Abstract
The opportunistic human pathogen Propionibacterium acnes is composed of a number of distinct phylogroups, designated types IA1, IA2, IB, IC, II, and III, which vary in their production of putative virulence factors, their inflammatory potential, and their biochemical, aggregative, and morphological characteristics. Although multilocus sequence typing (MLST) currently represents the gold standard for unambiguous phylogroup classification and individual strain identification, it is a labor-intensive and time-consuming technique. As a consequence, we developed a multiplex touchdown PCR assay that in a single reaction can confirm the species identity and phylogeny of an isolate based on its pattern of reaction with six primer sets that target the 16S rRNA gene (all isolates), ATPase (types IA1, IA2, and IC), sodA (types IA2 and IB), atpD (type II), and recA (type III) housekeeping genes, as well as a Fic family toxin gene (type IC). When applied to 312 P. acnes isolates previously characterized by MLST and representing types IA1 (n = 145), IA2 (n = 20), IB (n = 65), IC (n = 7), II (n = 45), and III (n = 30), the multiplex displayed 100% sensitivity and 100% specificity for detecting isolates within each targeted phylogroup. No cross-reactivity with isolates from other bacterial species was observed. This multiplex assay will provide researchers with a rapid, high-throughput, and technically undemanding typing method for epidemiological and phylogenetic investigations. It will facilitate studies investigating the association of lineages with various infections and clinical conditions, and it will serve as a prescreening tool to maximize the number of genetically diverse isolates selected for downstream higher-resolution sequence-based analyses.
INTRODUCTION
Propionibacterium acnes is an anaerobic-to-aerotolerant Gram-positive bacterium, which exists in nature as a human commensal and opportunistic pathogen. It is a major component of the human skin microbiota but can also be recovered from the oral cavity and the gastrointestinal and genitourinary tracts (1). Although P. acnes is the main cause of opportunistic human infections within the cutaneous group of propionibacteria and is well known for its association with the inflammatory skin condition acne vulgaris (2, 3), its roles in other human infections and clinical conditions are likely to have been significantly underestimated (4–6). Despite this, we now see a growing recognition that the bacterium is an important cause of human disease, especially in relation to indwelling medical device-related infections (7–12), and it may also play a role in chronic conditions that cause significant morbidity and mortality, including low back pain associated with modic type I changes (13), sarcoidosis (14, 15), and prostate cancer (16, 17).
Within the last 10 years, phylogenetic studies based on single and multilocus gene sequencing (18–21), as well as whole-genome analyses of isolates from the Human Microbiome Project (HMP) and other studies (22–28), have provided valuable insights into the genetic population structure of P. acnes, particularly in the context of health and disease. The bacterium has an overall clonal structure, and its isolates can be classified into a number of statistically significant clades or phylogroups designated types IA1, IA2, IB, IC, II, and III; these types appear to display differences in their associations with specific types of infections (20, 21) and vary in their production of putative virulence determinants (19, 20, 29–32), inflammatory potential (33–36), antibiotic resistances (21, 37), aggregative properties (16), and morphological characteristics (19). In particular, a number of independent epidemiological studies have shown a strong association between clonal complexes from the type IA1 phylogroup and moderate-to-severe acne, while the lineages from all other phylogroups appear more frequently to be isolated from medical device and soft tissue infections or be associated with health as true commensals (20, 21, 31, 38). Despite these associations, much uncertainty still exists regarding the exact clinical relevance of these phylogroups, particularly in the context of acne when skin sampling methods may not be optimal or appropriate (39), as well as the wider issue of whether isolates recovered from different clinical samples are truly representative of infection in all contexts or are simply skin contaminants/passive bystanders within a sample. These issues are common when dealing with an opportunistic pathogen that is also part of the normal microbiota, and untangling clinically relevant isolates from background contaminants can be a challenge. Future studies aimed at addressing such issues will undoubtedly provide a more solid platform on which we can make definite conclusions regarding the association of specific P. acnes phylogroups with human disease.
To date, a number of phenotypic and/or molecular approaches have been investigated as methods for phylogroup identification, ranging from very simple biochemical tests based on hemolysis (19) or fermentation profiles (40) to matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (41), monoclonal antibody (MAb) typing (18), and DNA-based analysis; DNA-based analysis includes ribotyping (23), DiversiLab analysis (42), direct PCR assays (43), and protein-coding gene sequencing (18, 20, 21, 44). Unfortunately, many of these approaches suffer from specificity or sensitivity limitations that constrain their diagnostic value. For example, previously described direct PCR assays do not differentiate IA1 from type IA2 or IC or target type III strains (43); they may also give ambiguous results (44). Single-locus nucleotide sequencing, which to date has been primarily based on the recA housekeeping and tly methyltransferase/hemolysin genes, is robust for identifying types I, II and III but displays reduced specificity for differentiating type IB from type IA2 and some strains within the type IA1 clade, as they contain identical alleles due to horizontal gene transfer (HGT) (20, 21, 23, 38). More recently, MAb typing with antibodies targeting types IA, IC, and II combined with recA sequence analysis has obviated this problem, facilitating the accurate differentiation of type IB from all type IA strains (45). MALDI-TOF MS has also been described as a valuable and powerful approach for rapid and high-throughput phylogroup identification but currently will not differentiate type IA1 from IA2 (41); furthermore, the technology is not available within all laboratories. At present, multilocus sequence typing (MLST) of P. acnes (20, 21) still represents the clear gold standard for unambiguous phylogroup identification, as well as individual strain resolution (see Table S1 in the supplemental material), and it offers significant advantages over comparable high-resolution gel-based typing methodologies, including random amplification of polymorphic DNA (RAPD) (46) and pulsed-field gel electrophoresis (PFGE) (47, 48), which have also been applied to this bacterium. The MLST method is, however, technically demanding, time-consuming, and expensive, especially when analyzing multiple isolates. Consequently, and as a result of the growing interest surrounding the role of this microbe in disease, there is a need for a rapid, less labor-intensive, and inexpensive method for the typing and stratification of P. acnes isolates, thus facilitating future molecular epidemiological and phylogenetic studies. Against this background, we describe here the development and validation of a multiplex touchdown PCR assay that can be used for quick, high-throughput, and accurate molecular confirmation of P. acnes isolates combined with a parallel disclosure of their phylogeny. This assay should prove valuable for researchers and, along with MLST, form part of a molecular typing toolbox that can be used for the analysis of P. acnes isolates.
MATERIALS AND METHODS
Bacterial strains and growth.
A total of 312 P. acnes isolates were used to validate the multiplex PCR (145 type IA1, 20 type IA2, 65 type IB, 7 type IC, 45 type II, and 30 type III isolates). These isolates were previously recovered from a wide range of clinical sources and healthy skin, and their phylogroup status was determined using an MLST scheme based on eight genes (38). Representative samples of isolates from each group were also analyzed by MAb typing, as previously described (18), and their reactivities were consistent with their phylogroup designations based on MLST. Genomic DNA from a panel of 49 isolates representing 34 medically relevant bacterial species, which also included other human Propionibacterium species, was also used in the assessment of multiplex specificity (see Table S2 in the supplemental material). All bacterial strains were maintained at −80°C in brain heart infusion (BHI) broth containing 12% (vol/vol) glycerol. The anaerobic isolates were cultured in BHI and on anaerobic horse blood agar (ABA) plates (Oxoid Ltd., Hampshire, United Kingdom) at 37°C in an anaerobic cabinet (Mark III; Don Whitley Scientific) under an atmosphere of 10% H2, 10% CO2, and 80% N2. The aerobic bacteria were cultured on horse blood agar at 37°C.
Development of phylogroup-specific primers.
Housekeeping gene sequences representing aroE (424 bp), atpD (453 bp), gmk (400 bp), guaA (493 bp), lepA (452 bp), and sodA (450 bp) were retrieved from the P. acnes MLST database (http://pubmlst.org/pacnes/). The sequences for each gene were then aligned using the MEGA version 5.1 software and inspected for phylogroup-specific polymorphisms. Phylogroup-specific genomic regions were also investigated using the progressiveMauve algorithm (version 2.3.1) and the Artemis Comparison Tool (ACT) (http://www.sanger.ac.uk/Software/ACT/) using whole-genome sequences (WGS) currently available as part of the HMP and other sequencing projects (http://www.ncbi.nlm.nih.gov/genome/genomes/1140) (see Table S1 in the supplemental material). Based on these analyses, phylogroup-specific primer sets were developed and are listed in Table 1.
TABLE 1.
Multiplex PCR primer characteristics
| Primera | Specificity | Gene(s) targeted | Sequence (5′ to 3′) | Positions | Concn (μM) | Annealing temp (°C) | Amplicon size (bp) |
|---|---|---|---|---|---|---|---|
| PArA-1 | All P. acnes | 16S rRNA | AAGCGTGAGTGACGGTAATGGGTA | 442–465 | 0.2 | 66 | 677 |
| PArA-2 | CCACCATAACGTGCTGGCAACAGT | 1118–1095 | |||||
| PAMp-1 | Type IA1/IA2/IC | ATPase | GCGTTGACCAAGTCCGCCGA | 451–470 | 0.25 | 66 | 494 |
| PAMp-2 | GCAAATTCGCACCGCGGAGC | 944–925 | |||||
| PAMp-3 | Type IA2/IB | sodA | CGGAACCATCAACAAACTCGAA | 168–189 | 0.6 | 62 | 145 |
| PAMp-4 | GAAGAACTCGTCAATCGCAGCA | 312–291 | |||||
| PAMp-5 | Type IC | Toxin, Fic family | AGGGCGAGGTCCTCTTCTACCAGCG | 17–41 | 0.1 | 66 | 305 |
| PAMp-6 | ACCCTCCAACTGCAACTCTCCGCCT | 321–297 | |||||
| PAMp-7 | Type II | atpD | TCCATCTGGCCGAATACCAGG | 339–360 | 0.15 | 66 | 351 |
| PAMp-8 | TCTTAACGCCGATCCCTCCAT | 689–669 | |||||
| PAMp-9 | Type III | recA | GCGCCCTCAAGTTCTACTCA | 641–660 | 0.25 | 66 | 225 |
| PAMp-10 | CGGATTTGGTGATAATGCCA | 865–846 |
For protein-coding housekeeping genes, the primers relate to positions within the open reading frame. For the 16S rRNA gene, the primers relate to positions within the sequence for P. acnes NCTC 737 (GenBank accession no. AB042288).
Multiplex PCR analysis.
Bacterial genomic DNA was prepared using an AquaGenomic kit (MultiTarget Pharmaceuticals). PCR amplification was carried out using a MultiGene thermocycler (Labnet International, Inc., United Kingdom). The samples contained 1× PCR buffer, 200 μM each deoxynucleoside triphosphate (Invitrogen Life Technologies, United Kingdom), six primer sets targeting each phylogroup at the concentrations described in Table 1, 1.5 mM MgCl2, 1× RediLoad (Invitrogen Life Technologies), 1.25 U Taq DNA polymerase (Invitrogen Life Technologies), and 50 ng of pure genomic DNA preparation in a total volume of 10 μl. The samples were initially heated at 94°C for 1 min, followed by 14 cycles consisting of 94°C for 30 s, 66°C (decreasing incrementally by 0.3°C per cycle) for 30 s, and 72°C for 1 min, followed by 11 cycles at 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min, culminating with a final cycle at 72°C for 10 min. A negative control (PCR water) and six positive-control samples representing all phylogroups were included in all experiments. The PCR products were analyzed by electrophoresis on 1.5% (wt/vol) agarose gels containing 1× Tris-acetate-EDTA buffer. The molecular size markers were run in parallel on all gels. The resolved DNA products were stained with 1× GelRed nucleic acid gel stain (Cambridge Biosciences, United Kingdom).
Nucleotide sequencing.
The sequencing reactions were performed using the BigDye Terminator cycle sequencing kit (version 1.1) (Life Technologies, United Kingdom), according to the manufacturer's instructions. The samples were then analyzed on an ABI Prism 3100 genetic analyzer capillary electrophoresis system (Life Technologies).
Split decomposition analysis.
Split decomposition analysis was performed using SplitsTree4 version 4.13.1 (49).
RESULTS
Primer design.
Polymorphisms in multiple aligned sequences of the sodA gene specific for types IA2 and IB, and the atpD and recA genes specific to types II and III, respectively, were identified as candidate regions for primer development (Table 1). Primers were also developed against the ATP-binding component (ATPase; GenBank accession no. ABB20821.1) of a previously described ABC-type peptide uptake operon (GenBank accession no. DQ208967) present in the closely related type IA1, IA2, and IC groups but absent in type IB, II, and III strains (Table 1) (43). This operon also includes genes encoding permeases (GenBank accession no. ABB20819.1 and ABB20820.1) and a solute binding protein (GenBank accession no. ABB20823.1) alongside genes for a glycoside hydrolase (GenBank accession no. ABB20818.1) and chitinase (GenBank accession no. ABB20824). For the type IC strains, we developed a primer set targeting a Fic family toxin gene located on an approximately 7.3-kb genomic fragment present in the draft genome sequences of the P. acnes type IC strains PRP-38 (TICEST70_07737) and HL097PA1 (HMPREF9344_02057) but not other phylogroups (Table 1). This genomic fragment also contained restriction enzyme-associated genes and a gene encoding a DEAD/DEAH box helicase (HMPREF9344_02061). Our previously described P. acnes-specific 16S rRNA gene-based primers were also included in the assay to confirm species identity (Table 1) (11, 50). The primer sets incorporated phylogroup-specific mismatches at the 3′ end, and elsewhere in the sequence when available, and were designed to have identical annealing temperatures where possible and to generate amplicons with characteristic size differences that would facilitate easy visual identification on a gel after multiplexing.
Multiplex PCR development and validation.
Each individual phylogroup-specific primer set was initially examined against a small panel of strains (n = 40) representing types IA1, IA2, IB, IC, II, and III. Amplicons of the predicted size were correctly generated from the targeted phylogroup, and no products were unexpectedly observed in the divisions outside those targeted by the primers (data not shown). The identity of each PCR product was confirmed by direct nucleotide sequence analysis (data not shown). Each primer set was then combined into a single multiplex touchdown PCR, which was optimized for final primer and MgCl2 concentrations as well as amplification cycles, as outlined in Materials and Methods. As the sodA primers PAMp-3/PAMp-4 had a lower annealing temperature (62°C) than that of all the other primer sets (66°C), a touchdown PCR approach was adopted to ensure satisfactory highly specific amplification of all gene targets within the assay. Using this approach, it proved possible to reliably determine the phylogeny of an isolate based on the combination of different phylogroup-specific amplification products, as illustrated in Fig. 1.
FIG 1.
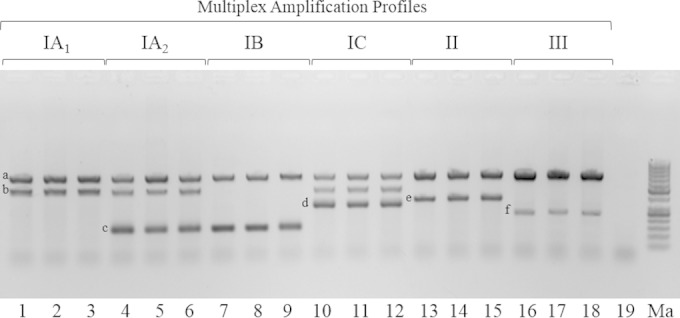
Multiplex PCR analysis of P. acnes strains (except lane 19) representing different phylogroups and STs. Lane 1, strain hdn-1 (ST1, type IA1); lane 2, strain PRP-60 (ST20, type IA1); lane 3, strain 76793 (ST101, type IA1); lane 4, strain Pacn33 (ST2, type IA2); lane 5, strain P.acn17 (ST2, type IA2); lane 6, strain P. acn31 (ST2, type IA2); lane 7, strain 6609 (ST5, type IB); lane 8, strain VA3/4 (ST78, type IB); lane 9, strain 74874 (ST43, type IB); lane 10, strain PRP-38 (ST70, type IC); lane 11, strain PV66 (ST85, type IC); lane 12, strain 5/1/3 (ST107, type IC); lane 13, strain ATCC 11828 (ST27, type II); lane 14, strain VA2/9N (ST28, type II); lane 15, strain 6187 (ST30, type II); lane 16, strain 12S (ST32, type III); lane 17, strain Asn12 (ST33, type III); lane 18, strain Asn10 (ST81, type III); lane 19, Propionibacterium avidum strain 44067. Ma, molecular size markers. ST is based on the eight-gene MLST scheme of McDowell et al. (21) and the database at http://pubmlst.org/pacnes/. Gene amplicons (left to right): a, 16S rRNA; b, ATPase; c, sodA; d, toxin; e, atpD; f, recA.
To assess the sensitivity and specificity of the multiplex assay, especially in relation to primers targeting genomic regions that were presumptively present/absent between phylogenetic divisions based on in silico analysis of the WGS data, we screened a large panel of 312 P. acnes isolates previously characterized by MLST and representing a total of 97 unique sequence types (ST) covering all the phylogroups. Based on this current sample cohort, the multiplex PCR displayed 100% specificity and 100% sensitivity for detecting isolates within each targeted phylogroup (Table 2); no cross-reactivity with isolates from a wide range of other medically relevant bacterial species was observed, including other cutaneous Propionibacterium and Staphylococcus species (Table 2; see also Table S2 in the supplemental material).
TABLE 2.
Multiplex PCR assay accuracy
| Phylogroupa | No. of isolates/total no. that wereb: |
Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Positive | Negative | |||
| IA1 | 145/145 | 0/145 | 100 | 100 |
| All others | 0/216 | 216/216 | ||
| IA2 | 20/20 | 0/20 | 100 | 100 |
| All others | 0/341 | 341/341 | ||
| IB | 65/65 | 0/65 | 100 | 100 |
| All others | 0/296 | 296/296 | ||
| IC | 7/7 | 0/7 | 100 | 100 |
| All others | 0/354 | 354/354 | ||
| II | 45/45 | 0/45 | 100 | 100 |
| All others | 0/316 | 316/316 | ||
| III | 30/30 | 0/30 | 100 | 100 |
| All others | 0/331 | 331/331 | ||
All others relates to P. acnes isolates outside the target phylogroup plus 49 isolates from other medically relevant species.
Positive relates to the detection of the expected amplification pattern under consideration, while negative indicates that one of the alternate phylogroup profiles was detected, or no reaction was observed in the case of other species.
DISCUSSION
Since the 16S rRNA gene of P. acnes has very high intraspecific sequence identity (18, 19), it afforded little opportunity for the design of phylogroup-specific primers on which we could base our multiplex assay. As an alternative, we examined various protein-coding housekeeping loci and interrogated available whole-genome sequences representing all known phylogroups for unique genetic regions that could act as platforms for assay development. By adopting this approach, we were able to design primers based on the atpD (PAMp-7/PAMp-8) and recA (PAMp-9/PAMp-10) housekeeping loci that specifically identified phylogroup II and III strains, respectively. Type IC strains were identified by their reaction with the primers PAMp-5/PAMp-6 that targeted a Fic family toxin gene present on a genomic region found in type IC strains only; such toxins form part of toxin-antitoxin (TA) systems, which are believed to be important in bacterial persistence in response to specific environmental stresses, as well as in pathogenicity (51). While types IA1, IA2, and IC all reacted with primers targeting an ATPase gene (PAMp-1/PAMp-2) that was part of an ABC-type peptide uptake operon, the differentiation of type IA2 and IC strains from IA1 and each other was achieved due to their separate reactions with the primers PAMp-3/PAMp-4 and PAMp-5/PAMp-6, respectively. The restriction of an ABC-type peptide uptake operon containing chitinase to type IA and IC divisions but not other phylogroups is of particular interest and is potentially advantageous for the cleavage of chitin from the cell walls of the fungus Malassezia and/or Demodex mites, which also colonize human skin (20). Since type IA2 isolates contain alleles of the sodA locus that are identical (allele 4) or very closely related (allele 5) to those present in all type IB strains (21, 38), both phylogenetic groups displayed a reaction with the sodA primer pair PAMp-3/PAMp-4. Their identities were, however, easily determined based on a differential reaction with the ATPase primers PAMp-1/PAMp-2; type IB isolates show no product with the PAMp-1/PAMp-2 primer set. Interestingly, as the type IA2 clade shares recA and tly alleles with type IB isolates, it provides evidence for HGT of large genomic fragments in the natural history of the bacterium (20, 21, 38).
By combining these different primer sets along with the 16S rRNA gene primers PArA-1 and PArA-2 into a single multiplex assay, we have been able to provide researchers with a robust method for the rapid and high-throughput molecular identification of presumptive P. acnes isolates, combined with valuable phylogenetic typing information. This assay should prove to be a useful tool for epidemiological studies and the stratification of isolates for various downstream analyses. It offers enhanced discrimination and specificity over comparable molecular (PCR and single-gene sequencing) and MAb typing approaches that have been described in the literature, and it is also less time-consuming, especially compared to methods that require multiple, separate analyses for each isolate. The multiplex PCR will facilitate future retrospective and prospective studies aimed at investigating the association of specific phylogenetic lineages with different human infections, clinical conditions, and antibiotic resistances and will now also provide a technically undemanding way to rapidly map multiple isolates from the same clinical sample so that the presence and pattern of mixed population types can be determined, especially at different body sites/niches. The method should also provide a useful complement to the more detailed and technically complicated study of P. acnes populations in complex microbiotas that is based on metagenomic analysis (23). In this study, although we utilized purified genomic DNA as the template for multiplex PCR, the future optimization of the method for direct analysis of bacterial colonies (colony PCR) would further enhance the rapid nature of the assay. Furthermore, of the P. acnes isolates tested, the numbers representing types IA2 and IC were lower than those from other phylogroups, especially type IC, which are infrequently recovered. As a consequence, the further analysis of additional isolates from these clusters will be important to confirm the multiplex PCR specificity and sensitivity results for these types.
To date, our understanding of the population structure of P. acnes within and between different body habitats of individuals is poor. These sites include not only various areas of, and regions within, the skin but also the oral cavity and genitourinary tract. Such data may prove especially valuable in our attempts to better understand the potential origin of different lineages associated with clinical samples, particularly in relation to blood culture, as well as whether the pattern of isolates recovered from primary surgical samples matches those on the overlying/surrounding skin, thus indicating potential contamination. In the surgical setting, the current methods used for preoperative skin antisepsis do not always prevent microbial contamination of surgical wounds with viable bacteria (52, 53). Although acute surgical site infections may not ensue due to effective intravenous (i.v.) prophylactic antibiotic administration, these bacteria may still cause downstream chronic biofilm-associated implant infections. It seems reasonable to assume that contamination from the skin would result in a mixture of different phylogenetic groups within a sample, while significant counts of one type may be more indicative of infection. Under such circumstances, the multiplex assay might prove to be a valuable and simple molecular screening tool to highlight such a scenario and thus aid in the diagnosis of biofilm-associated implant infections and bacteremia, etc. within a clinical setting (54). Furthermore, the detection of phylogroups with a potentially greater propensity to cause infection within a clinical sample, such as IA1, may also be more indicative of infection than those from phylogroups, such as types II and III, believed to be associated with a more commensal existence (21).
To date, two MLST schemes based on eight (MLST8) and nine (MLST9) different protein-coding genes have been described for P. acnes (20, 21); the methods are essentially concordant with respect to the clustering of strains into different clonal complexes (CCs), although more subtle differences in the resolution of particular lineages within these CCs exist (21, 38). While MLST provides high-resolution typing of P. acnes and generates not only phylogroup information but also sequence type (ST) data that are highly amenable to phylogenetic and evolutionary analyses, the method is laborious and time-consuming when investigating multiple isolates. The development of new approaches to help streamline the MLST workflow are very attractive, and recently, we described how cross-referencing a refined four-gene MLST allelic profile to the full eight-gene version available in the MLST database (see http://pubmlst.org/pacnes/) can be used to correctly predict and assign phylogroup, CC, and, in the vast majority of cases, ST for a P. acnes isolate (38). In this context, the rapid prescreening of isolates by multiplex PCR might also prove to be an extremely valuable way to maximize the number of genetically diverse isolates selected for downstream MLST analyses, thus reducing sequencing costs. Furthermore, MLST and whole-genome analyses have shown that types IA2, IB, IC, and III represent tight phylogenetic clusters, especially when compared to types IA1 and II (21, 22, 38) (see Table S1 in the supplemental material); this is reflected in a more restricted number of STs, some of which are highly dominant and widely disseminated. As a consequence, high-resolution MLST typing after multiplex PCR provides less-useful phylogenetic information for type IA2, IB, IC, and III isolates than that for types IA1 and II, which are genetically more heterogeneous and contain deeper levels of phylogenetic structure. In keeping with the desirability of a simpler approach to high-resolution typing of P. acnes, a single-locus typing scheme (SLST) for the bacterium based on nucleotide sequencing of an amplified target region (484 bp) immediately upstream of the camp1 gene (identified by a genome mining approach) was described during the preparation of this paper (55). While the MLST8 and MLST9 schemes resolve a greater number of genotypes than does SLST (see Table S1), SLST does correctly cluster isolates into phylogenetic groupings that are congruent with a core genome reference tree (55). In addition, there is little evidence of recombination within the locus based on a network tree analysis (phi test, P = 0.976) (see Fig. S1 in the supplemental material). The SLST method is, therefore, a valuable complement and technically simpler approach to current MLST methods for typing P. acnes.
The rapid screening of isolates by multiplex PCR will also aid the discovery of novel taxa via atypical PCRs. For example, a sole reaction with the 16S rRNA gene primer set PArA-1/PArA-2 may indicate a new closely related species of Propionibacterium with high 16S rRNA gene identity to P. acnes or a novel P. acnes phylogroup or ST that contains base mismatches at primer binding sites within the protein-coding genes of the assay. Indeed, as a direct result of multiplex PCR screening of skin-derived isolates, we recently came across such a scenario and identified a new Propionibacterium species that has very high 16S rRNA gene identity to P. acnes (and reacts with PArA-1/PArA-2) but is quite distinct from it, and other cutaneous propionibacteria, based on whole-genome analysis (J. Hunyadkürti, A. Vörös, B. Bálint, R. Herczeg, E. Urbán, M. Göker, M. Kostrzewa, A. McDowell, and I. Nagy, unpublished data).
In conclusion, the multiplex PCR described here facilitates the rapid molecular confirmation of presumptive P. acnes isolates along with parallel phylogenetic typing. It should provide researchers with a flexible typing tool that can be used in isolation or as an adjunct to more detailed sequence-based analysis, depending on the epidemiological questions being asked and the resolution required. It is also a technically simple methodology for the rapid analysis of mixed P. acnes populations and should therefore help improve our understanding of the roles of different P. acnes lineages in clinical conditions.
Supplementary Material
ACKNOWLEDGMENTS
A.M. was supported by a Health and Social Care Northern Ireland Research & Development Division grant awarded to S.P. (grant HSCNI RRG 9.41) and E.B. by a Prostate Cancer UK grant awarded to A.M. and S.P. (grant 110831). Funding to I.N. was from the French-Hungarian Associated European Laboratory (LEA) SkinChroma (grant OMFB-00272/2009); I.N. was also supported by the János Bólyai Research Scholarship of the Hungarian Academy of Sciences.
The Propionibacterium humerusii isolates were a kind gift from Susan Butler-Wu, University of Washington, USA. We also thank Derek Fairley (Belfast Health and Social Care Trust) for kindly providing genomic DNAs from a range of other bacterial species.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02460-14.
REFERENCES
- 1.Patrick S, McDowell A. 2011. Genus I. Propionibacterium, p 1138–1156. In Whitman W, Parte A, Goodfellow M, Kämpfer P, Busse HJ, Trujillo ME, Ludwig W, Suzuki K (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 5 Springer, New York, NY. [Google Scholar]
- 2.Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, McDowell A, Patrick S, Alexeyev OA. 2012. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol 167:50–58. doi: 10.1111/j.1365-2133.2012.10897.x. [DOI] [PubMed] [Google Scholar]
- 3.Beylot C, Auffret N, Poli F, Claudel J-P, Leccia M-T, Del Giudice P, Dreno B. 2014. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol 28:271–278. doi: 10.1111/jdv.12224. [DOI] [PubMed] [Google Scholar]
- 4.Levy O, Iyer S, Atoun E, Peter N, Hous N, Cash D, Musa F, Narvani AA. 2013. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 22:505–511. doi: 10.1016/j.jse.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Pan S-C, Wang J-T, Hsueh P-R, Chang S-C. 2005. Endocarditis caused by Propionibacterium acnes: an easily ignored pathogen. J Infect 51:e229–e31. doi: 10.1016/j.jinf.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Nisbet M, Briggs S, Ellis-Pegler R, Thomas M, Holland D. 2007. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother 60:1097–1103. doi: 10.1093/jac/dkm351. [DOI] [PubMed] [Google Scholar]
- 7.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. 1998. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br 80:568–572. doi: 10.1302/0301-620X.80B4.8473. [DOI] [PubMed] [Google Scholar]
- 8.Thompson TP, Albright AL. 1998. Propionibacterium [correction of Proprionibacterium] acnes infections of cerebrospinal fluid shunts. Childs Nerv Syst 14:378–380. doi: 10.1007/s003810050248. [DOI] [PubMed] [Google Scholar]
- 9.Clayton JJ, Baig W, Reynolds GW, Sandoe JAT. 2006. Endocarditis caused by Propionibacterium species: a report of three cases and a review of clinical features and diagnostic difficulties. J Med Microbiol 55:981–987. doi: 10.1099/jmm.0.46613-0. [DOI] [PubMed] [Google Scholar]
- 10.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. 2007. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect 55:119–124. doi: 10.1016/j.jinf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montano N, Sturiale C, Paternoster G, Lauretti L, Fernandez E, Pallini R. 2010. Massive ascites as unique sign of shunt infection by Propionibacterium acnes. Br J Neurosurg 24:221–223. doi: 10.3109/02688690903531067. [DOI] [PubMed] [Google Scholar]
- 13.Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, Nørgaard HS, Vernallis A, Busch F, Manniche C, Elliott T. 2013. Does nuclear tissue infected with bacteria following disc herniations lead to modic changes in the adjacent vertebrae? Eur Spine J 22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, Yokoyama T, Iwai K, Watanabe K, Shimizu S, Ishida N, Suzuki Y, Suzuki T, Yamada T, Ito T, Eishi Y. 2009. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog 46:80–87. doi: 10.1016/j.micpath.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Eishi Y. 2013. Etiologic link between sarcoidosis and Propionibacterium acnes. Respir Invest 51:56–68. doi: 10.1016/j.resinv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. 2005. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol 173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 17.Fassi Fehri L, Mak TN, Laube B, Brinkmann V, Ogilvie LA, Mollenkopf H, Lein M, Schmidt T, Meyer TF, Brüggemann H. 2011. Prevalence of Propionibacterium acnes in diseased prostates and its inflammatory and transforming activity on prostate epithelial cells. Int J Med Microbiol 301:69–78. doi: 10.1016/j.ijmm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 18.McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, Bhatia A, Maisonneuve J-F, Lodes M, Persing DH, Patrick S. 2005. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol 43:326–334. doi: 10.1128/JCM.43.1.326-334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDowell A, Perry AL, Lambert PA, Patrick S. 2008. A new phylogenetic group of Propionibacterium acnes. J Med Microbiol 57:218–224. doi: 10.1099/jmm.0.47489-0. [DOI] [PubMed] [Google Scholar]
- 20.Lomholt HB, Kilian M. 2010. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One 5:e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, Eady A, Cove J, Nord CE, Patrick S. 2012. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of “pathogenic,” “commensal” and antibiotic resistant strains. PLoS One 7:e41480. doi: 10.1371/journal.pone.0041480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilian M, Scholz CF, Lomholt HB. 2012. Multilocus sequence typing and phylogenetic analysis of Propionibacterium acnes. J Clin Microbiol 50:1158–1165. doi: 10.1128/JCM.r06129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitz-Gibbon S, Tomida S, Chiu B-H, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, Modlin RL, Miller JF, Sodergren E, Craft N, Weinstock GM, Li H. 2013. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Investig Dermatol 133:2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomida S, Nguyen L, Chiu BH, Liu J, Sodergren E, Weinstock GM, Li H. 2013. Pan-Genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. mBio 4(3):e00003–13. doi: 10.1128/mBio.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunyadkürti J, Feltóti Z, Horváth B, Nagymihály M, Vörös A, McDowell A, Patrick S, Urbán E, Nagy I. 2011. Complete genome sequence of Propionibacterium acnes type IB strain 6609. J Bacteriol 193:4561–4562. doi: 10.1128/JB.05372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horváth B, Hunyadkürti J, Vörös A, Fekete C, Urbán E, Kemény L, Nagy I. 2012. Genome sequence of Propionibacterium acnes type II strain ATCC 11828. J Bacteriol 194:202–203. doi: 10.1128/JB.06388-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vörös A, Horváth B, Hunyadkürti J, McDowell A, Barnard E, Patrick S, Nagy I. 2012. Complete genome sequences of three Propionibacterium acnes isolates from the type IA2 cluster. J Bacteriol 194:1621–1622. doi: 10.1128/JB.06758-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDowell A, Hunyadkürti J, Horváth B, Vörös A, Barnard E, Patrick S, Nagy I. 2012. Draft genome sequence of an antibiotic-resistant Propionibacterium acnes strain, PRP-38, from the novel type IC cluster. J Bacteriol 194:3260–3261. doi: 10.1128/JB.00479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O'Hagan S, Wisdom GB, Fairley D, Bhatia A, Maisonneuve J-F, Lodes M, Persing DH, Patrick S. 2005. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology 151:1369–1379. doi: 10.1099/mic.0.27788-0. [DOI] [PubMed] [Google Scholar]
- 30.Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, Brüggemann H. 2010. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol 10:230. doi: 10.1186/1471-2180-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, Nagy I, Lambert PA, Patrick S. 2011. A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface-associated antigens. Microbiology 157:1990–2003. doi: 10.1099/mic.0.049676-0. [DOI] [PubMed] [Google Scholar]
- 32.Brzuszkiewicz E, Weiner J, Wollherr A, Thürmer A, Hüpeden J, Lomholt HB, Kilian M, Gottschalk G, Daniel R, Mollenkopf H-J, Meyer TF, Brüggemann H. 2011. Comparative genomics and transcriptomics of Propionibacterium acnes. PLoS One 6:e21581. doi: 10.1371/journal.pone.0021581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JL, Cummins CS. 1972. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol 109:1047–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagy I, Pivarcsi A, Koreck A, Széll M, Urbán E, Kemény L. 2005. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Investig Dermatol 124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 35.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, Seltmann H, Patrick S, Zouboulis CC, Kemény L. 2006. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect 8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Lodes MJ, Secrist H, Benson DR, Jen S, Shanebeck KD, Guderian J, Maisonneuve J-F, Bhatia A, Persing D, Patrick S, Skeiky YA. 2006. Variable expression of immunoreactive surface proteins of Propionibacterium acnes. Microbiology 152:3667–3681. doi: 10.1099/mic.0.29219-0. [DOI] [PubMed] [Google Scholar]
- 37.Lomholt HB, Kilian M. 2014. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta Derm Venereol 94:534–538. doi: 10.2340/00015555-1794. [DOI] [PubMed] [Google Scholar]
- 38.McDowell A, Nagy I, Magyari M, Barnard E, Patrick S. 2013. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One 8:e70897. doi: 10.1371/journal.pone.0070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexeyev OA, Jahns AC. 2012. Sampling and detection of skin Propionibacterium acnes: current status. Anaerobe 18:479–483. doi: 10.1016/j.anaerobe.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Kishishita M, Ushijima T, Ozaki Y, Ito Y. 1979. Biotyping of Propionibacterium acnes isolated from normal human facial skin. Appl Environ Microbiol 38:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy E, Urbán E, Becker S, Kostrzewa M, Vörös A, Hunyadkürti J, Nagy I. 2013. MALDI-TOF MS fingerprinting facilitates rapid discrimination of phylotypes I, II and III of Propionibacterium acnes. Anaerobe 20:20–26. doi: 10.1016/j.anaerobe.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Davidsson S, Söderquist B, Elgh F, Olsson J, Andrén O, Unemo M, Mölling P. 2012. Multilocus sequence typing and repetitive-sequence-based PCR (DiversiLab) for molecular epidemiological characterization of Propionibacterium acnes isolates of heterogeneous origin. Anaerobe 18:392–399. doi: 10.1016/j.anaerobe.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Shannon BA, Cohen RJ, Garrett KL. 2006. Polymerase chain reaction-based identification of Propionibacterium acnes types isolated from the male urinary tract: evaluation of adolescents, normal adults and men with prostatic pathology. BJU Int 98:388–392. doi: 10.1111/j.1464-410X.2006.06273.x. [DOI] [PubMed] [Google Scholar]
- 44.Holmberg A, Lood R, Mörgelin M, Söderquist B, Holst E, Collin M, Christensson B, Rasmussen M. 2009. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect 15:787–795. doi: 10.1111/j.1469-0691.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 45.Rollason J, McDowell A, Albert HB, Barnard E, Worthington T, Hilton AC, Vernallis A, Patrick S, Elliott T, Lambert P. 2013. Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int 2013:530382. doi: 10.1155/2013/530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry AL, Worthington T, Hilton AC, Lambert PA, Stirling AJ, Elliott TSJ. 2003. Analysis of clinical isolates of Propionibacterium acnes by optimised RAPD. FEMS Microbiol Lett 228:51–55. doi: 10.1016/S0378-1097(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 47.Oprica C, Nord CE, ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria . 2005. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect 11:204–213. doi: 10.1111/j.1469-0691.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- 48.Unemo M, Friberg O, Enquist E, Källman J, Söderquist B. 2007. Genetic homogeneity/heterogeneity of Propionibacterium acnes isolated from patients during cardiothoracic reoperation. Anaerobe 13:121–126. doi: 10.1016/j.anaerobe.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 50.Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, Yaszemski MJ, Currier BL, Mandrekar JN, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Patel R. 2010. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 35:1218–1224. doi: 10.1097/BRS.0b013e3181c3b2f3. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi Y, Park JH, Inouye M. 2011. Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet 45:61–79. doi: 10.1146/annurev-genet-110410-132412. [DOI] [PubMed] [Google Scholar]
- 52.McLorinan GC, Glenn JV, McMullan MG, Patrick S. 2005. Propionibacterium acnes wound contamination at the time of spinal surgery. Clin Orthop Relat Res 67–73. doi: 10.1097/00003086-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Jonsson EÖ, Johannesdottir H, Robertsson O, Mogensen B. 2014. Bacterial contamination of the wound during primary total hip and knee replacement. Median 13 years of follow-up of 90 replacements. Acta Orthop 85:159–164. doi: 10.3109/17453674.2014.899848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrick S, McDowell A. 2013. Propionibacterium acnes: an emerging pathogen in biomaterial-associated infection, p 87–105. In Moriarty TF, Zaat SAJ, Busscher HJ (ed), Biomaterials associated infection. Springer, New York, NY. [Google Scholar]
- 55.Scholz CFP, Jensen A, Lomholt HB, Brüggemann H, Kilian M. 2014. A novel high-resolution single locus sequence typing scheme for mixed populations of Propionibacterium acnes in vivo. PLoS One 9:e104199. doi: 10.1371/journal.pone.0104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


