Abstract
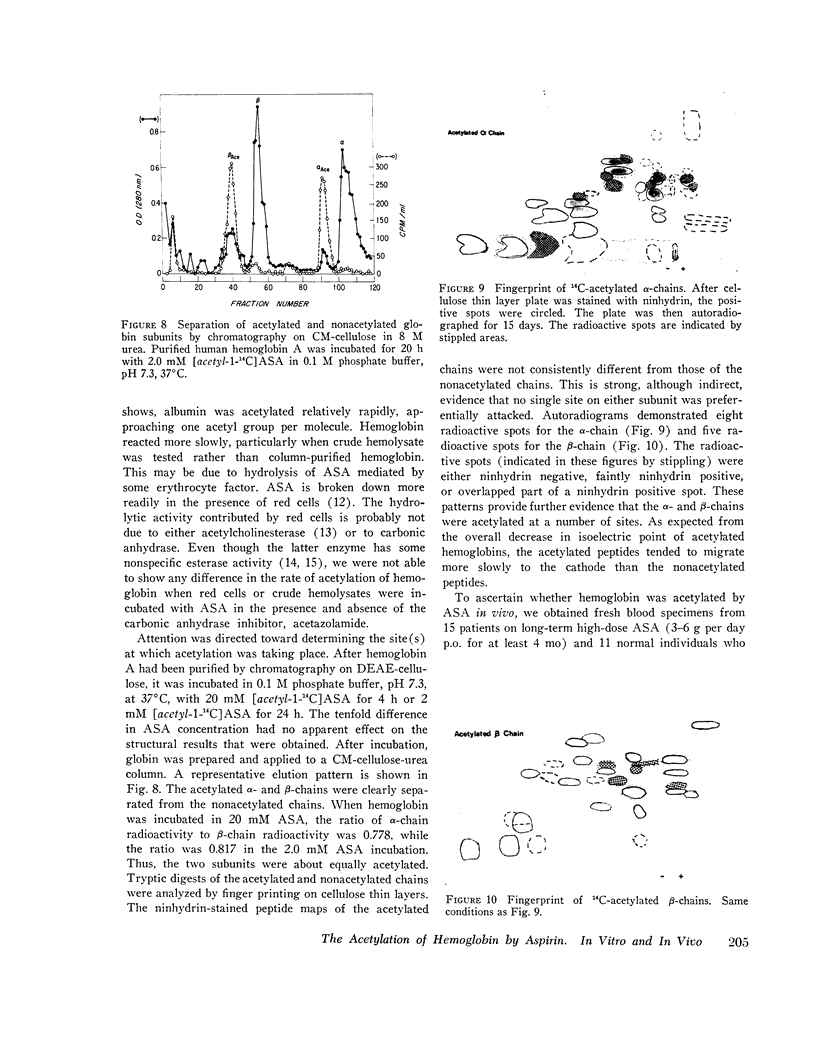
The chemical modification of hemoglobin by aspirin (ASA) has been studied, both in intact human red cells and in purified hemoglobin solutions. After incubation of red cells with 20 mM [acetyl-1minus14C]ASA, incorporation of radioactivity into hemoglobin was observed in agreement with the results of Klotz and Tam (1973. Proc. Natl. Acad. Sci. U. S. A. 70: 1313-1315). In contrast, no labeling of hemoglobin was seen when [carbosyl-14-C]ASA was used. These results indicate that ASA acetylates hemoglobin. The acetylated hemoglobin was readily separated from unmodified hemoglobin by both gel electrofocusing and by column chromatography. Quantitation of the extent of acetylation by densitometric scanning of gels agreed very well with estimates obtained from radioactivity measurements. Hemolysates prepared from red cells incubated with ASA showed normal oxygen affinity and heme-heme interaction. Purified acetylated hemoglobin had a slightly increased oxygen affinity and decreased heme-heme interaction. There was no difference in the rate of acetylation of oxy- and deoxyhemoglobin. ASA acetylated column-purified hemoglobin A more readily than hemoglobin in crude hemolysate, but less rapidly than purified human serum albumin. The rate of acetylation of hemoglobulin increased with pH up to approximately pH 8,5. Structural studies were done on hemoglobin incubated with 2.0 mM and 20 mM [acetyl-1-14-C]ASA. Alpha- and beta-chains were acetylated almost equally. Tryptic digests of purified acetylated subunits were fingerprinted on cellulose thin layer plates and autoradiographed. Both alpha- and beta-chains showed a number of radioactive spots that were either ninhydrin negative or weakly ninhydrin positive. These results indicate that hemoglobin is acetylated at a number of sites, probably at the epislon-amino group of lysine residues. To determine whether ASA acetylates hemoglobin in vivo, hemolysates of 14 patients on long-term high-dose ASA therapy were analyzed by gel electrofocusing and compared to specimens of individuals not receiving ASA. The ASA-treated group had a twofold increase in a minor hemoglobin component having an isoelectric point lower than that of hemoglobin A, and indistinguishable from the minoe component which appears when hemoglobin is incubated with ASA in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. B., Ranney H. M. Acquired disorders of hemoglobin. Prog Hematol. 1973;8:77–98. [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J. Fast hemoglobin in lead poisoning. Blood. 1966 Sep;28(3):377–386. [PubMed] [Google Scholar]
- De Furia F. G., Cerami A., Bunn H. F., Lu Y. S., Peterson C. M. The effect of aspirin on sickling and oxygen affinity of erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3707–3710. doi: 10.1073/pnas.70.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Harthon L., Hedström M. Hydrolysis of salicylsalicylic acid in human blood and plasma: a comparison with acetylsalicylic acid. Acta Pharmacol Toxicol (Copenh) 1971;29(23):155–163. doi: 10.1111/j.1600-0773.1971.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Hawkins D., Pinckard R. N., Crawford I. P., Farr R. S. Structural changes in human serum albumin induced by ingestion of acetylsalicylic acid. J Clin Invest. 1969 Mar;48(3):536–542. doi: 10.1172/JCI106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M., Nathan D. G., Bunn H. F. The reaction of cyanate with the alpha and beta subunits in hemoglobin. Effects of oxygenation, phosphates, and carbon dioxide. J Biol Chem. 1973 Dec 10;248(23):8057–8063. [PubMed] [Google Scholar]
- Kelly C. A. Determination of the decomposition of aspirin. J Pharm Sci. 1970 Aug;59(8):1053–1079. doi: 10.1002/jps.2600590802. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Tam J. W. Acetylation of sickle cell hemoglobin by aspirin. Proc Natl Acad Sci U S A. 1973 May;70(5):1313–1315. doi: 10.1073/pnas.70.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARDS J. R. Presence of acetylsalicylic acid in plasma following oral ingestion of aspirin. Proc Soc Exp Biol Med. 1962 Jun;110:304–308. doi: 10.3181/00379727-110-27499. [DOI] [PubMed] [Google Scholar]
- MANDEL H. G., CAMBOSOS N. M., SMITH P. K. The presence of aspirin in human plasma after oral administration. J Pharmacol Exp Ther. 1954 Dec;112(4):495–500. [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. 3. Kinetic studies of the enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1967 Mar;6(3):668–678. doi: 10.1021/bi00855a005. [DOI] [PubMed] [Google Scholar]
- Shamsuddin M., Mason R. G., Ritchey J. M., Honig G. R., Klotz I. M. Sites of acetylation of sickle cell hemoglobin by aspirin. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4693–4697. doi: 10.1073/pnas.71.12.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E. M., King T. P. Isoelectric heterogeneity of bovine plasma albumin. J Biol Chem. 1971 Jan 10;246(1):201–208. [PubMed] [Google Scholar]
- Tashian R. E., Douglas D. P., Yu Y. S. Esterase and hydrase activity of carbonic anhydrase. I. From primate erythrocytes. Biochem Biophys Res Commun. 1964;14:256–261. doi: 10.1016/0006-291x(64)90445-0. [DOI] [PubMed] [Google Scholar]
- VINCENT D., LAGREU Action comparée des sérums de diverses espèces sur l'acétylcholine et sur l'acide acétylsalicylique (pseudocholinestérase et aspirine-estérase). C R Seances Soc Biol Fil. 1950 May;144(9-10):641–643. [PubMed] [Google Scholar]
- Weiss H. J., Aledort L. M., Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest. 1968 Sep;47(9):2169–2180. doi: 10.1172/JCI105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Mondhiry H., Marcus A. J., Spaet T. H. On the mechanism of platelet function inhibition by acetylsalicylic acid. Proc Soc Exp Biol Med. 1970 Feb;133(2):632–636. doi: 10.3181/00379727-133-34533. [DOI] [PubMed] [Google Scholar]