Abstract
Multidrug and toxic compound extrusion (MATE) proteins are the most recently identified family of multidrug transporters. In plants, this family is remarkably large compared to the human and bacteria counterpart, highlighting the importance of MATE proteins in this kingdom. Here 33 Unigenes annotated as MATE transporters were found in the blueberry fruit transcriptome, of which eight full-length cDNA sequences were identified and cloned. These proteins are composed of 477–517 residues, with molecular masses ~54 kDa, and theoretical isoelectric points from 5.35 to 8.41. Bioinformatics analysis predicted 10–12 putative transmembrane segments for VcMATEs, and localization to the plasma membrane without an N-terminal signal peptide. All blueberry MATE proteins shared 32.1–84.4% identity, among which VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 were more similar to the MATE-type flavonoid transporters. Phylogenetic analysis showed VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8 and VcMATE9 clustered with MATE-type flavonoid transporters, indicating that they might be involved in flavonoid transport. VcMATE1 and VcMATE4 may be involved in the transport of secondary metabolites, the detoxification of xenobiotics, or the export of toxic cations. Real-time quantitative PCR demonstrated that the expression profile of the eight VcMATE genes varied spatially and temporally. Analysis of expression and anthocyanin accumulation indicated that there were some correlation between the expression profile and the accumulation of anthocyanins. These results showed VcMATEs might be involved in diverse physiological functions, and anthocyanins across the membranes might be mutually maintained by MATE-type flavonoid transporters and other mechanisms. This study will enrich the MATE-based transport mechanisms of secondary metabolite, and provide a new biotechonology strategy to develop better nutritional blueberry cultivars.
Introduction
Plants produce a large number of secondary metabolites that appear to have little function in their growth and development, but play important roles in reproduction and environmental adaptation. Secondary products are classified into three major groups: alkaloids, terpenoids, and phenolic compounds [1]. Flavonoids belong to a group of phenolic compounds and constitute one of the largest classes of secondary metabolites possessing a common three-ring chemical structure (C6–C3–C6). Predominant flavonoids forms include anthocyanins, proanthocyanidins (PAs, condensed tannins), flavonols, flavones, and flavanols. These compounds are widely distributed in different amounts according to the plant species, organ, developmental stage, and growth conditions [2]. Flavonols are thought to protect against UV-B irradiation in fruit skin [3] and function as an auxin transporter [4]. Anthocyanins have been demonstrated to give rise to the red, blue, and purple colors of many ripe fruits, vegetables, flowers, and other plant tissues or products [5], which attract frugivores and pollinators for seed dissemination and fertilization [6]. PAs in immature fruits help deter frugivores from consuming the fruit before they ripen due to their astringency and bitterness [7]. In addition, PAs are also thought to contribute to disease defense, stress resistance, and seed dormancy [8,9].
It is well known that flavonoids are synthesized along the general phenylpropanoid pathway by the activity of a cytosolic multienzyme complex that is loosely associated to the cytoplasmic face of the endoplasmic reticulum (ER) [10]. Because flavonoids with high chemical reactivity are toxic endogenous compounds, they must be removed expeditiously from the cytoplasm after synthesis and sequestered in the vacuole or cell wall. Additionally, it is also necessary for flavonoids to be sequestered in the proper compartment to prevent oxidation [11] and to perform its function as pigments. Accumulation within the appropriate compartments should be regulated in a highly sophisticated manner. Most of the structural genes and a number of regulatory genes related to the flavonoid pathway have been thoroughly described [12–14]. However, the mechanisms involved in the downstream steps of the pathway, such as transport and vacuolar accumulation, remain unclear.
In recent years, an increasing body of genetic, biochemical, and molecular biological evidence has implicated that multidrug and toxic compound extrusion (MATE) transporters are involved in flavonoid transport. The MATE family was originally discovered in prokaryotes and their function as a multiple drug efflux carrier was studied in detail. Thus far, putative MATE transporter sequences have been identified in the plant kingdoms, with some members reportedly involved in a much broader range of biological activities than previously thought (S1 Table), including protection of plant cells from inhibitory compounds [15], salicylic acid (SA)-dependent disease resistance signaling [16], aluminum tolerance [17,18], detoxification of heavy metals [19], maintenance of iron homoeostasis [20,21], alkaloids accumulation [22,23], plant development [24], and flavonoid vacuolar accumulation [25,26]. The connections between MATE transporter and flavonoid vacuolar accumulation have become topics of intensive research.
As a flavonoid/H+-antiporter, MATE transporters have a substrate preference for flavonoids with diverse chemical structures. The Arabidopsis gene TT12 acts as a transporter for the PA precursors epicatechin and catechin, while PA precursors undergo a glycosylation step prior to TT12-mediated transfer into seed coat vacuoles [2]. In vitro, TT12 exhibits an extended substrate specificity in vitro, accepting glycosylated anthocyanidin. Yeast vesicles expressing TT12 can transport anthocyanin-3-O-glucoside in the presence of MgATP, but not aglycones and epicatechin [27]. MATE1 from Medicago truncatula, an ortholog of TT12 expressed in yeast, preferentially transports the epicatechin 3’-O-glucoside, which has been proposed to be a precursor for PA biosynthesis [28,29]. Despite the high similarity between MATE1 and MATE2, the latter cannot efficiently transport PA precursors. In contrast, MATE2 shows higher transport capacity for anthocyanins and lower efficiency for other flavonoid glycosides [28,29]. MTP77 [30] and VvAM1/3 [25] are all anthocyanin transporters, but VvAM1/3 mediates acylated anthocyanin transport specifically rather than malvidin 3-O-glucoside or cyanidin 3-O-glucoside. However, plant MATEs have not been extensively analyzed to determine the relationship between their conformation, functional catalytic residues, and substrate preference.
Blueberry (Vaccinium corymbosum) is one of the most commercially significant berry crops. Blueberry fruit, which are rich in flavonoids, have received much attention due to their benefits to human health, including protection against liver injuries, significant blood pressure reduction, improved eyesight, strong anti-inflammatory and antimicrobial activities, inhibition of mutations caused by mutagens from cooked food, and suppression of proliferation of human cancer cells [31]. However, the existing researches on MATE-type flavonoid transporters were focused on model plants such as Arabidopsis, M. truncatula, and Vitis vinifera. Information regarding MATE transporters in blueberry has not been reported. A blueberry fruit transcriptome library allowed us to discover MATE transcripts as exhaustively as possible today. The objective of the present study was to clone MATE genes to provide basic data about VcMATE transporters and identify candidate genes from closely related species of the family Ericaceae. Moreover, the relationship between the expression specificity of VcMATEs and anthocyanin accumulation was investigated for predicting their physiological functions. This research on VcMATEs participating in flavonoid transport will fill a major gap in the complete picture of blueberry metabolic pathways, and also will provide us with better strategies for plant metabolic engineering of flavonoids to improve agronomic traits and the nutritional content of blueberries.
Materials and Methods
Plant materials
The Vaccinium spp. cultivar “Patriot” grown in the blueberry germplasm repository of Jilin Agricultural University was used for this study. Representative samples were taken from six stages: flower buds and leaf buds were collected at the germination stage (2012.5.5); flowers were collected at the full-bloom stage (2012.5.15); roots, stem, and young expanding leaves were all collected from vegetative organs during the rapid growth period (2012.6.1); fruits were collected at the green fruit period (2012.6.15), color turning period (2012.7.5), and fruit maturation period (2012.7.15). The separated exocarp, sarcocarp, and seed from the blueberry fruits were immediately frozen in liquid nitrogen and stored at −80°C.
Extraction of genomic DNA, total RNA, and cDNA synthesis
Genomic DNA was extracted from young blueberry leaves using the improved CTAB method [32]. Total RNA was isolated from 12 representative samples according to the method described in Jaakola, et al., 2001 [33]. The integrity of DNA and RNA was checked on a 1.2% agarose gel, and the purity was determined by spectrophotometry. RNA (1 μg) was used as a template for first-strand cDNA synthesis using a Superscript-II Reverse Transcriptase (TaKaRa) according to the manufacturer’s instructions.
cDNA cloning
Candidate MATE sequences were selected from the transcriptome database of the blueberry fruit. Based on Unigenes, specific primers were designed using the Primer Premier 5 software and are listed on S2 Table. The Genome Walking Kit (TaKaRa) was used to isolate the 5′-end of Unigene27, Unigene347, and Unigene17196, while the 5′-Full RACE Kit (TaKaRa) was used to isolate the 5′-end of Unigene34286 and Unigene34357. The 3′-end of Unigene34357 was obtained with a 3′-Full RACE Core Set Ver.2.0 Kit (TaKaRa). All manipulations were conducted according to the manufacturer's protocol. The amplified fragment was sequenced and compared with other plants to confirm whether it is a MATE homolog. The full-length MATE cDNA sequences were obtained by linking the 5′ fragment, Unigene, and 3′ fragment using CExpress software. In order to ensure that the amplified segments and Unigene were within the same sequence, eight pairs of specific primers were designed in the start codon and adjacent termination codon base on linking sequences to amplify full-length cDNAs (S3 Table). In the 20 μl standard rTaq (TaKaRa) PCR, 0.4 μl of first-strand total cDNA from the mature fruit was used as a template. The thermal cycling parameters were as follows: 94°C for 3 min; 35 cycles of 94°C for 1 min, (Tm-3)°C for 1 min, and 72°C for 2 min; followed by 72°C for 10 min. All target bands were recovered and cloned into the pMD18-T vector (TaKaRa) to transform E.coli TOP10 competent cells (TIANGEN). PCR-positive colonies were sequenced using the general primers, M13F and M13R.
Quantitative real-time PCR (qRT-PCR)
Gene-specific primers for qRT-PCR were designed by the Primer Express software and were listed in S4 Table. qRT-PCR was conducted with SYBR Premix Ex Tag (Tli RnaseH Plus) (TaKaRa) and 2 μl cDNA (diluted to 20X) as a template. Thermal cycling conditions were as follows: an initial enzyme activation of 30 s at 95°C, followed by 40 cycles of denaturation for 10 s at 95°C, annealing and extension for 20 s at 60°C, with a final melting gradient starting from 60°C and heating to 95°C for 15 s, 60°C for 1 min, and 95°C 15 s. The qRT-PCR reactions were conducted using a 7300 Fast Real Time PCR System (ABI). Primer specificity was confirmed by dissociation curves of the PCR amplification products. The mean Ct values were normalized against the reference, V. myrtillus glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NCBI accession number: AY123769.1). Since primer efficiencies were approximately equal, the expression was calculated by the 2–ΔCt method. To ensure the reliability of expression results, qRT-PCR was performed for all genes on three separate cDNA preparations for each organ and fruit developmental stage. For additional replication, all templates and standards were run in triplicate.
Identification of total anthocyanins
Total anthocyanins were identified according to the pH differential method [34]. Two dilutions of the crude blueberry extract were prepared for each sample, one with potassium chloride buffer (0.025M, pH 1.0) and the other with sodium acetate buffer (0.4 M, pH 4.5) using a predefined dilution factor. After equilibrating at room temperature for 1 h, the absorbance at 520 and 700 nm was measured by spectrophotometry. Anthocyanins content was calculated using a molar extinction coefficient for cyandin-3-glucoside of 26,900 and a molecular weight of 449.2. The quantity of anthocyanins on a fresh weight basis was expressed as mg/100g fresh weight. Cyanidin-3-glucoside was selected since it is the most common anthocyanin pigment in nature [35].
Statistical analysis
To determine the relationship between the VcMATE expression level and total anthocyanin accumulation during the fruit ripening process as well as in different organs, the coefficient of determination (R2) was calculated with the SPSS Statistics 17.0 software. A p-value of 0.05 was considered statistically significant.
In silico sequence analysis
Molecular weight and theoretical pI were predicted using the ProtParam tool (http://web.expasy.org/protparam/). Secondary structures of the eight blueberry MATE proteins were predicted by SOPMA (http://npsa-pbil.ibcp.fr/). Topological analysis for transmembrane regions was conducted using the HMMTOP program (http://www.enzim.Hu/hmmtop/index.html.). The PSORT program (http://psort.hgc.jp/form.html) was used for subcellular localization analysis. Signal peptides were predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/). Multiple sequence alignment of amino acid sequences was performed with Clustalx1.83. Formatting of aligned sequences was accomplished using the GENEDOC program. The nonrooted neighbor-joining tree was generated with the MEGA 5.0 program.
Accession numbers
Protein sequences in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases with the following accession numbers: M. truncatula MATE1, ACX37118; MATE2, HM856605. A. thaliana MATE transporters TT12, NP_191462; FFT, BAE98568; FRD3, NP_001154595; ALF5, AAK21273; EDS5, AAL27003; DXT1, NP_178496 and ZRIZI,NP_564731. Solanum lycopersicum (tomato) MTP77, AAQ55183. Nicotiana tabacum (tobacco) JAT1, CAQ51477; Nt MATE1, BAF47751; Nt MATE2, BAF47752. Hordeum vulgare (barley) Hv ACCT1, BAF75822. Sorghum bicolor (sorghum) MATE transporter Sb MATE1, ABS89149; V. vinifera MATE transporters AM1 and AM3, XP_002282932 and CAO69962, respectively.
Results
Identification and cloning of full-length VcMATE cDNA sequences
Because of the small number of available ESTs and lack of genomic information, identification of candidate MATE genes from blueberry is far behind that of other model plants. Recently, the complete of blueberry fruit transcriptome was sequenced using the Illumina RNA-Seq method, thus providing an opportunity for identification of candidate MATE transporters related to secondary metabolism. 64,312,746 raw reads longer than 75 bp were generated and made available in the Sequence Read Archive at the NCBI (http://www.ncbi.nlm.nih.gov/Traces/sra/sra.cgi) with accession number SRA046311. These relatively short reads can be effectively assembled, and a total of 34,464 All-unigenes with an average length of 735 bp were generated by de novo assembly. 8,193 differentially expressed genes (DEGs) between the exocarp and sarcocarp were categorized by molecular function, among which 516 DEGs were shown to be associated with transporter activity. Thirty-three available All-unigenes (6.4%) identified sequences encoding proteins with a significant similarity to MATE. Table 1 lists the blueberry MATE Unigenes identified in the blueberry fruit transcriptome database. Of these, 13 and 20 Unigenes were up-regulated in sarcocarp and exocarp, respectively. Among the up-regulated sarcocarp Unigenes, using TARAAPE rawreads >700, TARAAPE RPKM >10, and log2(TB/TA)>1 as a threshold, two sequences (Unigene27 and Unigene7401) were selected for cloning full-length cDNA sequences of VcMATE. Of the up-regulated exocarp Unigenes, using TBRAAPE raw reads >700, TBRAAPE RPKM >10, and |log2(TB/TA)|>1 as a threshold, six sequences (containing Unigene347, Unigene2915, Unigene17196, Unigene30398, Unigene34286, and Unigene34357) were selected. When the eight Unigenes were compared by Blaxtn to Genbank data, we found that Unigene2915, Unigene7401, and Unigene30398 covered the complete coding region, while the remaining Unigenes were missing sequence from the 3'-end, 5'-end, or both. Then, the corresponding full-length cDNA sequences of the five partial sequences were obtained by Genome walking and RACE technology. The eight full-length sequences of blueberry MATE transporters identified in this study were named VcMATE1, VcMATE2, VcMATE3, VcMATE4, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 as shown in Table 2, which also lists the accession number of their complete cDNA and the closest corresponding Arabidopsis genes.
Table 1. Unigenes with a significant similarity to MATE in the Illumina/Solexa sequencing library of blueberry.
| Unigene | Nr-ID | Gene length | TARAAPE rawreads | TBRAAPE rawreads | TARAAPE RPKM | TBRAAPE RPKM | log2(TB/TA) | Up/Down |
|---|---|---|---|---|---|---|---|---|
| Unigene27_All | NP_200058.1 | 1462 | 1573 | 853 | 71.9099 | 31.7664 | −1.1787 | Down |
| Unigene 347_All | ADO22709.1 | 1496 | 873 | 3212 | 39.0023 | 116.8988 | 1.5836 | Up |
| Unigene 1047_All | NP_194643.1 | 681 | 606 | 226 | 59.4748 | 18.0687 | −1.7188 | Down |
| Unigene 2475_All | NP_194643.1 | 388 | 571 | 196 | 98.3584 | 27.5037 | −1.8384 | Down |
| Unigene 2915_All | AAD46034.1 | 1878 | 95 | 1450 | 3.3809 | 42.0377 | 3.6362 | Up |
| Unigene 4510_All | AAK53040.1 | 592 | 226 | 99 | 25.5149 | 9.105 | −1.4866 | Down |
| Unigene 4079_All | ACC60274.1 | 2199 | 1677 | 1720 | 50.97 | 42.5862 | −0.2593 | Down |
| Unigene 4546_All | ADO22709.1 | 510 | 58 | 149 | 7.6009 | 15.9068 | 1.0654 | Up |
| Unigene 5999_All | NP_001057687.1 | 660 | 327 | 126 | 33.114 | 10.3942 | −1.6717 | Down |
| Unigene 7401_All | XP_002890090.1 | 1819 | 773 | 399 | 28.4023 | 11.9428 | −1.2499 | Down |
| Unigene 10025_All | NP_194034.1 | 212 | 138 | 61 | 43.5061 | 15.6661 | −1.4736 | Down |
| Unigene 13574_All | AAK53040.1 | 1136 | 608 | 263 | 35.7711 | 12.605 | −1.5048 | Down |
| Unigene 14339_All | ADO22709.1 | 389 | 48 | 122 | 8.2471 | 17.0756 | 1.05 | Up |
| Unigene 16852_All | NP_177270.1 | 910 | 1809 | 2027 | 132.8631 | 121.277 | −0.1316 | Down |
| Unigene 17196_All | NP_194294.2 | 1780 | 280 | 1267 | 10.5134 | 38.7545 | 1.8821 | Up |
| Unigene 17373_All | NP_001043893.1 | 227 | 230 | 308 | 67.7188 | 73.8739 | 0.1255 | Up |
| Unigene 19867_All | NP_200058.1 | 587 | 69 | 73 | 7.8563 | 6.71 | −0.2145 | Down |
| Unigene 20486_All | NP_001043893.1 | 227 | 41 | 119 | 12.0716 | 28.5422 | 1.2415 | Up |
| Unigene 23136_All | XP_002278724.1 | 372 | 25 | 7 | 4.4916 | 1.0245 | −2.1323 | Down |
| Unigene 25244_All | NP_194643.1 | 270 | 80 | 18 | 19.8031 | 3.6297 | −2.4478 | Down |
| Unigene 28730_All | AAK53040.1 | 368 | 0 | 41 | 0 | 6.066 | 12.5665 | Up |
| Unigene 28970_All | NP_566730.1 | 338 | 1 | 40 | 0.1977 | 6.4433 | 5.0264 | Up |
| Unigene 29667_All | BAF47751.1 | 437 | 0 | 57 | 0 | 7.1017 | 12.7939 | Up |
| Unigene 29812_All | XP_002278724.1 | 540 | 4 | 51 | 0.4951 | 5.1421 | 3.3766 | Up |
| Unigene 30026_All | NP_189291.1 | 1507 | 38 | 743 | 1.6853 | 12.3922 | 2.8784 | Up |
| Unigene 30398_All | NP_187012.2 | 1689 | 1 | 959 | 0.0396 | 30.914 | 9.6085 | Up |
| Unigene 31039_All | BAF47752.1 | 201 | 9 | 150 | 2.9926 | 40.6314 | 3.7631 | Up |
| Unigene 31994_All | XP_002278724.1 | 227 | 2 | 36 | 0.5889 | 8.6346 | 3.874 | Up |
| Unigene 32737_All | AAK53040.1 | 261 | 1 | 0.2561 | 19 | 3.9635 | 3.952 | Up |
| Unigene 33021_All | ADO22711.1 | 282 | 18 | 28 | 0.5889 | 8.6346 | 3.874 | Up |
| Unigene 33638_All | ABA96342.2 | 342 | 0 | 19 | 0 | 3.0248 | 11.5626 | Up |
| Unigene 34286_All | NP_187012.2 | 642 | 38 | 3144 | 3.956 | 266.6328 | 6.0747 | Up |
| Unigene 34357_All | BAF47752.1 | 786 | 34 | 2005 | 2.8911 | 138.8858 | 5.5861 | Up |
Note:TARAAPE and TBRAAPE rawreads represent the gene abundance of the sarcocarp and exocarp, respectively, TARAAPE RPKM (TA) and TBRAAPE RPKM (TB) is used for the expression level of sarcocarp and exocarp, respectively.
Table 2. Putative MATE transporters from blueberry and their closest Arabidopsis homologs.
| Unigene | Gene | Accession number | Arabidopsis closest homolog | Arabidopsis AGI gene |
|---|---|---|---|---|
| Unigene27 | VcMATE1 | KF875433 | AtDTX16 | AT5g52450 |
| Unigene347 | VcMATE2 | KF831422 | AtDTX41/AtTT12 | AT3g59030 |
| Unigene2915 | VcMATE3 | KF875434 | AtDTX33 | AT1g47530 |
| Unigene7401 | VcMATE4 | KF875435 | AtDTX12 | AT1g15170 |
| Unigene17196 | VcMATE5 | KF875436 | AtDTX34 | AT4g00350 |
| Unigene30398 | VcMATE7 | KF875438 | AtDTX24 | AT3g03620 |
| Unigene34286 | VcMATE8 | KF875439 | AtDTX24 | AT3g03620 |
| Unigene34357 | VcMATE9 | KF875440 | AtDTX40 | AT3g21690 |
Analysis of deduced MATE proteins in blueberry
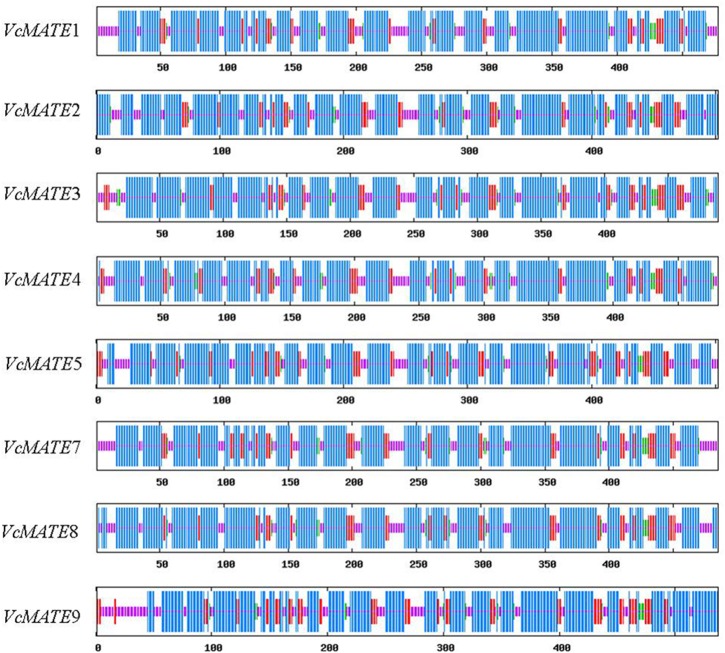
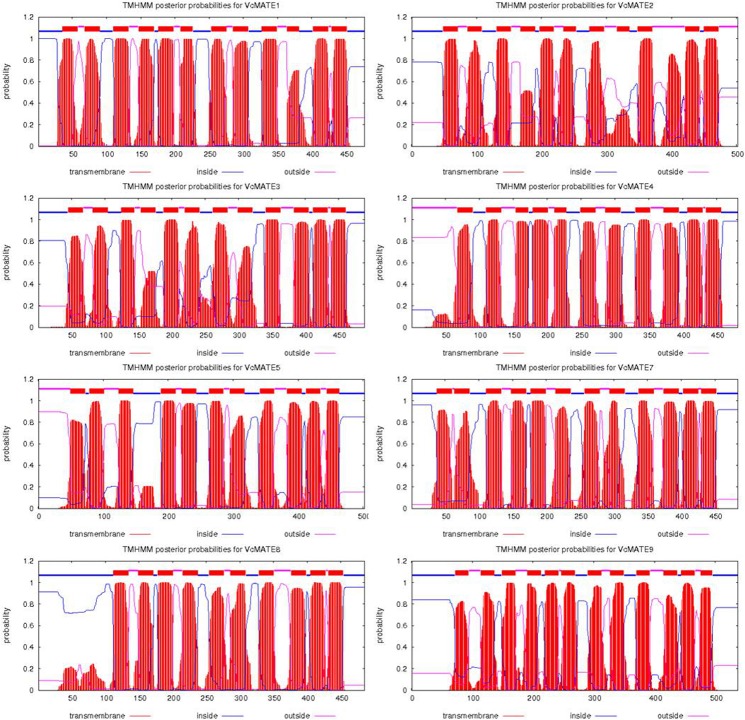
As presented in Table 3, VcMATE cDNA encoded proteins are composed of 477–537 residues, with predicted molecular masses around 54 kDa, theoretical isoelectric points from 4.89 to 8.41, aliphatic indices between 113.88 and 126.29, and grand average of hydropathicity around 0.7. All deduced VcMATE proteins were predicted as stable because their instability index is smaller than 40. When analyzed by SOPMA, the secondary structures of blueberry MATEs were predicted to be mainly composed of alpha helices (60.34–68.13%) interspersed with random coils (18.13–22.35%), extended strands (10.36–14.53%), and beta turns (2.39–4.09%) (Fig. 1). For each protein, there were more than 15 distinct alpha helices dispersed along the length of the protein. The prediction of transmembrane domains using HMMTOP suggested that there were 10–12 putative transmembrane (TM) segments in VcMATEs (Fig. 2). Prediction of the subcellular localization of VcMATEs was performed using the PSORT program. The results showed that the eight transporters were all localized to the plasma membrane (Table 3). Proteins are usually guided to their target membranes by signal peptides, but there were no signal peptides in the N-terminus according to SignalP. It is possible that signal peptides do not have strictly conserved amino acid sequences and are thus sometimes difficult to identify.
Table 3. Characteristics of the VcMATE cDNA deduced proteins.
| Number of amino acids | Formula | Molecular weight (kDa) | Theoretical pI | Instability index | Aliphatic index | Grand average of hydropathicity,GRAVY | Predicted protein localization | |
|---|---|---|---|---|---|---|---|---|
| VcMATE1 | 477 | C2362H3731N593O630S35 | 51.64 | 8.41 | 25.72 | 113.88 | 0.675 | plasma membrane |
| VcMATE2 | 502 | C2539H3961N603O675S25 | 54.54 | 5.57 | 28.05 | 126.29 | 0.842 | plasma membrane |
| VcMATE3 | 489 | C2418H3785N587O648S25 | 52.25 | 5.35 | 29.70 | 119.86 | 0.820 | plasma membrane |
| VcMATE4 | 481 | C2377H3768N586O650S30 | 51.92 | 6.87 | 29.00 | 116.96 | 0.701 | plasma membrane |
| VcMATE5 | 502 | C2527H3930N608O677S24 | 54.43 | 5.70 | 25.87 | 121.95 | 0.759 | Plasma membrane |
| VcMATE7 | 484 | C2472H3849N593O647S26 | 53.06 | 6.00 | 38.61 | 124.11 | 0.791 | plasma membrane |
| VcMATE8 | 485 | C2500H3875N589O662S23 | 53.48 | 4.89 | 33.05 | 125.67 | 0.786 | plasma membrane |
Fig 1. Secondary structures of the 8 blueberry MATE proteins predicted by SOPMA.
The α-helix, extended strand, β-turn, and random coil are denoted as the longest (blue), medium long (dark red), short (green), and the shortest (pink) vertical bars, respectively.
Fig 2. Topological analysis of VcMATEs.
The membrane-spanning domains of VcMATEs were determined using the TMHMM2 program.
VcMATE multiple sequence comparison
Multiple sequence alignment was conducted between the eight deduced VcMATE amino acid sequences and some representative MATE-type flavonoid transporters in other plant species (Fig. 3). The eight VcMATEs share 32.1–84.4% amino acid sequence identity amongst each other. The most striking finding in the sequence analysis was that VcMATE2 was more similar to the MATE-type flavonoid transporters, the V. vinifera MATE transporters AM1 (75.4% identity), the Arabidopsis TT12 (70.8% identity), Medicago MATE1 (70.4% identity) and MATE2 (40.8% identity), and tomato MTP77 (40.6% identity), than other VcMATEs. At less than 40%, VcMATE1 and VcMATE4 shared the lowest identity with known MATE-type flavonoid transporters. Five short stretches containing conserved amino acids in all 56 Arabidopsis MATE proteins also appeared to be particularly conserved in blueberry, which are highlighted by bars below the alignment and noted as D1-D5. The amino acid residue E290, which is critical to the proper functioning of AtTT12 [36], is also conserved in all VcMATEs except VcMATE7. Moreover, only VcMATE2 contains all the TT12-specific residues, such as KL(46–47), W50, A61, Q116, V118, IC132–133 Q173, R177, C190, N200, N203, G297, YYLNWDMQFMLGLS (319–332), F408, I413, KTS (449–451), A456, W460, and V466 in Arabidopsis [37].
Fig 3. Multiple sequence alignment of deduced amino acid sequences of VcMATEs with selected MATE transporter orthologs.
Protein sequence alignment was performed using Clustalx. Formatting of aligned sequences was accomplished with the box shade program. Amino acids that are identical in all 8 proteins are highlighted in black and conservative substitutions are highlighted in gray. Alignment of all 56 Arabidopsis MATE proteins resulted in five short stretches containing conserved amino acids, which are highlighted by thin lines above the alignment and noted as D1–5. The residue E290 (TT12), constituting the cation-binding site in the pore, is highlighted by a red box.
Phylogenetic analysis of VcMATEs
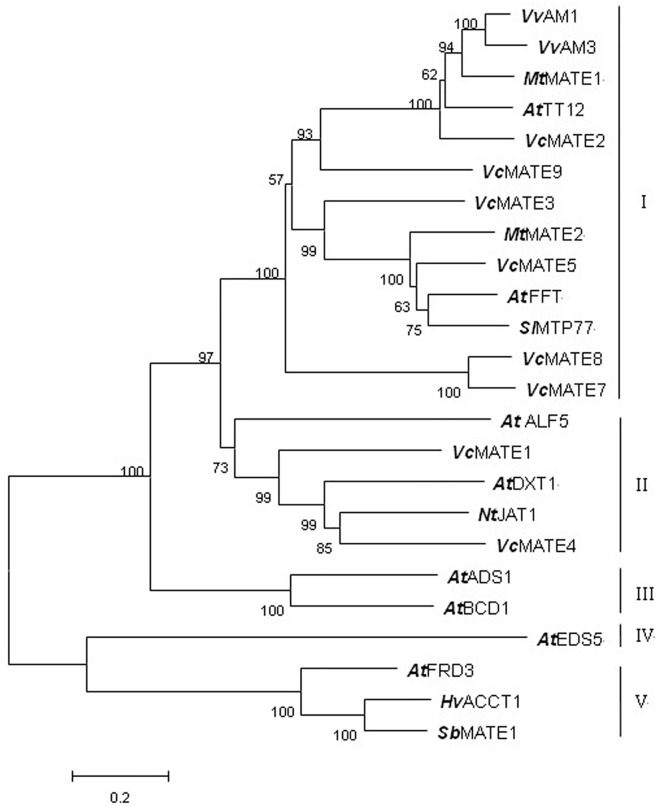
To further predict and distinguish the function of eight VcMATEs, phylogenetic analysis was performed between 18 known plant MATE protein sequences of different types and the putative VcMATEs. Phylogenetic analysis of these proteins revealed the presence of five distinct clusters (clusters I, II, III, IV, and V in Fig. 4), implying that the five clusters might differ functionally in some respect. The first clade contained VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, VcMATE9, and some known MATE-type flavonoid transporters. In addition, we found three subclades within the first clade: MtMATE1, AtTT12, VvAM1/3, and VcMATE2 were grouped into one subclade; VcMATE3 and VcMATE5 together with MtMATE2, AtFFT, and Ml MTP77 formed another subclade; while VcMATE7 and VcMATE8 were in a separate subclade. VcMATE1, VcMATE4, Arabidopsis toxin exporter DTX1, and ALF5, together with nicotine transporter JAT1 were grouped into the second clade. ADS1, which constitutively accumulates reactive oxygen intermediates (ROIs) to negatively regulate plant disease resistance, and BCD1, which enhances organ initiation, were grouped together in the third clade. The salicylic acid (SA) transporter EDS5 formed an individual clade. Three plasma membrane-localized citrate exporters, SbMATE from sorghum, HvAACT1 from barley, and FRD3 from Arabidopsis, were grouped into the last clade, which was much further removed from the eight VcMATE transporters. Transporter substrate specificity typically correlates with phylogeny, and hence such analyses provide a credible foundation for making functional predictions. This phylogenetic analysis suggested that VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 might function as flavonoid transporters to participate in the transport of anthocyanins, PAs, or flavonols, while VcMATE1 and VcMATE4 might be involved in the detoxification of xenobiotics or export of toxic cations.
Fig 4. Phylogenetic tree of MATE transporters.
Protein sequences of the characterized MATE transporters from Arabidopsis, TT12, FFT, ALF5, DXT1, ADS1, ZRIZI, EDS5, and FRD3; M. truncatula MATE1 and MATE2; sorghum Sb MATE1; grapevine AM1 and AM3; tomato MTP77; barley Hv AACT1; as well as tobacco JAT1 were aligned with Clustalx, and the nonrooted neighbor-joining tree was generated using the MEGA 5.0 program. Numbers at branch points indicate bootstrap support. The numbers above and below branches indicate bootstrap support percentages based on 1000 replicates.
Accumulation of anthocyanins in different organs and fruit developmental stages
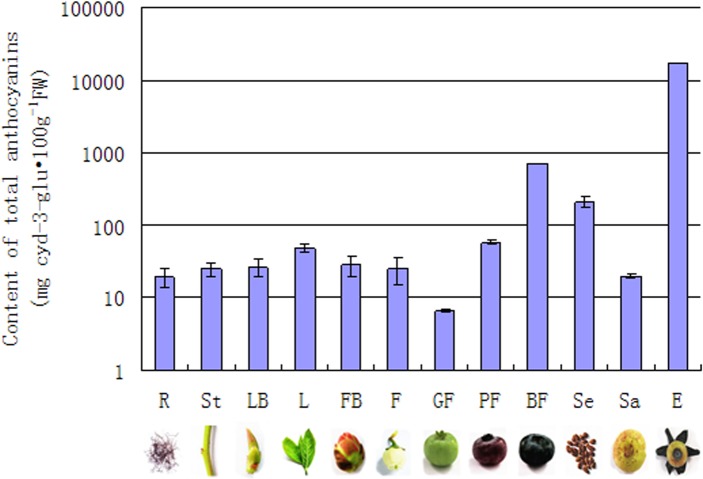
Blueberries are well known for their nutritional and beneficial health effects owing to their high anthocyanins content. The concentration of anthocyanins in different organs and fruit developmental stage was determined using a pH differential method. The result showed that anthocyanins were weekly detected in the vegetative organs. In fruit development, exocarp color will change from mostly green to partially pink, finally blue-purple. In the green fruit stages, anthocyanins were detected at low levels. In accordance with their deep coloration, the anthocyanins content increased, reaching the maximum in mature berries. Moreover, the anthocyanins content of exocarp in blue fruit was observed to be significantly higher than sarcocarp. Meanwhile, anthocyanins also accumulated in substantial quantities in seed. (Fig. 5)
Fig 5. Anthocyanin content in different organs and developmental stages of fruit.
The following 12 samples were analyzed: R-roots, S-stem, FB-flora buds, F-flowers, LB-leaf buds, L-young leaves, GF-green fruit, PF-pink fruit, BF-blue fruit, E-exocarp, Sa-sarcocarp, and Se-seed. Values represent the average ± SD of three biological replicates.
Blueberry organ and fruit developmental profiling of VcMATE transcripts
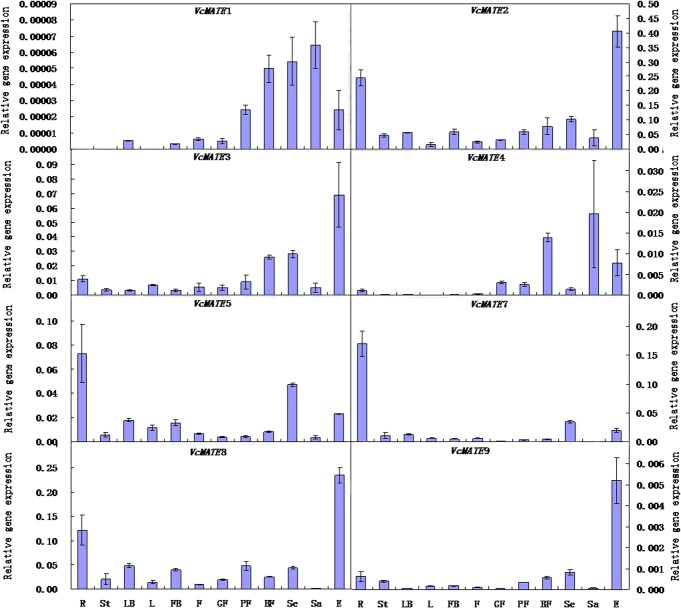
Anthocyanins content varied in the different organs, developmental stages of fruit, and parts of the mature fruit. This was reflected in the apparent concerted activation of anthocyanin biosynthesis gene expression. Next, we examined the expression pattern of VcMATE genes in accordance with anthocyanin accumulation. Quantitative real-time PCR was performed on eight MATE genes throughout fruit development and different organs (Fig. 6). The expression level of VcMATE2 was the highest among the eight genes. VcMATE2 transcript was found most strongly expressed in anthocyanin-rich exocarp, and followed by root. During fruit developmental stages, expression was low in green fruit stage and then increased after fruit coloring. The coefficient of determination (R2) between VcMATE2 expression and total anthocyanins accumulation was 0.852. VcMATE3, VcMATE8 and VcMATE9 followed a similar expression pattern with VcMATE2, and R2 values between the expression and anthocyanin accumulation were calculated as 0.913, 0.876 and 0.987, respectively. Contrast to VcMATE2, the transcript abundance of VcMATE8 in mature fruit was lower than pink fruit, while transcript abundance of VcMATE3 and VcMATE9 in root was not much higher than other organs. VcMATE5 and VcMATE7 were predominantly expressed in root. Moreover, both of VcMATE5 and VcMATE7 present at higher transcript abundance in seed than exocarp. Transcript level of VcMATE1 and VcMATE4 were much weaker than other genes. The relevance between expression and total anthocyanins accumulation was low, even negative correlation. Furthermore, no VcMATE1 transcripts were detected in root, steam, and leaf. There was another obvious difference between VcMATE1, VcMATE4 and other VcMATE genes, the highest expression site for the two genes was sarcocarp rather than exocarp. The expression level of sarcocarp and exocarp of all VcMATE genes obtained by qRT-PCR analysis were consistent with the expression found by in silico analysis. Gene expression patterns of MATE family genes varied in the different organs and fruit developmental stages, suggesting that different expression patterns reflect different functions. VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 may function as flavonoid transporters due to their relatively high expression level and correlation between expression profile and anthocyanin accumulation. VcMATE1 and VcMATE4 may be involved in other functions.
Fig 6. Relative gene expression levels of MATE-encoding genes in various blueberry organs and different developmental stages of fruit.
Total RNA was isolated from roots (R), stem(S), flora buds (FB), flowers (F), leaf buds (LB), young leaves (YL), green fruit (GF), pink fruit (PF), blue fruit (BF), exocarp (E), sarcocarp (Sa), and seed (Se). Gene expression was normalized with VmGAPDH. All data represent the mean of three replicates with error bars indicating SD.
Discussion
The MATE protein is an emerging member of the multidrug transporter superfamily that includes the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the resistance nodulation cell division (RND) family, and the ATP-binding cassette (ABC) family [38]. This family is widely distributed in all living organisms from prokaryotes to eukaryotes [39]. As a secondary active transporter, MATE is thought to couple transport of their target molecules across a membrane with an electrochemical gradient of H+ or Na+ ions, requiring the action of a plasma membrane P-type H+-ATPase, a vacuolar V-type H+-ATPase, or a vacuolar H+-pyrophosphatase [40]. In contrast to the relatively small number of MATE genes found in bacterial and animal species, this gene family has undergone a remarkable expansion in plants, with 58 MATEs in Arabidopsis, 57 in V. vinifera, 58 in Populus trichocarpa, and 38 in Zea mays and Brachypodium distachyon (ARAMEMNON plant membrane protein database). This finding highlights the importance of MATE proteins in this kingdom. The identification of genes specifying traits of interest through a candidate gene approach was hindered due to a lack of blueberry genomic information; however, the fruit transcriptome database makes this possible now. There were 33 Unigenes annotated as MATE efflux family proteins. In addition, eight differentially expressed VcMATEs between the exocarp and sarcocarp with higher gene abundance and expression level were obtained. We speculate that they may play a vital role in the detoxification of endogenous secondary metabolites and xenobiotics.
Structure of VcMATE transporter is related to its function
The length of proteins in the MATE family ranges from ~400 to ~700 amino acids, with most members consisting of 400–550 residues [41]. The yeast proteins are larger (up to about 700 residues), whereas the archaeal proteins are generally smaller. A large transporter size is characteristic of the eukaryotic domain, while a small size is characteristic of the archaeal domain [42]. The eight VcMATE transporters with 477–537 amino acids conform to the basic characteristics of MATE family members. MATE precursors were hypothesized to be complex carbohydrate exporters in prokaryotes with six TMs which underwent internal duplication to generate a 12 TM protein [43]. It is interesting to note that VcMATEs have 10–12 TMs predicted by the HMMTOP program rather than the usual 12 TMs that characterize the MATE family. MATE transporters possessing more or less than 12 TMs also have been reported in some species. For example, 8–13 TMs in the third cluster of genes was found in Arabidopsis MATE proteins [19], 9–11 in EDS5 [16], and 14 in the FRD3 protein [44]. The mammalian MATEs are generally predicted to have a 13th TM; however, TM13 has been confirmed to have little impact on ligand binding and the functional core of MATE1 consists of 12 (not 13) TMs [45]. Studies have shown that the few proteins possessing more than the usual 12 TM were derived from an internal gene duplication event, and that the “extra” regions are presumably nonessential for transport function [43]. However, other proteins have fewer than 12 TMs, possibly because the prediction of topology was performed using a different program with different computer methods. According to the TMHMM program, these data are only predictions based on computer analysis of protein sequences; therefore, they must be considered cautiously.
There were no consensus sequences to be conserved across all MATE members, which shared approximately 40% sequence similarity. However, we found that some TM domains were much more conserved than other sites, and some inter-TM linkers were remarkably conserved. Human MATE1 is a representative secondary structure for MATE-type transporters. Multiple sequence alignment has indicated that highly conserved regions are located in the vicinity of transmembrane helix 1 (TM1) and TM7, within the extracellular loops connecting TM1 with TM2 and TM7 with TM8, in cytoplasmic loops linking TM2 with TM3 and TM8 with TM9, and in loops connecting TM4 with TM5 and TM10 with TM11 [46]. Chai, et al. found two tandemly arranged MatE/NorM domains in plant TT12 proteins spanning from TM1 to TM5 and from TM7 to TM11 [37]. Conserved protein domains indicate they may be functional sites of MATE transporters. Quite possibly MATE proteins transport “cargo” via a pore maintained by the TM helices. It is necessary to maintain the secondary structure and topology of all the TM domains for normal MATE function, but conservation of some TMs and inter-TM linkers might be crucial for formation of the “pore” [37]. Alignment of all 56 Arabidopsis MATE proteins resulted in five short stretches (D1–D5) containing conserved amino acids, which also appear to be conserved in VcMATEs. Thus, we think that the five domains consisting of conserved amino acids may guide sequence-based identification of MATE transporters. Furthermore, E290, a conserved amino acid critical for transport and vacuolar targeting in Arabidopsis, was also present in VcMATEs. The E290 was hypothesized to exert a cation-binding function similar to E255 in NorM, which faces the internal cavity and is in proximity to the cavity-located cation [47]. However, until now, the structure-function relationships of plant MATEs has yet to be investigated. Thus, our results are in accordance with the hypothesis that E264 in VcMATE1, E285 in VcMATE2, E276 in VcMATE3, E267 in VcMATE4, E276 in VcMATE5, E265 in VcMATE8, and E308 in VcMATE9 may be involved in cation binding in VcMATEs.
Although MATE transporters are distributed widely across all kingdoms of living organisms, only model bacterial and human MATEs have been characterized in detail. An outward-facing conformation with two portals open to the outer leaflet of the membrane was reported in NorM from Vibrio cholerae [47]. Then, the structures of Pyrococcus furiosus reveal that a drug extrusion mechanism in which the protonation of Asp41 on the N-terminal lobe induces bending of TM1, which in turn collapses the N-lobe cavity, thereby extruding the substrate drug to the extracellular space [48]. In this study, we failed to predict information on the tertiary structure of the eight VcMATE proteins using the SWISS-MODEL because sequence identity was too low, implying that they might well have a unique tertiary structure distinct from that of the characterized NorM and Pyrococcus furiosus. However, all MATE proteins share similar transmembrane topologies. Further research is needed to determine whether flavonoid transporters have the opposite structure or a similar mechanism of action with efflux proteins.
Subcellular localization of MATE transporters
The subcellular localization of a transporter is crucial for its function. The plant MATE proteins characterized so far are localized to either the vacuolar membranes or plasma membranes, which are two primary sites for iron uptake and sequestration [49]. The substrate is transported out of the cells in exchange for the H+- influx when the transporter is localized to the plasma membrane. However, when the transporter is localized to the vacuolar membrane, it functions as an uptake transporter because the cytosolic pH (7.2–7.5) is higher than that of the vacuolar lumen, which is generally 5.5 [22]. Some MATE-type flavonoid transporters have been shown to localize in the tonoplast. It is necessary for most secondary metabolites to be delivered into vacuoles because most of them are toxic to the plant itself. Their vacuolar compartmentalization may improve the efficiency of their production and avoid harmful effects in the cells [50]. Unique among plant MATE transporters identified so far, ZRZ is localized to the membrane of a small organelle, possibly the mitochondria, suggesting that ZRZ may be involved in providing a complex network of communication about a leaf-borne signal that determines the rate of organ initiation [24]. Recently, BCD1 (previously named ZRZ) protein was observed to localize to the Golgi complex, suggesting that BCD1 is associated with excretion of excess iron, which would be produced in chlorotic cells under osmotic stress conditions and senescing leaf cells [51]. In this research, subcellular localization of VcMATEs has been predicted using the bioinformatics tool PSORT. The results revealed that all deduced proteins localize to the plasma membrane. However, VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 were phylogenetically related to the known flavonoid transporters MATE1, MATE2, TT12, AM1, and AM3, all of which appear to be localized to the vacuolar membrane on the basis of GFP fusion imaging. Our prediction about targeting information is different with vacuolar localization of MATE-type flavonoid transporter. This may result from some membrane transporters lacking discernible targeting information for bioinformatics tools to recognize. Therefore, the localization of VcMATEs requires further validation.
Functional diversity of VcMATE members
In recent years, plant MATEs have been genetically identified, characterized, and shown to have particular physiological functions. Transporter substrate specificity typically correlates with phylogeny. Hence such analyses provide a credible foundation for making functional predictions. In order to gain insight into the putative role played by VcMATE, phylogenetic analysis was studied between VcMATEs and other known MATEs. Clustering analysis showed VcMATE2, VcMATE3, VcMATE5, VcMATE7, VcMATE8, and VcMATE9 were clustered together with some MATE-type flavonoid transporters. Although VvAM1/3, MtMATE1/2, AtFFT, MlMTP77, and AtTT12 belong to MATE-type flavonoid transporter, no common substrate has been reported to the best of our knowledge. Generally, flavonoids are transported in the form of glycosides. MtMATE1 is a functional ortholog of AtTT12, and both of them transport E3'G with higher affinity and velocity than Cy3G [28,29]. Anthocyanins depositing in vacuoles are largely present in acylated forms. VvAM1/3, MtMATE2, and MlMTP77 are anthocyanin transporters, with VvAM1/3 involved specifically in transporting p-coumaroyl-acylated anthocyanidin glucosides (Gomez et al., 2009), while MtMATE2 specifically transports malonylated flavonoids. Two major types of acylation, aromatic acylation (such as addition of a p-coumaroyl group) and aliphatic acylation (such as addition of a malonyl group), have been suggested to have different physiological functions. Aromatic acylation enhances the color of anthocyanins, whereas aliphatic acylation (usually malonylation) may stabilize flavonoids and increase the resistance of flavonoid glucoside malonates to enzymatic degradation [52,53]. It is important to note that flavonoid composition is different in each plant species, such that the transport activity of the VcMATE transporters could be determined by transport studies. VcMATE1 and VcMATE4 clustered with some multidrug efflux transporters in another group. AtALF5 is expressed strongly in the root epidermis, and loss of ALF5 function results in increased sensitivity of the root to several compounds, including a contaminant of commercial agar [15]. AtDTX1 shows relatively broad substrate specificity, and confers norfloxacin, berberine, and cadmium tolerance when expressed in Escherichia coli [19]. Nt-JAT1 plays an important role in nicotine translocation, and is responsible for unloading of alkaloids in the aerial parts and deposition in the vacuoles [22,23]. Thus, we conclude that VcMATE1 and VcMATE4 may be involved in transport of alkaloids and detoxification of xenobiotics or toxic cations. It is interesting to find members in the same or different cluster that play the same or opposite role. Although the eight VcMATEs were not present in the other three clusters, we cannot exclude the possibility of other functions for other VcMATE transporters.
Expression patterns response to physiological processes
The eight VcMATE genes investigated in this study have different spatial and temporal expression patterns, possibly because of differences in transport substrates or the complex and widespread accumulation of (iso)flavonoid compounds in blueberry. VcMATE5 and VcMATE7 exhibit similar expression patterns with MtMATE2, which is strongly expressed in roots [28,29]. Malonylated flavonoid glycosides are abundant in root, and MtMATE2 preferentially transports malonylated flavonoids. Meanwhile, the two genes also appear to be highly expressed in seed of mature fruit. This is similar to AtTT12 and MtMATE1, which are transcribed mainly during the early stages of silique and young pod development after fertilization, especially in developing seeds [15]. The PAs found in the seed coat consist essentially of epicatechin units [54,55], and AtTT12 and MtMATE1 facilitate vacuolar uptake of epicatechin 3′-O-glucoside for PAs biosynthesis in Arabidopsis and Medicago. Expression levels of both genes did not increase with gradual deepening of the exocarp color until fruit maturation, possibly because PAs synthesis occurred primarily early in fruit development. There is competition between PAs and anthocyanin biosynthesis. Anthocyanin synthesis takes place rapidly following ripening initiation, primarily in the exocarp, which is likewise reflected in a concerted activation of anthocyanin transport gene expression. Correlation between transcript abundance of VcMATE2, VcMATE3, VcMATE8, and VcMATE9 with anthocyanin accumulation suggests that the four VcMATE gene products may be responsible for anthocyanidins vacuolar accumulation in the blueberry fruit. The expression patterns of VcMATE2, VcMATE3, VcMATE8, and VcMATE9 in blueberry fruit, which were also similar to that of VvAM1/3, parallels the expression patterns of five previously identified key enzyme-encoding genes associated with anthocyanin biosynthetic genes in blueberry, including Unigene938 (phenylalanine ammonia-lyase, PAL), Unigene23486 (chalcone synthase, CHS), Unigene7743 (flavonoid 3-hydroxylase, F3H), Unigene8103 (dihydroflavonol 4-reductase, DFR), and Unigene26381 (anthocyanidin synthase, ANS) [56]. VcMATE1 and VcMATE4 not only had the weakest transcript level, but also the transcript patterns were different from the other six VcMATE genes. What's more, they were most strongly expressed in sarcocarp instead of exocarp, root, or seed, suggesting that they may be involved in other physiological processes.
The flavonoids are products of phenylpropanoid metabolism constituting more than 10,000 structural variants known [57]. Anthocyanins constitute a major flavonoid group. After synthesis on ER, anthocyanins are then subjected to various modifications, such as methylation, acylation, hydroxylation, and glycosylation, yielding a wide variety of derivatives. These metabolites across the membranes in blueberry may be maintained by diverse mechanisms. Another secondary active transporter, bilirubin transporter bilitranslocase (BTL), primary transporter ATP-binding cassette (ABC) transporters, different forms of anthocyanin pigment-containing bodies the in vesicular transport (VT) model, and glutathione S-transferases (GSTs) supporting the ligandin transporter (LT) model, have all been demonstrated to be responsible for flavonoids vacuolar deposition in some plants. Thus, MATE transporters represent one route from the site of flavonoid biosynthesis towards the vacuole and other subcellular compartments in blueberry. Different mechanisms may cooperate with each other to form a highly sophisticated transport network. Therefore, no perfect correlation between expression pattern of VcMATE gene and accumulation of total anthocyanins was presented in this paper. It is interesting to clarify the relationship between preferred substrates and transport mechanism. In conclusion, the present study have set a foundation for further studies on functions of VcMATE genes, and may provide guidance for selection of target genes to improve agronomic traits and fruit quality of blueberry.
Supporting Information
Modified from Yazaki et al, 2008. *TT12-transparent testa; ALF5-aberrant lateral root formation; EDS5–enhanced disease susceptibility; FRD3–ferric reductase defective; FFT–flower flavonoid transporter; ADS1-Activated Disease Susceptibility; JAT1-jasmonate-inducible alkaloid transporter 1; FRDL1-FRD3 like 1; BCD1-bush and chlorotic dwarf n.d. not determined, PM-plasma membrane, Vac-vacuole, TMA-tetramethylammonium, PVP-polyvinylpyrrolidone, EtBr—ethidium bromide.
(XLS)
(XLS)
(XLS)
(XLS)
Acknowledgments
We thank Prof. Haiyan Li and Dr. Fawei Wang (Ministry of Education, Engineering Research Center of Bioreactor and Pharmaceutical Development, Jilin Agricultural University) for providing help in the experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 31301755), the Special Fund for Agro-scientific Research in the Public Interest of China (grant no. 201103037), the Doctoral Program Foundation of Institutions of Higher Education of China (grant no. 2013223120004), the Preferred Foundation of Scientific Research for Returned Overseas Chinese Scholar, the state human resource ministry of china, and the Breeding Foundation of Jilin Province Department of Finance (2012004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Hadacek F (2002) Secondary metabolites as plant traits: current assessment and future perspectives. Critical Reviews in Plant Sciences 21: 273–322. [Google Scholar]
- 2. Debeaujon I, Peeters AJ, Leon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13: 853–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solovchenko A, Schmitz-Eiberger M (2003) Significance of skin flavonoids for UV-B-protection in apple fruits. J Exp Bot 54: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 4. Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, et al. (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kocic B, Filipovic S, Nikolic M, Petrovic B (2011) Effects of anthocyanins and anthocyanin-rich extracts on the risk for cancers of the gastrointestinal tract. J BUON 16: 602–608. [PubMed] [Google Scholar]
- 6. Willson MF, Whelan CJ (1990) The evolution of fruit color in fleshy-fruited plants. American Naturalist: 790–809. [Google Scholar]
- 7. Sanoner P, Guyot S, Marnet N, Molle D, Drilleau JP (1999) Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J Agric Food Chem 47: 4847–4853. [DOI] [PubMed] [Google Scholar]
- 8. Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters DJ, Constabel CP (2002) Molecular analysis of herbivore-induced condensed tannin synthesis: cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J 32: 701–712. [DOI] [PubMed] [Google Scholar]
- 10. Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, et al. (2013) Plant flavonoids—biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14: 14950–14973. 10.3390/ijms140714950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrs KA, Alfenito MR, Lloyd AM, Walbot V (1995) A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 375: 397–400. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka Y, Brugliera F, Kalc G, Senior M, Dyson B, Nakamura N, et al. (2010) Flower color modification by engineering of the flavonoid biosynthetic pathway: practical perspectives. Biosci Biotechnol Biochem 74: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 13. Martens S, Preuss A, Matern U (2010) Multifunctional flavonoid dioxygenases: flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 71: 1040–1049. 10.1016/j.phytochem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 14. Broun P (2005) Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol 8: 272–279. [DOI] [PubMed] [Google Scholar]
- 15. Diener AC, Gaxiola RA, Fink GR (2001) Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13: 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nawrath C, Heck S, Parinthawong N, Métraux J-P (2002) EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. The Plant Cell Online 14: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magalhaes JV, Liu J, Guimaraes CT, Lana UG, Alves VM, Wang YH, et al. (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57: 389–399. 10.1111/j.1365-313X.2008.03696.x [DOI] [PubMed] [Google Scholar]
- 19. Li L, He Z, Pandey GK, Tsuchiya T, Luan S (2002) Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277: 5360–5368. [DOI] [PubMed] [Google Scholar]
- 20. Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers EE, Guerinot ML (2002) FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morita M, Shitan N, Sawada K, Van Montagu MC, Inze D, Rischer H, et al. (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci U S A 106: 2447–2452. 10.1073/pnas.0812512106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shoji T, Inai K, Yazaki Y, Sato Y, Takase H, Shitan N, et al. (2009) Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149: 708–718. 10.1104/pp.108.132811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burko Y, Geva Y, Refael-Cohen A, Shleizer-Burko S, Shani E, Berger Y, et al. (2011) From organelle to organ: ZRIZI MATE-type transporter is an organelle transporter that enhances organ initiation. Plant and cell physiology 52: 518–527. 10.1093/pcp/pcr007 [DOI] [PubMed] [Google Scholar]
- 25. Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier-Level A, Verriés C, et al. (2009) Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150: 402–415. 10.1104/pp.109.135624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson EP, Wilkins C, Demidchik V, Davies JM, Glover BJ (2010) An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. Journal of experimental botany 61: 439–451. 10.1093/jxb/erp312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, et al. (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. The Plant Cell Online 19: 2023–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao J, Dixon RA (2009) MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21: 2323–2340. 10.1105/tpc.109.067819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao J, Huhman D, Shadle G, He XZ, Sumner LW, Tang Y, et al. (2011) MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 23: 1536–1555. 10.1105/tpc.110.080804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N, et al. (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 15: 1689–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W (2004) Anthocyanins—More Than Nature's Colours. BioMed Research International 2004: 239–240.
- 32. Oakenfull RJ, Baxter R, Knight MR (2013) A C-repeat binding factor transcriptional activator (CBF/DREB1) from European bilberry (Vaccinium myrtillus) induces freezing tolerance when expressed in Arabidopsis thaliana. PLoS One 8: e54119 10.1371/journal.pone.0054119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaakola L, Pirttila AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19: 201–203. [DOI] [PubMed] [Google Scholar]
- 34. Lee J, Durst RW, Wrolstad RE (2005) Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC international 88: 1269–1278. [PubMed] [Google Scholar]
- 35. Francis FJ (1989) Food colorants: anthocyanins. Crit Rev Food Sci Nutr 28: 273–314. [DOI] [PubMed] [Google Scholar]
- 36.AI P (2011) Molecular characterization of the Arabidopsis TT12 MATE protein and functional analysis of other plant MATE transporters. Dissertation. University of Zurich.
- 37. Chai Y-R, Lei B, Huang H-L, Li J-N, Yin J-M, Tang ZL, et al. (2009) TRANSPARENT TESTA 12 genes from Brassica napus and parental species: cloning, evolution, and differential involvement in yellow seed trait. Molecular Genetics and Genomics 281: 109–123. 10.1007/s00438-008-0399-1 [DOI] [PubMed] [Google Scholar]
- 38. Brown MH, Paulsen IT, Skurray RA (1999) The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol 31: 394–395. [DOI] [PubMed] [Google Scholar]
- 39. Moriyama Y, Hiasa M, Matsumoto T, Omote H (2008) Multidrug and toxic compound extrusion (MATE)-type proteins as anchor transporters for the excretion of metabolic waste products and xenobiotics. Xenobiotica 38: 1107–1118. 10.1080/00498250701883753 [DOI] [PubMed] [Google Scholar]
- 40. Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581: 2204–2214. [DOI] [PubMed] [Google Scholar]
- 41. Yazaki K, Sugiyama A, Morita M, Shitan N (2008) Secondary transport as an efficient membrane transport mechanism for plant secondary metabolites. Phytochemistry Reviews 7: 513–524. [Google Scholar]
- 42. Chung YJ, Krueger C, Metzgar D, Saier MH Jr (2001) Size comparisons among integral membrane transport protein homologues in bacteria, archaea, and eucarya. Journal of bacteriology 183: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hvorup RN, Winnen B, Chang AB, Jiang Y, Zhou XF, Saier MH Jr, et al. (2003) The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) exporter superfamily. Eur J Biochem 270: 799–813. [DOI] [PubMed] [Google Scholar]
- 44. Green LS, Rogers EE (2004) FRD3 controls iron localization in Arabidopsis. Plant Physiol 136: 2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, He X, Baker J, Tama F, Chang G, Wright SH, et al. (2012) Twelve transmembrane helices form the functional core of mammalian MATE1 (multidrug and toxin extruder 1) protein. J Biol Chem 287: 27971–27982. 10.1074/jbc.M112.386979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y, et al. (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proceedings of the National Academy of Sciences of the United States of America 102: 17923–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang Q, et al. (2010) Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 467: 991–994. 10.1038/nature09408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka Y, Hipolito CJ, Maturana AD, Ito K, Kuroda T, Higuchi T, et al. (2013) Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 496: 247–251. 10.1038/nature12014 [DOI] [PubMed] [Google Scholar]
- 49. Jeong J, Guerinot ML (2009) Homing in on iron homeostasis in plants. Trends Plant Sci 14: 280–285. 10.1016/j.tplants.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 50. Roytrakul S, Verpoorte R (2007) Role of vacuolar transporter proteins in plant secondary metabolism: Catharanthus roseus cell culture. Phytochemistry Reviews 6: 383–396. [Google Scholar]
- 51. Seo PJ, Park J, Park MJ, Kim YS, Kim SG, Jung JH, et al. (2012) A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem J 442: 551–561. 10.1042/BJ20111311 [DOI] [PubMed] [Google Scholar]
- 52. Suzuki H, Nakayama T, Yonekura-Sakakibara K, Fukui Y, Nakamura N, Yamaguchi M, et al. (2002) cDNA cloning, heterologous expressions, and functional characterization of malonyl-coenzyme A: anthocyanidin 3-O-glucoside-6"-O-malonyltransferase from dahlia flowers. Plant Physiology 130: 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo J, Nishiyama Y, Fuell C, Taguchi G, Elliott K, Hill L, et al. (2007) Convergent evolution in the BAHD family of acyl transferases: identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. The Plant Journal 50: 678–695. [DOI] [PubMed] [Google Scholar]
- 54. Abrahams S, Tanner GJ, Larkin PJ, Ashton AR (2002) Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol 130: 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pang Y, Peel GJ, Wright E, Wang Z, Dixon RA (2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 145: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li X, Sun H, Pei J, Dong Y, Wang F, Chen H, et al. (2012) De novo sequencing and comparative analysis of the blueberry transcriptome to discover putative genes related to antioxidants. Gene 511: 54–61. 10.1016/j.gene.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 57. Tahara S (2007) A journey of twenty-five years through the ecological biochemistry of flavonoids. Bioscience, biotechnology, and biochemistry 71: 1387–1404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modified from Yazaki et al, 2008. *TT12-transparent testa; ALF5-aberrant lateral root formation; EDS5–enhanced disease susceptibility; FRD3–ferric reductase defective; FFT–flower flavonoid transporter; ADS1-Activated Disease Susceptibility; JAT1-jasmonate-inducible alkaloid transporter 1; FRDL1-FRD3 like 1; BCD1-bush and chlorotic dwarf n.d. not determined, PM-plasma membrane, Vac-vacuole, TMA-tetramethylammonium, PVP-polyvinylpyrrolidone, EtBr—ethidium bromide.
(XLS)
(XLS)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.