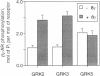
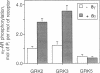
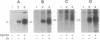Abstract
Phosphorylation of GTP-binding-regulatory (G)-protein-coupled receptors by specific G-protein-coupled receptor kinases (GRKs) is a major mechanism responsible for agonist-mediated desensitization of signal transduction processes. However, to date, studies of the specificity of these enzymes have been hampered by the difficulty of preparing the purified and reconstituted receptor preparations required as substrates. Here we describe an approach that obviates this problem by utilizing highly purified membrane preparations from Sf9 and 293 cells overexpressing G-protein-coupled receptors. We use this technique to demonstrate specificity of several GRKs with respect to both receptor substrates and the enhancing effects of G-protein beta gamma subunits on phosphorylation. Enriched membrane preparations of the beta 2- and alpha 2-C2-adrenergic receptors (ARs, where alpha 2-C2-AR refers to the AR whose gene is located on human chromosome 2) prepared by sucrose density gradient centrifugation from Sf9 or 293 cells contain the receptor at 100-300 pmol/mg of protein and serve as efficient substrates for agonist-dependent phosphorylation by beta-AR kinase 1 (GRK2), beta-AR kinase 2 (GRK3), or GRK5. Stoichiometries of agonist-mediated phosphorylation of the receptors by GRK2 (beta-AR kinase 1), in the absence and presence of G beta gamma, are 1 and 3 mol/mol, respectively. The rate of phosphorylation of the membrane receptors is 3 times faster than that of purified and reconstituted receptors. While phosphorylation of the beta 2-AR by GRK2, -3, and -5 is similar, the activity of GRK2 and -3 is enhanced by G beta gamma whereas that of GRK5 is not. In contrast, whereas GRK2 and -3 efficiently phosphorylate alpha 2-C2-AR, GRK5 is quite weak. The availability of a simple direct phosphorylation assay applicable to any cloned G-protein-coupled receptor should greatly facilitate elucidation of the mechanisms of regulation of these receptors by the expanding family of GRKs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., DeBlasi A., Stone W. C., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989 Oct 13;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Gomez J. Molecular cloning and expression of GRK6. A new member of the G protein-coupled receptor kinase family. J Biol Chem. 1993 Sep 15;268(26):19521–19527. [PubMed] [Google Scholar]
- Benovic J. L., Mayor F., Jr, Staniszewski C., Lefkowitz R. J., Caron M. G. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987 Jul 5;262(19):9026–9032. [PubMed] [Google Scholar]
- Benovic J. L., Onorato J. J., Arriza J. L., Stone W. C., Lohse M., Jenkins N. A., Gilbert D. J., Copeland N. G., Caron M. G., Lefkowitz R. J. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. J Biol Chem. 1991 Aug 15;266(23):14939–14946. [PubMed] [Google Scholar]
- Benovic J. L., Regan J. W., Matsui H., Mayor F., Jr, Cotecchia S., Leeb-Lundberg L. M., Caron M. G., Lefkowitz R. J. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987 Dec 25;262(36):17251–17253. [PubMed] [Google Scholar]
- Benovic J. L., Shorr R. G., Caron M. G., Lefkowitz R. J. The mammalian beta 2-adrenergic receptor: purification and characterization. Biochemistry. 1984 Sep 25;23(20):4510–4518. doi: 10.1021/bi00315a002. [DOI] [PubMed] [Google Scholar]
- Boekhoff I., Schleicher S., Strotmann J., Breer H. Odor-induced phosphorylation of olfactory cilia proteins. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11983–11987. doi: 10.1073/pnas.89.24.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Graziano M. P., Gilman A. G. G protein beta gamma subunits from bovine brain and retina: equivalent catalytic support of ADP-ribosylation of alpha subunits by pertussis toxin but differential interactions with Gs alpha. Biochemistry. 1989 Jan 24;28(2):611–616. doi: 10.1021/bi00428a029. [DOI] [PubMed] [Google Scholar]
- Chuang T. T., Sallese M., Ambrosini G., Parruti G., De Blasi A. High expression of beta-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992 Apr 5;267(10):6886–6892. [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Arriza J. L., Jaworsky D. E., Borisy F. F., Attramadal H., Lefkowitz R. J., Ronnett G. V. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993 Feb 5;259(5096):825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- Dolph P. J., Ranganathan R., Colley N. J., Hardy R. W., Socolich M., Zuker C. S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993 Jun 25;260(5116):1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Haribabu B., Snyderman R. Identification of additional members of human G-protein-coupled receptor kinase multigene family. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9398–9402. doi: 10.1073/pnas.90.20.9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Inglese J., Freedman N. J., Koch W. J., Lefkowitz R. J. Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem. 1993 Nov 15;268(32):23735–23738. [PubMed] [Google Scholar]
- Ishii K., Chen J., Ishii M., Koch W. J., Freedman N. J., Lefkowitz R. J., Coughlin S. R. Inhibition of thrombin receptor signaling by a G-protein coupled receptor kinase. Functional specificity among G-protein coupled receptor kinases. J Biol Chem. 1994 Jan 14;269(2):1125–1130. [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Kunapuli P., Benovic J. L. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H., Arriza J. L., Lefkowitz R. J. Characterization of alpha 2-adrenergic receptor subtype-specific antibodies. Mol Pharmacol. 1993 Mar;43(3):444–450. [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Leung E., Maan A. C., McMahon K. K., Ptasienski J., Green R. D., Hosey M. M. Correlation of agonist-induced phosphorylation of chick heart muscarinic receptors with receptor desensitization. J Biol Chem. 1987 Dec 5;262(34):16314–16321. [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lorenz W., Inglese J., Palczewski K., Onorato J. J., Caron M. G., Lefkowitz R. J. The receptor kinase family: primary structure of rhodopsin kinase reveals similarities to the beta-adrenergic receptor kinase. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8715–8719. doi: 10.1073/pnas.88.19.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J. A., Inglese J., Higgins J. B., Arriza J. L., Casey P. J., Kim C., Benovic J. L., Kwatra M. M., Caron M. G., Lefkowitz R. J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992 Aug 28;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Premont R. T., Koch W. J., Inglese J., Lefkowitz R. J. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994 Mar 4;269(9):6832–6841. [PubMed] [Google Scholar]
- Roth N. S., Campbell P. T., Caron M. G., Lefkowitz R. J., Lohse M. J. Comparative rates of desensitization of beta-adrenergic receptors by the beta-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993 Mar 5;268(7):4625–4636. [PubMed] [Google Scholar]