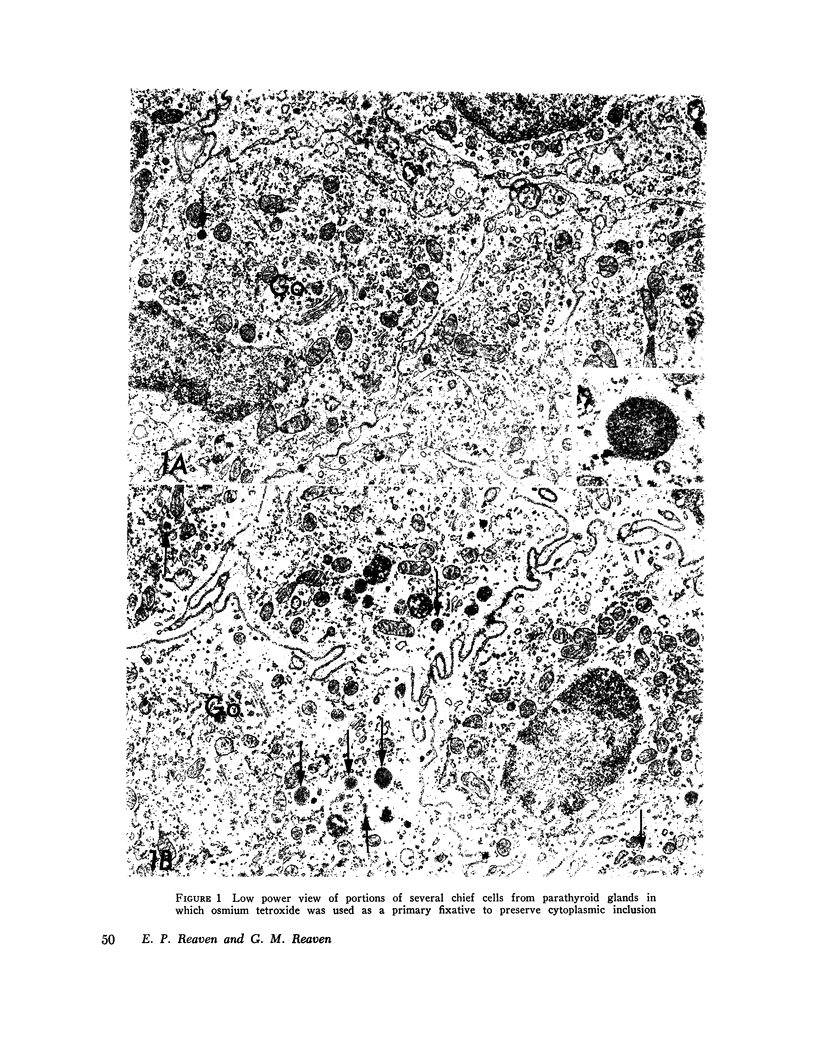
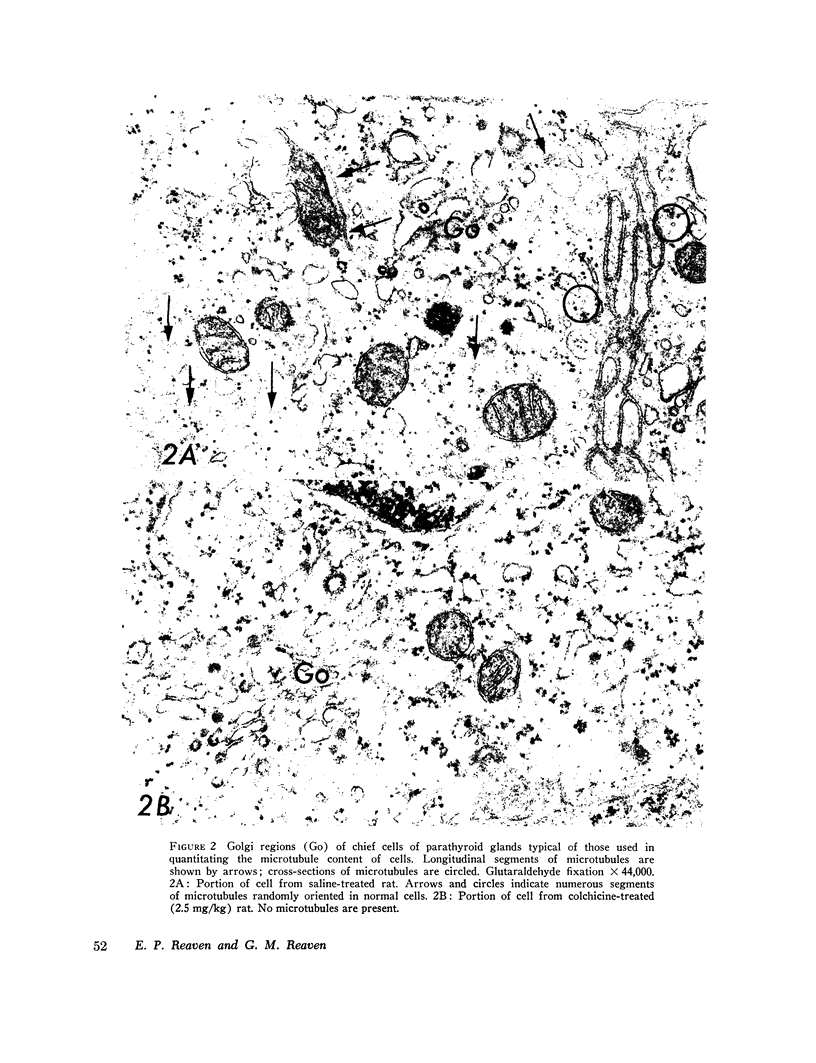
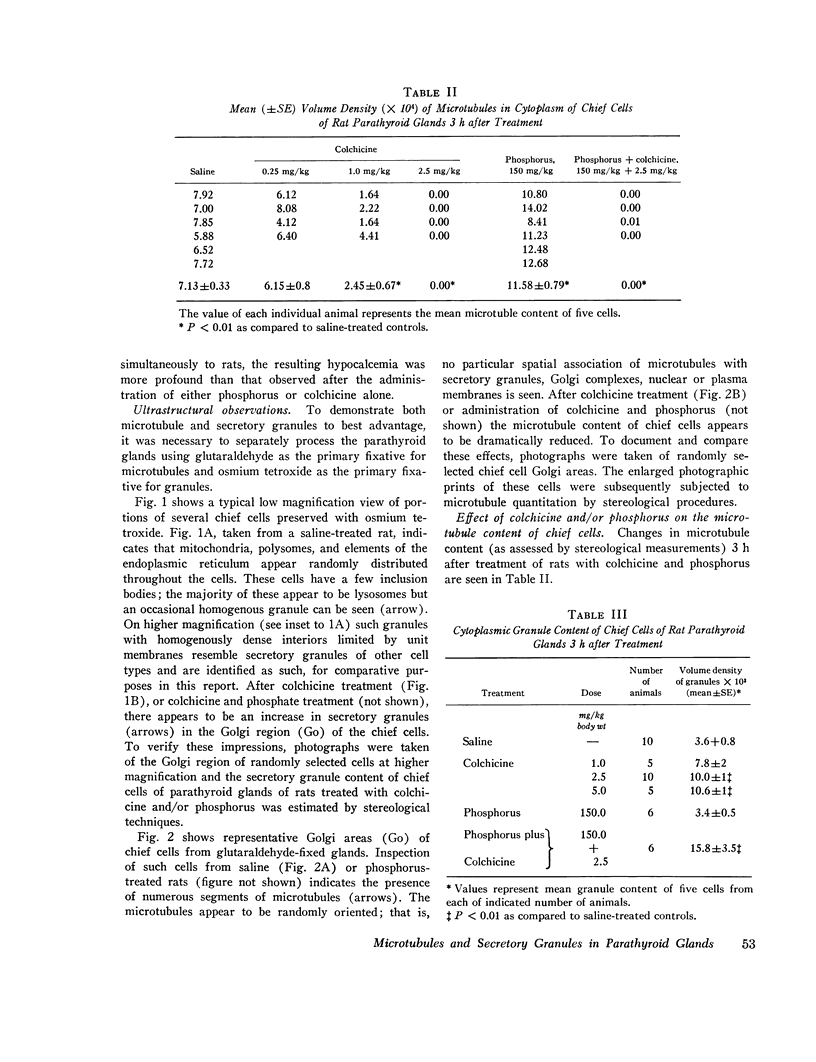
Abstract
Microtubule involvement in secretory events of the parathyroid gland was investigated in rats treated with colchicine and/or phosphorus, agents which have been shown to modify parathyroid secretion. Quantitative ultrastructural techniques were used in an effort to assess the cytoplasmic microtubule and secretory granule content of chief cells 3 h after treatment, when hypocalcemia was well established. After cochicine administration, the chief cells appeared to have lost all assembled microtubules and accumulated greater than normal amounts of cytoplasmic secretory granules. On the other hand, phosphorus treatment was associated with increased microtubule content although the cytoplasmic content of secretory granules remained unchanged. When colchicine and phosphorus were given concomitantly, microtubules were again absent, but the secretory granule content of the cells was markedly increased. These data provide direct evidence that colchicine disrupts assembled microtubules in chief cells of rat parathyroids; the consequence of this effect appears to be a blockage of hormone release which is reflected in the accumulation of secretory granules in the cell. The fact that microtubules also show a significant increase in content when hormone release from chief cells is presumed to increase, suggests that microtubules may participate in the physiological control of parathyroid hormone secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiraseveenuprapund P., Rosenberg I. N. Effects of colchicine on the formation of thyroid hormone. Endocrinology. 1974 Apr;94(4):1086–1093. doi: 10.1210/endo-94-4-1086. [DOI] [PubMed] [Google Scholar]
- Devis G., Van Obberghen E., Somers G., Malaisse-Lagae F., Orci L., Malaisse W. J. Dynamics of insulin release and microtubular-microfilamentous system. II. Effect of vincristine. Diabetologia. 1974 Feb;10(1):53–59. doi: 10.1007/BF00421414. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Effects of colchicine and vinblastine on the cellular action of vasopressin in mammalian kidney. A possible role of microtubules. J Clin Invest. 1974 Aug;54(2):252–262. doi: 10.1172/JCI107760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm R., Ericson L. E., Josefsson J. O., Melander A. In vivo action of vinblastine on thyroid ultrastructure and hormone secretion. Endocrinology. 1974 Mar;94(3):641–649. doi: 10.1210/endo-94-3-641. [DOI] [PubMed] [Google Scholar]
- Heath D. A., Palmer J. S., Aurbach G. D. The hypocalcemic action of colchicine. Endocrinology. 1972 Jun;90(6):1589–1593. doi: 10.1210/endo-90-6-1589. [DOI] [PubMed] [Google Scholar]
- Holtrop M. E., Raisz L. G., Simmons H. A. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol. 1974 Feb;60(2):346–355. doi: 10.1083/jcb.60.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Rich A., Potts J. T., Jr Microtubules and the intracellular conversion of proparathyroid hormone to parathyroid hormone. Endocrinology. 1975 Apr;96(4):903–912. doi: 10.1210/endo-96-4-903. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Rich A., Potts J. T., Jr Parathyroid secretion: discovery of a major calcium-dependent protein. Science. 1974 Apr 12;184(4133):167–169. doi: 10.1126/science.184.4133.167. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Walker M. M., Fink C. J. Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin. Diabetes. 1972 Oct;21(10):987–998. doi: 10.2337/diab.21.10.987. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Patzelt C., Assimacopoulos-Jeannet F., Loten E. G., Jeanrenaud B. Evidence for a role of the microtubular system in the secretion of newly synthesized albumin and other proteins by the liver. J Clin Invest. 1974 Jun;53(6):1512–1517. doi: 10.1172/JCI107701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Holtrop M. E., Simmons H. A. Inhibition of bone resorption by colchicine in organ culture. Endocrinology. 1973 Feb;92(2):556–562. doi: 10.1210/endo-92-2-556. [DOI] [PubMed] [Google Scholar]
- Stein O., Sanger L., Stein Y. Colchicine-induced inhibition of lipoprotein and protein secretion into the serum and lack of interference with secretion of biliary phospholipids and cholesterol by rat liver in vivo. J Cell Biol. 1974 Jul;62(1):90–103. doi: 10.1083/jcb.62.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. Colchicine-induced inhibition of very low density lipoprotein release by rat liver in vivo. Biochim Biophys Acta. 1973 Apr 13;306(1):142–147. doi: 10.1016/0005-2760(73)90219-1. [DOI] [PubMed] [Google Scholar]
- Taylor A., Maffly R., Wilson L., Reaven E. Evidence for involvement of microtubules in the action of vasopressin. Ann N Y Acad Sci. 1975 Jun 30;253:723–737. doi: 10.1111/j.1749-6632.1975.tb19241.x. [DOI] [PubMed] [Google Scholar]
- Taylor A., Mamelak M., Reaven E., Maffly R. Vasopressin: possible role of microtubules and microfilaments in its action. Science. 1973 Jul 27;181(4097):347–350. doi: 10.1126/science.181.4097.347. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Colchicine-binding protein and the secretion of thyroid hormone. J Cell Biol. 1972 Jul;54(1):157–165. doi: 10.1083/jcb.54.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]
- Wilson L., Meza I. The mechanism of action of colchicine. Colchicine binding properties of sea urchin sperm tail outer doublet tubulin. J Cell Biol. 1973 Sep;58(3):709–719. doi: 10.1083/jcb.58.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]