Abstract
Ion transporters are important in regulation of ionic homeostasis, cell volume, and cellular signal transduction under physiological conditions. They have recently emerged as important players in cancer progression. In this review, we discussed two important ion transporter proteins, sodium-potassium-chloride cotransporter isoform 1 (NKCC-1) and sodium-hydrogen exchanger isoform 1 (NHE-1) in Glioblastoma multiforme (GBM) and other malignant tumors. NKCC-1 is a Na+-dependent Cl− transporter that mediates the movement of Na+, K+, and Cl− ions across the plasma membrane and maintains cell volume and intracellular K+ and Cl− homeostasis. NHE-1 is a ubiquitously expressed cell membrane protein which regulates intracellular pH (pHi) and extracellular microdomain pH (pHe) homeostasis and cell volume. Here, we summarized recent pre-clinical experimental studies on NKCC-1 and NHE-1 in GBM and other malignant tumors, such as breast cancer, hepatocellular carcinoma, and lung cancer. These studies illustrated that pharmacological inhibition or down-regulation of these ion transporter proteins reduces proliferation, increases apoptosis, and suppresses migration and invasion of cancer cells. These new findings reveal the potentials of these ion transporters as new targets for cancer diagnosis and/or treatment.
Keywords: Bumetanide, cancer progression, cariporide, NKCC-1, NHE-1, targeted therapy
1. INTRODUCTION
Cancer is a leading cause of death worldwide (http://www.who.int.pitt.idm.oclc.org/mediacentre/factsheets/fs297/en/index.html). Malignant gliomas are the most common primary brain tumor with limited treatment options, and most patients diagnosed with glioblastomas survive less than a year [1]. Thus, understanding the mechanisms responsible for survival and progression of glioma and other malignant tumors may lead to identification of specific targets for a future treatment. The recent characterization of the genome and transcriptome of different cancers reveals the major structural and expression alterations in the development and progression of cancers and led to a fundamental shift on cancer therapy from systemic therapy to targeted therapy [2]. Many established targeted therapies focus on proteins that are involved in cell signaling pathways, which form complex communication systems governing basic cellular functions and activities. Accumulating evidence suggests that ion transporters and channels are involved in control of cancer progression. Targeting ion transporters and channels in cancer cells may represent an exciting new perspective for cancer therapy.
The pre-clinical experimental research on ion transporters and channels in cancer has only been developed for approximately 15 years. A number of recent reviews have discussed the roles of ion channels in certain human cancers [3–7]. Studies have shown that the expression and activity of different transporters and channels regulate specific stages of cancer progression. For instance, progression through the early G1 phase requires the activation of K+ channels in MCF-7 human breast cancer cells [8]. Decreased levels of the expression of Ca2+ permeable channels in rat insulinoma cells significantly suppress Ca2+ influx and cell death [9]. Voltage-gated Na+ channels were overexpressed in highly metastatic breast cancer and prostate cancer cells [10, 11]. The pharmacological blockade of these channels reduced migration, whereas the facilitation of channel opening by agonists enhanced migration of highly metastatic cell lines [7]. Recently, the essential roles of ion transporters and channels have also been highlighted in glioma progression. Liu et al. showed that transient receptor potential cation channel, subfamily M, member 7, also known as TRPM7, regulates glioma stem cell to promote glioma proliferation, migration and invasion through STAT3 and Notch signaling pathways [12]. Na+ independent K-Cl cotransporter isoform 3 (KCC3) was found highly expressed in Wistar C6 glioblastoma cells [13]. Genetic silencing of KCC3 with short hairpin interfering RNA significantly inhibited K+ influx and reduced C6 glioma cell motility [13]. Moreover, inhibition of Na+/K+-ATPase induced hybrid cell death and enhanced sensitivity to the chemotherapy drug temozolomide in human glioblastoma cells [14].
In this review, we discussed two important ion transporter proteins, sodium-potassium-chloride cotransporter isoform 1 (NKCC-1) and sodium-hydrogen exchanger isoform 1 (NHE-1), in glioma and other cancer cell proliferation, migration, invasion, metabolism and apoptosis, and their potentials as therapeutic targets.
2. NKCC-1 IN GLIOMA
2.1. Structure and function
Sodium-potassium-chloride cotransporter (NKCC) belongs to the SLC12 cation-chloride cotransporter (CCC) gene family [15]. There are two isoforms of NKCC, NKCC-1 and NKCC-2. Full-length clones of these two isoforms have been reported from different tissues of different species [15, 16], with wide distribution of NKCC-1 in all tissue types [17, 18] and expression of NKCC-2 only in the loop of Henle and juxtaglomerular apparatus in the medullary regions of the kidney [19]. Both isoforms are suggested to have twelve transmembrane domains, two putative sites for N-glycosylation on extracellular loop between transmembrane segments 7 and 8 and several phosphorylation sites in both N- and C- terminus [18].
NKCC acts in the concert with other transporters to maintain homeostasis of intracellular water and salt. Under physiological conditions, NKCC transports 1Na+, 1K+ and 2Cl− through cell membrane [15]. All three transported ions are required to be simultaneously present on the same side of the membrane during the NKCC mediated-ion translocation [15]. The operation of NKCC is a secondary active transport with the driving force at least in part supplied by Na+/K+-ATPase-maintained inward Na+ gradient [20]. In epithelial cells, NKCC plays an important role in epithelial salt absorptive and secretory process. It provides the driving force for Cl− to exit the cell by raising intracellular Cl− concentration ([Cl−]i) of the absorptive or secretory epithelial cells above their electrochemical equilibrium potentials [21]. In non-epithelial cells, the activity of NKCC-1 modulates intracellular K+ concentration ([K+]i) and [Cl−]i and maintains intracellular solute content and cellular volume against osmotic stress and excessive cell shrinkage [20]. As only NKCC-1 is expressed outside of the kidney, our discussion will focus on NKCC-1 in the following sections.
2.2. Regulation
NKCC-1 is activated by multiple stimuli, such as a decrease in [Cl−]i, hypertonic stress, an increase in intracellular Ca2+ concentration ([Ca2+]i), hypoxia and a wide range of hormones and growth factors [15, 20, 22–24]. Studies have suggested that protein phosphorylation and oxidative/nitrosative stress are involved in regulation of NKCC-1 activity [25].
Activity of NKCC-1 is precisely regulated by protein phosphorylation and dephosphorylation. Phosphorylation-associated NKCC-1 activation was first demonstrated in shark rectal gland epithelial cells after exposure to cAMP or hypertonic solution [26]. To date, several serine and threonine residues have been identified as phosphorus acceptors in both N- and C-terminus of duck, shark, mouse, rat and human NKCC-1. In particular, phosphorylation of Thr184 and Thr189 in shark NKCC-1, which corresponds to Thr212 and Thr217 in human, is required for maximal NKCC-1 activation. Moreover, these two threonine residues are conserved in the N-terminus of NKCC-1 among species [22].
Several kinases have been proposed to regulate NKCC-1 activity through phosphorylation. Among them, WNKs/SPAK/OSR1 cascade is extensively studied. With-no-K(Lys) kinase isoform 1–4 (WNK1-4) belong to a family of serine/threonine kinases that have the specific kinase domain. WNK kinases function as an “osmosensor” and regulate ion channels and transporters in response to hypertonic stress, low [Cl−]i or isotonic cell shrinkage [27]. WNK-mediated phosphorylation events are important to activity of ion transporters, and most of these events require intermediary kinases. Ste20/sps1-related proline alanine-rich kinase (SPAK0 and its homolog oxidative-stress responsive kinase 1 (OSR1) are two well-characterized WNK substrates, which act as intermediate kinases to connect WNK kinases and the effector such as NKCC-1 [28]. SPAK and OSR1 physically interact with and phosphorylate NKCC-1 at the same regulatory locus (Thr203/Thr207/Thr212/Thr217 on human NKCC-1) [29]. Under hypertonic stress, WNK-1 is rapidly activated by autophosphorylation at Ser382 within the T-loop of its kinase domain. The activated WNK-1 interacts with SPAK/OSR1 and phosphorylates them at two sites (Thr233/Ser373 in human SPAK, Thr185/Ser325 in human OSR1). Phosphorylation and activation of SPAK/OSR1 in turn stimulates NKCC-1 activity by phosphorylation to maintain intracellular ionic strength and cell volume [30]. In addition to the evolutionarily conserved WNK/SPAK/OSR1 signaling pathway, several other kinases have been suggested to play a role in regulation of NKCC, including protein kinase A, protein kinase C, Rho, Janus kinase 2 and CamKinase II [17, 22, 31, 32]. However, there is lack of evidence supporting a direct interaction between these kinases and NKCC-1 [20].
Net levels of NKCC-1 phosphorylation are determined by the balance between kinase and protein phosphatase activities. Studies have suggested that dephosphorylation of NKCC-1 is mediated by protein phosphatase 1 (PP1). In many tissues, NKCC-1 can be activated by phosphatase inhibitors such as okadaic acid and calyculin A in concentration ranges which inhibit PP1 activity [33]. Moreover, the N-terminus of NKCC-1 contains a binding motif for PP1 (RVXFXD). Introduction of point mutations into this motif significantly shifts the activation profile of NKCC-1 [33]. Taken together, phosphorylation/dephosphorylation is an important mechanism regulating NKCC-1 function.
Modification of proteins by free radicals and protein tyrosine nitration can affect protein structure and result in a gain or loss of their function. Studies have shown that oxidative/nitrosative stress affects NKCC-1 activity but whether the impact is positive or negative remains controversial. On one hand, exposure to oxidants, such as H2O2, tert-butylhydroperoxide (TBOH), and the nitric oxide donors, S-nitroso-N-acetyl penicillamine (SNAP) and 3-morpholinosydnoimine HCl (SIN-1), led to increased NKCC-1 oxidation and nitration as well as its activity in astorcytes [34]. Moreover, exposing astrocytes to antioxidants or nitric oxide synthase (NOS) inhibitors significantly reduced NKCC-1 activity induced by oxidants and nitric oxide (NO) donors [34]. On the other hand, inhibition of NKCC-1 activity was found in endothelial cells when they were exposed to TBOH [35]. Similarly, studies have demonstrated that endogenously produced NO inhibits NKCC activity in isolated thick ascending limb of the loop of Henle [36, 37], and in renal epithelial cells exposed to the NO donors SIN-1 and SNAP [38]. In summary, oxidation and nitration regulate NKCC-1 activity in various cell types. The different impact of oxidative/nitrosative stress on NKCC activity may depend on the levels of oxidants or NO donors in these cells.
2.3. Role of NKCC-1 in cancer cell proliferation
It has long been appreciated that NKCC-1 plays an important role in regulation of cell-cycle progression and proliferation through modulating cell volume. Checkpoint controls the correct cell cycle progress by monitoring DNA integrity and the progression of normal cell mitotic cycle [2]. Cell sizes are monitored for suitability for entry into the next phase of cell cycle at least at two checkpoints, G1 and metaphase checkpoint [39]. Stimulation of NKCC-1 in early G1 phase is essential for cell proliferation in certain cell types [15]. In the cell cycle of NIH3T3 cells, stimulation of NKCC-1 is required for the expansion of cell volume through an enhanced cell content of potassium and amino acids, and the concurrent osmotically obliged uptake of water. Inhibition of NKCC-1 with its potent inhibitor bumetanide hampers volume increase and delays cell cycle progression [40, 41]. Importantly, overexpression of NKCC-1 in Mouse Balb/c 3T3 cells leads to apparent cell transformation in a manner similar to some proto-oncogenes, e.g. loss of contact inhibition and formation of cell colonies in soft agar [42]. Moreover, NKCC-1 inhibitors bumetanide and furosemide reduced the clonogenic efficiency in these NKCC-1-transfected cells [42]. Taken together, these findings suggest a potential role of NKCC-1 in cancer proliferation, especially in cancers with high NKCC-1 expression, such as glioma.
2.4. NKCC-1 counteracts against AVD and apoptosis in glioma
Accumulating evidence suggests that cancer cells acquire the resistance to apoptosis [43]. The loss of cell volume during apoptosis, termed as apoptotic volume decrease (AVD), results from loss of intracellular K+ and Cl− and cell volume [44]. Traditionally, AVD has been viewed as a passive process to facilitate the breakdown of the cell into apoptotic bodies and their eventual engulfment by neighboring cells or macrophages [44]. However, recent studies suggest that AVD is an early prerequisite to apoptotic events leading to cell death [44, 45]. Loss of cell volume and reduction of total intracellular ionic strength (via loss of K+ and Cl−) occur prior to any other detectable characteristics of apoptosis [45, 46]. The reduction of intracellular ionic strength has been suggested to play a permissive role in activation of caspases and triggering the entire caspase cascade and apoptotic machinery [45].
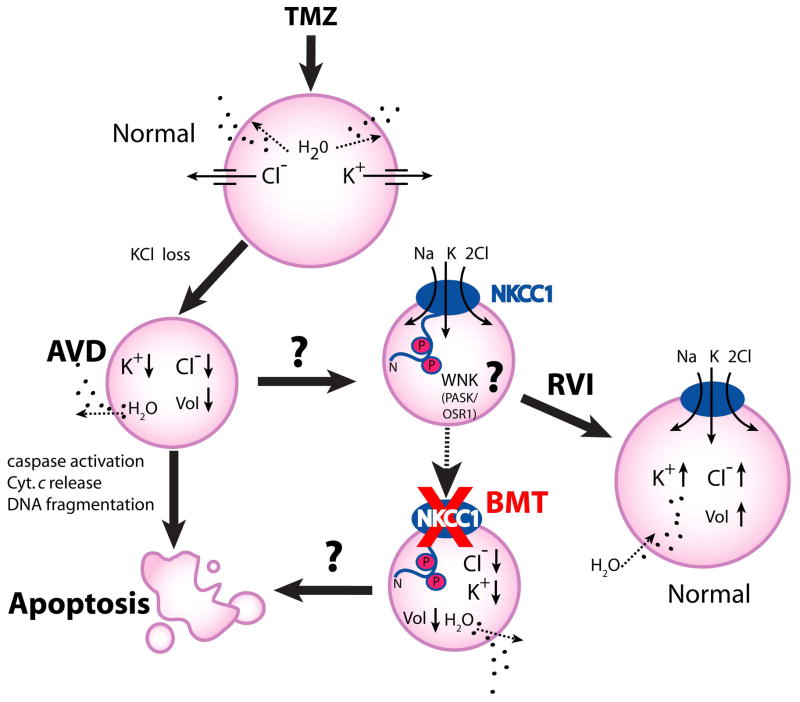
Normally, cells respond to volume perturbations by activating volume regulatory mechanisms such as regulatory volume increase (RVI). RVI can be regulated by the gain of osmotically active solutes such as Na+, K+ and Cl− through NKCC-1 activation [47]. However, impairment of RVI has been detected in HeLa cells in response to several apoptosis inducers such as staurosporine, tumor necrosis factor-α, or a Fas ligand [47]. In contrast, we recently demonstrated that NKCC-1-mediated RVI is intact in both glioma cells and glioma stem cell and plays an essential role in regulating cell volume homeostasis in response to osmotic stress [48]. Moreover, NKCC-1-mediated RVI in glioma cells remains active when glioma was treated with temozolomide, the first-line chemotherapeutic agent for glioma. TMZ causes a DNA methylation lesion, which triggers DNA repair, depletes the enzyme O6-methylguanine methyltransferase (MGMT), and leads to apoptotic cell death via extrinsic and/or intrinsic pathways [49]. Interestingly, as illustrated in Figure 1, we found that TMZ treatment not only led to significant reduction of [K+]i and [Cl−]i and AVD, but also concurrently stimulated NKCC1 and WNK1 activity in glioma cells [48]. Most importantly, inhibition of NKCC-1 with bumetanide accelerated TMZ-induced loss of intracellular K+ and Cl− and cell volume and facilitated the TMZ-triggered apoptosis [48].
Figure 1. Roles of NKCC-1 in counteract against the TMZ-mediated glioma apoptosis.
A schematic model illustrates that temozolomide (TMZ) triggers loss of K+i, Cli and apoptotic volume decrease (AVD), and leads to apoptotic cell death in glioblastoma cancer cells. In response to TMZ, the novel Cl−/volume-sensitive regulatory kinases WNK-mediated signaling transduction pathway is stimulated and activates NKCC-1 protein by phosphorylation. Activation of NKCC-1 accumulates intracellular Na+, K+, and Cl− and obligated water molecules (regulatory volume increase, RVI) to counteract ionic dysregulation and AVD and promote cell survival of the TMZ-treated cells. In contrast, inhibition of NKCC-1 activity with bumetanide facilitates loss of intracellular K+, Cl− and AVD, thus sensitizes glioma cells to the TMZ-mediated apoptosis (adopted from Algharabil, et al [48]).
Recently, several studies indicated that WNKs/SPAK/OSR1 are actively regulating NKCC-1 and play important roles in glioma progression. We identified WNK1 and OSR1 as the upstream kinases activating NKCC-1 in response to treatment TMZ in primary glioma cells [50, 51]. Reduced expression of these two kinases by siRNA significantly attenuated activation of NKCC-1 in the presence of TMZ [50, 51]. Taken together, these findings suggest that NKCC-1 activation in the TMZ-treated cells may elevate apoptotic cell death thresholds and promote cell survival [48]. The ability of a cancer cell to propagate is determined by equilibrium between the rate of cell proliferation and cell death. Therefore, NKCC-1 and its upstream regulatory kinases show great potentials as new therapeutic targets for facilitating apoptosis and reducing proliferation of glioma.
2.5. Role of NKCC-1 in brain tumor cell migration and invasion
The ability of cells to invade and spread through healthy tissue is one of the hallmarks of high-grade GBM [43]. Cell migration/invasion is a cyclical process involving the repetitive extension of lamellipodia/invadopodia at the leading edge of the cells, the formation of adhesion sites, the contraction of the cell body and the release of trailing adhesion sites [52]. A growing body of work suggests that cell migration/invasion is facilitated by ion channels and transporters through efficient regulation of volume in cell body and in cellular processes [53].
NKCC-1 modulates cellular volume and migration in both non-malignant and malignant cells. In normal neuroblasts, NKCC-1 serves as the uptake mechanism maintaining elevated [Cl−]i in migrating cells, thus NKCC-1 activity is necessary to maintain normal migration speed independently of GABAA-signaling [54]. In menigioma, extensive NKCC-1 protein expression was detected in meningioma cells and in capillaries [55]. In those tumors with dural or bone/soft tissue invasion, NKCC-1 immunoreactivity was detected in invading cells [55]. In human glioma, NKCC-1 protein expression was positively correlated with tumor grade [56]. NKCC-1 was localized to the leading edge of migrating glioma cells. Genetic ablation or pharmacalogic inhibition of NKCC-1 with bumetanide reduced glioma cell migration and invasion in in vitro and in vivo [56–58].
Accumulating evidence suggested that ion transporters can function as a plasma membrane anchor for actin filaments by directly binding to ezrin/radixin/moesin (ERM) proteins and affecting cell migration [59]. A recent study demonstrated that ezrin directly binds to two clusters of positive amino acids such as lysine (K) and arginine (R) in the juxtamembrane carboxy-terminus of NKCC1 in glioma cells [56]. Mutation of basic amino acids in these two clusters impaired the physical interaction between NKCC1 and ezrin [56]. Taken together, these findings suggest that NKCC1 may regulate glioma migration/invasion via its direct interactions with ezrin in anchoring to the cytoskeleton.
2.6. Pharmacological inhibitors of NKCC-1 and clinical application
NKCC is blocked by the FDA approved loop diuretics of the sulfamyl category such as furosemide and bumetanide [60]. Structure-function studies have shown that the second transmembrane region of NKCC is an important site in determining bumetanide binding [15]. At low concentrations (2–10 μM), bumetanide is a specific inhibitor of NKCC-1 and has well-established pharmacokinetic and pharmacodynamic properties in adult humans with few side effects [61]. In the healthy brain, NKCC-1 plays a significant role in setting a depolarizing GABAergic currents in certain interneurons [62, 63] and also in the axon initial segment of pyramidal neurons [64]. Inhibition NKCC-1 with bumetanide has been shown to be antiepileptic both in vitro and in vivo [60, 65]. Therefore, bumetanide is currently being studied in an ongoing clinical trial on newborn seizures (http://clinicaltrials.gov). However, because of its chemical structure, bumetanide is highly ionized at physiological pH and highly bound to plasma proteins so that it poorly penetrates into most organs, including the brain [66, 67]. Scientists started to seek bumetanide prodrugs with enhanced BBB penetration and prolonged maintenance in the brain. The most promising bumetanide prodrug is under testing for beneficial effects in mouse and rat models of seizures and epileptogenesis [60].
In summary, NKCC-1 is an essential ion cotransporter in glioma progression by counteracting TMZ-induced apoptosis and facilitating migration and invasion. Inhibition of NKCC-1 with bumetanide may be a feasible therapeutic strategy for glioma treatment through augmenting the chemo-radiotherapy cytotoxicity and reducing metastasis. Moreover, development of new inhibitors with higher NKCC-1 specificity and better BBB permeability may warrant a better therapeutic outcome.
3. NHE-1 IN GLIOMA AND OTHER CANCERS
3.1. Structure and function
The Na+/H+ exchanger (NHEs) family is a group of membrane proteins that transport one H+ out of cells in exchange with one Na+ into cells [68]. Nine NHE isoforms (NHE1-9) have been cloned [69]. NHE-1 is the first cloned plasma membrane isoform with the most widely tissue distribution and considered as a “housekeeping” isoform [70–72]. NHE-2-NHE-5 are also located on the plasma membrane, but with strong tissue specificity. NHE-2 and NHE-3 are highly expressed in intestine and kidney [73, 74] while NHE-4 is highly abundant in stomach, and also present in intestine, kidney, brain, uterus, and skeletal muscle [73]. NHE-5 is highly expressed in brain tissues [75]. NHE-6-NHE-9 are enriched in cellular organelles. NHE-6 is expression in early recycling endosomes. NHE-7 is in the trans-Golgi network. Location of NHE-8 is in the mid-to trans-Golgi and NHE-9 in late recycling endosomes [76].
Of all these isoforms, the most well-studied isoform in tumor cells is NHE-1. NHE-1 is an important regulator of both pHi and pHe in tumors [77]. NHE-1 protein comprises of 815 amino acids with a molecular weight of 85 kDa [68]. NHE-1 has two functional domains: hydrophobic N-terminus and hydrophilic C-terminus. Hydrophobic N-terminal domain contains 500-amino acids of transmembrane segments which are responsible for the exchange of intracellular H+ and extracellular Na+ [78]. The C-terminal functional domain is a cytoplasmic regulatory region of 315 amino acids with its distal serine and threonine residues which can be phosphorylated by several protein kinases such as extracellular signal-related kinase (ERK1/2), p90 ribosomal S kinase (p90rsk), p38 MAPK and an Nck-interacting kinase (NIK) [79].
A major role of NHE-1 in cells is regulation of the pHi. In addition, NHE-1 is one of the main mechanisms causing acidification of extracellular microenvironment [80]. In normal cells, acidification of the intracellular milieu activates NHE-1 which leads to increased H+ extrusion in exchange for Na+ influx. The Na+ energy gradient across the cell membrane generated by Na+/K+ ATPase catalyzes the electro-neutral exchange of one Na+ for one H+ to maintain the physiological pHi of 6.9 and 7.2 [81].
3.2. Regulation
Under normal physiological conditions, although Na+/K+-ATPase provides a large inward Na+ gradient, NHE-1 is essentially inactive. However, intracellular acidosis can make NHE-1 activation rapidly and make sure its effective response to a decreased pHi [81]. Besides the ion transport sites, a second H+ binding site in the protein has positive cooperative binding characteristics and functions as “H+ modifier” or “pH sensor” [82]. During intracellular acidification, allosteric modification at this proton binding site led to stimulation of NHE-1 activity. Interestingly, maintaining pHi via NHE-1 activation can simultaneously acidify pHe in the microdomains which promotes tumor cell invasion [81].
Activation of a variety of cell surface receptors can regulate the activity of NHE-1 by modulating the C-terminal tail region [83]. These regulatory proteins include carbonic anhydrase II, calmodulin and calcineurin homologous protein (CHP) 1 and 2, actin binding ERM proteins, heat shock protein 70, PI(4,5)P2, and the 14-3-3 adaptor protein [84]. The increase of NHE-1 activity is associated with multiple Ser/Thr residue phosphorylation in the C-terminal tail region [85] upon stimulation of Ca2+/calmodulin-dependent kinase II, ERK1/2, Janus-activated kinase 2, NCK-interacting kinase, p38 MAPK kinase, p90rsk and Rho kinase [80, 86]. Phosphorylation of amino acid residues Ser770 and Ser771 is responsible for ERK-mediated NHE-1 activation under acidosis conditions [87]. At Ser703, NHE-1 can be phosphorylated by p90rsk [88]. The activity of NHE-1 also can be modulated by recruiting 14-3-3 protein [89]. Through indirect phosphorylation, protein kinase C can regulate the activity of NHE-1 [80]. Through the actions of PP1, the activity of NHE-1 can be modulated by dephosphorylation [90].
3.3. NHE-1 in cancer cell pHi regulation
One of basic features of NHE-1 regulation is the exquisite regulation of pHi through its internal allosteric proton binding regulatory site. Many solid tumors and their blood vessels do not grow in parallel, resulting in the emergence of some anoxic zone in the solid tumor tissues with increased glycolysis and ATP decomposition. Therefore, tumor cells produce large amounts of lactate and H+ [91]. Intracellular acidosis can increase binding affinity of NHE-1 at the transmembrane domain for intracellular H+ and changes the pH set point higher (more alkaline) than the resting pHi [77]. NHE-1 protein is excessively expressed in a variety of tumor cells and pumps out H+ to maintain the optimal pHi of tumor cells [92, 93]. Moreover, NHE-1 is always in the activated state of cancer cells and leads to the acidification of the extracellular microenvironment [93]. Thus, NHE-1 activation results in both intracellular alkalization and extracellular acidification of tumor cells. The increased pHi of tumor cells can promote glycolysis which further aggravates the acidification of the extracellular microenvironment [94]. The extracellular acidic microenvironment will further increase the invasion and metastasis of the tumor cells [95].
3.4. Role of NHE-1 in cancer cell death
NHE-1 plays an important role in regulating apoptosis in a variety of cell types. The optimum pHi in the acidic range is required for death effectors, such as endonucleases, caspases, and cathepsins [96]. The major role of NHE-1 against apoptosis is limiting intracellular acidification which is an early event of cell death [96]. In some cells, endogenous inhibition or cleavage of NHE-1 via caspase-3 can lead to apoptotic cell death [97, 98]. In both cancer and non-cancer cells, pharmacological inhibition of NHE-1 can induce or aggravate apoptotic cell death [99, 100]. Both roles of scaffolding function and ion transport of NHE-1 are involved in regulation cell death [101]. NHE-1 may involve in ERM-protein-related scaffolding in control of apoptosis. It could be related to protection against loss of cortical ERM proteins and cytoskeletal degradation, which is an important process of apoptotic cell death [102]. Introduction of mutation residues 553–564 of NHE-1 or an NHE-1-defective construct containing a point mutation in the third cytoplasmic loop abolishes NHE-1 binding to ERM-protein and sensitizes cells to apoptosis [98]. Moreover, in LLC-PK1 pig kidney epithelial cells, binding of NHE-1 and ERM is required for NHE-1 mediated Akt activation. Mutation of NHE-1 on the ERM binding site impaired stimulation of Akt [103]. Therefore, NHE-1 activation may inhibit cell apoptosis via promoting the cell survival Akt signaling pathway [103].
Pharmacological inhibition of NHE-1 activity can lead to intracellular acidification and subsequent activation of apoptotic cell death [93]. Yang, et al [104] found that inhibition of the NHE-1 activity in hepatocellular carcinoma cell line by EIPA for 48 h can induce both early apoptosis and late apoptosis in a dose-dependent manner. Transfection of A549 cell line of human lung adenocarcinoma cells with NHE-1 antisense vector increased apoptosis [103]. Similar results were also detected in human H446/CDDP cell line of drug-resistant human small cell lung cancer.
3.5. Role of NHE-1 in cancer cell migration and invasion
Invasion and metastasis of tumor cells associated with neoplastic progression are the main causes of cancer death. Determining the ability of malignant cells invasion and migration and identifying the basic driving force in metastatic progression are the key issues of cancer research [105]. The invasion process of tumor cell is constituted by a series of complicated interactions with the host tissue. In addition to the tumor cells initially attached to the basement membrane or extracellular matrix, three other biochemical and physiological steps are important in invasion process: (a) local degradation of basement membrane or extracellular matrix resulting acid extrusion from both cytoplasm and intracellular organelles; (b) secretion of acid-dependent proteases; (c) increased cancer cell moving into the region where basement membrane or extracellular matrix have been degradated [91, 106]. The acidic extracellular microenvironment of tumor has been shown to play a critical role in increasing capacity of motility, invasive and subsequent malignant progression because it enhances the activity of one or more of the above steps [107]. Cells are in contact with the extracellular matrix when the microenvironment is acidic that can promote the secretion of acidic proteases by cancer cell [106, 107]. Lower pHe in the microdomains can also stimulate the migration of secretary vesicles towards the cell membrane of cancer cells and lead to the increased release of matrix metalloproteinases (MMP) [108].
Extracellular acidification promotes neoangiogenesis, anchorage-independent growth, genetic instability, and invasiveness [109] in metastatic breast cancer [110]. Increased expression and activity of NHE-1 is closely correlated to the invasion ability of the malignant cancer cells, locally or distantly [111]. NHE-1 contributes to the generation of cell surface H+ gradient. Increased H+ concentration at the leading edge can help migrating cell to establish contact with matrix [81]. Moreover, in the MDA-MB-231 breast cancer cells, NHE-1 can stimulate membrane-type matrix metalloproteinases (MT1-MMP) which is known to be an activator of MMP-2 and MMP-13 [112] and promote invasion through ERK1/2 and p38 MAPK signaling pathways [110, 111]
Cell migration depends on direct reorganization of cytoskeletal, recycling of ion transport membrane by endocytosis, and formation of focal adhesion sites with extracellular matrix through molecular receptors such as integrins and CD44 [113, 114]. NHE-1 is part of these focal adhesion sites through its indirect connection with integrins [115]. Some studies showed that NHE-1 played a major role of cell migration [116–118], via its function in cell volume regulation, cytoskeletal stability, and plasma membrane anchoring [83]. Therefore, NHE-1 plays an important role in cancer cell migration and invasion.
3.6. Pharmacological inhibitors and clinical application
In light of the important role of NHE-1 in the process of tumor progression, NHE-1 becomes one of the potential targets for anti-cancer therapeutics. Many inhibitors for NHE have been developed [111, 119]. Amiloride (3,5-diamino-6-chloro-N-(diaminomethylene)-2-pyrazinecarboxamide) is the first compound found to have inhibitory activity of NHE-1 with an IC50 of 17 μM [120]. Amiloride inhibits NHE-1 by binding to a site surrounding Glu346 in the extracellular domain. Although amiloride can inhibit NHE-1 at a relative low concentration, it can also inhibit the acid sensing cation channel-1 (ASIC-1) with similar concentration (IC50=0.2–20 μM) [121] and the Na+/Ca2+ exchanger (NCX) at a higher concentration (IC50=500 μM) [120]. Both amiloride and its more potent analogue 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) (IC50=0.19μM) can inhibit proliferation and migration, and promote apoptosis in a variety of cancer cell types through inhibition of NHE-1 [122, 123]. Newly developed NHE-1-selective acylguanidine-derived compounds such as cariporide (HOE-642) are much more selective towards NHE-1, with an IC50 of 0.85 μM [123].
Cariporide is extensively used to inhibit NHE-1 activity in pre-clinical and clinical experiments [124, 125]. For comprehensive review of NHE1 in malignant cancer cells and anti-cancer application of cariporide, please see the recent review by Harguindey et al [126]. Of note, cariporide has undergone a clinical trial “The Na+/H+ Exchanger Inhibition to Prevent Coronary Events in Acute Cardiac Conditions” (EXPEDITION) [127]. In this clinic trial, cariporide reduced myocardial infarcts but significantly increased the mortality rate due to an increase of cerebrovascular events which likely results from an abrupt withdrawal of cariporide and leads to a rebound effect in rapid activation of the exchanger and platelet hyperactivity [127]. Therefore, developing NHE inhibitors with improved selectivity and minimizing the systemic dose of the drug will reduce the adverse effects [128]. Moreover, combined strategies including blockade of NHE-1 activity in cancer cells may have promising potentials for development of pharmacogenetics and personalized cancer chemotherapy [77].
4. CONCLUSION
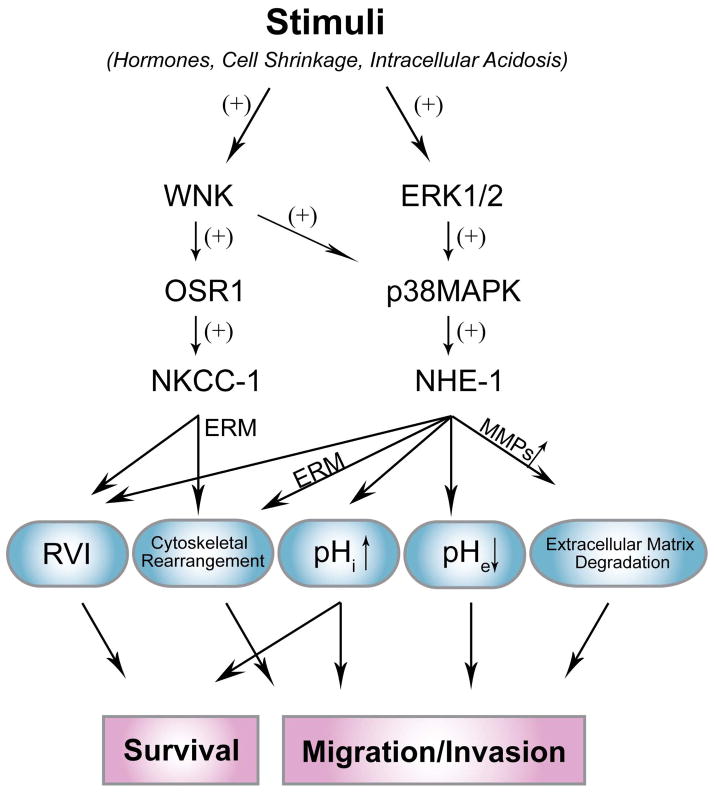
Ion transporters are important in regulation of ionic homeostasis, cell volume, and cellular signal transduction under physiological conditions. NKCC-1 and NHE-1 ion transporters have emerged as important players in cancer progression. Figure 2 summarizes signal transduction pathways which regulate NKCC-1 or NHE-1 in cancer cells. Their roles in regulation of cell volume, pHi, pHe environment and interactions with ERM protein complex may collectively promote cancer cell survival, increase resistance to apoptosis, and enhance migration and invasion. Therefore, these ion transporters present great potentials as new targets for cancer therapy including GBM.
Figure 2. Regulation of NKCC-1 and NHE-1 in cancer cell death and migration/invasion.
A schematic diagram illustrates important roles of NKCC-1 and NHE-1 in regulation of cancer cell survival and migration/invasion. In response to stimuli, WNKs/OSR1 are major upstream regulators of NKCC1, while ERK 1/2 and p38 MAPK signaling pathway is involved in activation of NHE-1. NKCC-1 and NHE-1 mediate regulatory volume increase (RVI) to against apoptosis and promote cell survival. Direct interaction between ERM-NKCC-1 or ERM-NHE-1 contributes to cytoskeletal rearrangement which affects cell migration. Activation of NHE-1 not only leads to cytosolic alkalinization and inhibits apoptosis, but also increases cell migration/invasion by mediating extracellular acidification and stimulating release of MMPs.
Acknowledgments
This work was supported in part by NIH grant R01NS75995 and R01NS38118 (D.S.), and the HEADRUSH Brain Tumor Research Professorship, the AANS-NREF Young Clinician Investigator Award, Loff Memorial Fund, Department of Neurological Surgery Brain Tumor Research Fund (J.S.K.) and Technology cooperation of Province and Chinese Academy of Sciences Program 2014 YS14C11 (S.H.).
LIST OF ABBREVIATIONS
- AKT
known as PKB (Protein Kinase B)
- AVD
apoptotic volume decrease
- CCC
cation-chloride cotransporter
- CHP
calcineurin homologous protein
- EIPA
5-(N-ethyl-N-isopropyl)-amiloride
- ERK1/2
extracellular signal-related kinase 1/2
- ERM
ezrin/radixin/moesin
- IC50
The half maximal inhibitory concentration
- MGMT
enzyme O6-methylguanine methyltransferase
- NHE
sodium-hydrogen exchanger
- NIK
Nck-interacting kinase
- NKCC
sodium-potassium-chloride cotransporter
- NO
nitric oxide
- NOS
nitric oxide synthase
- OSR1
oxidative-stress responsive kinase 1
- p38 MAPK
p38-mitogen-activated protein kinase
- p90rsk
p90 ribosomal S kinase
- pHe
extracellular pH
- pHi
intracellular pH
- PP1
protein phosphatase 1
- RVI
regulatory volume increase
- SIN-1
3-morpholinosydnoimine HCl
- SNAP
S-nitroso-N-acetyl penicillamine
- SPAK
Ste20-related proline alanine-rich kinase
- TBOH
tert-butylhydroperoxide
- WNK
With-No-Lysine kinase
References
- 1.Stupp R, Pavlidis N, Jelic S. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol. 2005;16(Suppl 1):i64–65. doi: 10.1093/annonc/mdi834. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16(1):66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 4.Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25(3):493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 5.Fraser SP, Pardo LA. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on Ion Channels and Cancer. EMBO Rep. 2008;9(6):512–515. doi: 10.1038/embor.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205(3):159–173. doi: 10.1007/s00232-005-0781-4. [DOI] [PubMed] [Google Scholar]
- 7.Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16(3):107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287(1):C125–134. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 9.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9(1):163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 10.Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101(2):149–160. doi: 10.1007/s10549-006-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8(3):266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Inoue K, Leng T, Guo S, Xiong ZG. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell Signal. 2014;26(12):2773–2781. doi: 10.1016/j.cellsig.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnon KB. High-grade glioma motility reduced by genetic knockdown of KCC3. Cell Physiol Biochem. 2012;30(2):466–476. doi: 10.1159/000339040. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Song M, Mohamad O, Yu SP. Inhibition of Na+/K+-ATPase induces hybrid cell death and enhanced sensitivity to chemotherapy in human glioblastoma cells. BMC Cancer. 2014;14(1):716. doi: 10.1186/1471-2407-14-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80(1):211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 16.Haas M, Forbush B., 3rd The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 17.Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B., 3rd Primary structure functional expression and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon. J Biol Chem. 1995;270(30):17977–17985. doi: 10.1074/jbc.270.30.17977. [DOI] [PubMed] [Google Scholar]
- 18.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush B., 3rd Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci U S A. 1994;91(6):2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne JA, Forbush B., 3rd Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci U S A. 1994;91(10):4544–4548. doi: 10.1073/pnas.91.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Sun D. The role of Na-K-Cl co-transporter in cerebral ischemia. Neurol Res. 2005;27(3):280–286. doi: 10.1179/016164105X25243. [DOI] [PubMed] [Google Scholar]
- 21.Greger R. The membrane transporters regulating epithelial NaCl secretion. Pflugers Arch. 1996;432(4):579–588. doi: 10.1007/s004240050173. [DOI] [PubMed] [Google Scholar]
- 22.Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem. 2002;277(40):37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- 23.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem. 2002;277(40):37551–37558. doi: 10.1074/jbc.M206294200. [DOI] [PubMed] [Google Scholar]
- 24.Foroutan S, Brillault J, Forbush B, O’Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl- cotransporter. Am J Physiol Cell Physiol. 2005;289(6):C1492–1501. doi: 10.1152/ajpcell.00257.2005. [DOI] [PubMed] [Google Scholar]
- 25.Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab Brain Dis. 2010;25(1):31–38. doi: 10.1007/s11011-010-9180-3. [DOI] [PubMed] [Google Scholar]
- 26.Lytle C, Forbush B., 3rd The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992;267(35):25438–25443. [PubMed] [Google Scholar]
- 27.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91(1):177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121(Pt 20):3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 29.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 30.Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012;441(1):325–337. doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem. 1994;269(41):25677–25683. [PubMed] [Google Scholar]
- 32.Di Ciano-Oliveira C, Sirokmany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol Cell Physiol. 2003;285(3):C555–566. doi: 10.1152/ajpcell.00086.2003. [DOI] [PubMed] [Google Scholar]
- 33.Darman RB, Flemmer A, Forbush B. Modulation of ion transport by direct targeting of protein phosphatase type 1 to the Na-K-Cl cotransporter. J Biol Chem. 2001;276(37):34359–34362. doi: 10.1074/jbc.C100368200. [DOI] [PubMed] [Google Scholar]
- 34.Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B, 3rd, Reddy PV, Norenberg MD. Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008;283(49):33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott SJ, Schilling WP. Oxidant stress alters Na+ pump and Na(+)-K(+)-Cl- cotransporter activities in vascular endothelial cells. Am J Physiol. 1992;263(1 Pt 2):H96–102. doi: 10.1152/ajpheart.1992.263.1.H96. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(−) cotransporter activity. Am J Physiol Renal Physiol. 2001;281(5):F819–825. doi: 10.1152/ajprenal.2001.281.5.F819. [DOI] [PubMed] [Google Scholar]
- 37.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol. 1999;276(1 Pt 2):F159–163. doi: 10.1152/ajprenal.1999.276.1.F159. [DOI] [PubMed] [Google Scholar]
- 38.He H, Podymow T, Zimpelmann J, Burns KD. NO inhibits Na+-K+-2Cl- cotransport via a cytochrome P-450-dependent pathway in renal epithelial cells (MMDD1) Am J Physiol Renal Physiol. 2003;284(6):F1235–1244. doi: 10.1152/ajprenal.00192.2002. [DOI] [PubMed] [Google Scholar]
- 39.Habela CW, Sontheimer H. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle. 2007;6(13):1613–1620. doi: 10.4161/cc.6.13.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bussolati O, Uggeri J, Belletti S, Dall’Asta V, Gazzola GC. The stimulation of Na,K,Cl cotransport and of system A for neutral amino acid transport is a mechanism for cell volume increase during the cell cycle. FASEB J. 1996;10(8):920–926. doi: 10.1096/fasebj.10.8.8666170. [DOI] [PubMed] [Google Scholar]
- 41.Jiang G, Klein JD, O’Neill WC. Growth factors stimulate the Na-K-2Cl cotransporter NKCC1 through a novel Cl(−)-dependent mechanism. Am J Physiol Cell Physiol. 2001;281(6):C1948–1953. doi: 10.1152/ajpcell.2001.281.6.C1948. [DOI] [PubMed] [Google Scholar]
- 42.Panet R, Marcus M, Atlan H. Overexpression of the Na(+)/K(+)/Cl(−) cotransporter gene induces cell proliferation and phenotypic transformation in mouse fibroblasts. J Cell Physiol. 2000;182(1):109–118. doi: 10.1002/(SICI)1097-4652(200001)182:1<109::AID-JCP12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2012;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Bortner CD, Cidlowski JA. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002;9(12):1307–1310. doi: 10.1038/sj.cdd.4401126. [DOI] [PubMed] [Google Scholar]
- 45.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci U S A. 2000;97(17):9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chimote AA, Adragna NC, Lauf PK. Ion transport in a human lens epithelial cell line exposed to hyposmotic and apoptotic stress. J Cell Physiol. 2010;223(1):110–122. doi: 10.1002/jcp.22015. [DOI] [PubMed] [Google Scholar]
- 47.Maeno E, Takahashi N, Okada Y. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett. 2006;580(27):6513–6517. doi: 10.1016/j.febslet.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 48.Algharabil J, Kintner DB, Wang Q, Begum G, Clark PA, Yang SS, Lin SH, Kahle KT, Kuo JS, Sun D. Inhibition of Na(+)-K(+)-2Cl(−) cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells. Cell Physiol Biochem. 2012;30(1):33–48. doi: 10.1159/000339047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 50.Zhu W, Wang Q, Clark PA, Yang S-S, Lin S-H, Kahle KT, Kuo JS, Sun D. Temozolomid activates upstream kinases of Na+-K+-Cl− cotransporter isoform 1 in glioblastoma cancer cells. Neuro-Oncology. 2012;14(suppl 6):vi7–vi20. [Google Scholar]
- 51.Zhu W, Begum G, Pointer K, Clark PA, Yang SS, Lin SH, Kahle KT, Kuo JS, Sun D. WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol Cancer. 2014;13:31. doi: 10.1186/1476-4598-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakab M, Ritter M. Cell volume regulatory ion transport in the regulation of cell migration. Contrib Nephrol. 2006;152:161–180. doi: 10.1159/000096322. [DOI] [PubMed] [Google Scholar]
- 53.Cuddapah VA, Sontheimer H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. 2011;301(3):C541–549. doi: 10.1152/ajpcell.00102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mejia-Gervacio S, Murray K, Lledo PM. NKCC1 controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev. 2011;6:4. doi: 10.1186/1749-8104-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson MD, O’Connell M. Na-K-2Cl cotransporter and aquaporin 1 in arachnoid granulations, meningiomas, and meningiomas invading dura. Hum Pathol. 2013 doi: 10.1016/j.humpath.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Garzon-Muvdi T, Schiapparelli P, ap Rhys C, Guerrero-Cazares H, Smith C, Kim DH, Kone L, Farber H, Lee DY, An SS, Levchenko A, Quinones-Hinojosa A. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 2012;10(5):e1001320. doi: 10.1371/journal.pbio.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas BR, Cuddapah VA, Watkins S, Rohn KJ, Dy TE, Sontheimer H. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301(5):C1150–1160. doi: 10.1152/ajpcell.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas BR, Sontheimer H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 reduces glioma invasion. Cancer Res. 2010;70(13):5597–5606. doi: 10.1158/0008-5472.CAN-09-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159(6):1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 61.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 62.Marty A, Llano I. Excitatory effects of GABA in established brain networks. Trends Neurosci. 2005;28(6):284–289. doi: 10.1016/j.tins.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28(18):4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahle KT, Staley K. Altered neuronal chloride homeostasis and excitatory GABAergic signaling in human temporal lobe epilepsy. Epilepsy Curr. 2008;8(2):51–53. doi: 10.1111/j.1535-7511.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brandt C, Nozadze M, Heuchert N, Rattka M, Loscher W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci. 2010;30(25):8602–8612. doi: 10.1523/JNEUROSCI.0633-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Cleary R, Kellogg M, Soul JS, Berry GT, Jensen FE. Sensitive isotope dilution liquid chromatography/tandem mass spectrometry method for quantitative analysis of bumetanide in serum and brain tissue. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(13–14):998–1002. doi: 10.1016/j.jchromb.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol. 2006;84(11):1081–1095. doi: 10.1139/y06-065. [DOI] [PubMed] [Google Scholar]
- 69.Fliegel L. The Na+/H+ exchanger isoform 1. Int J Biochem Cell Biol. 2005;37(1):33–37. doi: 10.1016/j.biocel.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Fliegel L, Sardet C, Pouyssegur J, Barr A. Identification of the protein and cDNA of the cardiac Na+/H+ exchanger. FEBS Lett. 1991;279(1):25–29. doi: 10.1016/0014-5793(91)80241-t. [DOI] [PubMed] [Google Scholar]
- 71.Luo J, Sun D. Physiology and pathophysiology of Na(+)/H(+) exchange isoform 1 in the central nervous system. Curr Neurovasc Res. 2007;4(3):205–215. doi: 10.2174/156720207781387178. [DOI] [PubMed] [Google Scholar]
- 72.Wakabayashi S, Sardet C, Fafournoux P, Counillon L, Meloche S, Pages G, Pouyssegur J. Structure function of the growth factor-activatable Na+/H+ exchanger (NHE1) Rev Physiol Biochem Pharmacol. 1992;119:157–186. doi: 10.1007/3540551921_6. [DOI] [PubMed] [Google Scholar]
- 73.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992;267(13):9331–9339. [PubMed] [Google Scholar]
- 74.Noel J, Roux D, Pouyssegur J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. J Cell Sci. 1996;109(Pt 5):929–939. doi: 10.1242/jcs.109.5.929. [DOI] [PubMed] [Google Scholar]
- 75.Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem. 1999;274(7):4383–4388. doi: 10.1074/jbc.274.7.4383. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280(2):1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 77.Reshkin SJ, Cardone RA, Harguindey S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov. 2012;8(1):85–99. doi: 10.2174/15748928130108. [DOI] [PubMed] [Google Scholar]
- 78.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 79.Khaled AR, Moor AN, Li A, Kim K, Ferris DK, Muegge K, Fisher RJ, Fliegel L, Durum SK. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol Cell Biol. 2001;21(22):7545–7557. doi: 10.1128/MCB.21.22.7545-7557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401(3):623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loo SY, Chang MK, Chua CS, Kumar AP, Pervaiz S, Clement MV. NHE-1: a promising target for novel anti-cancer therapeutics. Curr Pharm Des. 2012;18(10):1372–1382. doi: 10.2174/138161212799504885. [DOI] [PubMed] [Google Scholar]
- 82.Wakabayashi S, Hisamitsu T, Pang T, Shigekawa M. Kinetic dissection of two distinct proton binding sites in Na+/H+ exchangers by measurement of reverse mode reaction. J Biol Chem. 2003;278(44):43580–43585. doi: 10.1074/jbc.M306690200. [DOI] [PubMed] [Google Scholar]
- 83.Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- 84.Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci U S A. 1996;93(22):12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sardet C, Fafournoux P, Pouyssegur J. Alpha-thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem. 1991;266(29):19166–19171. [PubMed] [Google Scholar]
- 86.Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to alpha1-adrenergic stimulation. Mol Pharmacol. 2007;71(3):799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 87.Malo ME, Li L, Fliegel L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids Ser770 and Ser771. J Biol Chem. 2007;282(9):6292–6299. doi: 10.1074/jbc.M611073200. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem. 1999;274(29):20206–20214. doi: 10.1074/jbc.274.29.20206. [DOI] [PubMed] [Google Scholar]
- 89.Lehoux S, Abe J, Florian JA, Berk BC. 14-3-3 Binding to Na+/H+ exchanger isoform-1 is associated with serum-dependent activation of Na+/H+ exchange. J Biol Chem. 2001;276(19):15794–15800. doi: 10.1074/jbc.M100410200. [DOI] [PubMed] [Google Scholar]
- 90.Misik AJ, Perreault K, Holmes CF, Fliegel L. Protein phosphatase regulation of Na+/H+ exchanger isoform I. Biochemistry. 2005;44(15):5842–5852. doi: 10.1021/bi047659s. [DOI] [PubMed] [Google Scholar]
- 91.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim Biophys Acta. 2005;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Glunde K, Dussmann H, Juretschke HP, Leibfritz D. Na(+)/H(+) exchange subtype 1 inhibition during extracellular acidification and hypoxia in glioma cells. J Neurochem. 2002;80(1):36–44. doi: 10.1046/j.0022-3042.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- 93.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14(14):2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- 94.Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458(5):981–992. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 95.Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002;16(4):430–450. doi: 10.1002/jmri.10181. [DOI] [PubMed] [Google Scholar]
- 96.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2(6):318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 97.Lang F, Madlung J, Bock J, Lukewille U, Kaltenbach S, Lang KS, Belka C, Wagner CA, Lang HJ, Gulbins E, Lepple-Wienhues A. Inhibition of Jurkat-T-lymphocyte Na+/H+-exchanger by CD95(Fas/Apo-1)-receptor stimulation. Pflugers Arch. 2000;440(6):902–907. doi: 10.1007/s004240000358. [DOI] [PubMed] [Google Scholar]
- 98.Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol. 2003;284(4):F829–839. doi: 10.1152/ajprenal.00314.2002. [DOI] [PubMed] [Google Scholar]
- 99.Cho YL, Lee KS, Lee SJ, Namkoong S, Kim YM, Lee H, Ha KS, Han JA, Kwon YG. Amiloride potentiates TRAIL-induced tumor cell apoptosis by intracellular acidification-dependent Akt inactivation. Biochem Biophys Res Commun. 2005;326(4):752–758. doi: 10.1016/j.bbrc.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 100.Nylandsted J, Jaattela M, Hoffmann EK, Pedersen SF. Heat shock protein 70 inhibits shrinkage-induced programmed cell death via mechanisms independent of effects on cell volume-regulatory membrane transport proteins. Pflugers Arch. 2004;449(2):175–185. doi: 10.1007/s00424-004-1332-z. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen SF. The Na+/H+ exchanger NHE1 in stress-induced signal transduction: implications for cell proliferation and cell death. Pflugers Arch. 2006;452(3):249–259. doi: 10.1007/s00424-006-0044-y. [DOI] [PubMed] [Google Scholar]
- 102.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139(3):749–758. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279(25):26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 104.Yang X, Wang D, Dong W, Song Z, Dou K. Expression and modulation of Na(+)/H(+) exchanger 1 gene in hepatocellular carcinoma: A potential therapeutic target. J Gastroenterol Hepatol. 2011;26(2):364–370. doi: 10.1111/j.1440-1746.2010.06382.x. [DOI] [PubMed] [Google Scholar]
- 105.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 2003;63(14):3847–3854. [PubMed] [Google Scholar]
- 107.Webb SD, Sherratt JA, Fish RG. Modelling tumour acidity and invasion. Novartis Found Symp. 2001;240:169–181. doi: 10.1002/0470868716.ch12. discussion 181–165. [DOI] [PubMed] [Google Scholar]
- 108.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003;5(6):533–545. doi: 10.1016/s1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279(26):26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 110.Lin Y, Chang G, Wang J, Jin W, Wang L, Li H, Ma L, Li Q, Pang T. NHE1 mediates MDA-MB-231 cells invasion through the regulation of MT1-MMP. Exp Cell Res. 2011;317(14):2031–2040. doi: 10.1016/j.yexcr.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 111.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5(10):786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 112.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206(1):1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 113.Kostidou E, Koliakos G, Alamdari DH, Paletas K, Tsapas A, Kaloyianni M. Enhanced laminin carbonylation by monocytes in diabetes mellitus. Clin Biochem. 2007;40(9–10):671–679. doi: 10.1016/j.clinbiochem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 114.Verfaillie CM, Benis A, Iida J, McGlave PB, McCarthy JB. Adhesion of committed human hematopoietic progenitors to synthetic peptides from the C-terminal heparin-binding domain of fibronectin: cooperation between the integrin alpha 4 beta 1 and the CD44 adhesion receptor. Blood. 1994;84(6):1802–1811. [PubMed] [Google Scholar]
- 115.Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol. 2005;567(Pt 1):225–238. doi: 10.1113/jphysiol.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McHardy LM, Sinotte R, Troussard A, Sheldon C, Church J, Williams DE, Andersen RJ, Dedhar S, Roberge M, Roskelley CD. The tumor invasion inhibitor dihydromotuporamine C activates RHO, remodels stress fibers and focal adhesions, and stimulates sodium-proton exchange. Cancer Res. 2004;64(4):1468–1474. doi: 10.1158/0008-5472.can-03-2733. [DOI] [PubMed] [Google Scholar]
- 117.Paradiso A, Cardone RA, Bellizzi A, Bagorda A, Guerra L, Tommasino M, Casavola V, Reshkin SJ. The Na+-H+ exchanger-1 induces cytoskeletal changes involving reciprocal RhoA and Rac1 signaling, resulting in motility and invasion in MDA-MB-435 cells. Breast Cancer Res. 2004;6(6):R616–628. doi: 10.1186/bcr922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na(+)/H(+) exchange, motility, and invasion induced by serum deprivation. J Biol Chem. 2000;275(8):5361–5369. doi: 10.1074/jbc.275.8.5361. [DOI] [PubMed] [Google Scholar]
- 119.Thiry A, Supuran CT, Masereel B, Dogne JM. Recent developments of carbonic anhydrase inhibitors as potential anticancer drugs. J Med Chem. 2008;51(11):3051–3056. doi: 10.1021/jm701526d. [DOI] [PubMed] [Google Scholar]
- 120.Hegde M, Roscoe J, Cala P, Gorin F. Amiloride kills malignant glioma cells independent of its inhibition of the sodium-hydrogen exchanger. J Pharmacol Exp Ther. 2004;310(1):67–74. doi: 10.1124/jpet.103.065029. [DOI] [PubMed] [Google Scholar]
- 121.Paukert M, Babini E, Pusch M, Grunder S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol. 2004;124(4):383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Humphreys RA, Haist JV, Chakrabarti S, Feng Q, Arnold JM, Karmazyn M. Orally administered NHE1 inhibitor cariporide reduces acute responses to coronary occlusion and reperfusion. Am J Physiol. 1999;276(2 Pt 2):H749–757. doi: 10.1152/ajpheart.1999.276.2.H749. [DOI] [PubMed] [Google Scholar]
- 123.Pederson SF, Varming C, Christensen ST, Hoffmann EK. Mechanisms of activation of NHE by cell shrinkage and by calyculin A in Ehrlich ascites tumor cells. J Membr Biol. 2002;189(1):67–81. doi: 10.1007/s00232-001-0190-2. [DOI] [PubMed] [Google Scholar]
- 124.Matthews H, Ranson M, Kelso MJ. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int J Cancer. 2011;129(9):2051–2061. doi: 10.1002/ijc.26156. [DOI] [PubMed] [Google Scholar]
- 125.Rogister F, Laeckmann D, Plasman P, Van Eylen F, Ghyoot M, Maggetto C, Liegeois J, Geczy J, Herchuelz A, Delarge J, Masereel B. Novel inhibitors of the sodium-calcium exchanger: benzene ring analogues of N-guanidino substituted amiloride derivatives. Eur J Med Chem. 2001;36(7–8):597–614. doi: 10.1016/s0223-5234(01)01247-8. [DOI] [PubMed] [Google Scholar]
- 126.Harguindey S, Arranz JL, Polo Orozco JD, Rauch C, Fais S, Cardone RA, Reshkin SJ. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs--an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J Transl Med. 2013;11:282. doi: 10.1186/1479-5876-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mentzer RM, Jr, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasche P, Myers ML, Nicolau J, Simoons M, Thulin L, Weisel RD. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85(4):1261–1270. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 128.Avkiran M, Cook AR, Cuello F. Targeting Na+/H+ exchanger regulation for cardiac protection: a RSKy approach? Curr Opin Pharmacol. 2008;8(2):133–140. doi: 10.1016/j.coph.2007.12.007. [DOI] [PubMed] [Google Scholar]