SUMMARY
Adenosine analogues substituted at N6 with spacer arms designed for attachment to soluble macromolecules or to solid supports for affinity chromatography are agonists at the A2–adenosine receptor that mediates coronary vasodilation in the dog. The most active analogues had spacer arms terminating in −NH2, −NHCH3 or in a biotin residue. Comparisons of coronary vasoactivity with affinity" for brain A1 adenosine receptors identified one biotin–containing analogue as relatively selective for coronary A2 receptors. The complex of this analogue with avidin retained coronary vasoactivity.
Two classes of receptors, linked to adenylate cyclase, mediate many of the biological actions of adenosine: Activation of A1 receptors inhibits the enzyme while activation of A2 receptors stimulates the enzyme. The ligand binding peptide of the brain A1 receptor has been identified by photoaffinity radiolabelling (1,2), but as yet neither receptor has been isolated. As a first step toward the purification of these receptors, we have applied the “functionalized congener” approach to the design of adenosine receptor ligands suitable for affinity chromatography. The essential feature of this approach is the regioselective incorporation of a spacer chain on the pharmacophore, followed by incorporation of various moieties through a terminal carboxylic acid or amine on the spacer chain. The chain consists of an N6 substituent on the adenosine molecule or a C-8 substituent on a derivatized xanthine molecule (3,4). The binding of such analogues to rat brain A1 receptors can be influenced by both the nature of the spacer arm and by the nature of the distal moiety.
The present study extends the functionalized congener approach to the A2–adenosine receptor. We have used in vivo stimulation of coronary vasodilation (5) to measure potency of several analogues at the A2 receptor. The comparison of this assay with previous estimates of potency of the same analogues at brain A1 receptors is used to measure selectivity for each type of receptor. Included in this study are analogues which have a spacer arm terminating in a biotin residue. The complex of one such biotin–containing analogue with avidin effectively stimulates coronary vasodilation, demonstrating the retention of biological activity when the pharmacophore is attached to a macromolecule.
MATERIALS AND METHODS
Adenosine analogues 1-5, avidin, and dimethyl sulfoxide are commercially available (Research Biochemicals, Inc., Wayland, MA; Sigma, St. Louis, MO). Analogues 6 and 7 (6), 8-18 (3) and 19 (4) were synthesized and characterized as described.
Previous reports describe in detail the assay of coronary vasoactivitv in the anesthetized, open-chest dog (6,7). Such an assay estimates an EC-50, the concentration of analogue which produces a half maximum change in coronary conductance (reciprocal resistance). To reduce between-animal variability activity has been expressed as a molar potency ratio (MPR) the value of the EC–50 of adenosine divided by that of the analogue. Owing to the low water solubility of some of the analogues, solutions for intracoronary infusion were prepared by diluting stock solutions in dimethyl sulfoxide. In such instances, solutions of adenosine used to establish a standard of potency also contained dimethyl sulfoxide.
Assays of the inhibition of [3H]N6-cyclohexyladenosine to rat brain A1 receptors by analogs 8-19 have been reported (3,4). Preparation of the complexes of analogue 18 or 19 with avidin for intracoronary infusion consisted of the dropwise addition of 1 mL of an 0.33 mM solution of 19 in dimethyl sulfoxide to a stirred solution of 20 mg (0.33 μmole) of avidin in 19 mL of 0.14 M NaCl. Because avidin consists of 4 subunits, each of which contains a biotin binding site, employing a 1:1 stoichiometry resulted in a fractional occupancy of 0.25. The avidin complexes were purified on columns of Sephadex G-10. The control infusate was a solution of 15 mg of avidin and 0.75 mL of dimethyl sulfoxide in 14 mL of 0.14 M NaCl.
RESULTS AND DISCUSSION
Table 1 summarizes the experimental observations and comparisons of potency at A1 and A2 adenosine receptors. The potency rank order of analogues 1 - 5 identifies the coronary adenosine receptor as an A2 receptor (6). The results with analogues 6 and 7 show that N6-phenyladenosines can exhibit substantial activity at this A2-receptor. Accordingly, functionalized analogues having spacer arms extending from an N6-phenyl group should be appropriate A2 receptor agonists.
Table 1.
Potency of adenosine analogues at a coronary A2-adenosine receptor and at a brain A1-receptor
| Analogue | A2 Receptor |
A1 Receptor | EC50(A2) |
|
|---|---|---|---|---|
| MPRa Relative to Ado |
Estimated Potencyb EC50(nM) |
Ki(nM)c | Ki(A1) | |
| 1 NECA | 150 | 8 | 5.1 | 1.6 |
| 2 2-chloroadenosine | 27 | 44 | 6.7 | 6.6 |
| 3N6-methyladenosine | 0.05 | 24,000 | 60 | 400 |
| 4 R-PIA | 4.3 | 280 | 1.2 | 230 |
| 5 CHA | 1.6 | 750 | 0.85 | 880 |
| 6 N6-phenyladenosine | 1.4 | 860 | 3.3 | 260 |
| 7 N6-p-tolyladenosine | 1.35 | 890 | 2.5 | 360 |
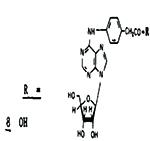
|
[27% @ 21μM]d | 210 | - | |
| 9 NHCH3 | 4.9±1.3 | 240 | 16 | 15 |
|
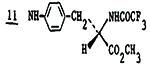
|
2.1±1.3 | 570 | 1.7 | 340 |
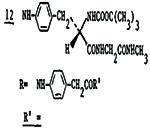
|
3.0±0.7 | 400 | 18 | 22 |
|
4.4±0.4 | 270 | 13 | 21 |
| 13 OCH3 | 3.2±1.6 | 380 | 2.5 | 150 |
| 14 NHCH3 | 7.2±1.3 | 170 | 6.7 | 25 |
| 15 NHNH2 | 3.5±0.8 | 340 | 4.5 | 80 |
| 16 NH(CH2)2NH2 | 7.8±2.5 | 150 | 0.85 | 180 |
|
|
2.8±0.1 | 430 | 4.5 | 96 |
| 18 NH(CH2)2NH-CO–biotin | 3.0±0.2 | 400 | 11.4 | 35 |
| 19 NH(CH2)2NHCO(CH2)5NH-CO–biotin | 10.2±7.3 | 120 | 18 | 6.7 |
Molar potency ratio relative to adenosine, which is set equal to 1.0.
Estimated IC50 values based on potency of adenosine (MPR = 1.0) of 1,200±150 nM (6) .
Ki values for antagonism of binding of 1nM [3H]N6-cyclohexyladenosine to rat cerebral cortical membranes (data from 3, 4, 12).
Highest concentration of analogue did not raise coronary blood flow to a level × 50% of maximum possible increase. In such a case we report % increase in flow over control at the plasma nucleoside concentration achieved during infusion.
All of the functionalized ligands except N6-(4-carboxymethylphenyl)adenosine, 8, were potent coronary vasodiators, having MPRs vs adenosine ranging between 2 and 10. Although the most active analogue, 19, also had the largest N6 substituent, there was no correlation between activity and size of the substituent. The spacer arms of the three most active analogues 14, 16 and 19, terminated, respectively, in an −NH2 group, in an −NHCH3 group, and in a biotin residue. Coronary vasoactivity appeared to be independent of whether the spacer arm contained one phenyl residue (9) or two (10-19).
Certain of the functionalized analogues are among the most potent N6-substituted adenosines in the coronary system. Comparisons of their relative selectivity for A1 and A2, adenosine receptors are tentative since at present the only A1 receptor data are from an in vitro binding assay (3,4), and the only A2 receptor data are from an in vivo physiological assay. A variety of factors including efficacy, penetration to sites of action, and non-specific binding to proteins complicate interpretation of such comparisons between dissimilar systems. Nonetheless, comparison of estimated EC50 values (Table 1, see footnote) in the coronary blood flow system with Ki values in the A1-brain membrane binding assay reveals that certain of the functionalized congeners (9, 11, 12, 14, 18, 19) have relatively low selectivity for A1 receptors (A2/A1 ratios 6.7-35) compared to such A1 selective analogues as 4 - 6 (A2/A1 ratios 230-280). Indeed compound 19 is comparable in selectivity to 2-chloroadenosine.
The intracoronary infusion of the complex of analogue 19 with avidin caused dose-dependent coronary vasodilation in each of two dogs. In contrast, the complex of analogue 18 with avidin was inactive in this system, suggesting the requirement of the ε-aminocaproyl spacer unit for accessibility of the pharmacophore at the A2-receptor site. Avidin by itself lacked coronary vasoactivity. The MPR vs adenosine of the analogue 19-avidin complex was 2.1 ± 0.40, somewhat lower than that of 19, which was 10.2 ± 7.3, Possible reasons for the reduced potency of 19 when bound to avidin include poor penetration of the avidin molecule (MW 66,000) into the cardiac intersitial space, steric hindrance exerted by the avidin molecule that impairs interaction of 19 with the receptor and loss of the contribution to binding affinity made by the spacer arm which, when anchored to the avidin molecule, may not be able to interact with the receptor. Unlike 19, which is practically insoluble in water and precipitated when stock solutions in the dimethyl sulfoxide were diluted for intracoronary infusion, the complex of 19 with avidin is quite soluble. Following intracoronary infusion of the 19-avidin complex, vasodilation was evident within 1-2 minutes, an nset of activation somewhat slower than the 20-30 seconds observed for 19. The retarded onset doubtless reflects the reduced rate at which avidin crosses the coronary capillary wall. Unlike the prolonged vasodilation caused by 19, which often lasted for an hour or more, the effect of the 19-avidin complex dissipated within 20 min. The vasoactivity of this macromolecular conjugate supports previous studies which showed an adenosine receptor to be located on the surface of coronary smooth muscle (8,9).
Certain analogues contain functionalization designed for direct coupling to polymers (10). Thus, compounds 15 and 16 may be coupled to appropriately activated sepharose via acylation or aldehyde condensation, compound 16 by alkvlation, compound 17 by electrophilic attack, compound 8 by amine condensation, and compounds 18 and 19 by protein complexation.
This study shows that the coronary artery A2-adenosine receptor recognizes adenosines having N6 substituents a great deal larger than those examined hitherto (6). Although an N6-phenyl substituent nearly doubles the coronary vasoactivity of adenosine (11), the still higher activity of analogues 9 and 11-19, whose MPRs range between 3 and 10, show that bulky substituents distal to the N6-phenyl moiety also contribute to activity, in some instances remarkably so. Structure-activity correlations reveal that certain functionalized N6-phenyl substituents increase affinity for A1 receptors far more than a phenyl group (3,4) and, likewise, other functionalized N6-phenyl substituents can enhance Aagonist potency and selectivity. Thus, the N6-phenyladenosines described here may be only the first generation in a family of functionalized congener ligands selective for adenosine receptors.
Supplementary Material
ABBREVIATIONS
- MPR
molar potency ratio
- Ado
adenosine
- CHA
N6-cyclohexylyladenosine
- NECA
5′N-ethylcarboxamidoadenosine
- PIA
N6-phenylisopropyladenosine
REFERENCES
- 1.Stiles GL, Daly DT, Olsson RA. J. Biol. Chem. 1985;260:10806–10811. [PubMed] [Google Scholar]
- 2.Choca JI, Kwatra MM, Hosey MM, Green RD. Biochem. Biophys. Res. Comm. 1985;131:115–121. doi: 10.1016/0006-291x(85)91778-4. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson KA, Kirk KL, Padgett WL, Daly JW. J. Med. Chem. 1985;28:1341–1346. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson KA, Kirk KL, Padgett WL, Daly JW. FEBS Letters. 1985;184:30–35. doi: 10.1016/0014-5793(85)80646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusachi S, Thompson RD, Olsson RA. J. Pharmacol. Exp. Ther. 1983;227:316–321. [PubMed] [Google Scholar]
- 6.Kusachi S, Thompson RD, Bugni WJ, Yamada N, Olsson RA. J. Med. Chem. 1985;28:1636–1643. doi: 10.1021/jm00149a016. [DOI] [PubMed] [Google Scholar]
- 7.Olsson RA, Khouri EM, Bedyneck JL, Jr., McLean J. Circ. Res. 1979;45:468–478. doi: 10.1161/01.res.45.4.468. [DOI] [PubMed] [Google Scholar]
- 8.Olsson RA, Davis CJ, Khouri EM, Patterson RE. Circ. Res. 1976;39:93–98. doi: 10.1161/01.res.39.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Schrader J, Nees S, Gerlach E. Pfliigers Arch. 1977;369:251–257. doi: 10.1007/BF00582192. [DOI] [PubMed] [Google Scholar]
- 10.Lowe CR. An Introduction to Affinity Chromatography. Elsevier; Amsterdam: 1979. pp. 344–400. [Google Scholar]
- 11.Kusachi S, Thompson RD, Daly DT, Yamada N, Olsson RA. J. Med. Chem. doi: 10.1021/jm00156a016. in press. [DOI] [PubMed] [Google Scholar]
- 12.Daly JW, Padgett W, Thompson RD, Kusachi S, Bugni W, Olsson RA. Biochem. Pharmacol. doi: 10.1016/0006-2952(86)90042-0. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


