Abstract
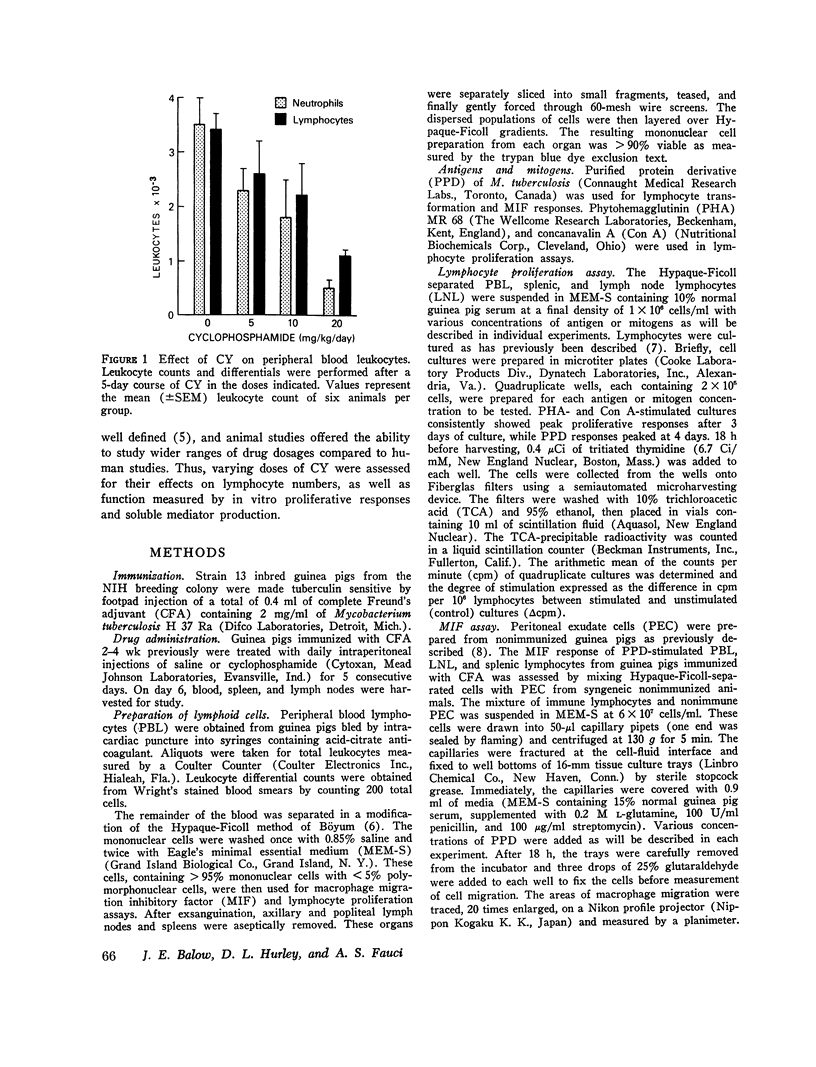
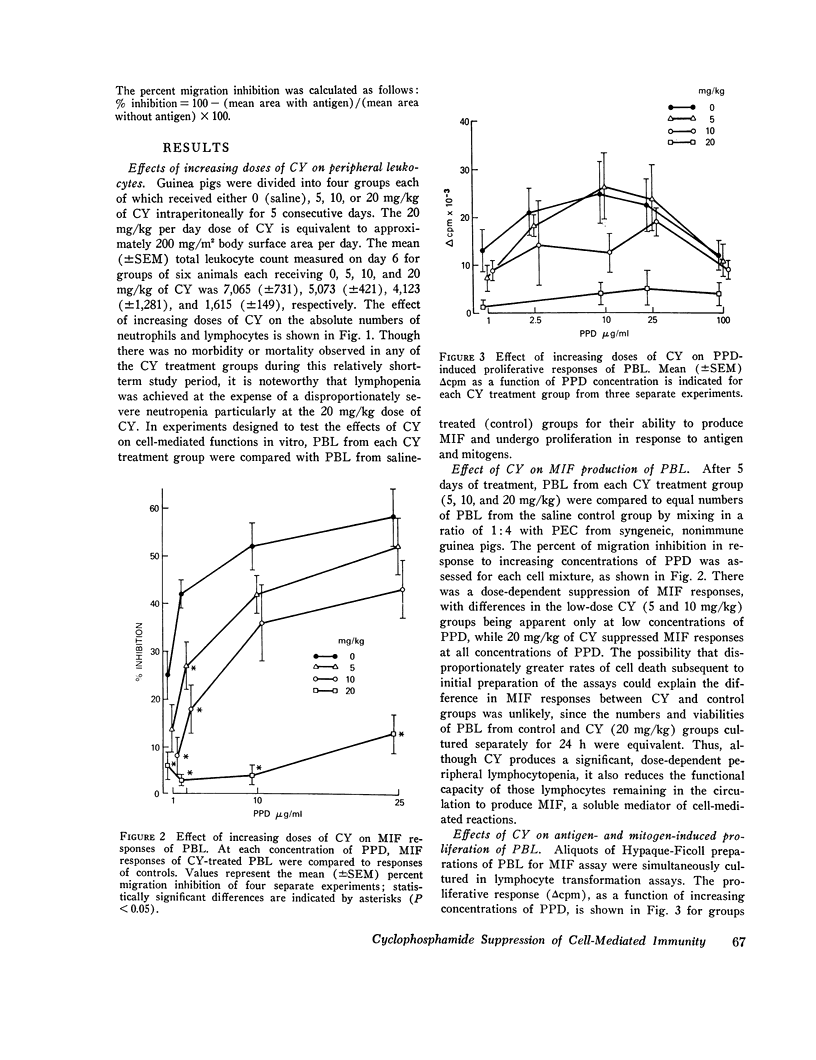
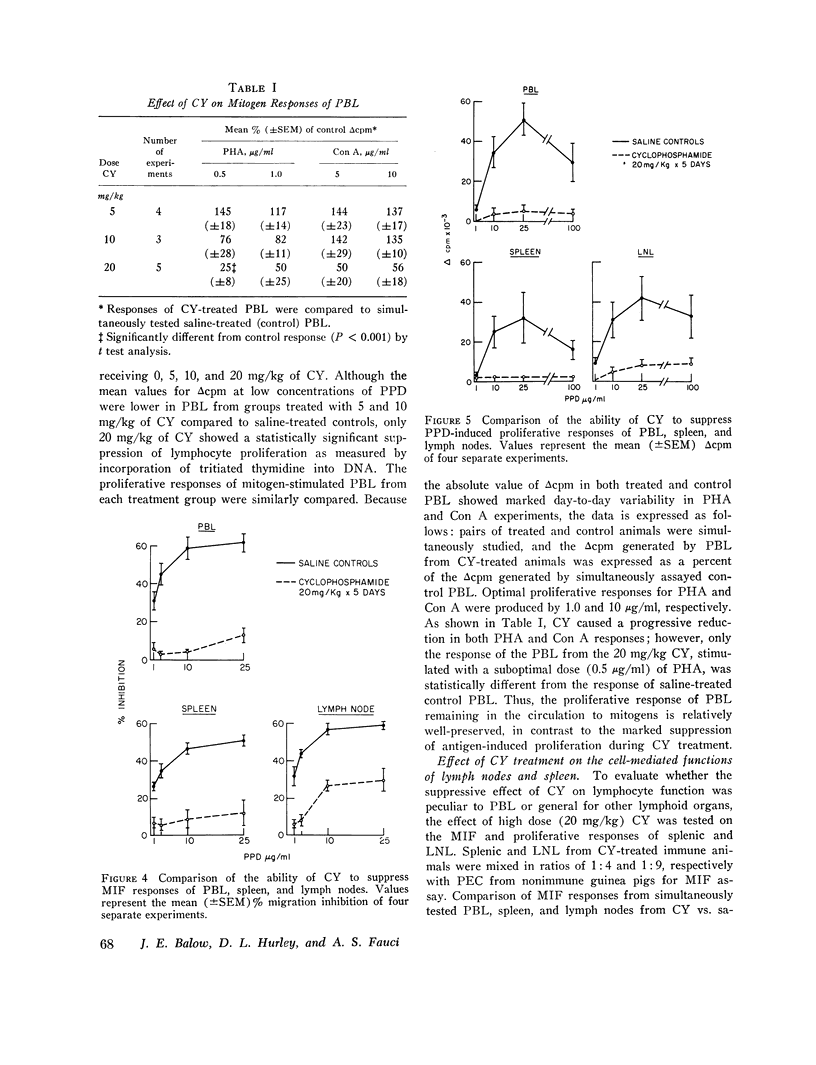
The characteristics of cyclophosphamide-induced suppression of established ccll mediated immunity were studied in guinea pigs previously senstized to tuberculin. Cyclophosphamide treatment for 5 days produced a dose-dependent peripheral lymphoctopenia and disproportionatley greater neutrophenia which was particularly striking at high doses of 20 mg/kg per day(approximaetly 200 mg/kg-2 per day). Lymphoctes remianing in the circulation of cyclophosphamide treeated aniamls showed a doses-dependent reduction to both in vitro proliferactive and macrophage migration inhibitory factor responses to tuberculin compared to lymphocte responses of controls. Proliferative responses to phytohemaggultinin and concanavalin a were not significatly suppressed. Additional studies showed that cyclophosphamide suppressed the porliferactive and migration inhibitroy factor responses to tuberculin of lymph node and splenic as well as cirulating lymphocte populations. These studies showed that relatively short-term cyclophospamide administration produced immunosuppresion by quantitative as well as qualitative changes in lymphocyte populations. Significant suppresion of lymphocte function, howerver, was achived only with doses of cyclophoshamide which also produced a severe neutropenia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alepa F. P., Zvaifler N. J., Sliwinski A. J. Immunologic effects of cyclophosphamide treatment in rheumatoid arthritis. Arthritis Rheum. 1970 Nov-Dec;13(6):754–760. doi: 10.1002/art.1780130604. [DOI] [PubMed] [Google Scholar]
- Arinoviche R., Loewi G. Comparison of the effects of two cytotoxic drugs and of antilymphocytic serum on immune and non-immune inflammation in experimental animals. Ann Rheum Dis. 1970 Jan;29(1):32–39. doi: 10.1136/ard.29.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Hurley D. L., Fauci A. S. Immunosuppressive effects of glucocorticosteroids: differential effects of acute vs chronic administration on cell-mediated immunity. J Immunol. 1975 Mar;114(3):1072–1076. [PubMed] [Google Scholar]
- Balow J. E., Rosenthal A. S. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973 Apr 1;137(4):1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Gaffney J., Jimenez L. Dissociation of MIF production and cell proliferation. J Immunol. 1972 Dec;109(6):1395–1398. [PubMed] [Google Scholar]
- Clements P. J., Yu D. T., Levy J., Paulus H. E., Barnett E. V. Effects of cyclophosphamide on B- and T-lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1974 Jul-Aug;17(4):347–353. doi: 10.1002/art.1780170403. [DOI] [PubMed] [Google Scholar]
- Curtis J. E., Sharp J. T., Lidsky M. D., Hersh E. M. Immune response of patients with rheumatoid arthritis during cyclophosphamide treatment. Arthritis Rheum. 1973 Jan-Feb;16(1):34–42. doi: 10.1002/art.1780160106. [DOI] [PubMed] [Google Scholar]
- David J. R., David R. R. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300–449. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Wolff S. M. Cyclophosphamide and lymphocyte subpopulations in Wegener's granulomatosis. Arthritis Rheum. 1974 Jul-Aug;17(4):355–361. doi: 10.1002/art.1780170404. [DOI] [PubMed] [Google Scholar]
- Fink M. P., Cloud C. L. Graft-versus-host disease in rats after donor treatment with cyclophosphamide and spleen cells of host origin. Transplantation. 1974 May;17(5):508–512. doi: 10.1097/00007890-197405000-00011. [DOI] [PubMed] [Google Scholar]
- Gelfand M. C., Steinberg A. D. Therapeutic studies in NZB-W mice. II. Relative efficacy of azathioprine, cyclophosphamide and methylprednisolone. Arthritis Rheum. 1972 May-Jun;15(3):247–252. doi: 10.1002/art.1780150305. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Goetzl E. J., Steinberg A. D. Cyclophosphamide: use in practice. Ann Intern Med. 1974 Apr;80(4):531–540. doi: 10.7326/0003-4819-80-4-531. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A. Selective depletion of Ig-bearing lymphocytes by cyclophosphamide in rheumatoid arthritis and systemic lupus erythematosus. Guidelines for dosage. Arthritis Rheum. 1974 Jul-Aug;17(4):363–374. doi: 10.1002/art.1780170405. [DOI] [PubMed] [Google Scholar]
- Hurd E. R. Immunosuppressive and antiinflammatory properties of cyclophosphamide, azathioprine and methotrexate. Arthritis Rheum. 1973 Jan-Feb;16(1):84–88. doi: 10.1002/art.1780160114. [DOI] [PubMed] [Google Scholar]
- Hurd E. R., Ziff M. Parameters of improvement in patients with rheumatoid arthritis treated with cyclophosphamide. Arthritis Rheum. 1974 Jan-Feb;17(1):72–78. doi: 10.1002/art.1780170111. [DOI] [PubMed] [Google Scholar]
- Johansen K. S., Johansen T. S., Talmage D. W. T cell rosette formation in primates, pigs, and guinea pigs. The influence of immunosuppresive agents. J Allergy Clin Immunol. 1974 Aug;54(2):86–93. doi: 10.1016/0091-6749(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Jokipii A. M., Jokipii L. Suppression of cell-mediated immunity by cyclophosphamide: its independence of concomitant B cell response. Cell Immunol. 1973 Dec;9(3):477–481. doi: 10.1016/0008-8749(73)90063-4. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. Suppression of thyroid lesions in rabbits by treatment with cyclophosphamide after the induction of thyroiditis. Clin Exp Immunol. 1970 Oct;7(4):541–550. [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y. Cyclophosphamide treatment of experimental allergic encephalomyelitis in Lewis rats. J Immunol. 1971 Jun;106(6):1473–1479. [PubMed] [Google Scholar]
- Rocklin R. E. Production of migration inhibitory factor by non-dividing lymphocytes. J Immunol. 1973 Mar;110(3):674–678. [PubMed] [Google Scholar]
- Salvin S. B., Liauw H. L. Immunologic unresponsiveness to allergic thyroiditis in guinea pigs. J Immunol. 1967 Mar;98(3):432–441. [PubMed] [Google Scholar]
- Scheiffarth F., Leykam D., Warnatz H., Baenkler H. W. Effect of cytostatic drugs on blast cell transformation of lymph node and spleen cells. Res Exp Med (Berl) 1973 May 21;160(3):196–205. doi: 10.1007/BF01856783. [DOI] [PubMed] [Google Scholar]
- Skinner M. D., Schwartz R. S. Immunosuppressive therapy. 1. N Engl J Med. 1972 Aug 3;287(5):221–227. doi: 10.1056/NEJM197208032870504. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein A. Differential effects of immunosuppressants on lymphocyte function. J Clin Invest. 1973 Sep;52(9):2293–2299. doi: 10.1172/JCI107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein A. Mechanisms of immunosuppression: effects of cyclophosphamide on cellular immunity. Blood. 1973 Feb;41(2):273–284. [PubMed] [Google Scholar]
- Winkelstein A., Mikulla J. M., Nankin H. R., Pollock B. H., Stolzer B. L. Mechanisms of immunosuppression: effects of cyclophosphamide on lymphocytes. J Lab Clin Med. 1972 Oct;80(4):506–513. [PubMed] [Google Scholar]


