Abstract
Antibody drug conjugates (ADCs) have emerged as an important pharmaceutical class of drugs designed to harness the specificity of antibodies with the potency of small molecule therapeutics. The three main components of ADCs are the antibody, the linker, and the payload; the majority of early work focused intensely on improving the functionality of these pieces. Recently, considerable attention has been focused on developing methods to control the site and number of linker/drug conjugated to the antibody, with the aim of producing more homogenous ADCs. In this article, we review popular conjugation methods and highlight recent approaches including “click” conjugation and enzymatic ligation. We discuss current linker technology, contrasting the characteristics of cleavable and non-cleavable linkers, and summarize the essential properties of ADC payload, centering on chemotherapeutics. In addition, we report on the progress in characterizing to determine physicochemical properties and on advances in purifying to obtain homogenous products. Establishing a set of selection and analytical criteria will facilitate the translation of novel ADCs and ensure the production of effective biosimilars.
KEY WORDS: ADC, antibody drug conjugate, biopharmaceutics, enzymatic ligation, therapeutics
Antibody drug conjugates (ADCs) couple the highly desirable pharmacokinetic (PK) profile and targetability of monoclonal antibodies (mAbs) with the potent cytotoxicity of small molecule drugs. Such a combination can potentially minimize dose-limiting toxicities while maximizing desired therapeutic effects. Yet, initial ADCs pairing standard anti-cancer agents, such as doxorubicin, were ineffective in clinical trials (1). These failures were linked to (1) the limited number of drug molecules that can be conjugated to one antibody without affecting antigen binding and (2) the limited number of antigens on target cell surfaces, preventing therapeutic levels of drug accumulation in cells. To date, the most successful approaches to overcome these challenges are improved linker technology and the selection of extremely potent drugs to the pair with the antibody (e.g., ado-trastuzumab emtansine and brentuximab vedotin) (2,3). Innovations in linker design are focused on multiple issues ranging from serum stability to mechanism of release to drug to antibody ratio (DAR). As linkers become increasingly sophisticated, more emphasis is being placed on the methods of bioconjugation between linker and antibody, with the goal of producing homogeneous ADC populations. Several methods of characterization are now employed to assess the composition of such conjugates and to increase our understanding of correlations between ADC structure and efficacy. These many facets of ADC synthesis will be addressed in this review.
CONJUGATION
The majority of ADCs are built on IgG1 scaffolds. These complex, ~150-kDa biomolecules contain multiple native sites for conjugation and can be modified to include additional reactive sites. Most conjugation methods involve nucleophilic residues, while others use special genetic engineering techniques to introduce electrophilic handles such as aldehydes or ketones. In any approach, chemical reactive sites at the scaffold surface must be utilized and conjugation must not affect biophysical integrity.
Non-specific Conjugation Through Native Residues
Reactive side chains of naturally occurring amino acids such as lysine and cysteine are attractive sites of conjugation. The main advantage of linkage through native residues is facile reactivity that does not require preliminary processing/modification of the antibody. The main disadvantages of these methods are the variability and heterogeneity of the resulting products (4,5).
The IgG scaffold has over 80 lysines. With over 20 residues found at highly solvent-accessible sites, conjugation to lysines leads to a wide range of possible drug to DARs at varying conjugation sites (6). For example, amidation of lysines to produce trastuzumab emtansine (T-DM1) results in an average of 3.5 drugs attached per antibody molecule (2). Separately, Acchione et al. reported average DARs between 6 and 14 depending on the equivalents of a model linker used (4). The heterogeneous mixtures produced contain several species that are difficult to purify and characterize (7).
Non-specific conjugation often alters the electrostatic properties (isolectric points) and hydrophobicity of the parent antibody, which influence ADC stability and PK (5). An in-depth analysis of antibody stability by Wankakar and co-workers found that most solvent-accessible lysines are located in the CH2 domain and that conjugation in this region destabilizes the antibody and leads to aggregation (8).
Cysteines are less prevalent in IgGs than free amines and are more uniformly distributed. There are 16 cysteine pairs in a full IgG scaffold: 12 intra-chain and 4 inter-chain disulfide bonds. Due to greater solvent accessibility, the four inter-chain disulfide bonds are the main targets for conjugation (9). Disulfide bonds are integral structural components of the IgG scaffold but must be reduced prior to modification. Therefore, reduction–oxidation conditions must be carefully controlled to allow for conjugation while ensuring overall structure integrity. Despite improved dispersity, cysteine conjugation still results in a mixture of products with varying sites and number of drugs attached (Fig. 1) (9,10). Due to the limited number of potential sites (DAR ≤ 8), this method produces ADCs that are easier to characterize than the lysine coupling method with lower DARs, a feature that has been correlated with increased efficacy (11,12).
Fig. 1.
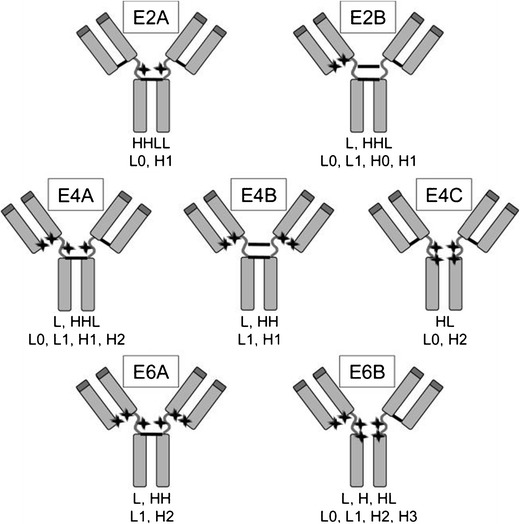
Potential isomers from native cysteine conjugation. The locations of conjugation are indicated by stars and intact disulfide bonds are shown as bars. Below the isomer are the chain compositions under denaturing conditions (first line, nonreducing; second line, reducing). For denaturing and nonreducing conditions, the possible species formed are L, H, HL, HH, HHL, and LHHL. For denaturing and reducing, the possible species formed are L0, L1, H0, H1, H2, and H3, in which the numbers indicate how many drug molecules are attached to the light or heavy chain (adapted with permission from Bioconjug. Chem. 16, 1282–90. Copyright 2005 American Chemical Society)
To further direct the site of attachment, McDonagh et al. systematically replaced cysteine residues with serine to limit the available disulfide bridges for conjugation (13). They reported high conversion rates (89–96%), creating mixtures of conjugates with DARs of 0, 2, 4, and 8. The products were purified by hydrophobic interaction chromatography (HIC), affording nearly homogeneous mixtures (80% DAR = 4).
Overall, utilizing cysteines from inter-chain disulfide bridges is an effective strategy that does not require expensive and time-consuming reengineering of the antibody structure. The major limitation is the inability to produce truly homogenous populations without extensive purification. Disrupting inter-chain disulfide bonds may also compromise the physical stability of antibodies (14).
An innovative approach by Godwin and co-workers addressed the instability associated with reducing disulfide bonds by utilizing a bis-thiol linker to maintain inter-chain bridging while incorporating a drug payload. In this method, the linker is inserted between two reduced bridging cysteines (Fig. 2) (15). Thus, the DAR can be controlled by the concentration of the reducing agent and the stoichiometry of reactants. For example, a fully reduced trastuzumab antibody was treated with six equivalents of bridging linker, resulting in a mixture containing 78% of a conjugate with a DAR of 4. Milder reducing conditions and fewer equivalents of linker yielded a DAR of 2.8. Although this method does not necessarily lead to more homogeneous ADCs, it does provide the ability to reliably prepare ADCs with lower DAR while maintaining the intact antibody structure.
Fig. 2.
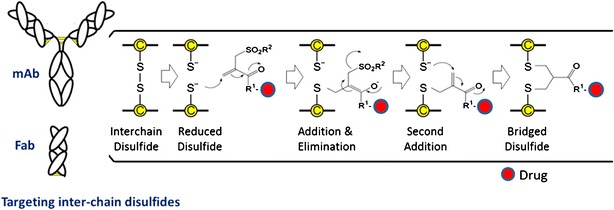
A bis-thiol reactive linker was used to cross-link reduced disulfide bonds and simultaneously incorporate a drug. The method required a PEG25 chain due to low solubility of the linker and payload (shown as R 1) (adapted with permission from Bioconjug. Chem. 25, 1124–36. Copyright 2014 American Chemical Society)
Although the majority of reported work is focused on lysine and cysteine nucleophiles, any reactive side chain of native amino acids, such as the hydroxyl group of tyrosine, could serve as a target for conjugation (16). The advantages of using native residues are facile expression and minimal purification prior to conjugation; however, product heterogeneity can lead to inconsistent PK profiles (17). Despite the inherent dispersity, all three FDA-approved ADCs to date utilize native lysines or cysteines.
Site-specific conjugation through genetically engineered sites
To increase the site specificity of ADC conjugation, reactive handles can be introduced by altering amino acid sequences. A simple approach pioneered by Lyons et al. and later built upon by Kull and co-workers introduces a single solvent-accessible cysteine into an IgG4 scaffold, providing a site-specific handle for conjugation (18,19). After expression and reduction to remove cysteine or glutathione adducts, the mutant antibodies were functionalized using a bromoacetyl-linked payload. Junutula and co-workers improved this method by screening for more suitable modification sites, naming the resulting ADCs “THIOMABs” (20). Using carefully optimized reduction conditions, conjugates with as high as 92% DAR = 2 and conversions of over 98% were achieved. This efficiency makes this technology more amenable to industrial-scale production; however, the required reduction prior to conjugation remains a liability. In addition, cysteine incorporation often leads to aggregation caused by disulfide bridging between antibodies.
Several limitations of this method may be avoided by incorporating more discriminate residues, especially unnatural amino acids. Alternatively, ligating enzymes can be used to catalyze bond formation between specific sequences or chemical groups.
Unnatural Amino Acids
The standard genetic code has been allocated to include unnatural amino acids in proteins (21). Axup et al. used this approach in successfully incorporating a p-acetylphenylalanine (pAcPhe) group into the heavy-chain Fab region of an anti-Her2 antibody (22). A drug molecule bearing a terminal alkoxyamine or hydrazide can be coupled to the keto group by analine-catalyzed condensation to form a stable oxime or hydrazone bond. They generated ADCs linking alkoxy-amine functional auristatin molecules to pAcPhe (Fig. 3). This site-specific and highly efficient conjugation did not interfere with antigen binding, and the resulting ADCs were shown to be homogeneous (DAR = 2) by SDS page and ESI/MS with >95% coupling. This method is limited, however, by the requirement for extensive genetic engineering and the notorious inefficiency of unnatural amino acid incorporation (23). In addition, oxime formation requires long reaction times of 1–4 days.
Fig. 3.
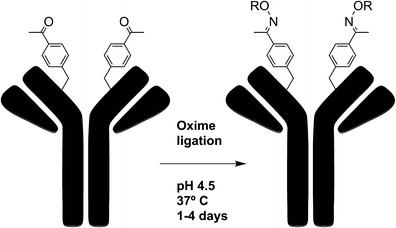
Site-specific conjugation of alkoxy-amine-derivatized auristatin to anti-Her2 Fab and IgG with pAcPhe. The IgG is coupled by oxime ligation to drug derivitized with a terminal alkoxy-amine through pAcPhe residues (adapted from Proc. Natl. Acad. Sci. U. S. A. 109, 16101–6)
Selenocysteine (Sec) is a rare but naturally occurring amino acid that features a highly nucleophilic selenol group. Insertion of selenocysteine residues into proteins does not require synthetic tRNA, but only that the 3′ end of cDNA is modified to include a selenocysteine insertion sequence (24). Once a Sec-containing antibody is isolated, maleimide or iodoacetamide can be used to create selenoether conjugates (25). One group sought to increase the DAR of Sec antibodies by inserting multiple Sec residues into the C termini of full IgGs and fragments (26). Similar to cysteine conjugation, selenocysteines require reduction for nucleophilic activity, raising concerns about concomitant reduction of disulfide bridges within the antibody.
In the field of bioconjugation, there are a multitude of unnatural amino acid strategies to explore, each with distinctive reactivity and properties. For ADC development, systematic evaluation is imperative to establish criteria to pair the location and functionality of a given unnatural amino acid with a desired outcome (e.g., DAR, release, etc.).
Enzymatic Ligation
Recently, Sortase A (SortA)-mediated peptide coupling has seen increasing utility in various bioconjugation applications. SortA recognizes a C-terminal pentapeptide sequence (LPXTG) and creates an amide bond between threonine within the sequence and glycine in the N-terminus of the conjugation partner (Fig. 4) (27).
Fig. 4.
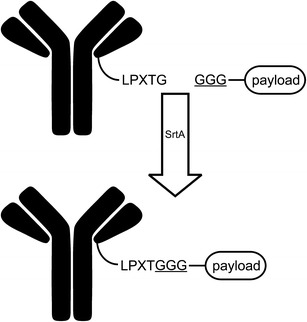
Structures of N and C-terminal fusion partners denoting site and sequence of sortase A recognition motifs of each domain (adapted from Levary DA, Parthasarathy R, Boder ET, Ackerman ME (2011) Protein–Protein Fusion Catalyzed by Sortase A. PLoS ONE 6(4): e18342. Copyright 2011 Levary et al.)
There are several examples of SortA ligations in the development of next-generation ADCs (28–30). NBE-Therapeutics, for example, recently announced a patent-pending Sortase-mediated antibody conjugation (SMACTM)-Technology. This technique has been used to conjugate biotin functional handles to the C-terminus of a single-chain Fv fragment derived from an anti-EGFR antibody (27). A more recent account demonstrates the fusion between the heavy-chain C terminus of an anti-Her2 Fab and the 30-kDA plant toxin gelonin (29). Levary and co-workers verified the flexibility of SortA by ligating a variety of oligo-glycine-tagged biomolecules to IgGs without disrupting antigen binding (28).
One advantage of using SortA is that the recognition sequence can be incorporated to either conjugation partner. Further, the LPXTG sequence does not require any unnatural amino acids, allowing expression to be carried out under a wide variety of conditions. A potential disadvantage is that SortA ligation is currently limited to C and N termini.
Another example of enzymatic ligation employs bacterial transglutaminases (mTGs) that catalyze the coupling of glutamine side chains to alkyl primary amines, such as lysine. Conveniently, bacterial mTGs are unable to modify glutamine residues in native IgG1s. Schibli and co-workers reported, however, that deglycosylating IgGs at N297 exposed a glutamine residue at the 295 position to enzymatic ligation to create ADCs with a DAR of 2 (31). Further, by producing a N297 to Q297 mutant IgG1, they were able to introduce two viable sites for enzymatic labeling to create ADCs with a DAR of 4 (31,32).
This method has also been used to modify native IgGs through the development of a unique “glutamine tag” (LLQG) that can be incorporated at variable locations in the antibody scaffold (Fig. 5) (33). Strop et al identified as many as 12 sites within an anti-EGFR antibody amenable to efficient conjugation (as high as 99% with a DAR = 2). Several of these sites were verified for compatibility and broad applicability using an anti-Her2 or anti-M1S1 antibody.
Fig. 5.
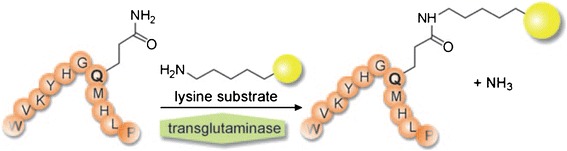
A glutamic acid side chain found in a conserved amino acid sequence is ligated to a lysine side chain by transglutaminase (adapted with permission from Jeger, S., Zimmermann, K., Blanc, A., Grünberg, J., Honer, M., Hunziker, P., Struthers, H. and Schibli, R. (2010) Angew. Chem. Int. Ed., 49: 9995–9997. Copyright 2010 Wiley Periodicals Inc.)
Rabuka and co-workers offer an interesting example that combines enzymatic ligation with unnatural amino acids (34). By encoding a short consensus sequence, CXPXR (where X is serine, threonine, alanine, or glycine), a formylglycine residue, can be introduced by co-expressing the gene with formylglycine-generating enzyme (FGE). FGE oxidizes cysteine residues within the consensus sequence to formylglycine residues (fGly) with conversion efficiencies of up to 98%. Drugs can be attached through the aldehyde functionality of fGly by means of a Hydrazino-iso-Pictet-Spengler (HIPS) ligation. HIPS proceeds with efficiencies above 90% and creates a carbon-bonded heterocyclic linkage that is stable under physiological conditions.
Guided by the X-ray crystal structure of a human IgG1, these researchers used in silico analysis to identify potential locations for aldehyde tag incorporation based on maximum solvent accessibility and minimal immunogenicity. Using a library approach, eight model antibodies were assessed for their propensity for aggregation. Based on these results, three trastuzumab mutants were expressed and conjugated to a maytansine derivative and characterized in vitro and in vivo. Interestingly, each ADC variant exhibited a unique PK profile, although minimal changes to FcRn binding were observed.
The varying properties that result from each type and site of enzymatic ligation portend the questions of how to correlate ligation location with desired PK. The versatility in location of consensus sequence incorporation could increase the potential to include advanced linker features.
Oligosaccharides
All native mAbs exhibit some level of post-translation glycosylation, bestowing an additional site-specific target for conjugation (35). Glyco-conjugation is advantageous if limited conjugation sites for drugs are desired as a DAR of around 2 is easily achievable. Importantly, the antibody scaffolds often require minimal derivitization to achieve efficient conjugation. Initial accounts of carbohydrate-based conjugation involved the oxidation of terminal sugar residues using periodate to yield aldehyde functionalities. Strong oxidizing conditions can lead to undesired oxidation of protein residues that can potentially disrupt antibody binding and stability (36). Zhao and co-workers were able to use milder conditions alongside glycoremodeling to produce ADCs with DAR of 1.5–1.7 (37). Using a two-step process, native sugars are modified with terminal sialic acid residues that are oxidized to generate aldehydes, which can then be coupled with primary amines by reductive amination or with hydrazides in aniline-catalyzed condensation to create acid-labeled hydrazones or with aminooxy groups to form oximes (Fig. 6) (37–39). Nevertheless, even under mild conditions, off-site oxidation may interfere with native antibody structure and function as observed during the development of gemtuzumab ozogamicin (Mylotarg) (36).
Fig. 6.
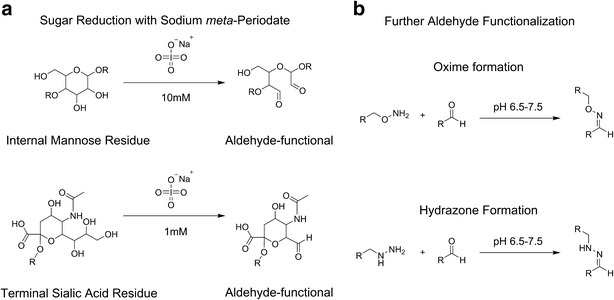
Native sugars in the glycosylated sites of antibodies can be conjugated to functional linkers in a two-step process: a internal mannose or terminal sialic acid residues are reduced to aldehydes and b aldehydes react with linkers containing hydroxylamine or hydrazines to produce oxime and hydrazones, respectively
Qasba and colleagues circumvented detrimental oxidizing conditions by introducing bioorthogonal functional sites. They first homogenize mAb glycoforms via galactosidase digestion and subsequently integrate keto- or azide-modified galactose residues using mutant galactosyltransferases (40,41). Similarly, a recent report by Senter and co-workers exploits the promiscuity of native fucosyltransferases to incorporate thiol-functional fucose analogues for thiol-maleimide conjugation to MMAE (42). This method, however, requires reduction/oxidation steps to selectively liberate active thiol nucleophiles from cysteine adducts and suffers from limited integration efficiency (60–70%). Regardless, fucosyl engineering is of interest due to evidence that afucosylated IgGs exhibit significant enhancements in antibody-dependent cell-mediated cytotoxicity (ADCC) (43,44).
Current examples of glyco-conjugated ADCs often fail to discuss the effects of conjugation on the physical stability of the IgG structure. It has been suggested that this method prevents aggregation by producing a more homogeneous ADC population with lower DARs (37). In theory, structural integrity may be unaffected by drug conjugation provided that stabilizing interactions between the inner core sugars and the CH2 domain are not disrupted. These weak interactions serve to promote an optimal “open” conformation within the Fc. Conversely, complete deglycosylation closes the CH2– CH2 gap, leading to IgG destabilization, aggregation, and therefore diminished effector functions (45,46). Unfortunately, it is currently difficult to predict how glyco-modification will affect Fc-receptor binding and related activity, although studies are reported to be forthcoming.
UV Cross-Linking
Bilgicer et al. reported a unique example of site-specific functionalization that does not require genetic engineering or pre-activated scaffolds (47,48). Payloads featuring a UV-reactive indole-3-butyric acid (IBA) moiety can be covalently linked to an IgG at a known “nucleotide binding site” (NBS) upon exposure to 254-nm light (Fig. 7). A computational analysis suggests that a phenylalanine residue at position 42 within the aromatic NBS region is the site of cross-linking. Conjugates of rituximab with an average DAR of 1.5 were synthesized using this method, confirming that each antibody is able to cross-link one or two IBA molecules in a concentration-dependent manner. The optimal UV intensity was found to be between 0.5 and 5 J/cm2, above which damage to the CDR can occur.
Fig. 7.
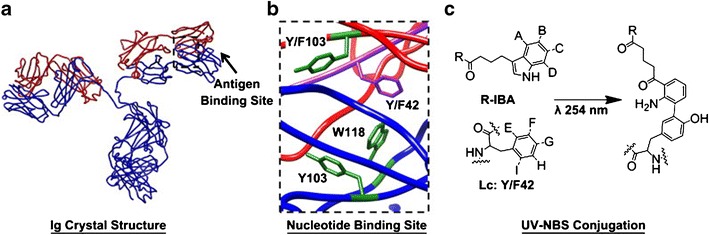
a IgG antibody crystal structure: light chains (red), heavy chains (blue), nucleotide binding site (NBS, boxed). b Rituximab (PDB: 2OSL) with the four NBS residue side chains depicted, two on the light chain and two on the heavy chain; site of conjugation highlighted in purple. c Proposed UV-NBS cross-linking mechanism between the IBA-ligand (R-IBA) and NBS light chain residue Y/F42 (reprinted from Alves, N. J.; Champion, M. M.; Stefanick, J. F.; Handlogten, M. W.; Moustakas, D. T.; Shi, Y.; Shaw, B. F.; Navari, R. M.; Kiziltepe, T.; Bilgicer, B. Biomaterials 2013, 34, 5700–5710, with permission from Elsevier)
LINKER
Although many drugs are amenable to direct conjugation to an antibody scaffold, heterobifunctional linkers often facilitate ADC bioconjugation. At the most basic level, linkers provide a functional handle for efficient conjugation to antibodies through methods described in the previous section. More sophisticated linkers increase effector solubility, improve stability throughout the production process, prevent premature drug release, and facilitate the liberation of active drug at the target. Critical aspects of linker chemistry include the functionality that allows conjugation to antibody, the mechanism for drug release, and the physical properties of the linker itself.
Reactive handles often featured in linker chemistry are grouped based on the site of conjugation. N-hydroxysuccinimide esters are the most common choice for functionalizing amines, especially when coupling to ε-lysine residues. For conjugation to cysteines, thiol-reactive maleimide is the most applied handle, although it is also possible to create a disulfide bridge by oxidation with a linker bearing a sulfhydryl group. Aldehyde or keto functional groups such as oxidized sugar groups or pAcPhe unnatural amino acids can be reacted with hydrazides and alkoxyamines to yield acid-labile hydrazones or oxime bonds. In addition, a hydrazine can be coupled with an aldehyde via HIPS ligation to generate a stable C–C linkage. As additional forms of copper-free click chemistry and unnatural amino acid incorporation become more efficient, it is possible that related methods will see more utility in the future.
The mechanism of drug release is an important consideration in linker selection. Non-cleavable linkers rely on degradation of the scaffold within the lysosome after internalization. Alternatively, cleavable linkers respond to physiological stimuli such as low pH, high glutathione concentrations, and proteolytic cleavage. Each strategy has inherent advantages and disadvantages, but ultimately the optimal combination of linker and conjugation chemistry must be uniquely tailored to correlate each unique facet: the antibody, the drug molecule, and the profile of the disease to be treated.
Non-cleavable Linkers
Several non-cleavable alkyl and polymeric linkers have been explored in ADC development. A notable example is the MCC amine-to-sulfhydryl bifunctional cross-linker featured in T-DM1 (Fig. 8a) (49). This linker is especially useful as the cyclohexane ring provides steric hindrance that decreases the rate of hydrolysis of the resulting thioether.
Fig. 8.
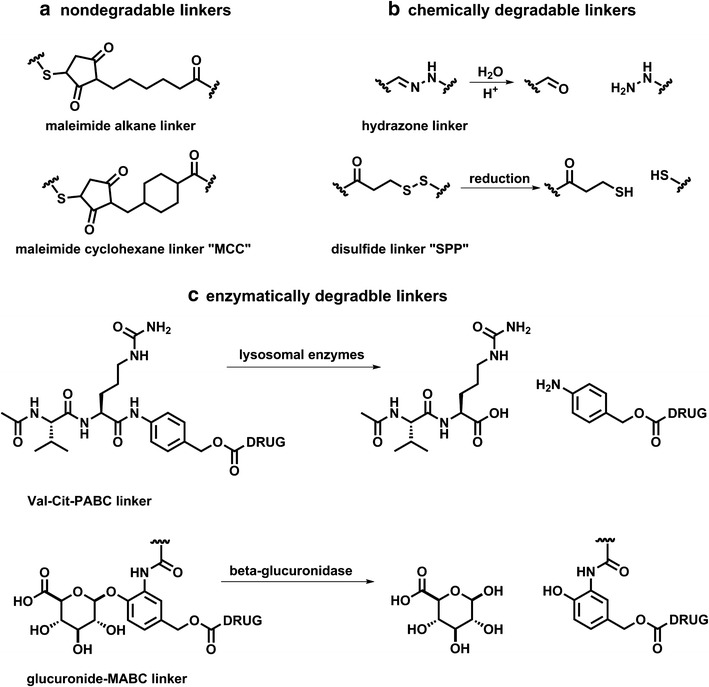
Linkers provide a functional handle to conjugate drug payloads to the antibody scaffold. One important aspect of linker chemistry is the mechanism of drug release. Representative examples of each type are shown: a nondegradable linkers, b chemically degradable linkers can be cleaved by hydrolysis or reduction, and c enzymatically degradable linkers are first cleaved and may further degrade by inclusion of self-immolative benzyl-alcohol spacers
The greatest advantage of using non-cleavable linkers is their increased plasma stability when compared to many cleavable linkers. Despite the limited “bystander” effect, the resistance to cleavage outside of target cells may actually increase the specificity of drug release. Several in vivo studies and clinical data, for example, have shown that non-cleavable linked ADCs outperform their cleavable counterparts in vivo (2).
Non-cleavable linkers require mAb degradation within the lysosome after ADC internalization to release active drug. With this mechanism, differences between parent drug and potential ADC metabolites must be taken into consideration. For example, MMAE, a protein-based anti-mitotic drug, is most potent in its native form and is therefore poorly suited for derivitization with non-cleavable linkers. Conversely, MMAF retained its potency even when linked with a simple alkyl chain in vitro and in vivo (50). One proposed mechanism for the decreased efficacy of non-cleavable linked ADCs is that drugs bearing charged amino acids suffer from decreased membrane permeability, limiting their ability to kill nearby cells. A major motivation for employing cleavable linkers is to improve this “bystander” effect (51).
Chemically Labile Linkers
Cleavable linkers are popular in the ADC clinical pipeline with acid-sensitive linkers such as hydrazones and silyl ethers at the forefront (52). Hydrazones are easily synthesized and have a plasma half-life of 183 h at pH 7 and 4.4 h at pH 5, suggesting that they are selectively cleavable under acidic conditions such as those found in the lysosome (Fig. 8b) (10). The first-generation ADC gemtuzumab ozogamicin (Mylotarg) contains a hydrazone linker that was deemed necessary for drug potency (36). Acidic conditions, however, are often found in various places in the body, increasing the potential for nonspecific drug release. Mylotarg was recently withdrawn from the US market due to toxicities that were attributed in part to poor plasma stability of hydrazone, spawning the need to further tailor acid-labile linkers (53).
Disulfide bridges are envisioned to take advantage of the cellular reducing environment (54). After internalization and degradation, disulfide bridges can release drugs in the lysosome. Erickson et al. established that lysosomal processing is necessary for drug activation and found a significant population of lysine-bound, disulfide-linked drug among the metabolites of ADC degradation (55). These results suggest that the majority of disulfide-linked drugs are first liberated intact by proteolytic degradation of the antibody and only then released as active metabolites through disulfide exchange or by reducing agents such as glutathione. The methylated drug metabolite is then able to diffuse through the lipid membranes to the relevant site of action (56).
Although several side-by-side studies have shown that steric hindrance enhances the plasma stability of disulfide-linked conjugates, the factors governing disulfide-linked metabolite processing are poorly understood (57). This fact is exemplified by an account where a trastuzumab-MCC-DM1 conjugate outperforms several disulfide-linked conjugates, including trastuzumab-SPP-DM1 in in vivo models, a clear reversal of the proposed trend (2). Interestingly, the SPP linker caused significant weight loss compared to the MCC analogue, hinting at potential toxicity of such conjugates. As such, the exact mechanism of disulfide-linked drug release remains elusive and should be elucidated to improve the efficacy of these linkers.
Enzymatically Cleavable Linkers
Enzymatically cleavable linkers are gaining significant attention in ADC development due to superior plasma stability and release mechanism. The most popular enzymatic cleavage sequence is the dipeptide valine-citrulline, combined with a self-immolative linker p-aminobenzyl alcohol (PAB). Cleavage of an amide-linked PAB triggers a 1,6-elimination of carbon dioxide and concomitant release of the free drug in parent amine form (Fig. 8c) (58).
A library of dipeptide linkers was screened by Debowchik and co-workers to measure the rate of doxorubicin release by enzymatic hydrolysis (59,60). They found that Phe-Lys was cleaved most rapidly with a half-life of 8 min, followed closely by Val-Lys with a half-life of 9 min. In stark contrast, Val-Cit showed a half-life of 240 min. They also found that removal of the PAB group reduced the cleavage rate, presumably through steric interference with enzyme binding. Substituting the PAB group for a glycine residue provides adequate spacing for cleavage but does not allow the release of free drug.
Another study compared the potency of auristatin derivative MMAE linked by dipeptide linkers Phe-Lys and Val-Cit and an analogous hydrazone linker. The Val-Cit linker proved to be over 100 times as stable as the hydrazone linker in human plasma. Most significantly, the Phe-Lys linker was substantially less stable than Val-Cit in human plasma, which accounts for its current popularity (10).
Non-peptide cleavable linkers are also being investigated. A glucuronide linker incorporates a hydrophilic sugar group that is cleaved by the lysosomal enzyme beta glucuronidase. Once the sugar is cleaved from the phenolic backbone, self-immolation of the PAB group releases the free drug. Initially, this linker was used to conjugate MMAE, MMAF, and doxorubicin propyloxazoline to various antibodies to create ADCs (61). In a subsequent study, glucuronide- and Val-Cit-PAB-linked ADCs were evaluated side by side for aggregation and efficacy. The glucuronide-linked conjugates show minimal aggregation (<5%) compared to dipeptide-linked conjugates, which show up to 80% aggregation. Though in vitro efficacy results were similar for the two ADCs, the glucuronide linker exhibited greater efficacy in vivo; however, the glucuronide-linked ADC was not well tolerated in vivo compared to Val-Cit-PAB (58).
Overall, enzymatically cleavable linkers provide antibody drug conjugates with plasma stabilities comparable to that of non-cleavable linkers while boasting a more defined method of drug release compared to disulfide-linked or acid-labile linkers. The ability to pair these linkers with self-immolative chemical groups bestows the release of free drugs with minimal derivation; the main constraint being the requirement for the drug to bear an amine or hydroxyl group to conjugate with PAB.
In concert with the numerous methods for bioconjugation to antibodies, the linker is an integral aspect of ADC development. The selection of linker should depend on the application and conditions a given antibody is likely to encounter.
PAYLOAD
The basic criteria for selecting the ADC payload are solubility, amenability to conjugation, and stability (62). In addition, the poor clinical efficacy of first-generation ADCs is attributed to sub-therapeutic levels of drug reaching the target. As such, drug potency is also a vital criterion for current ADC delivery mechanisms.
Lipophilic drugs readily pass cell membranes and therefore have a greater potential to escape the lysosome after release. Conversely, a potential payload must be sufficiently soluble to allow for conjugation to the antibody in aqueous buffers as high concentrations of organic solvent lead to antibody scaffold denaturing. The low solubility of many candidate payloads may be balanced by hydrophilic linkers, such as those containing sulfonates or poly(ethylene glycol), allowing for higher DAR than hydrophobic linkers such as SMCC (63).
Many potent drugs lack chemically functional handles that are necessary for conjugation. Modification to incorporate such handles can have deleterious effects on drug action. Likewise, conjugated drugs that are not released as the free, parent form may suffer decreased efficacy as is often seen when paired with non-cleavable linkers. Self-immolative linkers such as PAB facilitate the release of appended drugs back to the original unconjugated form; however, the PAB moiety itself is hydrophobic and may limit the use of certain payloads. As with solubility, a balance must be found between amending candidate drugs to allow conjugation and maintain efficacy.
Following conjugation, the payload must remain stable in circulation, through cellular processing and release, to reach the cytosolic target. Acid-sensitive drugs may degrade in the lysosome prior to reaching the site of action; disulfide-, alkene-, and epoxide-containing drugs may be reduced or transformed by cellular enzymes. Such drugs must be protected or modified.
Presently, the vast majority of ADC payloads in clinical trials fall into two categories: anti-mitotic or DNA damaging. The two most recently approved ADCs both contain anti-mitotic payloads (64,65). The effector in trastuzumab emtansine is a maytansinoid, and brentuximab vedotin employs an auristatin. The IC50s of each of these families of drugs are in the sub-nanomolar range. Additional anti-mitotic drugs investigated as ADC payloads include the taxanes and vinca alkaloids (66,67). DNA-damaging drugs including duocarmycin, pyrrolobenzodiazepine, calicheamicins, and doxorubicin have also been explored as ADC payloads. The first FDA-approved ADC, gemtuzumab ozogamicin, carries a derivative of calicheamicin with IC50 values in the low nanomolar range (36). Several calicheamicin-based ADCs, including inotuzumab ozogamicin, are currently being tested in clinical trials.
The physicochemical properties of ADCs limit the choice in payloads. Typically, a DAR >4 can diminish ADC solubility, impair binding, and influence PK. In addition, cellular trafficking of ADCs is restricted by the target antigen. Until these challenges can be circumvented, ADC payloads will be confined to extremely potent drugs with narrow therapeutic indices.
CHARACTERIZATION
Each of the components discussed can influence the stability, PK, and pharmacodynamics of an ADC. Significant efforts have been made in monitoring the physicochemical characteristics of ADCs (68); unfortunately, there are no standardized guidelines that correlate a specific characteristic with a given outcome. The major analytical techniques currently used to evaluate ADCs are mainly focused on DAR and dispersity. Several less-implemented techniques are also being explored for the routine evaluation of ADC properties, including dynamic light scattering (DLS) to measure aggregation (69), differential scanning calorimetry to measure changes in higher-order structure (4,8), IR-MALDI (70) to determine DAR, and immunoassays to detect disrupted binding of the Fc region.
UV/Vis
Possibly the most reported characteristic of ADCs is the DAR. UV/vis spectroscopy is the most commonly implemented technique to determine DAR. This molar ratio can be derived from the Beer–Lambert law by comparing the IgG absorbance maxima at 280 nm to that of a drug molecule in simultaneous equations (34,67,71–74). ADCs with a variety of payloads have been evaluated using this method, including DM1, methotrexate, calicheamicin analogues, and auristatins (11,75,76). Consideration should be given for any other contributions to the 280-nm absorbance and for the variance in extinction coefficients that result from different buffers (70,77). Moreover, UV/vis can only be used to determine the average DAR for the whole sample, unless orthogonal methods are employed to delineate the sample composition.
Chromatography
ADC synthesis often yields mixed products that differ in the number and site of conjugated payload. An assortment of chromatographic techniques can be applied to resolve ADCs based on characteristics specific to conjugation, linker, and payload. Capillary electrophoresis (CE) and HIC are especially useful in resolving native ADCs (9,78,79). Although size exclusion chromatography is most useful as a means of ADC purification, it can be used to characterize variants in ADC samples, particularly when aggregates are suspected; however, the hydrophobicity of the payload may lead to non-specific interactions with the stationary phase of columns. Importantly, conjugation of small drugs may not provide a large enough shift in hydrodynamic radius to be resolved by SEC (34,37,63,80,81).
Conjugating drugs onto the mAb structure can greatly alter their electrostatic characteristics, making charge-based separations such as CE useful in the characterization of ADCs (8,9,78). For example, conjugating through surface lysine residues can result in decreased positive surface charge when compared to the unconjugated mAb (82). Ion exchange chromatography and isoelectric focusing can offer information about such changes in ADC isoelectric point (5,83). These methods are not applicable to heterogeneous products with only small differences in isoelectric point.
Unnatural amino acid-linked conjugates and other site-specifically linked ADCs are well suited for characterization by standard reverse-phase high performance liquid chromatography (HPLC) using C18 columns (20). Direct characterization of intact inter-chain cysteine-linked ADCs is difficult, however. Disruption of the inter-chain disulfide bridges leads to instability under denaturing HPLC conditions due to incomplete covalent linkage between subunits; nevertheless, the heavy and light chains can be characterized separately (13).
HIC in particular is advantageous for characterization of intact ADCs because of the pH-neutral and non-denaturing salt gradients used for separation (79). This method is most amenable for relatively homogeneous conjugations such as conjugates formed by inter-chain disulfide linkage and those formed by other site-specific conjugation methods such as glycoengineering (9,11,20,37).
The information regarding ADC composition from chromatography is substantially enhanced by coupling separation with other analyses such as UV/vis and mass spectrometry. For example, using HIC-UV/Vis, Hamblett and co-workers were able to separate and identify individual peaks corresponding to mAb species with zero to eight molecules of conjugated vc-MMAE (11).
Mass Spectrometry
Innovations in ionization techniques such as MALDI and ESI have expanded the utility of mass spectrometry (MS) to analyze larger macromolecules, including antibodies (68). These systems allow access to mass ranges over 4000 mass-to-charge ratio when paired with TOF or quadrupole detectors (84,85). The acquisition of mass spectra is relatively straightforward; however, it is important to remove all salts and other excipients that may interfere with protein ionization. Tandem liquid chromatography-mass spectrometry is often employed to simplify desalting and to improve resolution (7,86,87). In addition, the deglycosylation of mAb samples using enzymes such as PNGase is common practice and beneficial to simplify MS analysis.
MALDI-TOF MS can be used to detect mass shifts between parent mAb and ADC that correlate with payload conjugation. Although MALDI-TOF lacks mass accuracy to resolve individual species with different numbers of conjugated drugs, the obtained peak spectra can be analyzed to confirm conjugation and to determine the average DAR of the ADC. For example, Safavy and co-workers used MALDI-TOF to assess the DAR of paclitaxel-conjugated anti-EGFR antibodies (88).
ESI-MS produces “charge envelopes” of multiple-charged ionized molecules. This increases the accuracy and resolution of different DAR variants. Lazar and coworkers were able to analyze intact lysine-linked huC242-DM4 conjugates using ESI-MS. The complex chromatograms can be deconvoluted to reveal peaks of individual species. ESI-MS is also amenable to analyze intact disulfide-linked ADC, as demonstrated by Vallerie-Douglass et al. Using mild buffer conditions and by deconvoluting the resulting spectra, they were able to resolve individual forms of MMAF-conjugated antibodies (Fig. 9) (89). The observed DAR results were consistent with results from an orthogonal HIC analysis.
Fig. 9.
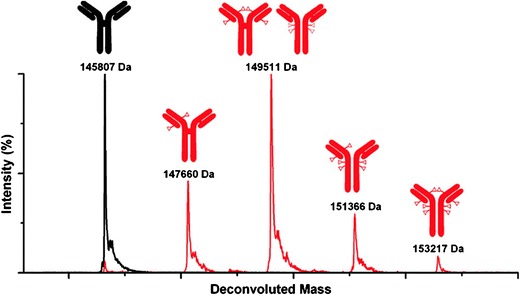
ESI-MS is used to produce these deconvoluted mass spectra of a deglycosylated ADC in red. The ADC mixture of six different species in red is compared to that of the unconjugated mAb in black (reprinted with permission from Anal. Chem. 84, 2843–2849. Copyright 2012 American Chemical Society)
The combination of multiple analytical techniques can elucidate detailed information on ADC structure. Wang and colleagues paired enzymatic digestion with protein mapping to identify individual sites of conjugation within a huN901-DM1 conjugate (78). In addition to deglycosylation, ADCs were reduced by DTT, after which heavy and light chains were purified by SEC and subsequently digested and analyzed by ESI-MS. Through this method, they were able to identify seven light chain and 13 heavy chain modifications. Molecular modeling verified that the identified lysine sites are generally located at solvent-accessible regions with structural flexibility.
Many bioanalytical techniques are well established for the characterization of antibody drug conjugates, but these methods require precise selection and optimization for a specific mAb, drug, and linker chemistry. Well-defined and standardized analysis will become increasingly important as ADCs and many other biologics come under growing quality control scrutiny and standardization (90).
CONCLUSIONS AND PROSPECTUS
The versatility of antibodies and the increasing sophistication of bioconjugation methods have moved ADCs into the forefront of next-generation therapies for a widening pool of diseases. The content of this review demonstrates the vast diversity of strategies in ADC development, many of which are featuring a heavy focus on linker and conjugation chemistry. A systematic evaluation of the site and type of modification is essential to better understand the relationships between ADC structure, mechanism of action, and efficacy. Questions probing conjugation’s effects on stability, the rate and specificity of payload release, and the resulting effects on PK must be thoroughly addressed. As site-specific conjugation becomes increasingly reliable, so will the opportunities for elegant linking systems be. Finally, advanced methods of characterization will be extremely important as this class of biologics is translated to industrial-scale production.
Future work in this area must address the limitations posed by the narrow therapeutic indices of highly potent drugs such as ematansine and vedotin. Control of cellular internalization, the targeting of multiple pathways, and the attachment of multiple orthogonal payloads may be promising areas of interest in the development of next-generation ADCs. As our understanding of antigen biochemistry increases, ADCs may see more utility in disease areas outside of cancer.
REFERENCES
- 1.Jaracz S, Chen J, Kuznetsova LV, Ojima L. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13(17):5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 2.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 3.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 4.Acchione M, Kwon H, Jochheim CM, Atkins WM. Impact of linker and conjugation chemistry on antigen binding, Fc receptor binding and thermal stability of model antibody-drug conjugates. MAbs. 2012;4(3):362–372. doi: 10.4161/mabs.19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan NJ, Zhou W, Proos RJ, Tolbert TJ, Wolfe JL, Laurence JS. Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates. Bioconjug Chem. 2013;24(6):1008–1016. doi: 10.1021/bc4000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chari RVJ. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 2007;41(1):98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- 7.Lazar AC, Wang L, Blättler WA, Amphlett G, Lambert JM, Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun Mass Spectrom RCM. 2005;19(13):1806–1814. doi: 10.1002/rcm.1987. [DOI] [PubMed] [Google Scholar]
- 8.Aa W, Feeney MB, Rivera J, Chen Y, Kim M, Sharma VK, et al. Physicochemical stability of the antibody-drug conjugate Trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjug Chem. 2010;21(9):1588–1595. doi: 10.1021/bc900434c. [DOI] [PubMed] [Google Scholar]
- 9.Sun MMC, Beam KS, Cerveny CG, Hamblett KJ, Blackmore RS, Torgov MY, et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides. Bioconjug Chem. 2005;16(5):1282–1290. doi: 10.1021/bc050201y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21(7):778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 11.Hamblett KJ, Senter PD, Chace DF. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 12.Jackson D, Atkinson J, Guevara CI, Zhang C, Kery V, Moon S-J, et al. In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates. PLoS One. 2014;9(1):e83865. doi: 10.1371/journal.pone.0083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonagh CF, Turcott E, Westendorf L, Webster JB, Alley SC, Kim K, et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel PEDS. 2006;19(7):299–307. doi: 10.1093/protein/gzl013. [DOI] [PubMed] [Google Scholar]
- 14.Michaelsen TE, Brekke OH, Aase A, Sandin RH, Bremnes B, Sandlie I. One disulfide bond in front of the second heavy chain constant region is necessary and sufficient for effector functions of human IgG3 without a genetic hinge. Proc Natl Acad Sci U S A. 1994;91(20):9243–9247. doi: 10.1073/pnas.91.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badescu G, Bryant P, Bird M, Henseleit K, Swierkosz J, Parekh V, et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjug Chem. 2014;25(6):1124–1136. doi: 10.1021/bc500148x. [DOI] [PubMed] [Google Scholar]
- 16.Ban H, Gavrilyuk J, Barbas CF. Tyrosine bioconjugation through aqueous ene-type reactions: a click-like reaction for tyrosine. J Am Chem Soc. 2010;132(5):1523–1525. doi: 10.1021/ja909062q. [DOI] [PubMed] [Google Scholar]
- 17.Boswell CA, Mundo EE, Zhang C, Bumbaca D, Valle NR, Kozak KR, et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-STEAP1 antibody-drug conjugates in rats. Bioconjug Chem. 2011;22(10):1994–2004. doi: 10.1021/bc200212a. [DOI] [PubMed] [Google Scholar]
- 18.Lyons A, King DJ, Owens RJ, Yarranton GT, Millican A, Whittle NR, et al. Site-specific attachment to recombinant antibodies via introduced surface cysteine residues. Protein Eng. 1990;3(8):703–708. doi: 10.1093/protein/3.8.703. [DOI] [PubMed] [Google Scholar]
- 19.Stimmel JB, Merrill BM, Kuyper LF, Moxham CP, Hutchins JT, Fling ME, et al. Site-specific conjugation on serine right-arrow cysteine variant monoclonal antibodies. J Biol Chem. 2000;275(39):30445–30450. doi: 10.1074/jbc.M001672200. [DOI] [PubMed] [Google Scholar]
- 20.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 21.Young TS, Schultz PG. Beyond the canonical 20 amino acids: expanding the genetic lexicon. J Biol Chem. 2010;285(15):11039–11044. doi: 10.1074/jbc.R109.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109(40):16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takimoto JK, Adams KL, Xiang Z, Wang L. Improving orthogonal tRNA-synthetase recognition for efficient unnatural amino acid incorporation and application in mammalian cells. Mol BioSyst. 2009;5(9):931–934. doi: 10.1039/b904228h. [DOI] [PubMed] [Google Scholar]
- 24.Hofer T, Thomas JD, Burke TR, Rader C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc Natl Acad Sci U S A. 2008;105(34):12451–12456. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofer T, Skeffington LR, Chapman CM, Rader C. Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry. 2009;48(50):12047–12057. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Yang J, Rader C. Antibody conjugation via one and two C-terminal selenocysteines. Methods (San Diego, Calif) 2014;65(1):133–138. doi: 10.1016/j.ymeth.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madej MP, Coia G, Williams CC, Caine JM, Pearce LA, Attwood R, et al. Engineering of an anti-epidermal growth factor receptor antibody to single chain format and labeling by Sortase A-mediated protein ligation. Biotechnol Bioeng. 2012;109(6):1461–1470. doi: 10.1002/bit.24407. [DOI] [PubMed] [Google Scholar]
- 28.Levary DA, Parthasarathy R, Boder ET, Ackerman ME. Protein-protein fusion catalyzed by sortase A. PLoS One. 2011;6(4):e18342. doi: 10.1371/journal.pone.0018342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornberger P, Skerra A. Sortase-catalyzed in vitro functionalization of a HER2-specific recombinant Fab for tumor targeting of the plant cytotoxin gelonin. mAbs. 2014;6(2):354–366. doi: 10.4161/mabs.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swee LK, Guimaraes CP, Sehrawat S, Spooner E, Barrasa MI, Ploegh HL. Sortase-mediated modification of α DEC205 affords optimization of antigen presentation and immunization against a set of viral epitopes. Proc Natl Acad Sci U S A. 2013;110(4):1428–33. [DOI] [PMC free article] [PubMed]
- 31.Jeger S, Zimmermann K, Blanc A, Honer M, Hunziker P, Struthers H, et al. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem (Int Engl) 2010;49:9995–9997. doi: 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- 32.Dennler P, Chiotellis A, Fischer E, Brégeon D, Belmant C, Gauthier L, et al. Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody–drug conjugates. Bioconjug Chem. 2014;25(3):569–578. doi: 10.1021/bc400574z. [DOI] [PubMed] [Google Scholar]
- 33.Strop P, Liu S-H, Dorywalska M, Delaria K, Dushin RG, Tran T-T, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20(2):161–167. doi: 10.1016/j.chembiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Drake PM, Albers AE, Baker J, Banas S, Barfield RM, Bhat AS, et al. Aldehyde tag coupled with hips chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjug Chem. 2014;25(7):1331–1341. doi: 10.1021/bc500189z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- 36.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13(1):47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Stefano JE, Manning C, Kyazike J, Chen B, Gianolio DA, et al. Site-specific antibody-drug conjugation through glycoengineering. Bioconjug Chem. 2014;25(3):510–520. doi: 10.1021/bc400505q. [DOI] [PubMed] [Google Scholar]
- 38.Hinman LM, Hamann PR, Wallace R, Menendez AT, Dã FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent fam. 1993;3336–42. [PubMed]
- 39.Rodwell JD, Alvarez VL, Lee C, Lopes AD, Goers JW, King HD, et al. Site-specific covalent modification of monoclonal antibodies: in vitro and in vivo evaluations. Proc Natl Acad Sci U S A. 1986;83(8):2632–2636. doi: 10.1073/pnas.83.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishnan B, Boeggeman E, Pasek M, Qasba PK. Bioconjugation using mutant glycosyltransferases for the site-specific labeling of biomolecules with sugars carrying chemical handles. In: Mark SS, editor. Totowa: Humana Press; 2011. p. 281–96. [DOI] [PubMed]
- 41.Boeggeman E, Ramakrishnan B, Pasek M, Manzoni M, Puri A, Loomis KH, et al. Site specific conjugation of fluoroprobes to the remodeled Fc N-glycans of monoclonal antibodies using mutant glycosyltransferases: application for cell surface antigen detection. Bioconjug Chem. 2009;20(6):1228–1236. doi: 10.1021/bc900103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okeley NM, Toki BE, Zhang X, Je SC, Burke PJ, Alley SC, et al. Metabolic engineering of monoclonal antibody carbohydrates for antibody − drug conjugation. 2013. [DOI] [PubMed]
- 43.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336(5):1239–1249. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325(5):979–989. doi: 10.1016/S0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 46.Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc : properties of a series of truncated glycoforms. Mol Immunol. 2001;37(2000):697–706. doi: 10.1016/s0161-5890(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 47.Alves NJ, Mustafaoglu N, Bilgicer B. Conjugation of a reactive thiol at the nucleotide binding site for site-specific antibody functionalization. Bioconjug Chem. 2014;25(7):1198–1202. doi: 10.1021/bc500211d. [DOI] [PubMed] [Google Scholar]
- 48.Alves NJ, Champion MM, Stefanick JF, Handlogten MW, Moustakas DT, Shi Y, et al. Selective photocrosslinking of functional ligands to antibodies via the conserved nucleotide binding site. Biomaterials. 2013;34(22):5700–5710. doi: 10.1016/j.biomaterials.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 49.Lambert JM, Chari RVJ. Ado-trastuzumab Emtansine (T-DM1): An Antibody–Drug Conjugate (ADC) for HER2-Positive Breast Cancer. J Med Chem. 2014;57(16):6949–64. [DOI] [PubMed]
- 50.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17(1):114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 51.Kovtun YV, Goldmacher VS. Cell killing by antibody-drug conjugates. Cancer Lett. 2007;255(2):232–240. doi: 10.1016/j.canlet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody-drug conjugates: current status and future directions. Drug Discov Today. 2013;1–13. [DOI] [PubMed]
- 53.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21(1):5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 54.Saito G, Swanson JA, Lee K-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55(2):199–215. doi: 10.1016/S0169-409X(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 55.Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- 56.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 57.Thorpe PE, Wallace PM, Knowles PP, Reif MG, Brown ANF, Watson GJ, et al. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin a-chain and hindered disulfide linkages improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered bisulfide linkages. 1988;6396–403. [PubMed]
- 58.Burke PJ, Senter PD, Meyer DW, Miyamoto JB, Anderson M, Toki BE, et al. Design, synthesis, and biological evaluation of antibody-drug conjugates comprised of potent camptothecin analogues. Bioconjug Chem. 2009;20(6):1242–1250. doi: 10.1021/bc9001097. [DOI] [PubMed] [Google Scholar]
- 59.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13(4):855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 60.Dubowchik GM, Radia S, Mastalerz H, Walker MA, Firestone RA, Dalton King H, et al. Doxorubicin immunoconjugates containing bivalent, lysosomally-cleavable dipeptide linkages. Bioorg Med Chem Lett. 2002;12(11):1529–1532. doi: 10.1016/S0960-894X(02)00194-4. [DOI] [PubMed] [Google Scholar]
- 61.Jeffrey SC, Andreyka JB, Bernhardt SX, Kissler KM, Kline T, Lenox JS, et al. Development and properties of beta-glucuronide linkers for monoclonal antibody-drug conjugates. Bioconjug Chem. 2006;17(3):831–840. doi: 10.1021/bc0600214. [DOI] [PubMed] [Google Scholar]
- 62.Widdison W, Chari RJ. Factors involved in the design of cytotoxic payloads for antibody–drug conjugates. In: Phillips GL, editor. Antibody-drug conjugates and immunotoxins. New York: Springer; 2013. pp. 93–115. [Google Scholar]
- 63.Zhao RY, Wilhelm SD, Audette C, Jones G, Leece BA, Lazar AC, et al. Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates. J Med Chem. 2011;54(10):3606–3623. doi: 10.1021/jm2002958. [DOI] [PubMed] [Google Scholar]
- 64.Fishkin N, Maloney EK, Chari RVJ, Singh R. A novel pathway for maytansinoid release from thioether linked antibody-drug conjugates (ADCs) under oxidative conditions. Chem Commun. 2011;47(38):10752–10754. doi: 10.1039/c1cc14164c. [DOI] [PubMed] [Google Scholar]
- 65.Sanderson RJ, Hering MA, James SF, Sun MMC, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11(2):843–852. [PubMed] [Google Scholar]
- 66.Quiles S, Raisch KP, Sanford LL, Bonner JA, Safavy A. Synthesis and preliminary biological evaluation of high-drug load paclitaxel-antibody conjugates for tumor-targeted chemotherapy. J Med Chem. 2010;53(2):586–594. doi: 10.1021/jm900899g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laguzza BC, Nichols CL. New antitumor monoclonal antibody-vinca conjugates LY203725 and related compounds: design, preparation, and representative in vivo activity. J Med Chem. 1989;32(11):548–555. doi: 10.1021/jm00123a007. [DOI] [PubMed] [Google Scholar]
- 68.Wakankar A, Chen Y, Gokarn Y, Jacobson FS. Analytical methods for physicochemical characterization of antibody drug conjugates. MAb0073. 2011;3(2):161–172. doi: 10.4161/mabs.3.2.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nobbmann U, Connah M, Fish B, Varley P, Gee C, Mulot S, et al. Dynamic light scattering as a relative tool for assessing the molecular integrity and stability of monoclonal antibodies. Biotechnol Genet Eng Rev. 2007;24(1):117–128. doi: 10.1080/02648725.2007.10648095. [DOI] [PubMed] [Google Scholar]
- 70.Siegel MM, Tabei K, Kunz A, Hollander IJ, Hamann RR, Bell DH, et al. Calicheamicin derivatives conjugated to monoclonal antibodies: determination of loading values and distributions by infrared and UV matrix-assisted laser desorption/ionization mass spectrometry and electrospray ionization mass spectrometry. Anal Chem. 1997;69(14):2716–2726. doi: 10.1021/ac970035q. [DOI] [PubMed] [Google Scholar]
- 71.Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem. 2002;13(1):40–46. doi: 10.1021/bc0100206. [DOI] [PubMed] [Google Scholar]
- 72.Vandongen G, Visser G, Vrouenraets M. Photosensitizer-antibody conjugates for detection and therapy of cancer. Adv Drug Deliv Rev. 2004;56(1):31–52. doi: 10.1016/j.addr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Kim KM, McDonagh CF, Westendorf L, Brown LL, Sussman D, Feist T, et al. Anti-CD30 diabody-drug conjugates with potent antitumor activity. Mol Cancer Ther. 2008;7(8):2486–2497. doi: 10.1158/1535-7163.MCT-08-0388. [DOI] [PubMed] [Google Scholar]
- 74.Hutchins BM, Kazane SA, Staflin K, Forsyth JS, Felding-Habermann B, Schultz PG, et al. Site-specific coupling and sterically controlled formation of multimeric antibody fab fragments with unnatural amino acids. J Mol Biol. 2011;406(4):595–603. doi: 10.1016/j.jmb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Amphlett G, Blättler WA. Structural characterization of the maytansinoid–monoclonal antibody immunoconjugate, huN901–DM1, by mass spectrometry. Protein Sci. 2005;14:2436–2446. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garnett MC, Embleton MJ, Jacobs E, Baldwin RW. Preparation and properties of a drug-carrier-antibody conjugate showing selective antibody-directed cytotoxicity in vitro. Int J Cancer. 1983;31(5):661–670. doi: 10.1002/ijc.2910310520. [DOI] [PubMed] [Google Scholar]
- 77.Greenfield RS, Kaneko T, Daues A, Edson MA, Fitzgerald KA, Olech LJ, et al. Evaluation in vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. 1990;5(19):6600–8. [PubMed]
- 78.Liu J, Zhao H, Volk KJ, Klohr SE, Kerns EH, Lee MS. Analysis of monoclonal antibody and immunoconjugate digests by capillary electrophoresis and capillary liquid chromatography. J Chromatogr A. 1996;735(1–2):357–366. doi: 10.1016/0021-9673(95)01054-8. [DOI] [PubMed] [Google Scholar]
- 79.Valliere-Douglass J, Wallace A, Balland A. Separation of populations of antibody variants by fine tuning of hydrophobic-interaction chromatography operating conditions. J Chromatogr A. 2008;1214(1–2):81–89. doi: 10.1016/j.chroma.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 80.Shen B-Q, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30(2):184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 81.King HD, Dubowchik GM, Mastalerz H, Willner D, Hofstead SJ, Firestone RA, et al. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: inhibition of aggregation by methoxytriethyleneglycol chains. J Med Chem. 2002;45(19):4336–4343. doi: 10.1021/jm020149g. [DOI] [PubMed] [Google Scholar]
- 82.Boswell CA, Tesar DB, Mukhyala K, Theil F-P, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21(12):2153–2163. doi: 10.1021/bc100261d. [DOI] [PubMed] [Google Scholar]
- 83.Righetti PG. Determination of the isoelectric point of proteins by capillary isoelectric focusing. J Chromatogr A. 2004;1037(1–2):491–499. doi: 10.1016/j.chroma.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 84.Rosati S, Thompson NJ, Heck AJR, Rosati S, Thompson NJ, Heck AJR. Tackling the increasing complexity of therapeutic monoclonal antibodies with mass spectrometry. TrAC Trends Anal Chem. 2013;48:72–80. doi: 10.1016/j.trac.2013.02.013. [DOI] [Google Scholar]
- 85.Rosati S, Yang Y, Barendregt A, Heck AJR. Detailed mass analysis of structural heterogeneity in monoclonal antibodies using native mass spectrometry. Nat Protocol. 2014;9(4):967–976. doi: 10.1038/nprot.2014.057. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Lu Q, Wu S-L, Karger BL, Hancock WS. Characterization and comparison of disulfide linkages and using LC-MS with electron transfer dissociation. Anal Chem. 2011;(83)8:3133–40. [DOI] [PMC free article] [PubMed]
- 87.Lyubarskaya Y, Houde D, Woodard J, Murphy D, Mhatre R. Analysis of recombinant monoclonal antibody isoforms by electrospray ionization mass spectrometry as a strategy for streamlining characterization of recombinant monoclonal antibody charge heterogeneity. Anal Biochem. 2006;348(1):24–39. doi: 10.1016/j.ab.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Safavy A, Bonner JA, Waksal HW, Buchsbaum DJ, Gillespie GY, Arani R, et al. Synthesis and biological evaluation of paclitaxel−C225 conjugate as a model for targeted drug delivery. Bioconjug Chem. 2003;14(2):302–310. doi: 10.1021/bc020033z. [DOI] [PubMed] [Google Scholar]
- 89.Valliere-Douglass JF, McFee WA, Salas-Solano O. Native intact mass determination of antibodies conjugated with monometyl auristatin E and F at interchain cysteine residues. Anal Chem. 2012;84(6):2843–2849. doi: 10.1021/ac203346c. [DOI] [PubMed] [Google Scholar]
- 90.Beck A, Sanglier-Cianférani S, Van Dorsselaer A. Biosimilar, biobetter, and next generation antibody characterization by mass spectrometry. Anal Chem. 2012;84(11):4637–4646. doi: 10.1021/ac3002885. [DOI] [PubMed] [Google Scholar]


