Abstract
Language production is a complex neural process that requires the interplay between multiple specialized cortical regions. We investigated modulations in large‐scale cortical networks underlying preparation for speech production by contrasting cortico‐cortical coherence for overt and silent picture naming in an all‐to‐all connectivity analysis. To capture transient, frequency‐specific changes in functional connectivity we analyzed the magnetoencephalography data in two consecutive 300‐ms time windows. Within the first 300 ms following picture onset beta frequency coherence was increased for overt naming in a network of regions comprising the bilateral parieto‐temporal junction and medial cortices, suggesting that overt articulation modifies selection processes involved in speech planning. In the late time window (300–600 ms after picture onset) beta‐range coherence was enhanced in a network that included the ventral sensorimotor and temporal cortices. Coherence in the gamma band was simultaneously reduced between the ventral motor cortex and supplementary motor area, bilaterally. The results suggest functionally distinct roles for beta (facilitatory) and gamma (suppressive) band interactions in speech production, with strong involvement of the motor cortex in both frequency bands. Overall, a striking difference in functional connectivity between the early and late time windows was observed, revealing the dynamic nature of large‐scale cortical networks that support language and speech. Our results demonstrate that as the naming task evolves in time, the global connectivity patterns change, and that these changes occur (at least) on the time‐scale of a few hundred milliseconds. More generally, these results bear implications for how we view large‐scale neural networks underlying task performance. Hum Brain Mapp 36:1202–1216, 2015. © 2014 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: functional connectivity, coherence, picture naming, magnetoencephalography, speech production, task networks, gamma, beta, oscillations
INTRODUCTION
Language production is a complex neural process that requires transforming a concept that the speaker wishes to express into a linguistic form, and translating this word‐form into a rapid, precisely co‐ordinated, articulatory motor sequence [Hickok, 2012; Levelt et al., 1999; Postma, 2000]. Neuroimaging studies have shown that language production involves multiple functionally specialized brain regions, with core language regions situated within the left inferior frontal cortex and the left posterior temporal cortex [Price, 2012]. The motor control of language production is primarily mediated by the ventral motor cortex [Bouchard et al., 2013; Brown et al., 2009; Penfield and Rasmussen, 1949; Simonyan et al., 2009], projecting to subcortical structures [Jurgens, 2002]. In addition, speech production is thought to involve the supplementary motor area (SMA), anterior cingulate cortex, and insula for selection, sequencing, and initiation of motor speech [Dronkers, 1996; Jurgens, 2009; Tremblay and Gracco, 2009], as well as premotor and parietal areas for planning of purposeful utterances [Grabski et al., 2012; Jurgens, 2002]. Together, these cortical regions form a large‐scale neural network supporting language and speech. In this study, we set out to address the dynamic integration of speech production processes at the level of such large‐scale cortical circuits.
Although neuroimaging studies have been successful in mapping functionally specialized cortical regions involved in language production, less is known about the integration of information across these multiple regions. Analysis of effective connectivity among prespecified anatomical regions have delineated the interplay between several brain regions specialized in motor speech production [Eickhoff et al., 2009; Heim et al., 2009]. These studies have used the slowly fluctuating hemodynamic responses to study connectivity, but for capturing transient, short‐lasting changes in functional connectivity underlying language production a different approach is needed.
One suggested mechanism for how information is integrated across spatially distributed neural populations is through coherence of oscillatory neural activity [Engel et al., 1999; Fries, 2005; Singer and Gray, 1995]. Oscillatory activity in neural populations is a prominent feature in noninvasive electrophysiological recordings [Buzsaki and Draguhn, 2004; Pfurtscheller and Lopes da Silva, 1999; Salmelin and Hari, 1994], and functional coupling between brain regions may occur through the synchronization of these distant oscillators [Engel et al., 1999; Fries, 2005]. Such a mechanism can provide temporal windows for efficient information transfer between neural populations [Fries, 2005]. At the large‐scale network level, coherence can be studied using noninvasive electrophysiological measurement techniques, such as magnetoencephalography (MEG). Here, we used MEG together with a spatial filtering technique to track global, task‐dependent, changes in functional connectivity patterns in human language production.
We used picture naming as a lead‐in process to study functional connectivity underlying word production. Naming a picture is a complex cognitive process which, prior to onset of speech, involves visual recognition of the presented object(s), accessing the meaning (lexical‐semantics) of the object, finding the correct word‐form, and finally motor programming and preparation for articulation of the word [Indefrey and Levelt, 2004; Levelt et al., 1999]. How connections are recruited within the language network during picture naming, and how such connections are modulated over time as the speech production process evolves from perception of the presented image to motor onset is not known. To address this issue, we used time‐sensitive MEG recordings that allowed us to focus on different time windows that preceded naming. In particular, we focused on connectivity modulations underlying motor preparation for speech by contrasting overt and silent naming.
Silent (or inner) speech is often considered as a truncated version of overt speech, the two differing only in that overt naming comprises articulation. Thus, neuroimaging studies have often used silent (or delayed) speech as a substitute for overt speech, thereby avoiding problems with speech artefacts or auditory feed‐back [Barch et al., 1999; Laganaro and Perret, 2011; Liljeström et al., 2008; Sahin et al., 2009]. Indeed, brain activations are highly similar for the two conditions, with activation levels differing mainly within inferior frontal, premotor and motor areas [Palmer et al., 2001; Salmelin et al., 1994; Shuster and Lemieux, 2005; Vihla et al., 2006]. Behavioral data also suggest that the processes that underlie silent and overt speech are decidedly similar. In silent speech, people make speech errors that are qualitatively similar to errors made during overt speech [Postma and Noordanus, 1996]. For example, both silent and overt speech display a lexical bias effect, that is, in making speech errors, speakers are more likely to produce other real words than nonwords [Oppenheim and Dell, 2008, 2010; Postma and Noordanus, 1996]. Notably, however, only when speakers are asked to articulate a word (either by silently mouthing the word, or overtly), do they also make errors with a tendency of phonemic similarity [Oppenheim and Dell, 2008, 2010]. Such results suggest that motor articulation affects also earlier stages of speech planning [Oppenheim and Dell, 2010], although this view has not been without controversy [Corley et al., 2011]. In neuroimaging, similarity between the two conditions has been assessed in activation studies. Although activation studies generally have not captured differences between overt and silent naming in early time windows [Salmelin et al., 1994; Vihla et al., 2006], it is possible that differences between the two conditions could be observed in the elicited connectivity patterns.
In this study, we investigated modulations in long‐range cortico‐cortical interactions when subjects named an item overtly as compared to silent naming in two different time windows. Our time‐sensitive approach permitted the usage of time windows as short as 300 ms, resulting in one time window that followed stimulus onset (0–300 ms), and a second time window that preceded speech onset (300–600 ms after stimulus presentation). In terms of models proposed for speech production [Indefrey, 2011; Indefrey and Levelt, 2004], differences between the two tasks in the early time window would be associated primarily with perceptual or lexical processing, while processes related to motor planning of speech should be captured within the late time window. In addition, retrieval of the phonological word form could be reflected in either of the two time windows [Indefrey, 2011].
Of particular interest was whether the various spectral components in the theta, alpha, beta, and gamma frequency ranges would contribute differently to the network dynamics in speech production. In a given brain region, oscillations of different frequencies can be generated through multiple cellular mechanisms [Ainsworth et al., 2011; Roopun et al., 2008a, 2008b], and within specific cortical layers [Buffalo et al., 2011]. Accordingly, different functional roles have been hypothesized both for local oscillations within distinct frequency bands [Donner and Siegel, 2011; Engel and Fries, 2010; Wang, 2010], as well as for interactions of these rhythms across long distances [Cannon et al., 2014; Fries et al., 2008; Lee et al., 2013]. Previous work on motor planning and execution has demonstrated cortico‐muscular and cortico‐cortical coherence within the motor system at highly varying frequencies [Gross et al., 2001, 2002; Jerbi et al., 2007; Ruspantini et al., 2012; Salenius et al., 1997; Schoffelen et al., 2005]. Although most previous studies have focused on hand movements, modulations of rhythmicity and cortico‐muscular coherence are key features observed also for mouth movements underlying speech [Ruspantini et al., 2012; Saarinen et al., 2006]. We examined whether different functional roles could be attributed to the distinct frequency bands, and whether the time‐dependent coherence analysis would allow capturing transient changes in large‐scale functional connectivity underlying language production.
MATERIALS AND METHODS
Subjects and Experimental Design
We recorded MEG data from 11 healthy human subjects (ten right‐handed, one ambidextrous) performing overt and silent naming. All participants were native Finnish speakers, had no history of neurological or psychiatric disorders, and had normal or corrected‐to‐normal vision (mean age 27, age range 20–33; four females, seven males). The subjects viewed simple line drawings and were asked to name the actions or objects depicted in the images, either silently (300 images) or overtly (300 images). Stimuli were presented in 30‐s blocks (10 images were shown within each block with an inter‐stimulus interval (ISI) of 1.8–4.2 s, stimulus duration 300 ms), with a 21‐s rest period between blocks (Fig. 1). The stimulus materials were derived from a previous fMRI study [Liljeström et al., 2008]. In the current MEG study, the subjects performed the overt and silent naming tasks in four sessions (two of each, Fig. 1), the order of which was randomized across subjects. We obtained informed consent from all subjects, in agreement with the prior approval of the Helsinki and Uusimaa Ethics Committee. Results on active cortical regions in the silent naming conditions have been reported elsewhere [Liljeström et al., 2009]. Here, we collapsed the data across action/object naming conditions and focused on the distinction in functional connectivity between overt and silent naming. In collapsing over all naming categories, we made the specific assumption that there are differences between overt and silent naming that are similar for both action and object naming.
Figure 1.
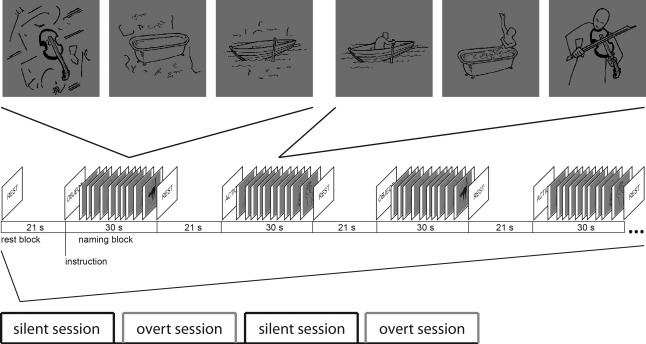
Experimental design. Stimuli were presented in 30‐s blocks (10 images were shown within each block), with a 21‐s rest period between blocks. The subjects named the actions or objects depicted in the images, either silently (300 images) or overtly (300 images). Subjects performed the overt and silent naming tasks in four sessions, the order of which was randomized across subjects.
Prior to collapsing the data across conditions, the reaction times for the three different categories were calculated for the overt naming condition using the recorded electromyogram (EMG). To this end, the EMG signal was band‐pass filtered to 90‐Hz, rectified, and thresholded. The mean reaction times were: action naming from action images 678 ± 78 ms (mean ± SD), object naming from action images 715 ± 88 ms, and object naming for object images 660 ± 71 ms. While the differences in reaction times between object naming from action images and the two other categories were significant [t(10) = 4.22, P = 0.002, compared to action naming; t(10) = 3.89, P = 0.003, compared to object naming from object images], the relatively long 300‐ms time windows allowed for collapsing across categories. The mean reaction time over all conditions was 684 ± 76 ms, while the lower and upper quartiles were 539 and 826 ms, respectively.
MEG and MR Recordings
MEG data were measured using a Vectorview whole‐head MEG device (Elekta Oy, Helsinki, Finland) comprising 306 channels, organized in 102 triplet sensor elements in a helmet‐shaped array (two planar gradiometers and one magnetometer at each triplet site). Vertical and horizontal electro‐oculogram (EOG) signals were recorded for identifying and rejecting epochs contaminated by eye blinks and saccades. EMG signals were measured to monitor mouth movements with two electrodes placed near the upper and lower lip margins. The overt speech responses were recorded. Four head position indicator coils were used to determine the head position with respect to the sensor array, and the position of these coils with respect to three anatomical landmarks (the preauricular points and nasion) were found with a 3D digitizer (Polhemus, Colchester, VT). This information was then used to align the MEG results with the individual anatomical magnetic resonance images (MRIs). Head position was measured at the beginning of each session (i.e., four times during the course of the experiment). The MEG signals were filtered at 0.03–200 Hz and sampled at 600 Hz.
MRIs were acquired with a Signa VH/i 3.0 T MRI scanner (GE Healthcare, Chalfont St Giles, UK) using a standard T1‐weighted 3D spoiled‐gradient echo sequence. If available, previously acquired T1‐weighted anatomical images were used.
Preprocessing
For removal of external disturbances and artefacts the spatiotemporal signal space separation method [tSSS; Taulu and Simola, 2006] was applied. To compensate for head movements between measurement sessions the measured (noncontinuous) head positioning data was used to transform the MEG data for each subject to the same reference position (Elekta Maxfilter software package). Epochs containing eye blinks or saccades were rejected (based on the recorded EOG, rejection limit 150 µV).
A Common‐Subject Gray‐Matter Grid
To obtain grid points that were spatially equivalent across subjects a regular grid with 7‐mm spacing was created in an atlas brain (limited to 2 cm from the surface of the brain, not including the cerebellum) and transformed to each subject's brain using an elastic transformation [Schormann et al., 1996]. Grid points within the most anterior frontal cortex and temporal pole areas that are sensitive to eye movement artefacts were excluded from the analysis.
Implementation of the Spatial Filter
To identify task‐specific modulations in connectivity between experimental conditions [Kujala et al., 2012] we applied event‐related dynamic imaging of coherent sources [erDICS; Laaksonen et al., 2008]. DICS is based on finding brain areas that act in synchrony, with the help of a spatial filter [Gross et al., 2001]. Here, we used the spatial filter to perform an all‐to‐all connectivity analysis on the common‐subject gray‐matter grid. To reduce the effect of spurious connectivity differences [Schoffelen and Gross, 2009, 2011] we used a power‐matched control condition. Task‐specific modulations in connectivity were identified by contrasting the all‐to‐all connectivity results for overt and silent naming. Our results therefore describe modulations of connectivity within the language network between overt and silent naming, rather than the entire underlying network.
At the sensor level, a time‐frequency representation for each epoch was first calculated using Morlet wavelets of width 7. The (time‐dependent) cross‐spectral density (CSD) matrix was obtained by calculating the product of the time‐frequency representations of the trial time series (from −100 to 900 ms) with respect to stimulus onset, for all sensor combinations. The single‐trial CSDs were averaged together into a mean CSD matrix. The sensor‐level data, represented by the CSD matrix, were then transformed into a cortical representation using the spatial filter. A spherically symmetric head conductor model (specified individually for each subject) was used to represent the conduction profile of the head. In this project, an implementation of the DICS spatial filter was used in which coherence estimation is based on numerical maximization of coherence for a discrete set of source‐orientation combinations. For each cortico‐cortical connection the source orientation configuration was determined by identifying the orientation combination for the two sources that maximizes their mutual coherence. The possible orientation pairs contained all possible combinations between 50 regularly spaced orientations at both ends of the connection that spanned the tangential source space with respect to a sphere centered at the center of the brain. A minimum connection distance criterion of 4 cm between grid points was applied to avoid spurious coherence detection due to field spread effects [Schoffelen and Gross, 2009].
Selection of Frequency Bands and Time Windows
The frequency bands were selected to cover the frequency spectra between 3 and 90 Hz, and divided into frequency bands based on the extensive literature on electrophysiologically measured brain rhythms [e.g., Gross et al., 2013; Jensen et al., 2012; Palva and Palva, 2007; Pfurtscheller and Lopes da Silva, 1999; Salmelin and Hari, 1994; van Wijk et al., 2012]. For theta frequency oscillations (3–7 Hz), and alpha frequency oscillations (7–13 Hz), a single frequency band was chosen. The beta frequency band (13–31 Hz) was divided into a single low‐beta (13–17 Hz), and two high‐beta (17–25 Hz, 25–31 Hz) frequency bands. The gamma frequency range (31–90 Hz) was within the range of frequencies often observed for pyramidal‐interneuronal network gamma [Whittington et al., 2000] and divided into four frequency bands (low gamma: 31–39 Hz, 39–47 Hz; high gamma: 52–70 Hz, 70–90 Hz; 50‐Hz line noise excluded). The analysis was performed in 2‐Hz frequency bins for the frequencies ranging from 3 to 90 Hz and averaged across the predefined frequency bands.
Using the event‐related version of DICS we were able to restrict the analysis to two equal time windows (0–300 ms and 300–600 ms, time‐locked to stimulus presentation) within the time period that preceded speech execution (mean reaction time 684 ± 76 ms, see stimulus and experimental design above). In the following, we will refer to the early time window as the window following stimulus presentation, whereas the late window will be referred to as the time window preceding speech onset; note however, that both time windows were averaged with respect to stimulus onset. The time windows were, to a high degree, chosen on grounds of methodological constraints; muscle artefacts related to speech onset were the main limiting factor. Another important limiting factor was the need to obtain enough data points for a stable estimation of the data covariance matrix; this precluded the usage of time windows shorter than 300 ms. In terms of models proposed for speech production [Indefrey 2011; Indefrey and Levelt, 2004], the chosen early time window would reflect processes such as conceptual preparation and lemma selection, whereas the late time window would encompass processes such as syllabification and phonetic encoding. Phonological code retrieval (starting around 275 ms in the Indefrey and Levelt models) could take place in either of the time windows.
Power differences between conditions can introduce spurious differences in the coherence estimates. We therefore checked, separately at each sensor and each frequency band, that the power difference between the overt and silent naming conditions did not exceed 10%, and that there were no significant power‐level differences between the two conditions (across subjects paired t‐test, P < 0.01) at 99% of the sensors (max 3 sensors with a difference). The 7–13 Hz frequency band during the early (0–300 ms) time window did not survive these exclusion criteria, and was therefore not included in the analysis.
To ensure that the selected time windows did not contain artefacts related to mouth movements we tested, separately for each frequency band, that there was no amplitude difference in the EMG signal between the selected time windows and the baseline (−300 to 0 ms) (paired t‐test, P < 0.05). In the early (0–300 ms) time window, we observed an increase in the EMG amplitude as compared to the baseline (300‐ms time window preceding stimulus onset) in the 3–7 Hz frequency band and this frequency band was therefore excluded from the analysis for the early time window. However, as no results survived corrections in this frequency band (nor in the 7–13 Hz frequency band excluded based on power differences) in the further analysis our exclusion criteria did not affect any of the results.
Statistical Evaluation of all‐to‐all Connectivity
We approached the statistical evaluation of the all‐to‐all cortico‐cortical connectivity via an extensive set of simulations that were designed to address the possibility of chance findings specifically for the spatial profile of the evaluated connectivity and the subjects within the study. In the evaluation, we generated 200 sets of simulated data for each subject and two conditions, and estimated the probability that a random data‐set would yield a significant finding at different statistical and cluster thresholds when the two conditions were compared at the group‐level. Hierarchical clustering was used to determine the grouping of individual connections. The clustering was conducted using a weighted distance (across both end‐points of the connections) algorithm for linking the connections, and an average Euclidian distance of 12.2 mm (the 3D diagonal of a single voxel) was used as the cutoff threshold. For each simulation, we generated, separately for each subject and condition, data that consisted of 20 randomly placed cortical sources each of which was active at a random frequency between 0 and 75 Hz; the number of trials and their lengths were matched to the real data. For each subject, the cortico‐cortical connectivity was evaluated for these data using the same grids and minimum connection length as for the real data. We then calculated, using group‐level paired samples t‐tests, the statistical significance of modulation of coherence across the two conditions for each connection. Here, any statistical metric could be used, and the testing does not require, for example, the data to be normally distributed. We evaluated, for t‐scores ranging from 4.5 to 8.5 (0.25 step‐size) and with cluster sizes ranging from 1 to 15 (1‐connection step‐size) whether the simulation would have yielded any significant connections. From the 200 simulations we obtained a distribution of findings (0 indicating no significant connections, 1 that at least one connection had survived) at each threshold pair (significance and cluster‐size) and computed the likelihood that a random data‐set would yield a finding at the threshold (Fig. 2). As Figure 2 shows, there is a range of parameters where the probability of chance findings is 0.05 or less. For the analysis of the real data, we selected a parameter set at P = 0.05 in approximately middle of this range (t‐score = 6.75, cluster size = 5) and used it in all the subsequent analyses of the real data.
Figure 2.
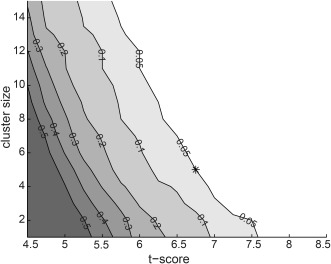
Estimation of appropriate statistical threshold. From 200 sets of simulated data (see Methods for a description) we obtained a distribution of findings at each threshold pair (t‐score and cluster size). Each contour line depicts the likelihood level that a random data‐set would yield a chance finding. The selected parameter set (t‐score = 6.75, cluster size = 5), with a probability of chance findings of 0.05 or less, is indicated by a star.
Visualization of all‐to‐all Connectivity and Graph‐Theoretical Network Analysis
Group‐level clusters with significant task‐dependent modulation of interareal coupling were visualized on an atlas brain using FreeSurfer [Dale et al., 1999; Fischl et al., 1999]. For visualization of the connectivity pattern, we used a circular plotting design. The nodes were based on an anatomical parcellation scheme [AAL; Tzourio‐Mazoyer et al., 2002]. MNI (Montreal Neurological Institute) coordinates were obtained by applying a linear transformation (12‐parameter affine, obtained from FreeSurfer) from the FreeSurfer MRI of an atlas brain to MNI Talairach co‐ordinates [Liljeström et al., 2009]. The data were represented by the adjacency matrix Cij, which was constructed by assigning an anatomical label to the connection start and end point, such that the value of matrix element Cij(i,j) corresponded to the number of significant connections between anatomical areas i and j. The weights of the adjacency matrix thus represent the number of connections between the parcellated regions.
To define hub regions within the observed networks we used two graph theoretical measures, degree and betweenness centrality. The degree of a node reflects the total number of connections to that node, whereas the betweenness centrality of a node is equal to the fraction of the shortest paths between all pairs of nodes that pass through that specific node [Sporns et al., 2007]. The adjacency matrix was binarized for the betweenness centrality, whereas the weight of each connection (edge) was included in the degree calculations. To calculate betweenness centrality we used a Matlab toolbox developed by Rubinov and Sporns [2010]. We defined high‐degree nodes as nodes with a degree greater than the network mean plus one standard deviation [Sporns et al., 2007].
RESULTS
The all‐to‐all cortex‐wide connectivity analysis identified numerous task‐relevant long‐range connections with significant modulations between tasks. Figure 3 illustrates the spatial structure of the overall network modulations in the early (0–300 ms) and late (300–600 ms) time windows, across all frequency bands. Figure 4 visualizes the interconnected regions in a circular plotting scheme parcellated according to the AAL atlas. Figure 5 shows the AAL‐parcellated regions ranked according to degree and betweenness centrality. Frequency‐resolved degree is shown in Figure 5C.
Figure 3.
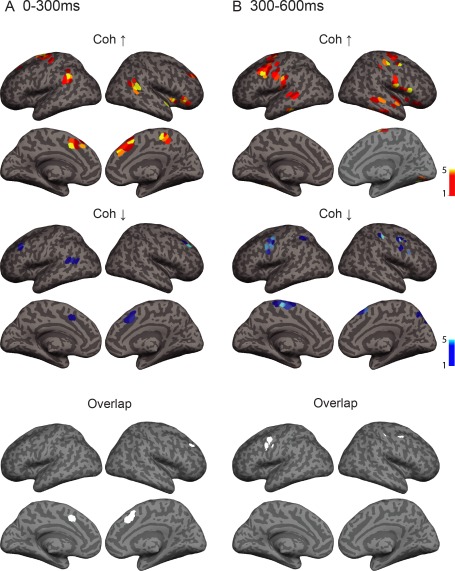
Spatial structure of overall network modulations between overt and silent naming across all frequency bands at (A) 0–300 ms and (B) 300–600 ms after image onset. Enhanced coherence between cortical regions is shown in yellow‐red colorscale (top) and reduced coherence is shown in blue (middle). Images are colorcoded according to connection density (number of significant connections connecting to a specific grid point). Overlap between grid points showing increased vs. decreased coherence in the early (left) and late (right) time window is shown in white (bottom). Results are shown at a significance level of P = 0.05 (corrected). Coh ↑ = task‐related increase in coherence, Coh ↓ = task‐related decrease in coherence.
Figure 4.
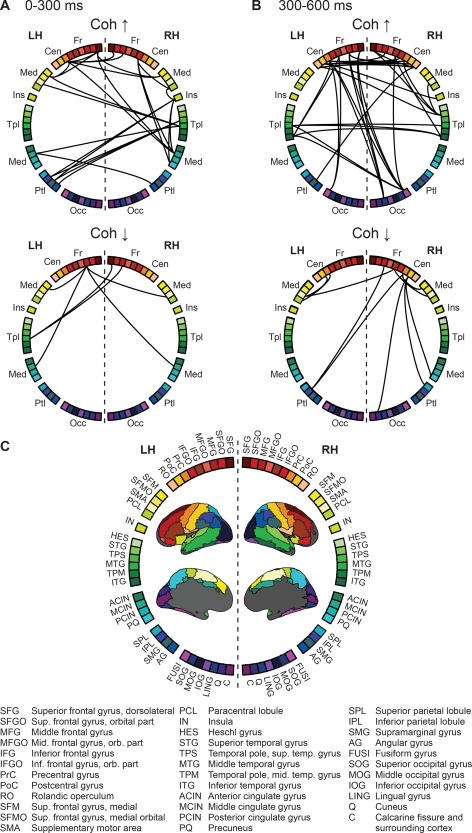
Task‐dependent modulation of interareal coupling at (A) 0–300 ms and (B) 300–600 ms after image onset. The adjacency matrix is visualized using a (C) circular plotting scheme with nodes based on the AAL parcellation scheme. Coh ↑ = increased coherence, Coh ↓ = decreased coherence, Fr = frontal regions, Cen = central, Med = medial, Ins = insula, Tpl = temporal, Ptl = parietal, Occ = occipital, LH = left hemisphere, RH = right hemisphere. Results are shown at a significance level of P = 0.05 (corrected).
Figure 5.
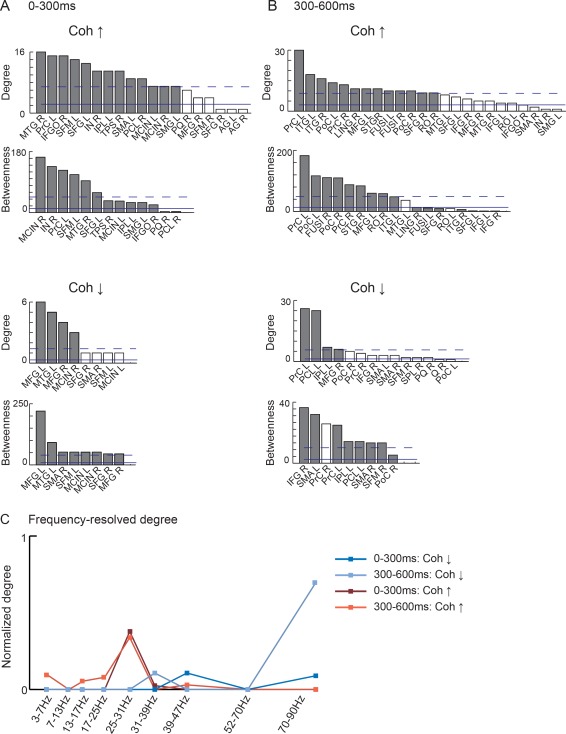
Degree and betweenness centrality at (A) 0–300 ms and (B) 300–600 ms after image onset. High‐degree nodes (dark gray bars, same coloring used also in the betweenness plot) are defined as mean (filled line) + 1 SD (dashed line). Network nodes are labelled according to the AAL parcellation scheme (see Fig. 3C), and according to hemisphere (e.g., SMA R = supplementary motor area, right; SMA L = supplementary motor area, left). (C) Normalized frequency‐resolved degree calculated over all significant connections.
Early Time Window (0–300ms)
Within the time window immediately following the onset of the visual image we observed modulation of connectivity within two separate networks, revealing either enhanced, or reduced, connectivity for preparation for overt vs. silent speech. As illustrated in Figures 3A and 5A, enhanced connectivity was observed in a network consisting of the parieto‐temporal junction [left hemisphere: supramarginal gyrus (SMG, cf. abbreviations in the Figures); right hemisphere: posterior middle temporal gyrus (MTG)], the medial cortex [medial frontal cortex (SFM); SMA; middle cingulate cortex (MCIN)], the right inferior frontal gyrus (IFG), and the adjacent anterior insula (IN). The most highly interconnected regions, as defined by connection density, were the right posterior middle temporal gyrus (MTG) and the left SMA, as well as the left primary motor cortex (PrC) and medial frontal cortex (SFM) (Fig. 4A).
At the same time, coherence was reduced in a network involving the bilateral dorsolateral and dorsomedial frontal cortex, and the left posterior middle temporal gyrus (Figs. 3A and 5A). Modulation in connectivity was observed (Fig. 4A) between the right superior and middle frontal gyri (SFG, MFG) and the left posterior MTG, as well as between the left MFG and the medial cortex (SFM, MCIN, and SMA).
Nodes showing increased coherence vs. nodes showing decreased coherence overlapped in the medial frontal cortex and the right dorsolateral frontal cortex (Fig. 3).
Late Time Window (300–600 ms)
Within the late time window that preceded naming onset (300–600 ms, Fig. 3B and 5B), increased coherence was seen in a network involving the bilateral primary sensorimotor cortex, premotor cortex and middle/inferior temporal gyri. The importance of the sensorimotor cortex within this network was highlighted in betweenness centrality and connection density (Fig. 5B). Long‐range anterior–posterior connections were observed between the frontal cortex and the fusiform/inferior temporal cortex (Fig. 4B). In addition, the right superior temporal and frontal regions were highly interconnected.
Within the same time window, decreased coherence was observed in a frontoparietal network involving the bilateral sensorimotor cortex, SMA, medial frontal, and parietal cortex (Figs. 3B, 4B, and 5B). In both hemispheres, modulation in connectivity was observed between the sensorimotor cortex and ipsilateral SMA.
Overlap between areas showing increased and decreased coherence was observed in the ventral sensorimotor cortex in both hemispheres (Fig. 3).
Frequency‐Specificity of the Network Modulations
The spectral specificity of the observed network modulations was investigated by calculating the frequency‐resolved degree over all significant connections within each frequency band. Figure 5C depicts the normalized degree for coherence enhancements and reductions. We observed increased coherence primarily within the high beta frequency band (25–31 Hz) in both the early and the late time window. In contrast, decreased coherence was seen exclusively in the low and high gamma bands (31–39 Hz, 39–47 Hz, and 70–90 Hz).
Discussion
We studied the dynamic integration of speech production processes at the level of large‐scale neural circuits. Our goal was to identify, in a data‐driven all‐to‐all connectivity analysis, the global connectivity patterns underlying preparation for overt speech. The time‐dependent MEG measurements allowed us to track, with high temporal and spatial accuracy, how connections are modulated within the language network, and how they change over time.
Connectivity Modulations in the Early Time Window
In the time window immediately after stimulus onset, we identified network hubs in the bilateral parieto‐temporal junction (left SMG; right pMTG). Several studies have suggested that the left pMTG [Indefrey and Levelt, 2004], or SMG [Binder et al., 2009; Hultén et al., 2014], is involved in lexical selection, and that such processing takes place around 200–400 ms after picture presentation [Indefrey and Levelt, 2004]. While lexical selection is required in both silent and overt naming, the need to translate the lexical word form into a motor command may be greater in overt speech. In particular, overt speech requires the selection, initiation and sequencing of appropriate speech movements. The observed interaction between the SMG/pMTG and contralateral SMA/pre‐SMA (associated with motor selection/sequencing [Tremblay and Gracco, 2009]), insula (associated with coordination of articulatory gestures [Dronkers, 1996] and singing [Ackermann and Riecker, 2004]), and mid cingulate (associated with initiation of emotional utterances [Jurgens, 2002]) within the first 300 ms following stimulus onset might thus reflect the transformation of the lexical word form into a motor command in the overt speech condition. These results support the suggestion that both lexical and motor selection processes are involved in speech production [Tremblay and Small, 2011]. Our results further suggest that these two processes interact and that such processing starts during the first 300 ms following stimulus onset. Whether motor execution affects speech planning processes [Oppenheim and Dell, 2010], or not [Corley et al., 2011; Postma and Noordanus, 1996], is a matter of controversy. Our results clearly favor an account in which overt articulation modifies early processes involved in speech planning, indicating that preparation for overt speech begins at an earlier stage than predicted by models of word production [Indefrey and Levelt, 2004; Indefrey, 2011].
In the overt naming condition the subject is required to initiate a goal‐directed motor action (speech), while this action should be suppressed in the silent condition. The observed changes in the gamma band coherence between bilateral prefrontal cortex, the medial frontal cortex and the pMTG in the early time window could reflect a modulatory role of cognitive controlling mechanisms [Kerns et al., 2004] at the onset of the task. This prediction could be further explored in experiments that more explicitly target the role and timing of goal‐oriented or domain‐general processes in language production [Fedorenko and Thompson‐Schill, 2014].
Connectivity Modulations in the Late Time Window
The observed interactions changed dramatically between the early and the late time window. Within the 300–600 ms time window that preceded speech onset, preparation for overt speech modulated connection strengths within the cortical motor network, primarily seen as enhanced beta‐band coherence in a network involving the bilateral sensorimotor and premotor cortices. Interactions within this cortical network in motor speech is in close agreement with activation studies on vocal production [Bohland and Guenther, 2006; Brown et al., 2009; Hartwigsen et al., 2013; Simonyan and Horwitz, 2011], and known structural connections from the ventral motor cortex in humans [Simonyan et al., 2009], and nonhuman primates [Simonyan and Jurgens, 2002, 2005]. The 20‐Hz beta rhythm is an intrinsic cortical rhythm found in the motor cortex, and modulations of the beta rhythm typically show a somatotopical organization [Salmelin et al., 1995; van Wijk et al., 2012]. Our results suggest that information transfer during preparation for overt speech between the primary motor cortex and cortical regions within the motor network are facilitated by rhythms that are intrinsic to the motor cortex. The neuronal circuitry may be tuned to these frequencies, enabling more efficient synchronization across distant neural populations within the motor network.
In the same 300–600 ms time window, we identified increased coherence between the inferior frontal cortex and fusiform cortex, consistent with anatomical connections within the inferior fronto‐occipital fasciculus [Martino et al., 2010; Sarubbo et al., 2013]. Electrical stimulations of this pathway lead to semantic paraphasia or naming deficits [Duffau et al., 2002, 2005; Mandonnet et al., 2007]. The importance of an anterior–posterior pathway in language production is here reflected in the modulation of coherent coupling in preparation for speech.
We observed decreased coherence in the gamma band in a frontoparietal network including the bilateral sensorimotor cortex and SMA in the late time window. Before onset of speech, the appropriate motor sequences should be selected for execution. Such a selection may be performed by enhancing activation for the appropriate motor sequence, while actively suppressing inappropriate motor sequences. During silent naming the motor output should be entirely suppressed: this could be viewed as an active process [cf. Sedley and Cunningham, 2013] in the form of stronger gamma band coherence between the motor cortex and the SMA/pre‐SMA during silent naming. The pre‐SMA has previously been associated with inhibition of speech [Xue et al., 2008], and the gamma‐band interactions between the SMA/pre‐SMA and the motor cortex observed in this study provide one candidate mechanism through which this could occur.
Frequency‐Specificity of the Identified Networks
Overall, the network modulations identified in the beta and gamma frequency bands differed widely, both regarding functional effect (increase or decrease), and network connections. In particular, we observed increased coherence for overt naming mostly in the beta band, whereas decreases were exclusive to the gamma band. These results suggest a functional differentiation between long‐range beta and gamma synchronizations, analogous to the suggestion of distinct roles for local oscillations in the beta and gamma frequency band [Donner and Siegel, 2011]. Low‐frequency beta synchronizations have been deemed more suitable for long‐range cortico‐cortical interactions [Kopell et al., 2000] than gamma band synchronizations, which might be more related to local interactions. Our results suggest a facilitatory role for beta‐band synchronizations, and a suppressive role for gamma‐band synchronizations in preparation for motor output, with involvement of the ventral motor cortex observed in both frequency bands.
Methodological Considerations
Identification of time‐dependent cortex‐wide connectivity patterns was made feasible through a number of methodological considerations. MEG measurements of neural oscillations provide a more direct link to the underlying neural activity than hemodynamic measures do. The event‐related modification of the DICS spatial filter [Laaksonen et al., 2008] used for MEG source reconstruction allowed us to focus on specific, reasonably narrow time‐windows, thus capturing the dynamic, time‐dependent rearrangement of functional connectivity patterns. Importantly, we were able to avoid muscle artefacts present during speech by limiting the analysis to an artefact‐free time window. For identifying the cortical networks we used a data‐driven all‐to‐all connectivity analysis which does not constrain the analysis to preselected seed regions.
Although this approach enables a cortex‐wide analysis of the cortical interactions underlying preparation for speech, it does not provide a complete description of the networks underlying language production. Due to spatial blurring of the MEG signal, we focused on long‐range connectivity. We used a power‐matched control condition (silent speech), which allowed us to focus on modulations related to motor preparation for speech. Moreover, with this approach we were able to control for spurious connectivity due to power differences [Schoffelen and Gross, 2009]. In future studies, this approach can be further extended by introducing a battery of control conditions which may permit identification of several more subnetworks involved in for example, visual object recognition, lexical‐semantic processing, distinctions in grammatical category, or motor execution.
In estimating interactions between brain regions we used coherence as a measure for connectivity. Current theories propose that coherence among brain regions constitutes a mechanism through which information is transferred within cortical networks [Bressler and Kelso, 2001; Fries, 2005]. In support for this view, coherence estimates of intracranial recordings in monkeys have revealed task‐related long‐range interactions between cortical regions in several different tasks (attention, motor preparation/decision) and in multiple frequency bands [Buschman and Miller, 2007; Pesaran et al., 2008; Gregoriou et al., 2009]. In addition to being a neurophysiologically well motivated measure of connectivity, coherence allows for direct whole‐cortex mapping of connectivity at different frequencies without the need to estimate time series at the level of cortical sources [Kujala et al., 2008]. However, it does not allow for differentiation between feed‐forward/feed‐back vs. reciprocal interactions—estimation of such effects would require a directed measure of connectivity, for example, Granger causality [Geweke, 1982; Barnett and Seth, 2014], which has been used to investigate connectivity between pre‐defined seed regions. The current results could thus be integrated with both serial [Levelt et al., 1999] and interactive accounts of speech production [Dell, 1986]. Once task‐relevant cortical nodes have been identified, future studies could target more specific questions related to the manner in which these nodes are integrated into the network, and the directionality of information flow amongst these regions.
Notably, the left IFG did not appear prominently in our analysis. The left IFG plays an established role in speech production, seen similarly in broadband MEG recordings of silent and overt naming [Liljeström et al., 2009; Salmelin et al., 1994]. Different task manipulations may be necessary to capture modulation of interactions between the left IFG and other regions within the speech production network.
A Reconfiguration of Large‐Scale Connectivity Patterns during Preparation for Speech
Functional connectivity in large‐scale cortical networks has been studied extensively in task‐free conditions, utilizing the slow fluctuations in the hemodynamic response [Fox and Raichle, 2007]. Those studies systematically reveal large‐scale connectivity patterns, and correlated networks that agree with known functional systems (e.g. visual, attention, language, sensorimotor) in the brain [Biswal et al., 1995; Deco et al., 2011; Greicius et al., 2003; Smith et al., 2009]. Recent work has shown that large‐scale global networks can be identified also from noninvasive electrophysiological recordings of ongoing spontaneous activity [Brookes et al., 2011; de Pasquale et al., 2010, 2012; Hipp et al., 2012]. Importantly, global connectivity patterns are not static, but show a remarkable task‐dependent spatial rearrangement [Betti et al., 2013]. In goal‐oriented tasks, with varying task requirements, different brain regions (or possibly network modules), specialized in various aspects of the task, must be dynamically, and flexibly, recruited to facilitate task performance [Crossley et al., 2013; van den Heuvel et al., 2012]. Transient reconfigurations of existing cortical networks allow such flexible behavior. In this study, we observed a striking difference in functional connectivity between the early and late time windows, revealing a dynamic reconfiguration of large‐scale connectivity within the language network during preparation for overt speech. Our results demonstrate that as the naming task evolves in time, the global connectivity pattern changes, and that this change occurs (at least) on the time‐scale of a few hundred milliseconds. Moreover, the observed functional differentiation between specific frequency bands (here, beta and gamma) in long‐range synchronizations suggests that multiple neural mechanisms may contribute to network patterns involved in speech production. Overall, the transient nature of global functional networks should be taken into account when assessing task‐relevant brain networks.
ACKNOWLEDGMENT
We thank Dr. Annika Hultén for her help at various stages of the project. The authors declare no competing financial interests.
REFERENCES
- Ackermann H, Riecker A (2004): The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang 89:320–328. [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Roopun AK, Traub RD, Kopell NJ, Whittington MA (2011): Dual gamma rhythm generators control interlaminar synchrony in auditory cortex. J Neurosci 31:17040–17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett L, Seth AK (2014): The MVGC multivariate Granger causality toolbox: A new approach to Granger‐causal inference. J Neurosci Methods 223:50–68. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD (1999): Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage 10:642–657. [DOI] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, Romani GL, Corbetta M (2013): Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron 79:782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH (2006): An fMRI investigation of syllable sequence production. Neuroimage 32:821–841. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF (2013): Functional organization of human sensorimotor cortex for speech articulation. Nature 495:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Kelso JA (2001): Cortical coordination dynamics and cognition. Trends Cogn Sci 5:26–36. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Barnes GR, Smith SM, Morris PG (2011): Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA 108:16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S , Laird, AR , Pfordresher, PQ , Thelen, SM , Turkeltaub, P , Liotti, M (2009): The somatotopy of speech: Phonation and articulation in the human motor cortex. Brain Cogn 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R (2011): Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA 108:11262–11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK (2007): Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science 315:1860–1862. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Cannon J, McCarthy MM, Lee S, Lee J, Borgers C, Whittington MA, Kopell N (2014): Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci 39:705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley M, Brocklehurst PH, Moat HS (2011): Error biases in inner and overt speech: evidence from tongue twisters. J Exp Psychol Learn Mem Cogn 37:162–175. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vertes PE, Winton‐Brown TT, Patel AX, Ginestet CE, McGuire P, Bullmore ET (2013): Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci USA 110:11583–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dell GS (1986): A spreading‐activation theory of retrieval in sentence production. Psychol Rev 93:283–321. [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M (2010): Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA 107:6040–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, Corbetta M (2012): A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 74:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR (2011): Emerging concepts for the dynamical organization of resting‐state activity in the brain. Nat Rev Neurosci 12:43–56. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M (2011): A framework for local cortical oscillation patterns. Trends Cogn Sci 15:191–199. [DOI] [PubMed] [Google Scholar]
- Dronkers NF (1996): A new brain region for coordinating speech articulation. Nature 384:159–161. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D (2002): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125:199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L (2005): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128:797–810. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2009): A systems perspective on the effective connectivity of overt speech production. Philos Trans A Math Phys Eng Sci 367:2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P (2010): Beta‐band oscillations‐‐signalling the status quo? Curr Opin Neurobiol 20:156–165. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Konig P, Brecht M, Singer W (1999): Temporal binding, binocular rivalry, and consciousness. Conscious Cogn 8:128–151. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Thompson‐Schill SL (2014): Reworking the language network. Trends Cogn Sci 18:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fries P (2005): A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R (2008): The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci 28:4823–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geweke J, (1982): Measurement of linear‐dependence and feedback between multiple time‐series. J Am Stat Assoc 77:304–313. [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallee N, Tropres I, Baciu M, Le Bas JF, Sato M (2012): Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33:2306–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R (2009): High‐frequency, long‐range coupling between prefrontal and visual cortex during attention. Science 324:1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, MD , Krasnow, B , Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R (2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A (2002): The neural basis of intermittent motor control in humans. Proc Natl Acad Sci USA 99:2299–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Hoogenboom N, Thut G, Schyns P, Panzeri S, Belin P, Garrod S (2013): Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol 11:e1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Saur D, Price CJ, Baumgaertner A, Ulmer S, Siebner HR (2013): Increased facilitatory connectivity from the pre‐SMA to the left dorsal premotor cortex during pseudoword repetition. J Cogn Neurosci 25:580–594. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Amunts K (2009): Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. Neuroimage 48:616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G (2012): Computational neuroanatomy of speech production. Nat Rev Neurosci 13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK (2012): Large‐scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci 15:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén A, Karvonen L, Laine M, Salmelin R (2014): Producing speech with a newly learned morphosyntax and vocabulary: An magnetoencephalography study. J Cogn Neurosci 26:1721–1735. [DOI] [PubMed] [Google Scholar]
- Indefrey P (2011): The spatial and temporal signatures of word production components: A critical update. Front Psychol 2:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ (2004): The spatial and temporal signatures of word production components. Cognition 92:101–44. [DOI] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M, VanRullen R (2012): An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn Sci 16:200–206. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Lachaux JP, N'Diaye K, Pantazis D, Leahy RM, Garnero L, Baillet S (2007): Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc Natl Acad Sci USA 104:7676–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens U (2002): Neural pathways underlying vocal control. Neurosci Biobehav Rev 26:235–258. [DOI] [PubMed] [Google Scholar]
- Jurgens U (2009): The neural control of vocalization in mammals: A review. J Voice 23:1–10. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023‐1026. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD (2000): Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97:1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala J, Gross J, Salmelin R (2008): Localization of correlated network activity at the cortical level with MEG. Neuroimage 39:1706–1720. [DOI] [PubMed] [Google Scholar]
- Kujala J, Vartiainen J, Laaksonen H, Salmelin R (2012): Neural interactions at the core of phonological and semantic priming of written words. Cereb Cortex 22:2305–2312. [DOI] [PubMed] [Google Scholar]
- Laaksonen H, Kujala J, Salmelin R (2008): A method for spatiotemporal mapping of event‐related modulation of cortical rhythmic activity. Neuroimage 42:207–217. [DOI] [PubMed] [Google Scholar]
- Laganaro M, Perret C, (2011): Comparing electrophysiological correlates of word production in immediate and delayed naming through the analysis of word age of acquisition effects. Brain Topogr 24:19–29. [DOI] [PubMed] [Google Scholar]
- Lee JH, Whittington MA, Kopell NJ (2013): Top‐down beta rhythms support selective attention via interlaminar interaction: a model. PLoS Comput Biol 9:e1003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS (1999): A theory of lexical access in speech production. Behav Brain Sci 22:1–38; discussion 38–75. [DOI] [PubMed] [Google Scholar]
- Liljeström M, Tarkiainen A, Parviainen T, Kujala J, Numminen J, Hiltunen J, Laine M, Salmelin R (2008): Perceiving and naming actions and objects. Neuroimage 41:1132–1141. [DOI] [PubMed] [Google Scholar]
- Liljeström M, Hultén A, Parkkonen L, Salmelin R (2009): Comparing MEG and fMRI views to naming actions and objects. Hum Brain Mapp 30:1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H (2007): Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130:623–629. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2010): Anatomic dissection of the inferior fronto‐occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699. [DOI] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS (2008): Inner speech slips exhibit lexical bias, but not the phonemic similarity effect. Cognition 106:528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS (2010): Motor movement matters: the flexible abstractness of inner speech. Mem Cognit 38:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE (2001): An event‐related fMRI study of overt and covert word stem completion. Neuroimage 14:182–193. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM (2007): New vistas for alpha‐frequency band oscillations. Trends Neurosci 30:150–158. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T (1949): Vocalization and arrest of speech. Arch Neurol Psychiatry 61:21–27. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA (2008): Free choice activates a decision circuit between frontal and parietal cortex. Nature 453:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH (1999): Event‐related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857. [DOI] [PubMed] [Google Scholar]
- Postma A (2000): Detection of errors during speech production: A review of speech monitoring models. Cognition 77:97–132. [DOI] [PubMed] [Google Scholar]
- Postma A, Noordanus C (1996): Production and detection of speech errors in silent, mouthed, noise‐masked, and normal auditory feedback speech. Lang Speech 39:375–392. [Google Scholar]
- Price CJ (2012): A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62:816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Kramer MA, Carracedo LM, Kaiser M, Davies CH, Traub RD, Kopell NJ, Whittington MA (2008a): Period concatenation underlies interactions between gamma and beta rhythms in neocortex. Front Cellular Neurosci 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Kramer MA, Carracedo LM, Kaiser M, Davies CH, Traub RD, Kopell NJ, Whittington MA (2008b): Temporal interactions between cortical rhythms. Front Neurosci 2:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruspantini I, Saarinen T, Belardinelli P, Jalava A, Parviainen T, Kujala J, Salmelin R (2012): Corticomuscular coherence is tuned to the spontaneous rhythmicity of speech at 2–3 Hz. J Neurosci 32:3786–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen T, Laaksonen H, Parviainen T, Salmelin R (2006): Motor cortex dynamics in visuomotor production of speech and non‐speech mouth movements. Cereb Cortex 16:212–222. [DOI] [PubMed] [Google Scholar]
- Sahin NT, Pinker S, Cash SS, Schomer D, Halgren E (2009): Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science 326:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R (1997): Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77:3401–3405. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R (1994): Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr Clin Neurophysiol 91:237–248. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R, Lounasmaa OV, Sams M (1994): Dynamics of brain activation during picture naming. Nature 368:463–465. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R (1995): Functional segregation of movement‐related rhythmic activity in the human brain. Neuroimage 2:237–243. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H (2013): Frontal terminations for the inferior fronto‐occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi‐component bundle. Brain Struct Funct 218:21–37. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Gross J (2009): Source connectivity analysis with MEG and EEG. Hum Brain Mapp 30:1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, Gross J (2011): Improving the interpretability of all‐to‐all pairwise source connectivity analysis in MEG with nonhomogeneous smoothing. Hum Brain Mapp 32:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P (2005): Neuronal coherence as a mechanism of effective corticospinal interaction. Science 308:111–113. [DOI] [PubMed] [Google Scholar]
- Schormann T, Henn S, Zilles K (1996): A new approach to fast elastic alignment with applications to human brains. Lect Notes Comput Sci 1131:337–342. [Google Scholar]
- Sedley W, Cunningham MO (2013): Do cortical gamma oscillations promote or suppress perception? An under‐asked question with an over‐assumed answer. Front Hum Neurosci 7:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LI, Lemieux SK (2005): An fMRI investigation of covertly and overtly produced mono‐ and multisyllabic words. Brain Lang 93:20–31. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B (2011): Laryngeal motor cortex and control of speech in humans. Neuroscientist 17:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U (2002): Cortico‐cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res 949:23–31. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U (2005): Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neuroscience 130:133–49. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ostuni J, Ludlow CL, Horwitz B (2009): Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci 29:14912–14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Gray CM (1995): Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18:555–586. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R (2007): Identification and classification of hubs in brain networks. PloS One 2:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL (2009): Contribution of the pre‐SMA to the production of words and non‐speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS). Brain Res 1268:112–124. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Small SL (2011): On the context‐dependent nature of the contribution of the ventral premotor cortex to speech perception. Neuroimage 57:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O (2012): High‐cost, high‐capacity backbone for global brain communication. Proc Natl Acad Sci USA 109:11372–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BC, Beek PJ, Daffertshofer A (2012): Neural synchrony within the motor system: What have we learned so far? Front Hum Neurosci 6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ (2010): Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH (2000): Inhibition‐based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol 38:315–336. [DOI] [PubMed] [Google Scholar]
- Vihla M, Laine M, Salmelin R (2006): Cortical dynamics of visual/semantic vs. phonological analysis in picture confrontation. Neuroimage 33:732–738. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA (2008): Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18:1923–1932. [DOI] [PubMed] [Google Scholar]


