Abstract
Reports in recent years indicate that the increasing emergence of resistance to drugs be using to TB treatment. The resistance to them severely affects to options for effective treatment. The emergence of multidrug-resistant tuberculosis has increased interest in understanding the mechanism of drug resistance in M. tuberculosis and the development of new therapeutics, diagnostics and vaccines. In this study, a label-free quantitative proteomics approach has been used to analyze proteome of multidrug-resistant and susceptible clinical isolates of M. tuberculosis and identify differences in protein abundance between the two groups. With this approach, we were able to identify a total of 1,583 proteins. The majority of identified proteins have predicted roles in lipid metabolism, intermediary metabolism, cell wall and cell processes. Comparative analysis revealed that 68 proteins identified by at least two peptides showed significant differences of at least twofolds in relative abundance between two groups. In all protein differences, the increase of some considering proteins such as NADH dehydrogenase, probable aldehyde dehydrogenase, cyclopropane mycolic acid synthase 3, probable arabinosyltransferase A, putative lipoprotein, uncharacterized oxidoreductase and six membrane proteins in resistant isolates might be involved in the drug resistance and to be potential diagnostic protein targets. The decrease in abundance of proteins related to secretion system and immunogenicity (ESAT-6-like proteins, ESX-1 secretion system associated proteins, O-antigen export system and MPT63) in the multidrug-resistant strains can be a defensive mechanism undertaken by the resistant cell.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-015-0511-2) contains supplementary material, which is available to authorized users.
Keywords: Tuberculosis (TB), Mycobacterium tuberculosis, Multidrug-resistance, Label-free quantitation, Liquid chromatography mass spectrometry
Introduction
Tuberculosis (TB) is a serious infectious disease caused by Mycobacterium tuberculosis and remains one of the leading causes of infectious disease-related morbidity and mortality worldwide, particularly in developing countries. Infection of M. tuberculosis leads to 8.6 million people fell ill every year [1]. Multidrug-resistant TB (MDR-TB) is caused by a mycobacterium that is resistant to at least isoniazid (INH) and rifampicin (RIF), the two most powerful first-line TB drugs to treat persons with TB disease. Multidrug-resistant TB is increasing and about 3.6 % of new TB patients in the world have multidrug-resistant strains. The WHO reported that MDR-TB was present in virtually all countries surveyed and there were 480,000 new MDR-TB cases in the world in 2013 [2].
The mechanism of resistance to various anti-TB drugs is attributed primarily to specific mutations in target genes [3–6]. However, a proportion of drug resistant clinical M. tuberculosis isolates has not been found to have mutations [7]. Furthermore, several evidences demonstrated that antibacterial drugs can target multiple proteins/enzymes in M. tuberculosis [8, 9]. Therefore, it is possible that other mechanisms could contribute to drug resistance. Some discussed mechanisms such as the production of drug-modifying and -inactivating enzymes, low cell wall permeability (restricted influx of drugs) and efflux-related mechanisms [10–12] could be considered as alternative ways for drug resistance in mycobacteria.
Proteomics is a powerful tool for the large-scale protein identification of complex biological samples. Investigations of protein expression profiles of M. tuberculosis by proteomics approach under various growth conditions, geographic distribution, genetic backgrounds, subcellular fraction have been performed [13–29]. Especially, recent researches on comparative analysis of monodrug-resistant and susceptible M. tuberculosis by 2-DE combined with MS have been reported to reveal proteins associated with resistance [30–32]. The global study of the protein profile of multidrug-resistant and susceptible strains by proteomic approach could help in further revealing of resistance mechanisms and determining multidrug resistance-associated biomarkers. The obtained findings support to develop newer drugs, development of vaccine and probably a rapid diagnosis tool for multidrug-resistance tuberculosis.
In the present study, a combination of orbitrap mass spectrometry and relative protein expression abundance calculations was performed to compare the proteome of multidrug-resistant and susceptible M. tuberculosis strains. The aim of this study was to identify multidrug-resistant associated proteins to support further understanding of drug resistance in M. tuberculosis and provide potential protein biomarkers for development of novel diagnostic tools and vaccines against MDR-TB as well.
Materials and Methods
Mycobacterial Growth
Three resistant and sensitive (Isoniazid and Rifampicin) M. tuberculosis clinical isolates were obtained from National Institute of Hygiene and Epidemiology (NIHE). Bacteria were grown in Middlebrook 7H9 broth (Difco) supplemented with 0.2 % glycerol, 0.05 % Tween 80, and 1× OADC (0.5 % bovine serum albumin, 0.2 % Dextrose, 0.85 % NaCl, 0.0004 % catalase, 0.005 % oleic acid) at 37 °C for 4 weeks (107–108 cfu/ml).
Protein Preparation of M. tuberculosis
Mycobacterial cell extract was prepared according to modified protocol of [31]. Briefly, cells were washed three times with phosphate saline buffer (1× PBS buffer, pH 7.4) and then suspended in UT buffer containing 8 M urea, 2 M thiourea. The cell suspension was broken by intermittent sonication (15 s ON, 15 s OFF) for 4 min on ice at 80 % energy using sonicator (Sonics & Materials Inc, USA). Subsequently, the lysate was clarified by centrifugation at 16,000×g for 1 h at 4 °C. The supernatant was collected in a new reaction tube and protein concentration of the supernatant was determined using a Bradford assay kit (Sigma-Aldrich, USA). Sample aliquots were stored at −80 °C for later use.
Peptide Preparation for MS Analysis
Protein samples were proteolytically digested in solution as the following procedure: 4 µg protein of each sample, (three technical and two biological replicates per sample), were reduced by incubation with a finial concentration of 25 mM DTT and alkylated with 100 mM iodoacetamide. Proteolysis was carried out with trypsin (Promega) in the ratio of 1:25 overnight (16 h) at 37 °C. The digestion was quenched by the addition of acetic acid to a final concentration of 1 %. The digested peptides were purified on µC18-ZipTip columns (Merck Millipore).
Relative Quantitation of R and S Type Dependent Differences by ESI-LC Tandem Mass Spectrometry
The purified peptides were separated on an Acclaim PepMap 100 reverse phase column with an EASY-nLC (Thermo Electron) using a 86 min non-linear gradient ranging from 2 to 100 % ACN in 0.1 % acetic acid at a flow rate of 0.3 µL/min before full MS data and data-dependent fragment ion spectra were recorded with a LTQ-Orbitrap-Velos mass spectrometer (Thermo Electron). Raw data were analyzed with the Refiner software (GeneData) employing alignment across all 12 MS runs with subsequent peak identification and annotation. Identification of peptides and assignment to proteins was performed via automated Mascot search (rel. 2.3, Matrix Science) against the Uniprot/Trembl reference proteome database for M. tuberculosis with a peptide mass tolerance of 10 ppm and 0.6 Da for fragment ions. Proteins identified with at least two rank one peptides with an ion score >20 were considered in relative quantitation based on summed protein intensities.
The data were log10 transformed to gain normal distributions and median normalized across all samples and categories before mean values across the three technical replicates were calculated.
Analysis of Identified Proteins
The physicochemical properties of all identified proteins of interest were analyzed by using the following software. The theoretical molecular weight, pI value and GRAVY scores were obtained from calculation in http://www.bioinformatics.org/sms2/. Protein transmembrane domains (TMDs) were predicted TMHMM server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/), SOSUI (Batch) engine ver. 1.10 (http://harrier.nagahama-i-bio.ac.jp/sosui/sosuiG/sosuigsubmit.html), Phobius (http://phobius.sbc.su.se/). Possible signal peptide and lipoprotein signal peptide sequence prediction were predicted using the SignalP and LipoP programs, respectively (http://www.cbs.dtu.dk/services/). Prediction of subcellular localization of the identified proteins was carried out using the PSORT v3.0 program available at http://www.psort.org/psortb/. Lipoproteins were identified by comparing the identified proteins with the list of M. tuberculosis predicted lipoproteins in [33]. Functional classifications of all proteins were determined according to the TubercuList database [34].
Results
Identification of the M. tuberculosis Proteins by ESI-LC Tandem Mass Spectrometry
The resulting MS/MS spectra were searched using automated Mascot search against the Uniprot/Trembl reference proteome database for M. tuberculosis. Applying the filtering criteria described in method section, a total of 6,110 peptide sequences were obtained. The complete list of peptides identified in our study is provided in Supplementary Table S1. LC-ESI-LTQ ORBITRAP-VelosMS analysis resulted in identification of a total of 1,583 mycobacterial proteins in both susceptible and multidrug-resistant group (Supplementary Table S2). Of those, 448 proteins were identified with one peptide only and 1,135 proteins were identified with two and more peptides. Peptide identification by MS was considered as evidence for the existence of the gene products predicted by genome annotation [35, 36].
Functional Classification of Identified Proteins
All identified proteins were grouped by functional category as defined by [34]. Totally 1,583 identified proteins can be classified into ten different functional categories (Fig. 1a). The majority of identified proteins have predicted roles in lipid metabolism (153—10 %), intermediary metabolism (508—32 %), cell wall and cell processes (272—17 %). A large list of conserved hypothetical proteins of unknown function was also identified in this study (352—22 %). This pattern of functional category is similar to previous reports in proteomic analysis of the M. tuberculosis [23, 25, 29]. Functional classification of predicted membrane proteins (Fig. 1b) showed that 175 (52 %) proteins are associated with cell wall processes (group 4) and 59 (18 %) proteins are related to intermediary metabolism. In addition, 10 % are conserved hypothetical proteins. This data indicates that 90 % of identified membrane proteins had known functions. This observation is coincidence with [24].
Fig. 1.
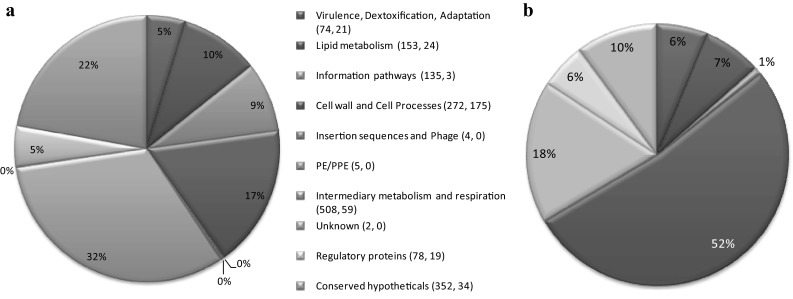
Functional assignment of proteins in M. tuberculosis identified by LC–MS/MS. Proteins were assigned to functional categories based on the TubercuList database. The pie charts (a) and (b) represent the distribution of all identified M. tuberculosis proteins and predicted membrane proteins respectively according to functional categories in percentage. Numbers in parenthesis indicate the number of proteins among total and membrane proteins respectively in each category
Comparison of Susceptible and Multidrug-Resistant M. tuberculosis Proteomes
Quantitative analysis of the proteins was done on the basis of summed peptide intensities. Totally, 1,583 proteins were considered for quantitative analysis. Of these, proteins with a ratio of 2.0-fold and a technical q value <0.05 were considered as displaying significant differences. These filter criteria identified 68 proteins with at least two peptides including 28 and 40 proteins showing increased and decreased amounts in resistant strains in comparison to the susceptible strains, respectively (Table 1). The list of different abundant proteins identified with one peptide is provided in Supplementary Table S3.
Table 1.
List of proteins with different abundance in multidrug-resistant and susceptible M. tuberculosis isolates (q value for all was <0.05)
| Rv number | Proteins description | Gene name | Functional categoriesa | Fold difference |
|---|---|---|---|---|
| Higher abundance in resistant isolates | ||||
| Rv1398c | Putative antitoxin | vapB10 | 0 | 2.7 |
| Rv3648c | Probable cold shock protein A | cspA | 0 | 2.8 |
| Rv0470c | Cyclopropane mycolic acid synthase 3 | pcaA | 1 | 4.3 |
| Rv2187 | Long-chain-fatty-acid–CoA ligase | fadD15 | 1 | 4.5 |
| Rv1912c | Possible oxidoreductase | fadB5 | 1 | 5.9 |
| Rv2831 | Enoyl-CoA hydratase/isomerase family protein | echA16 | 1 | 6.2 |
| Rv1080c | Transcription elongation factor | greA | 2 | 2.0 |
| Rv1234 | Probable transmembrane protein | 3 | 2.1 | |
| Rv3690 | Probable conserved membrane protein | 3 | 2.1 | |
| Rv1249c | Possible membrane protein | 3 | 2.6 | |
| Rv3794 | Probable arabinosyltransferase A | embA | 3 | 2.9 |
| Rv2873 | Cell surface lipoprotein | mpt83 | 3 | 3.7 |
| Rv2945c | Putative lipoprotein | lppX | 3 | 4.2 |
| Rv1899c | Uncharacterized protein | 3 | 4.7 | |
| Rv0341 | Isoniazid-induced protein | iniB | 3 | 4.8 |
| Rv3841 | Ferritin | bfrB | 7 | 2.1 |
| Rv2344c | Deoxyguanosinetriphosphate triphosphohydrolase-like protein | dgt | 7 | 2.2 |
| Rv2971 | Uncharacterized oxidoreductase | 7 | 2.9 | |
| Rv2713 | Probable soluble pyridine nucleotide transhydrogenase | sthA | 7 | 2.9 |
| Rv2858c | Probable aldehyde dehydrogenase | aldC | 7 | 3.5 |
| Rv0392c | NADH dehydrogenase | ndh-1 | 7 | 9.0 |
| Rv2259 | S-nitrosomycothiol reductase | mscR | 7 | 536 |
| Rv3133c | Transcriptional regulatory protein | devR | 9 | 2.6 |
| Rv0566c | UPF0234 protein | 10 | 2.2 | |
| Rv1156 | Conserved protein | 10 | 2.6 | |
| Rv1770 | Conserved protein | 10 | 3.0 | |
| Rv0577 | 27 kDa antigen | cfp30B | 10 | 3.0 |
| Rv2337c | Uncharacterized protein | 10 | 3.3 | |
| Lower abundance in resistant isolates | ||||
| Rv0456c | Enoyl-CoA hydratase | echA2 | 1 | 13.4 |
| Rv0672 | Acyl-CoA dehydrogenase, putative | fadE8 | 1 | 3.1 |
| Rv2524c | Probable fatty acid synthase | fas | 1 | 2.1 |
| Rv1708 | Uncharacterized protein | 3 | 11.8 | |
| Rv0347 | Probable conserved membrane protein | 3 | 4.5 | |
| Rv3868 | ESX-1 secretion system protein | eccA1 | 3 | 3.4 |
| Rv1038c | Putative ESAT-6-like protein | 3 | 3.3 | |
| Rv3620c | Putative ESAT-6-like protein 10 | 3 | 3.3 | |
| Rv3781 | O-antigen export system | rfbE | 3 | 2.9 |
| Rv1197 | Putative ESAT-6-like protein | 3 | 2.9 | |
| Rv2347c | Putative ESAT-6-like protein 7 | 3 | 2.9 | |
| Rv1793 | ESAT-6-like protein | esxN | 3 | 2.6 |
| Rv0932c | Phosphate-binding protein | pstS2 | 3 | 2.5 |
| Rv2462c | Trigger factor | tig | 3 | 2.4 |
| Rv2155c | UDP-n-acetylmuramoylalanine–d-glutamate ligase | murD | 3 | 2.4 |
| Rv2691 | CeoB | ceoB | 3 | 2.3 |
| Rv1368 | Putative lipoprotein | lprF | 3 | 2.2 |
| Rv1926c | Immunogenic protein | mpt63 | 3 | 2.0 |
| Rv3614c | ESX-1 secretion-associated protein | espD | 3 | 2.0 |
| Rv2964 | Formyltetrahydrofolate deformylase | purU | 7 | 43.9 |
| Rv2280 | Uncharacterized FAD-linked oxidoreductase | 7 | 13.6 | |
| Rv2161c | Conserved protein | 7 | 13.5 | |
| Rv2952 | Phthiotriol/phenolphthiotriol dimycocerosates methyltransferase | 7 | 11.4 | |
| Rv3086 | Putative alcohol dehydrogenase d | adhD | 7 | 9.1 |
| Rv2391 | Sulfite reductase [ferredoxin] | sir | 7 | 5.4 |
| Rv2682c | 1-deoxy-d-xylulose-5-phosphate synthase | dxs | 7 | 4.8 |
| Rv3710 | 2-isopropylmalate synthase | leuA | 7 | 4.5 |
| Rv1895 | Probable zinc-binding alcohol dehydrogenase | 7 | 4.1 | |
| Rv3257c | Phosphomannomutase | manB-1 | 7 | 3.5 |
| Rv2363 | Putative amidase | amiA2 | 7 | 2.3 |
| Rv0814c | Conserved protein | sseC1 | 7 | 2.2 |
| Rv3118 | Uncharacterized protein | sseC1 | 7 | 2.2 |
| Rv3324c | Cyclic pyranopterin monophosphate synthase accessory protein 3 | moaC3 | 7 | 2.1 |
| Rv0844c | Probable transcriptional regulatory protein | narL | 9 | 43.6 |
| Rv2159c | Conserved protein | 10 | 14.3 | |
| Rv0825c | Conserved protein | 10 | 10.2 | |
| Rv3168 | Putative aminoglycoside phosphotransferase | 10 | 4.1 | |
| Rv1444c | Uncharacterized protein | 10 | 2.5 | |
| Rv2623 | Universal stress protein | 10 | 2.5 | |
| Rv3169 | Conserved protein | 10 | 2.1 | |
aFunctional categories 0—virulence, detoxification, adaptation; 1—lipid metabolism; 2—information pathways; 3—cell wall and cell processes; 7—intermediary metabolism and respiration; 9—regulatory proteins; 10—conserved hypotheticals
Functional classification of different abundant proteins showed that proteins with lower abundance in the resistant strains were assigned to five categories, whereas proteins with higher abundance were assigned to seven categories including virulence, detoxification, adaptation; lipid metabolism; information pathways; cell wall and cell processes; intermediary metabolism and respiration; regulatory proteins; and conserved hypotheticals.
Using the predicted protein sequences, a series of parameters were calculated that included physiochemical characteristics such as molecular mass, pI, hydrophobicity (GRAVY), and predicted subcellular location. The theoretical Mr distribution for the identified proteins ranged from 7.37 kDa (a probable cold shock protein A) to 326.3 kDa (a probable fatty acid synthase). The majority of identified proteins were 10–50 kDa in molecular weight, representing approximately 77.9 % of all predicted in this range (Fig. 2a). Similarly the theoretical pI values for the identified proteins ranged from 3.73 (ESX-1 secretion-associated protein) to 12.32 (a possible membrane protein) and were mainly distributed in the range 4.0–7.0 (Fig. 2b). It is notably that a higher proportion of basic proteins (pI > 8.0) were observed with higher abundant in multidrug-resistant group in comparison with susceptible group. The GRAVY score is calculated by the hydropathic indices of each amino acid in the protein based on Kyte and Doolittle algorithms, with a more positive score indicating higher hydrophobicity [37]. In the present study, the majority of differential abundant proteins were hydrophilic proteins and there were only 10 % of proteins with GRAVY score ≥0.2 (Fig. 2c), which hints to hydrophobicity. Subcellular localizations of these proteins were predicted by loading their protein sequences to the pSORTb v3.0.2 program. The subcellular localization information for the identified proteins is displayed in Fig. 2d, showing that the majority of identified proteins were determined as cytoplasmic proteins. Approximately 16 % proteins were predicted as cytoplasmic membrane proteins. There were also a large proportion of identified proteins (31 %) with unknown localization.
Fig. 2.
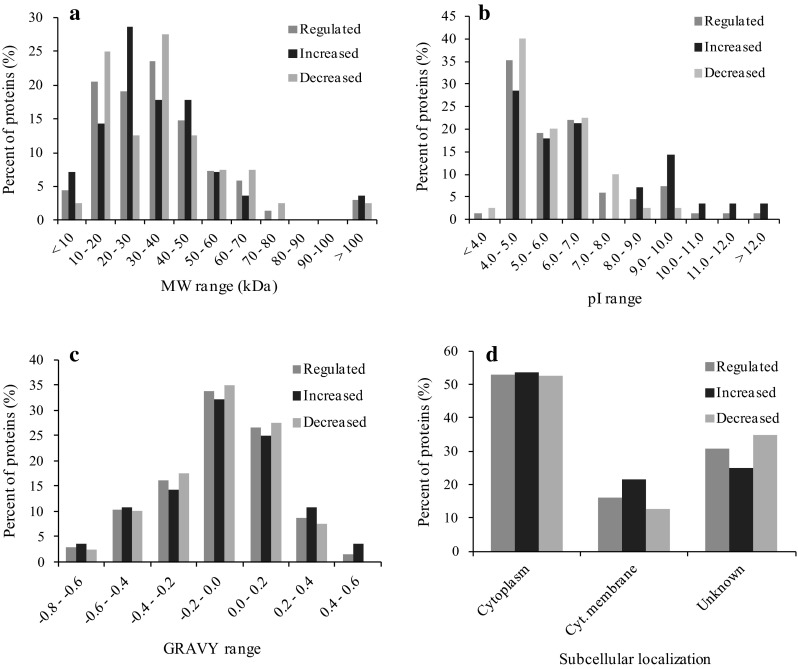
Characteristics of proteins with different abundances (higher and lower abundant proteins) in susceptible and resistant M. tuberculosis isolates. These physiochemical properties of identified proteins were determined based on databases as mentioned in the text
The obtained data was also searched for membrane and membrane-associated proteins by using the TMHMM v2.0 algorithm. Nine proteins were predicted to have at least one transmembrane helix (TMH) regions for integration into the cytoplasmic membrane in which six proteins were observed with higher abundance and only three proteins were observed with lower abundance in the resistant strains. The predicted TMH numbers of these proteins ranged from 1 to 13. Remarkably, an important enzyme for the biosynthesis of the mycobacterial cell wall (probable arabinosyltransferase A) was predicted to have 13 TMHs and observed increased in the resistant strains. Furthermore, another interesting group of proteins that are associated with the membrane is lipoproteins. Mycobacterial lipoproteins have been illustrated in forming a functionally diverse class of membrane-anchored or associated proteins [33, 38, 39] and are predicted to be involved in host-pathogen interactions [40]. In the present study, five proteins in which two proteins with higher abundance and three proteins with lower abundance in the resistant isolates were predicted by LipoP program as lipoproteins or containing lipoprotein signal peptide that predicts that these proteins could be lipidated during the process of export [41]. In addition, two proteins with lower abundance and one protein with higher abundance in the resistant isolates were also predicted by SignalP program to have a cleavable signal peptide for export across the cytoplasmic membrane.
Discussion
The emergence of multidrug-resistant TB (resistance with at least the first-line drugs isoniazid and rifampicin [42]) is a major concern in the control of TB at all countries in the world. As mentioned above, resistance to various anti-TB drugs has been reported to be associated with specific mutations in structural or regulatory regions of target genes [3–6]. However, the minireview [7] reported that the approximately 30 % of clinical INH-resistant, and 5 % of RIF-resistant M. tuberculosis isolates respectively do not have mutations in any of the known genes. Furthermore, anti-TB drugs have been shown to be targeting several different protein/enzymes in M. tuberculosis [8, 9]. There were evidences to indicate that resistance can be explained by other mechanisms such as the upregulation of drug–disabling enzymes, the improvement of cell wall integrity (lower influx and higher efflux of drugs) and overexpression of drug sensitive target proteins [10–12, 43].
The aim of this study was to analyze the changes in protein abundance between multidrug-resistant and susceptible clinical isolates by free gel approach for exploring multidrug-resistant associated proteins. The study included whole cell lysate proteins which will cover all proteins present in the cells. A total of 1,583 proteins were identified by free-gel approach that much higher than the number of proteins identified by 2-DE approach so far. Relative quantitation of resistant and susceptible type dependent differences based on summed protein intensities has revealed a total of 68 proteins with more than twofold difference. The majority of different abundant proteins were assigned to the categories of lipid metabolism, cell wall and cell processes, intermediary metabolism and respiration. The genome of M. tuberculosis although was well-characterized [35], the understanding of the function of mycobacterial proteins is still missing. Therefore, some identified proteins of interest will be discussed further below.
Proteins with Higher Abundance in Multidrug-Resistant Strains Associated with NADH
It had long been known that oxidation of INH in the presence of NADH led to covalent INH-NADH adducts—powerful inhibitors of InhA and other cellular processes. InhA is an enoyl acyl carrier protein reductase involved in the synthesis of mycolic acids—an important component of mycobacterial cell wall. Therefore, the capture of these INH adducts by proteins could be a way to resist INH in mycobacteria.
Rv2971 is an oxidoreductase of the aldo/keto reductase family and probably involved in cellular metabolism [34]. rv2971 gene has been demonstrated as an essential gene for growth and survival in M. tuberculosis [44] and seem to be playing a potential role in the detoxification of toxic metabolites [45, 46]. This protein was identified by MS in the membrane fraction, Triton X-114 extracts of M. tuberculosis H37Rv [19, 23, 26, 29] and was also considered as a membrane-associated protein [19]. Previous study also found that this protein was different in the pI and molecular weight between BCG and H37Rv [47], and was also considered as a candidate antigen for development of novel vaccine [48, 49]. Interestingly, this protein has also been reported to be higher abundant in isoniazid (INH)-monoresistant strains in comparison with susceptible strains [30] and in streptomycin resistant strain of M. tuberculosis [31]. Proteome-wide profiling approach for isoniazid targets in M. tuberculosis has shown that Rv2971 was one of the targets of INH adducts [9]. Recently, the crystal structure of Rv2971 in its unliganded form has been successfully determined and revealed the complex of this protein with the INH-NADH adduct [50]. Therefore, it is possible that the up-regulation of this protein in multidrug-resistant strains observed in the present study is necessary for resistance by binding and neutralizing the drug.
Rv0392c is a membrane-bound NADH dehydrogenase (NdhA) with one predicted transmembrane domain. This enzyme participates in energy metabolism by transferring electrons from NADH to the respiratory chain. By sequencing, a single nucleotide substitution of G to C located 44 bp upstream of the coding region of ndhA (Rv0392c) was identified in mutants resistant to 2-mercapto-quinazolinone. This mutation resulted in a significant expression of ndhA gene by 40- to 80-fold compared to standard strain H37Rv [51]. It is interesting to note that the NADH dehydrogenase uses pyridine nucleotides as cofactor and therefore this enzyme can easily bind to INH adducts. In the present study this protein was found with higher abundance in multidrug-resistant strains. It could be hypothesized that the increase of this protein could cause resistance by binding and sequestering the compound. This hypothesis is supported by observation in [8]. Furthermore, it is also possible that drug (such as INH adducts) acts by inhibiting NADH-dependent targets [9] and therefore the overexpression of NdhA is necessary to overcome this deficiency.
Similarly, aldehyde dehydrogenase AldC (Rv2858c), probable soluble pyridine nucleotide transhydrogenase (Rv2713), and S-nitrosomycothiol reductase (Rv2259) observed in higher abundance in multidrug-resistant strains in this study are enzymes using pyridine nucleotides as cofactor [52] and therefore they can bind to INH adducts. Interestingly, the experimental evidence showed that aldehyde dehydrogenase AldC bind INH adducts with high affinity [9]. It is likely that the mechanism for drug resistance of these proteins is similar to that of two proteins mentioned above. Further studies need to be conducted to address this hypothesis.
Proteins with Higher Abundance in Multidrug-Resistant Strains Involved in the Construction of the Cell Envelope of M. tuberculosis
The prevention of influx of drugs by improving the integrity of the cell wall or the active export of drugs out of the cells could be regarded as the main physiological mechanisms of intrinsic resistance. Therefore the development of this intrinsic resistance could be also considered as an effective and useful resolution for multidrug-resistant M. tuberculosis against toxic compounds. Mycobacterial cell wall was known as the primary permeability barrier responsible for resistance to antibiotics [53]. The higher thickness of the cell wall was observed in multidrug-resistant M. tuberculosis [54] and therefore some proteins involved in the construction of the cell envelope of M. tuberculosis will be discussed below.
Rv0470c-cyclopropane mycolic acid synthase 3 (mycolic acid methyltransferase) participates in lipid metabolism by catalyzing the transfer of a methylene group from S-adenosyl-l-methionine to form a cyclopropane ring at the proximal position of a fatty acid. Several different fatty acids can be used as substrates for this enzyme such as cis, cis 11,14-eicosadienoic acid and linoelaidic acid. This enzyme was shown to be required for the synthesis of mycolic acid cyclopropane ring in the cell wall of both M. bovis BCG and M. tuberculosis [55]. Cyclopropanated mycolic acids are important factors involved in cell wall permeability and a previous study demonstrated that inactivation of multiple mycolic acid methyltransferase activity resulted in loss of mycolic acid cyclopropanation, loss of acid fastness, and concomitantly cell death when exposed to antibiotics [10].
Rv3794 (probable arabinosyltransferase A) is an integral membrane protein with 13 predicted transmembrane domains [26, 29]. Arabinosyl transferase is responsible for of the attachment of arabinose molecules to the arabinan of arabinogalactan. Mycolic acids are then covalently linked to the 5′-hydroxyl groups of d-arabinose residues of arabinogalactan and form mycolyl-arabinogalactan-peptidoglycan complex in the cell wall of M. tuberculosis [5, 55]. Therefore this enzyme plays an important role in the biosynthesis of the mycobacterial cell wall. Previous study also indicated that this enzyme was the drug target of ethambutol (Emb)—an antimycobacterial drug usually used in combination with rifampicin and isoniazid for tuberculosis treatment and its overexpression in mycobacteria leaded to Emb resistance [43].
Rv2945c (putative lipoprotein LppX) is a lipoprotein required for the translocation of the phthiocerol dimycocerosates (DIM)—a complex lipid to the outer membrane of M. tuberculosis [56]. As a component of the cell wall, DIM has been shown to be involved in the cell wall permeability barrier and thus play important roles in the resistance of M. tuberculosis to reactive nitrogen intermediates (possibly derived from INH [57]) [58]. Interestingly, a recent study demonstrated that DIM deficient mutant of Mycobacterium marinum was an increase in cell wall permeability and more sensitive to rifampicin [59].
A functional category of proteins that was significantly altered in abundance was related to the lipid metabolism. They were long-chain-fatty-acid-CoA ligase FadD15 (Rv2187), possible oxidoreductase FadB5 (Rv1912) and enoyl-CoA hydratase/isomerase family protein (Rv2831). Rv2187 catalyzes the activation of long-chain fatty acids as acyl-coenzyme A (acyl-CoA), which are then transferred to the multifunctional polyketide synthase (PKS) type III for further chain extension [60]. Previous study indicated that Rv1912 was significantly induced by treatment with the analogs of isoniazid [61]. Although, no evidence indicated that there was direct interaction between these enzymes and INH, rifampicin and other drugs. However, it is possible that these enzymes might play role in drug resistance by improving the integrity of mycobacterial cell wall through lipid metabolism.
The increase in abundance of a group of membrane proteins including Rv3690, Rv1234 and 1249c with 1, 2, 3 predicted transmembrane domains respectively, and Rv1899 (possible lipoprotein LppD) was observed in resistant group. The function of these proteins are unknown so far [34] and the role of these proteins in drug resistance need to be investigated in further works. However, it has been shown that the membrane proteins of M. tuberculosis play critical roles in vital cell processes including nutrient transport, energy metabolism, signal transduction and especially cell-wall synthesis [62, 63]. Furthermore, mycobacterial membrane proteins can stimulate immune responses [64] therefore identification of multidrug-resistant associated membrane proteins might be valuable for development of novel vaccines and diagnostics against multidrug-resistant M. tuberculosis strains.
Rv0341 (isoniazid-induced protein iniB) is encoded by iniB gene in the iniBAC operon containing three genes of iniB, iniA and iniC. This protein is very Gly-, Ala- rich and predicted similar to cell wall proteins [34]. Its function was unknown however the transcription of this operon was specifically induced by a broad range of antibiotics that inhibit the synthesis of peptidoglycan (ampicillin), arabinogalactan (ethambutol), mycolic acids (isoniazid, ethionamide) and fatty acids (5-chloropyrazinamide) [69–71]. Furthermore, iniBAC operon was shown to be essential for activity of an efflux pump [72]. The observation suggests that these proteins might participate in the regulation of cell wall biosynthesis and confer resistance to antibiotics.
Rv2873 (MPT83) is a cell wall-associated lipo-glycoprotein of M. tuberculosis and whose function is unknown. However, this protein was identified as a seroreactive antigen of M. tuberculosis [65, 66]. Previous studies also demonstrated that rMPT83 stimulated strong T cell responses (IFN-γ T cell and antigen-specific T cell responses) [67, 68] and significant levels of protection in lungs and spleens during experimental murine M. tuberculosis infection [68]. Thus MPT83 should be considered as a candidate for TB subunit vaccines in future. It is an interesting case because we found that this protein with higher abundance in multidrug-resistant than in susceptible strains. The success in development of this subunit vaccine will strongly worth for prevention of infection of resistant M. tuberculosis.
Rv1080c is a putative gre factor (transcription elongation factor GreA) in the genome of M. tuberculosis. This proteins was demonstrated to enhance the efficiency of promoter clearance and also rescue arrested and paused elongation complexes [73]. Decrease in level of GreA reduced the bacterial survival therefore this protein plays a vital role in mycobacteria. As described in [73], Gre factors can modify the properties of the RNA polymerase (RNAP) by projecting its N-terminal coiled-coil domain into the active center of RNAP through the secondary channel. With this function, it is possible that GreA protein can help to recover the rifampicin-inhibited activity of RNAP (RpoB), leading to rifampicin resistance. A recombinant M. tuberculosis overexpressing GreA should be conducted to further confirm this mechanism.
In the present study, a set of proteins was also found with lower abundance in multidrug-resistant strains. They were classified into five functional categories including lipid metabolism, intermediary metabolism and respiration, cell wall and cell processes, and regulatory proteins. In which majority of identified proteins was belong to two functional categories of number 2, 3.
It has been described that M. tuberculosis exports virulence factors such as EsxA (Esat-6), EsxB (CFP-10), and EspB, through the ESX-1 (ESAT-6 system 1) type 7 protein secretion system [74]. The gene cluster of espA-espC-espD (rv3616c-rv3615c-rv3614c) is also essential for ESX-1-dependent protein secretion and virulence of M. tuberculosis [74, 75]. In the present study, a group of five proteins with significant homology in amino acid composition (putative ESAT-6-like proteins) was found to be lower abundance in the multidrug-resistant strains. It is known that these proteins are highly abundant and regarded as virulence factors in M. tuberculosis [76]. These proteins were also found decreased in the hypervirulent strain compared to a hypovirulent strain [25]. Furthermore, our data also showed that the decrease in abundance of EspD and EccA1 protein (a functional component of ESX-1 system), O-antigen export system and immunogenic protein MTP63 was observed in the multidrug-resistant strains. Drug resistance and immune escape mechanism (reduced virulence) has been illustrated to co-evolve in Staphylococcus aureus [77]. Thus, the reduction in the presence of ESAT-6-like proteins, MPT63 and ESX-1 secretion system associated proteins in the multidrug-resistant strains indicated that changes in the repertoire of highly immunogenic proteins can be a defensive mechanism undertaken by resistant cells.
Conclusions
We used a label-free quantitative approach to identify proteins and compare protein abundance in multidrug-resistant and susceptible clinical isolates of M. tuberculosis. This study is being considered as the first report applying free-gel approach for comparative analysis of multidrug-resistant and susceptible M. tuberculosis. The differentially expressed proteins from multidrug-resistant M. tuberculosis might be considered as potential markers of multidrug-resistance and novel drug targets for drug-resistant tuberculosis. Furthermore, the proteins identified may provide additional useful information for more understanding the mechanism of drug resistance in M. tuberculosis.
Electronic Supplementary Material
Acknowledgments
The research was supported by the National Foundation for Science & Technology Development (NAFOSTED), Vietnam.
References
- 1.WHO (2014) Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/
- 2.WHO (2013) Multidrug-resistant tuberculosis (MDR-TB). http://www.who.int/tb/challenges/mdr/en/
- 3.Taniguchi H, Aramaki H, Nikaido Y, Mizuguchi Y, Nakamura M, Koga T, Yoshida S (1996) Rifampicin resistance and mutation of the rpoB gene in Mycobacterium tuberculosis. FEMS Microbiol Lett 144:103–108. doi:10.1016/0378-1097(96)00346-1 [DOI] [PubMed]
- 4.Khadka JB, Rai SK, Shrestha S, Maharjan B, Bhatta DR, Ghimire P. Study of rifampicin and isoniazid resistance mutation genes of M. tuberculosis isolates in Nepal. Nepal Med Coll J. 2011;13:147–151. [PubMed] [Google Scholar]
- 5.Rattan A, Kalia A, Ahmad N. Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg Infect Dis. 1998;4:195–209. doi: 10.3201/eid0402.980207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JM, Uplekar S, Gordon SV, Cole ST. A point mutation in cycA partially contributes to the D-cycloserine resistance trait of Mycobacterium bovis BCG vaccine strains. PLoS One. 2012;7:e43467. doi: 10.1371/journal.pone.0043467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC (2009) A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother 53:3181–3189. doi:10.1128/AAC.01577-08 [DOI] [PMC free article] [PubMed]
- 8.Chen P, Bishai WR. Novel selection for isoniazid (INH) resistance genes supports a role for NAD+ -binding proteins in mycobacterial INH resistance. Infect Immun. 1998;66:5099–5106. doi: 10.1128/iai.66.11.5099-5106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyrou A, Jin L, Siconilfi-Baez L, Angeletti RH, Blanchard JS. Proteome-wide profiling of isoniazid targets in Mycobacterium tuberculosis. Biochemistry. 2006;45:13947–13953. doi: 10.1021/bi061874m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkan D, Liu Z, Sacchettini JC, Glickman MS (2009) Mycolic acid cyclopropanation is essential for viability, drug resistance, and cell wall integrity of Mycobacterium tuberculosis. Chem Biol 16:499–509. doi:10.1016/j.chembiol.2009.04.001 [DOI] [PMC free article] [PubMed]
- 11.Wright GD (1999) Aminoglycoside-modifying enzymes. Curr Opin Microbiol 2:499–503. doi:10.1016/S1369-5274(99)00007-7 [DOI] [PubMed]
- 12.da Silva PE, Von Groll A, Martin A, Palomino JC (2011) Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol 63:1–9. doi:10.1111/j.1574-695X.2011.00831.x [DOI] [PubMed]
- 13.Mattow J, Schaible UE, Schmidt F, Hagens K, Siejak F, Brestrich G, Haeselbarth G, Muller EC, Jungblut PR, Kaufmann SH. Comparative proteome analysis of culture supernatant proteins from virulent Mycobacterium tuberculosis H37Rv and attenuated M. bovis BCG Copenhagen. Electrophoresis. 2003;24:3405–3420. doi: 10.1002/elps.200305601. [DOI] [PubMed] [Google Scholar]
- 14.Betts JC, Dodson P, Quan S, Lewis AP, Thomas PJ, Duncan K, McAdam RA. Comparison of the proteome of Mycobacterium tuberculosis strain H37Rv with clinical isolate CDC 1551. Microbiology. 2000;146:3205–3216. doi: 10.1099/00221287-146-12-3205. [DOI] [PubMed] [Google Scholar]
- 15.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K (2002) Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi:10.1046/j.1365-2958.2002.02779.x [DOI] [PubMed]
- 16.Starck J, Kallenius G, Marklund BI, Andersson DI, Akerlund T (2004) Comparative proteome analysis of Mycobacterium tuberculosis grown under aerobic and anaerobic conditions. Microbiology 150:3821–3829. doi:10.1099/mic.0.27284-0 [DOI] [PubMed]
- 17.Mawuenyega KG, Forst CV, Dobos KM, Belisle JT, Chen J, Bradbury EM, Bradbury AR, Chen X (2005) Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol Biol Cell 16:396–404. doi:10.1091/mbc.E04-04-0329 [DOI] [PMC free article] [PubMed]
- 18.Pheiffer C, Betts JC, Flynn HR, Lukey PT, van Helden P (2005) Protein expression by a Beijing strain differs from that of another clinical isolate and Mycobacterium tuberculosis H37Rv. Microbiology 151:1139–1150. doi:10.1099/mic.0.27518-0 [DOI] [PubMed]
- 19.Gu S, Chen J, Dobos KM, Bradbury EM, Belisle JT, Chen X. Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol Cell Proteomics. 2003;2:1284–1296. doi: 10.1074/mcp.M300060-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Y, Chalmers MJ, Gao FP, Cross TA, Marshall AG. Identification of Mycobacterium tuberculosis H37Rv integral membrane proteins by one-dimensional gel electrophoresis and liquid chromatography electrospray ionization tandem mass spectrometry. J Proteome Res. 2005;4:855–861. doi: 10.1021/pr0500049. [DOI] [PubMed] [Google Scholar]
- 21.Sinha S, Kosalai K, Arora S, Namane A, Sharma P, Gaikwad AN, Brodin P, Cole ST (2005) Immunogenic membrane-associated proteins of Mycobacterium tuberculosis revealed by proteomics. Microbiology 151:2411–2419. doi:10.1099/mic.0.27799-0 [DOI] [PubMed]
- 22.Malen H, Berven FS, Fladmark KE, Wiker HG. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics. 2007;7:1702–1718. doi: 10.1002/pmic.200600853. [DOI] [PubMed] [Google Scholar]
- 23.Malen H, Pathak S, Softeland T, de Souza GA, Wiker HG (2010) Definition of novel cell envelope associated proteins in Triton X-114 extracts of Mycobacterium tuberculosis H37Rv. BMC Microbiol 10:132. doi:10.1186/1471-2180-10-132 [DOI] [PMC free article] [PubMed]
- 24.Wolfe LM, Mahaffey SB, Kruh NA, Dobos KM. Proteomic definition of the cell wall of Mycobacterium tuberculosis. J Proteome Res. 2010;9:5816–5826. doi: 10.1021/pr1005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souza GA, Fortuin S, Aguilar D, Pando RH, McEvoy CR, van Helden PD, Koehler CJ, Thiede B, Warren RM, Wiker HG (2010) Using a label-free proteomics method to identify differentially abundant proteins in closely related hypo- and hypervirulent clinical Mycobacterium tuberculosis Beijing isolates. Mol Cell Proteomics 9:2414–2423. doi:10.1074/mcp.M900422-MCP200 [DOI] [PMC free article] [PubMed]
- 26.Malen H, De Souza GA, Pathak S, Softeland T, Wiker HG (2011) Comparison of membrane proteins of Mycobacterium tuberculosis H37Rv and H37Ra strains. BMC Microbiol 11:18. doi:10.1186/1471-2180-11-18 [DOI] [PMC free article] [PubMed]
- 27.Kelkar DS, Kumar D, Kumar P, Balakrishnan L, Muthusamy B, Yadav AK, Shrivastava P, Marimuthu A, Anand S, Sundaram H, Kingsbury R, Harsha HC, Nair B, Prasad TS, Chauhan DS, Katoch K, Katoch VM, Chaerkady R, Ramachandran S, Dash D, Pandey A (2011) Proteogenomic analysis of Mycobacterium tuberculosis by high resolution mass spectrometry. Mol Cell Proteomics 10:M111 011627. doi:10.1074/mcp.M111.011445 [DOI] [PMC free article] [PubMed]
- 28.Bell C, Smith GT, Sweredoski MJ, Hess S. Characterization of the Mycobacterium tuberculosis proteome by liquid chromatography mass spectrometry-based proteomics techniques: a comprehensive resource for tuberculosis research. J Proteome Res. 2012;11:119–130. doi: 10.1021/pr2007939. [DOI] [PubMed] [Google Scholar]
- 29.Gunawardena HP, Feltcher ME, Wrobel JA, Gu S, Braunstein M, Chen X. Comparison of the membrane proteome of virulent Mycobacterium tuberculosis and the attenuated Mycobacterium bovis BCG vaccine strain by label-free quantitative proteomics. J Proteome Res. 2013;12:5463–5474. doi: 10.1021/pr400334k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Zhang W, Gao F, Huang Y, Lv C, Wang H. Comparison of the proteome of isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis. Microb Drug Resist. 2006;12:231–238. doi: 10.1089/mdr.2006.12.231. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Kumar B, Gupta Y, Singhal N, Katoch VM, Venkatesan K, Bisht D (2010) Proteomic analysis of streptomycin resistant and sensitive clinical isolates of Mycobacterium tuberculosis. Proteome Sci 8:59. doi:10.1186/1477-5956-8-59 [DOI] [PMC free article] [PubMed]
- 32.Kumar B, Sharma D, Sharma P, Katoch VM, Venkatesan K, Bisht D (2013) Proteomic analysis of Mycobacterium tuberculosis isolates resistant to kanamycin and amikacin. J Proteomics 94:68–77. doi:10.1016/j.jprot.2013.08.025 [DOI] [PubMed]
- 33.Sutcliffe IC, Harrington DJ (2004) Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol Rev 28:645–659. doi:10.1016/j.femsre.2004.06.002 [DOI] [PubMed]
- 34.Lew JM, Kapopoulou A, Jones LM, Cole ST (2011) TubercuList–10 years after. Tuberculosis (Edinb) 91:1–7. doi:10.1016/j.tube.2010.09.008 [DOI] [PubMed]
- 35.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 36.Camus JC, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148:2967–2973. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- 37.Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132. doi:10.1016/0022-2836(82)90515-0 [DOI] [PubMed]
- 38.Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, Pecora ND, Ziady AG, Shank S, Harding CV, Boom WH, Rojas RE (2011) Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via toll-like receptors 1 and 2. Infect Immun 79:663–673. doi:10.1128/IAI.00806-10 [DOI] [PMC free article] [PubMed]
- 39.Seshadri C, Turner MT, Lewinsohn DM, Moody DB, Van Rhijn I (2013) Lipoproteins are major targets of the polyclonal human T cell response to Mycobacterium tuberculosis. J Immunol 190:278–284. doi:10.4049/jimmunol.1201667 [DOI] [PMC free article] [PubMed]
- 40.Sander P, Rezwan M, Walker B, Rampini SK, Kroppenstedt RM, Ehlers S, Keller C, Keeble JR, Hagemeier M, Colston MJ, Springer B, Bottger EC. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;52:1543–1552. doi: 10.1111/j.1365-2958.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 41.Juncker AS, Willenbrock H, Von Heijne G, Brunak S, Nielsen H, Krogh A. Prediction of lipoprotein signal peptides in gram-negative bacteria. Protein Sci. 2003;12:1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ormerod LP (2005) Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. Br Med Bull 73–74:17–24. doi:10.1093/bmb/ldh047 [DOI] [PubMed]
- 43.Belanger AE, Besra GS, Ford ME, Mikusova K, Belisle JT, Brennan PJ, Inamine JM. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sassetti CM, Boyd DH, Rubin EJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi:10.1046/j.1365-2958.2003.03425.x [DOI] [PubMed]
- 45.Barski OA, Tipparaju SM, Bhatnagar A (2008) The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev 40:553–624. doi:10.1080/03602530802431439 [DOI] [PMC free article] [PubMed]
- 46.Grimshaw CE. Aldose reductase: model for a new paradigm of enzymic perfection in detoxification catalysts. Biochemistry. 1992;31:10139–10145. doi: 10.1021/bi00157a001. [DOI] [PubMed] [Google Scholar]
- 47.Jungblut PR, Schaible UE, Mollenkopf HJ, Zimny-Arndt U, Raupach B, Mattow J, Halada P, Lamer S, Hagens K, Kaufmann SH (1999) Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol Microbiol 33:1103–1117. doi:10.1046/j.1365-2958.1999.01549.x [DOI] [PubMed]
- 48.Mollenkopf HJ, Grode L, Mattow J, Stein M, Mann P, Knapp B, Ulmer J, Kaufmann SH (2004) Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect Immun 72:6471–6479. doi:10.1128/IAI.72.11.6471-6479.2004 [DOI] [PMC free article] [PubMed]
- 49.Schmidt F, Donahoe S, Hagens K, Mattow J, Schaible UE, Kaufmann SH, Aebersold R, Jungblut PR. Complementary analysis of the Mycobacterium tuberculosis proteome by two-dimensional electrophoresis and isotope-coded affinity tag technology. Mol Cell Proteomics. 2004;3:24–42. doi: 10.1074/mcp.M300074-MCP200. [DOI] [PubMed] [Google Scholar]
- 50.Shahine A, Prasetyoputri A, Rossjohn J, Beddoe T (2014) A structural characterization of the isoniazid Mycobacterium tuberculosis drug target, Rv2971, in its unliganded form. Acta Crystallogr F Struct Biol Commun 70:572–577. doi:10.1107/S2053230X14007158 [DOI] [PMC free article] [PubMed]
- 51.Ioerger TR, O’Malley T, Liao R, Guinn KM, Hickey MJ, Mohaideen N, Murphy KC, Boshoff HI, Mizrahi V, Rubin EJ, Sassetti CM, Barry CE, 3rd, Sherman DR, Parish T, Sacchettini JC. Identification of new drug targets and resistance mechanisms in Mycobacterium tuberculosis. PLoS One. 2013;8:e75245. doi: 10.1371/journal.pone.0075245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geertz-Hansen HM, Blom N, Feist AM, Brunak S, Petersen TN. Cofactory: sequence-based prediction of cofactor specificity of Rossmann folds. Proteins. 2014 doi: 10.1002/prot.24536. [DOI] [PubMed] [Google Scholar]
- 53.Barry CE 3rd, Mdluli K (1996) Drug sensitivity and environmental adaptation of mycobacterial cell wall components. Trends Microbiol 4:275–281. doi:10.1016/0966-842X(96)10031-7 [DOI] [PubMed]
- 54.Velayati AA, Farnia P, Ibrahim TA, Haroun RZ, Kuan HO, Ghanavi J, Kabarei AN, Tabarsi P, Omar AR, Varahram M, Masjedi MR (2009) Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy 55:303–307. doi:10.1159/000226425 [DOI] [PubMed]
- 55.Glickman MS, Cox JS, Jacobs WR Jr (2000) A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell 5:717–727. doi:10.1016/S1097-2765(00)80250-6 [DOI] [PubMed]
- 56.Sulzenbacher G, Canaan S, Bordat Y, Neyrolles O, Stadthagen G, Roig-Zamboni V, Rauzier J, Maurin D, Laval F, Daffe M, Cambillau C, Gicquel B, Bourne Y, Jackson M (2006) LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J 25:1436–1444. doi:10.1038/sj.emboj.7601048 [DOI] [PMC free article] [PubMed]
- 57.Timmins GS, Master S, Rusnak F, Deretic V. Requirements for nitric oxide generation from isoniazid activation in vitro and inhibition of mycobacterial respiration in vivo. J Bacteriol. 2004;186:5427–5431. doi: 10.1128/JB.186.16.5427-5431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rousseau C, Winter N, Pivert E, Bordat Y, Neyrolles O, Ave P, Huerre M, Gicquel B, Jackson M (2004) Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol 6:277–287. doi:10.1046/j.1462-5822.2004.00368.x [DOI] [PubMed]
- 59.Yu J, Tran V, Li M, Huang X, Niu C, Wang D, Zhu J, Wang J, Gao Q, Liu J (2012) Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect Immun 80:1381–1389. doi:10.1128/IAI.06370-11 [DOI] [PMC free article] [PubMed]
- 60.Trivedi OA, Arora P, Sridharan V, Tickoo R, Mohanty D, Gokhale RS. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature. 2004;428:441–445. doi: 10.1038/nature02384. [DOI] [PubMed] [Google Scholar]
- 61.Boyne ME, Sullivan TJ, amEnde CW, Lu H, Gruppo V, Heaslip D, Amin AG, Chatterjee D, Lenaerts A, Tonge PJ, Slayden RA (2007) Targeting fatty acid biosynthesis for the development of novel chemotherapeutics against Mycobacterium tuberculosis: evaluation of A-ring-modified diphenyl ethers as high-affinity InhA inhibitors. Antimicrob Agents Chemother 51:3562–3567. doi:10.1128/AAC.00383-07 [DOI] [PMC free article] [PubMed]
- 62.Niederweis M (2008) Nutrient acquisition by mycobacteria. Microbiology 154:679–692. doi:10.1099/mic.0.2007/012872-0 [DOI] [PubMed]
- 63.Calva E, Oropeza R (2006) Two-component signal transduction systems, environmental signals, and virulence. Microb Ecol 51:166–176. doi:10.1007/s00248-005-0087-1 [DOI] [PubMed]
- 64.Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, Felgner PL, Liang X, Gennaro ML (2010) Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci USA 107:14703–14708. doi:10.1073/pnas.1009080107 [DOI] [PMC free article] [PubMed]
- 65.Hewinson RG, Michell SL, Russell WP, McAdam RA, Jacobs WR., Jr Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 66.Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG (2010) Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol 17:1539–1547. doi:10.1128/CVI.00198-10 [DOI] [PMC free article] [PubMed]
- 67.Mustafa AS (2011) Comparative evaluation of MPT83 (Rv2873) for T helper-1 cell reactivity and identification of HLA-promiscuous peptides in Mycobacterium bovis BCG-vaccinated healthy subjects. Clin Vaccine Immunol 18:1752–1759. doi:10.1128/CVI.05260-11 [DOI] [PMC free article] [PubMed]
- 68.Kao FF, Mahmuda S, Pinto R, Triccas JA, West NP, Britton WJ. The secreted lipoprotein, MPT83, of Mycobacterium tuberculosis is recognized during human tuberculosis and stimulates protective immunity in mice. PLoS One. 2012;7:e34991. doi: 10.1371/journal.pone.0034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alland D, Steyn AJ, Weisbrod T, Aldrich K, Jacobs WR., Jr Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition. J Bacteriol. 2000;182:1802–1811. doi: 10.1128/JB.182.7.1802-1811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alland D, Kramnik I, Weisbrod TR, Otsubo L, Cerny R, Miller LP, Jacobs WR, Jr, Bloom BR. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:13227–13232. doi: 10.1073/pnas.95.22.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE 3rd (2004) The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279:40174–40184. doi:10.1074/jbc.M406796200 [DOI] [PubMed]
- 72.Colangeli R, Helb D, Sridharan S, Sun J, Varma-Basil M, Hazbon MH, Harbacheuski R, Megjugorac NJ, Jacobs WR Jr, Holzenburg A, Sacchettini JC, Alland D (2005) The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol Microbiol 55:1829–1840. doi:10.1111/j.1365-2958.2005.04510.x [DOI] [PubMed]
- 73.China A, Mishra S, Nagaraja V. A transcript cleavage factor of Mycobacterium tuberculosis important for its survival. PLoS One. 2011;6:e21941. doi: 10.1371/journal.pone.0021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen JM, Boy-Rottger S, Dhar N, Sweeney N, Buxton RS, Pojer F, Rosenkrands I, Cole ST (2012) EspD is critical for the virulence-mediating ESX-1 secretion system in Mycobacterium tuberculosis. J Bacteriol 194:884–893. doi:10.1128/JB.06417-11 [DOI] [PMC free article] [PubMed]
- 75.Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ (2005) Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA 102:10676–10681. doi:10.1073/pnas.0504922102 [DOI] [PMC free article] [PubMed]
- 76.Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R (2004) ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12:500–508. doi:10.1016/j.tim.2004.09.007 [DOI] [PubMed]
- 77.Gao W, Cameron DR, Davies JK, Kostoulias X, Stepnell J, Tuck KL, Yeaman MR, Peleg AY, Stinear TP, Howden BP (2013) The RpoB H(4)(8)(1)Y rifampicin resistance mutation and an active stringent response reduce virulence and increase resistance to innate immune responses in Staphylococcus aureus. J Infect Dis 207:929–939. doi:10.1093/infdis/jis772 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


