [PubMed] [Google Scholar]
- 48.Dorresteijn JA, Schrover IM, Visseren FL, Scheffer PG, Oey PL, Danser AH, Spiering W. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: a double-blind, placebo-controlled cross-over trial. J Hypertens. 2013;31:393–403. doi: 10.1097/HJH.0b013e32835b6c02. [DOI] [PubMed] [Google Scholar]
- 49.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 50.Brenner BM, Cooper ME, de ZD, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 51.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 52.de SF, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 53.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247. doi: 10.1146/annurev-med-042711-135929. [DOI] [PubMed] [Google Scholar]
- 54.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25:1148–1155. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol. 2001;281:F639–F648. doi: 10.1152/ajprenal.2001.281.4.F639. [DOI] [PubMed] [Google Scholar]
- 56.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 57.Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ., Jr Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol. 1995;269:H629–H637. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 58.Lohmeier TE, Iliescu R. The sympathetic nervous system in obesity hypertension. Curr Hypertens Rep. 2013;15:409–416. doi: 10.1007/s11906-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–R225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 60.Hirsch J, Leibel RL, Mackintosh R, Aguirre A. Heart rate variability as a measure of autonomic function during weight change in humans. Am J Physiol. 1991;261:R1418–R1423. doi: 10.1152/ajpregu.1991.261.6.R1418. [DOI] [PubMed] [Google Scholar]
- 61.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 62.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 63.Wofford MR, Anderson DC, Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14:694–698. doi: 10.1016/s0895-7061(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 64.Julius S, Valentini M, Palatini P. Overweight and hypertension : a 2-way street? Hypertension. 2000;35:807–813. doi: 10.1161/01.hyp.35.3.807. [DOI] [PubMed] [Google Scholar]
- 65.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 66.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 67.Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Granger JP. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 1998;31:397–402. doi: 10.1161/01.hyp.31.1.397. [DOI] [PubMed] [Google Scholar]
- 68.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henegar JR, Zhang Y, Rama RD, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 71.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64:118–124. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- 72.Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 73.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 74.Goldblatt H, Gross J, Hanzal RF. STUDIES ON EXPERIMENTAL HYPERTENSION : II. THE EFFECT OF RESECTION OF SPLANCHNIC NERVES ON EXPERIMENTAL RENAL HYPERTENSION. J Exp Med. 1937;65:233–241. doi: 10.1084/jem.65.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norman RA, Jr, Murphy WR, Dzielak DJ, Khraibi AA, Carroll RG. Role of the renal nerves in one-kidney, one clip hypertension in rats. Hypertension. 1984;6:622–626. doi: 10.1161/01.hyp.6.5.622. [DOI] [PubMed] [Google Scholar]
- 76.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 77.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 78.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 79.Zappe DH, Capel WT, Keen HL, Shek EW, Brands MW, Hall JE. Role of renal afferent nerves in obesity-induced hypertension. Am J Hypertens. 1996:9. [Google Scholar]
- 80.da Silva AA, do Carmo JM, Wang Z, Hall JE. The brain melanocortin system, sympathetic control, and obesity hypertension. Physiology (Bethesda) 2014;29:196–202. doi: 10.1152/physiol.00061.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life. 2013;65:692–698. doi: 10.1002/iub.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens. 2013;22:135–140. doi: 10.1097/MNH.0b013e32835d0c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Snitker S, Pratley RE, Nicolson M, Tataranni PA, Ravussin E. Relationship between muscle sympathetic nerve activity and plasma leptin concentration. Obes Res. 1997;5:338–340. doi: 10.1002/j.1550-8528.1997.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 84.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machleidt F, Simon P, Krapalis AF, Hallschmid M, Lehnert H, Sayk F. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab. 2013;98:E491–E496. doi: 10.1210/jc.2012-3009. [DOI] [PubMed] [Google Scholar]
- 86.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension. 2001;37:670–676. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- 87.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 88.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zelissen PM, Stenlof K, Lean ME, Fogteloo J, Keulen ET, Wilding J, Finer N, Rossner S, Lawrence E, Fletcher C, McCamish M. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2005;7:755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 91.Brook RD, Bard RL, Bodary PF, Eitzman DT, Rajagopalan S, Sun Y, Depaoli AM. Blood pressure and vascular effects of leptin in humans. Metab Syndr Relat Disord. 2007;5:270–274. doi: 10.1089/met.2006.0023. [DOI] [PubMed] [Google Scholar]
- 92.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 93.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 95.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 96.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith PM, Ferguson AV. Cardiovascular actions of leptin in the subfornical organ are abolished by diet-induced obesity. J Neuroendocrinol. 2012;24:504–510. doi: 10.1111/j.1365-2826.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 100.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dubinion JH, da Silva AA, Hall JE. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens. 2011;29:758–762. doi: 10.1097/HJH.0b013e328344280b. [DOI] [PubMed] [Google Scholar]
- 102.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 103.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 104.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 105.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dubinion JH, do Carmo JM, Adi A, Hamza S, da Silva AA, Hall JE. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension. 2013;61:1066–1074. doi: 10.1161/HYPERTENSIONAHA.111.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 108.do Carmo J, da Silva A, Hall J. Leptin reduces food intake but fails to raise blood pressure in mice with deficiency of insulin receptor substrate 2 (IRS2) in the entire brain or specifically in POMC neurons. Hypertension. 2012;60:A27. [Google Scholar]
- 109.Krajewska M, Banares S, Zhang EE, Huang X, Scadeng M, Jhala US, Feng GS, Krajewski S. Development of diabesity in mice with neuronal deletion of Shp2 tyrosine phosphatase. Am J Pathol. 2008;172:1312–1324. doi: 10.2353/ajpath.2008.070594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, Davis MT, Hall JE. Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes (Lond) 2014;38:775–783. doi: 10.1038/ijo.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 112.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 113.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 115.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 116.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 117.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 118.Martinelli CE, Keogh JM, Greenfield JR, Henning E, van der Klaauw AA, Blackwood A, O’Rahilly S, Roelfsema F, Camacho-Hubner C, Pijl H, Farooqi IS. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–E188. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 119.Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta. 2014;1842:482–494. doi: 10.1016/j.bbadis.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41:768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 122.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43:370–375. doi: 10.1161/01.HYP.0000111836.54204.93. [DOI] [PubMed] [Google Scholar]
- 123.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51:884–890. doi: 10.1161/HYPERTENSIONAHA.107.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 125.Greenfield JR. Melanocortin signalling and the regulation of blood pressure in human obesity. J Neuroendocrinol. 2011;23:186–193. doi: 10.1111/j.1365-2826.2010.02088.x. [DOI] [PubMed] [Google Scholar]
- 126.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 127.do Carmo JM, da Silva AA, Rushing JS, Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R561–R567. doi: 10.1152/ajpregu.00392.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yosten GL, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptors. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1194–R1199. doi: 10.1152/ajpregu.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.da Silva AA, do Carmo JM, Dubinion JH, Bassi M, Mokhtarpouriani K, Hamza SM, Hall JE. Chronic Central Nervous System MC3/4R Blockade Attenuates Hypertension Induced by Nitric Oxide Synthase Inhibition but not by Angiotensin II Infusion. Hypertension. 2015;65:171–177. doi: 10.1161/HYPERTENSIONAHA.114.03999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 132.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol. 2013;98:435–443. doi: 10.1113/expphysiol.2012.067256. [DOI] [PubMed] [Google Scholar]
- 134.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol. 2013;305:R359–R368. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–982. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 139.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150:2646–2653. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 140.Burton JO, Gray LJ, Webb DR, Davies MJ, Khunti K, Crasto W, Carr SJ, Brunskill NJ. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant. 2012;27:1860–1866. doi: 10.1093/ndt/gfr574. [DOI] [PubMed] [Google Scholar]
- 141.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 142.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33:23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 143.Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11:41–54. doi: 10.1053/j.arrt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 144.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 145.Egan BM, Li J. Role of aldosterone blockade in resistant hypertension. Semin Nephrol. 2014;34:273–284. doi: 10.1016/j.semnephrol.2014.04.004. [DOI] [PubMed] [Google Scholar]