Abstract
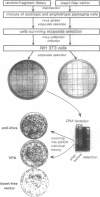
We describe a general strategy for cloning mammalian genes whose downregulation results in a selectable phenotype. This strategy is based on expression selection of genetic suppressor elements (GSEs), cDNA fragments encoding either specific peptides that act as dominant inhibitors of protein function or antisense RNA segments that efficiently inhibit gene expression. Since GSEs counteract the gene from which they are derived, they can be used as dominant selectable markers for the phenotype associated with downregulation of the corresponding gene. A retroviral library containing random fragments of normalized (uniform abundance) cDNA expressed in mouse NIH 3T3 cells was used to select for GSEs inducing resistance to the anticancer drug etoposide. Three GSEs were isolated, two of which are derived from unknown genes and the third encodes antisense RNA for the heavy chain of a motor protein kinesin. The kinesin-derived GSE induces resistance to several DNA-damaging drugs and immortalizes senescent mouse embryo fibroblasts, indicating that kinesin is involved in the mechanisms of drug sensitivity and in vitro senescence. Expression of the human kinesin heavy-chain gene was decreased in four of four etoposide-resistant HeLa cell lines, derived by conventional drug selection, indicating that downregulation of kinesin represents a natural mechanism of drug resistance in mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Borst P. Genetic mechanisms of drug resistance. A review. Acta Oncol. 1991;30(1):87–105. doi: 10.3109/02841869109091819. [DOI] [PubMed] [Google Scholar]
- Deiss L. P., Kimchi A. A genetic tool used to identify thioredoxin as a mediator of a growth inhibitory signal. Science. 1991 Apr 5;252(5002):117–120. doi: 10.1126/science.1901424. [DOI] [PubMed] [Google Scholar]
- Endow S. A. The emerging kinesin family of microtubule motor proteins. Trends Biochem Sci. 1991 Jun;16(6):221–225. doi: 10.1016/0968-0004(91)90089-e. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov A. V., Zelnick C. R., Kazarov A. R., Thimmapaya R., Suttle D. P., Beck W. T., Roninson I. B. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3231–3235. doi: 10.1073/pnas.90.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmayer T. A., Pestov D. G., Roninson I. B. Isolation of dominant negative mutants and inhibitory antisense RNA sequences by expression selection of random DNA fragments. Nucleic Acids Res. 1992 Feb 25;20(4):711–717. doi: 10.1093/nar/20.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Kikuya. A Collection of cDNA Clones with Specific Expression Patterns in Mouse Brain. Eur J Neurosci. 1990;2(8):704–711. doi: 10.1111/j.1460-9568.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Ko M. S. An 'equalized cDNA library' by the reassociation of short double-stranded cDNAs. Nucleic Acids Res. 1990 Oct 11;18(19):5705–5711. doi: 10.1093/nar/18.19.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Schnapp B., Inouye H., Neve R. L. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990 Feb 25;265(6):3278–3283. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Navone F., Niclas J., Hom-Booher N., Sparks L., Bernstein H. D., McCaffrey G., Vale R. D. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol. 1992 Jun;117(6):1263–1275. doi: 10.1083/jcb.117.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Roninson I. B. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Dorit R. L., Gilbert W. One-sided polymerase chain reaction: the amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5673–5677. doi: 10.1073/pnas.86.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanjali S. R., Parimoo S., Weissman S. M. Construction of a uniform-abundance (normalized) cDNA library. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1943–1947. doi: 10.1073/pnas.88.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Perry M. E., Rolfe M., McIntyre P., Commane M., Stark G. R. Induction of gene amplification by 5-aza-2'-deoxycytidine. Mutat Res. 1992 May;276(3):189–197. doi: 10.1016/0165-1110(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Wright B. D., Henson J. H., Wedaman K. P., Willy P. J., Morand J. N., Scholey J. M. Subcellular localization and sequence of sea urchin kinesin heavy chain: evidence for its association with membranes in the mitotic apparatus and interphase cytoplasm. J Cell Biol. 1991 May;113(4):817–833. doi: 10.1083/jcb.113.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]