Abstract
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease with a high prevalence of hypertension and cardiovascular disease. Because SLE predominantly affects women, estrogen is commonly implicated as a contributor to SLE disease progression. Utilizing an established mouse model of SLE (female NZBWF1) we tested whether estrogen has a causal role in the development of hypertension in adulthood. Thirty-week-old SLE and control mice (NZW/LacJ) underwent either a sham or ovariectomy (OVX) procedure. 17β-estradiol (E2; 5μg/mouse, twice/week, s.c.) was administered to a subset of OVX mice. Mean arterial pressure (in mmHg) was increased in SLE mice (134±4 versus 119±3 in controls). Contrary to our hypothesis, OVX exacerbated the hypertension in female SLE mice (153±3; p<0.05 vs. SLE sham), and repletion of E2 prevented the OVX induced increase in blood pressure (132±2). The prevalence of albuminuria was increased in SLE mice in comparison to controls (37% vs. 0%). OVX increased the prevalence in SLE mice (70% versus 37% in SLE shams). Repletion of E2 completely prevented albuminuria in OVX SLE mice. Renal cortical TNF-α was increased in SLE mice compared to controls and was further increased in OVX SLE. The OVX induced increase in renal TNF-α expression was prevented by repletion of E2. Treatment of OVX SLE mice with the TNF-α inhibitor, etanercept, blunted the OVX induced increase in blood pressure (140±2) and prevalence of albuminuria (22%). These data suggest that 17β-estradiol protects against the progression of hypertension during adulthood in SLE, in part, by reducing TNF-α.
Keywords: pressure, immune, systemic lupus erythematosus, estrogen
Introduction
Cardiovascular disease is the leading cause of mortality in patients with systemic lupus erythematosus (SLE), a chronic autoimmune inflammatory disorder that predominantly affects women of reproductive age (1–3). The prevalence of hypertension, a major cardiovascular risk factor, is much higher in women with SLE than age matched healthy women (4–8). However, the factors that underlie the increased prevalence of hypertension during SLE remain unclear.
Due to the partiality towards women, estrogen is commonly implicated as an important contributor to SLE disease progression. In addition, estrogens can promote humoral (antibody) mediated immunity (9, 10) that is essential to the formation of immune complexes resulting in the tissue injury and inflammation characteristic of SLE. A role for estrogens in SLE is commonly supported by studies in experimental animal models where removal of estrogens early in life (typically <6–8 weeks of age) delays the production of autoantibodies and the subsequent renal injury and mortality (11, 12). While these early life manipulations of estrogen suggest that they are important for the onset of SLE, the impact of estrogens on SLE in adulthood has not been widely studied, particularly with regard to their role in the development of hypertension.
Understanding the influence of estrogens on cardiovascular risk factors like hypertension in adulthood is important for women with SLE because autoimmunity accounts for up to 50% of premature ovarian failure, and women with SLE have increased risk for early menopause (13), an important risk factor for hypertension and cardiovascular disease. Using an established experimental model of SLE (female NZBWF1 mice), this study was initially designed to test the hypothesis that estrogen, commonly considered an important factor in the development of SLE, has a causal role in the development of hypertension in adulthood. However, contrary to the hypothesis, the data suggest that estrogens may have an anti-inflammatory role that protects against the further development of hypertension.
Methods and Materials
Animals
Adult (30 week old) female NZBWF1 (SLE) and NZW/LacJ (Ctrl) (Jackson Laboratories, Bar Harbor, ME) mice were used in this study. Mice were maintained on a 12 hour light/dark cycle in temperature controlled rooms with access to chow and water ad libitum. All studies were performed with the approval of the University of Mississippi Medical Center Institutional Animal Care and Use Committee and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ovariectomy
Ovariectomy (OVX) was performed through a dorsal midline incision. The abdominal muscle and peritoneum were incised, and a silk ligature was tied below the ovaries to remove them. In order to confirm the efficacy of OVX, the uterus was collected and weighed at the conclusion of the study.
17β-estradiol repletion
17β-estradiol 17-valerate (E2: 5μg/mouse; Sigma, St. Louis, MO, USA, Cat. # E1631) dissolved in 0.1 ml corn oil (14–18) was injected subcutaneously twice per week for 4 weeks. Mice not receiving E2 were injected with 0.1 ml corn oil.
Etanercept Administration
A subset of mice was administered the TNF-α inhibitor etanercept (Wyeth, Thousand Oaks, CA, 0.8mg/kg) or sterile water by subcutaneous injection once weekly for 4 weeks as we previously published (19).
Blood Pressure Measurements
Mean arterial pressure (MAP in mmHg) was recorded via indwelling carotid artery catheters in conscious mice as previously described by our lab (20).
Autoantibody Production
Plasma anti-dsDNA antibodies, a hallmark of SLE disease, were measured by commercial ELISA (Alpha Diagnostic International, San Antonio, TX) as previously described (21).
Albuminuria
Urinary albumin was monitored weekly by dipstick analysis (Albustix, Tarrytown, NY) of overnight urine samples. Animals were considered to be positive for albuminuria at ≥100mg/dL as previously described (21). Urinary albumin was confirmed by ELISA (Alpha Diagnostic International, San Antonio, TX) in urine samples collected at the end of the study as previously published by our laboratory (21).
Renal TNF-α
Renal cortical protein expression of TNF-α was determined utilizing standard Western blot technique as previously published by our laboratory (19). A mouse monoclonal anti-TNF-α antibody (1:250; Santa Cruz Biotechnology, Dallas, TX) and rabbit anti-β-actin (1:4000; Abcam Inc, Cambridge, MA) were used. Proteins were visualized using an IR700-conjugated donkey anti-mouse IgG (1:2000) and IR800-conjugated donkey anti-rabbit IgG (1:2000; Rockland Immunologicals, Gilbertsville, PA). Blots were analyzed using the Odyssey Infrared Scanner (LI-COR Biosciences, Lincoln, NE). Data are presented as units of protein, based on band optical density, normalized to β-actin.
Protocol 1
30 week old SLE and control mice underwent either OVX or sham operation. Both SLE and control mice were assigned to one of the following treatment groups: Sham, OVX, or OVX+E2. Sham and OVX mice in the E2 repletion study that were injected with oil were grouped with all other Sham or OVX respectively because vehicle had no effect on any parameters measured. Body weight was measured weekly. Urine was collected weekly and assessed for the presence of albumin. Mean arterial pressure was measured at 34 weeks of age, and tissues were collected.
Protocol 2
A subset of 30 week old female OVX SLE and OVX Ctrl mice was administered etanercept via subcutaneous injection once a week for 4 weeks. Mice were followed to 34 weeks of age for measurement of mean arterial pressure and tissue collection.
Statistical Analysis
Data are presented as mean±SEM. Statistical analyses were performed using GraphPad Prism 6 software. A one way ANOVA was used to test for treatment interactions with a Tukey’s multiple comparisons post hoc test. p<0.05 was considered statistically significant.
Results
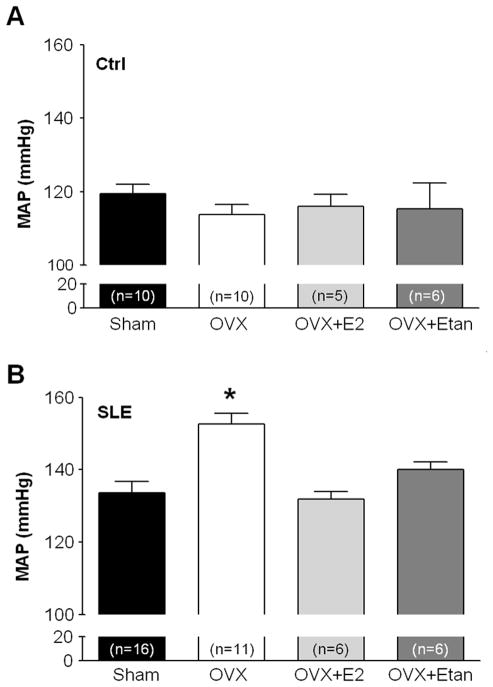
In order to test the hypothesis that estrogen, which is commonly considered an important factor in the development of SLE, has a causal role in the development of hypertension in adulthood, 30 week old control and NZBWF1 mice were subjected to either OVX or sham operation. Consistent with our previously published work, MAP was increased in SLE shams compared to control shams (134±4 mmHg vs. 119±3 mmHg, p<0.05 SLE vs. Ctrl, 2 way ANOVA). Contrary to our hypothesis, MAP was significantly increased in OVX SLE mice in comparison to SLE shams (Figure 1B; 153±3 mmHg, p<0.05). MAP was not altered by OVX in control mice (Figure 1A; 114±3 mmHg). In a subset of animals, E2 was administered to OVX SLE and control mice to determine whether the loss of E2, rather than other ovarian hormones such as progesterone, was responsible for the further increase in blood pressure. Repletion of E2 in OVX SLE mice prevented the increase in MAP caused by OVX (Figure 1B; 132±2 mmHg). Repletion of E2 in OVX control mice did not alter MAP (Figure 1A).
Figure 1.
Effect of ovariectomy (OVX), estradiol (E2) repletion, and etanercept (Etan) on MAP in control mice (A) and SLE mice (B). A, Neither OVX, E2 repletion, nor etanercept altered MAP in comparison to control Sham mice (n≥5).
B, MAP was significantly higher in OVX SLE mice (n=11) compared with Sham (n=16). Repletion of E2 prevented the OVX induced increase in MAP in SLE mice (n=6). Treatment with etanercept blunted the exaggerated increase in MAP in OVX SLE mice (n=6). * p<0.05 vs. SLE sham
Because repletion of E2 prevented the OVX induced increase in MAP, the question of the mechanism by which this protection occurs was examined. Estradiol has known anti-inflammatory effects and reduces proinflammatory cytokines including TNF-α (22–24), previously shown by us to mechanistically contribute to the hypertension in female NZBWF1 (19). Renal cortical TNF-α expression was significantly increased in SLE Sham mice compared to controls (Figure 2; 1.8±0.4 vs. 1.0±0.1, p<0.05). In OVX SLE mice, renal cortical TNF-α expression was increased (3.2±0.8), and repletion of E2 after OVX prevented the OVX induced increase in renal cortical TNF-α expression (2.2±0.3). Renal TNF-α expression was not altered in controls by OVX or repletion of E2 after OVX.
Figure 2.
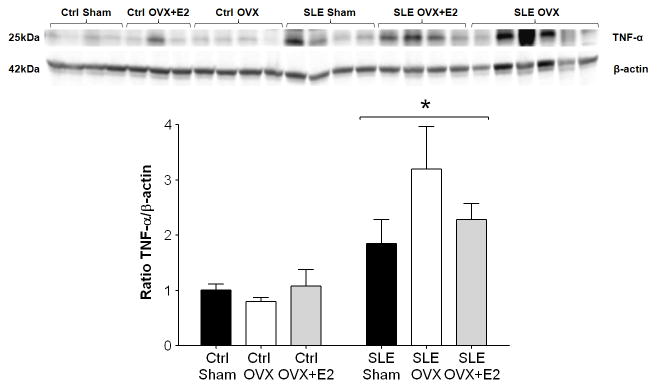
Effect of estradiol on renal cortical TNF-α protein expression in control and SLE mice. TNF-α protein expression was significantly increased in the renal cortex of SLE mice compared with controls. OVX increased TNF-α expression in SLE mice, and repletion of estradiol blunted this increase. * p<0.05 vs. Ctrl
To determine whether the increased TNF-α after OVX contributes to the OVX induced increase in blood pressure in adult SLE mice, OVX SLE and control mice were treated with etanercept, an inhibitor of TNF-α biological activity. Treatment with etanercept in OVX SLE mice ameliorated the OVX induced increase in MAP in female SLE mice (Figure 1B; 140±2 mmHg), suggesting that the exacerbated hypertension caused by OVX was mediated, in part, by TNF-α. This is consistent with our previously published work related to the impact of etanercept on blood pressure during SLE (19). Treatment with etanercept did not alter MAP in controls.
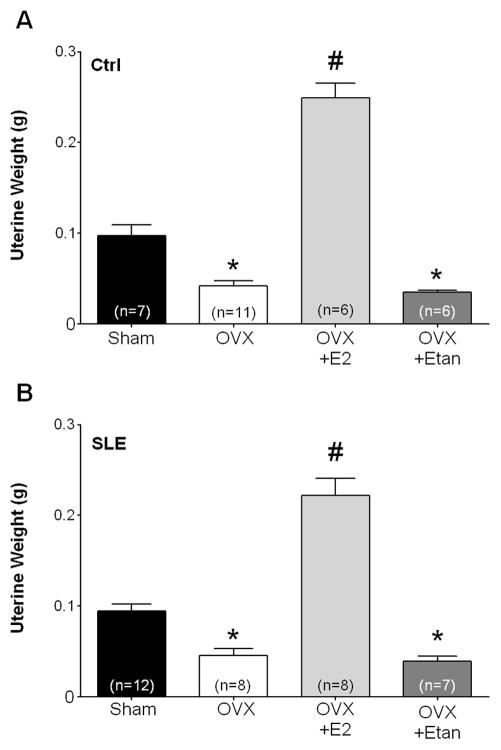
The efficacy of OVX and E2 repletion was confirmed in these studies by measuring uterine weight at the time of sacrifice. As expected after OVX in adult mice, uterine weight was significantly reduced in both control (Figure 3A; Ctrl Sham: 0.097±0.012 g vs. Ctrl OVX: 0.042±0.006 g; p<0.05) and SLE mice (Figure 3B; SLE Sham: 0.094±0.008 g; SLE OVX: 0.045±0.008 g; p<0.05). Uterine weight in mice replete with estradiol was significantly greater than corresponding sham controls (Ctrl OVX+E2: 0.25±0.02 g; SLE OVX+E2: 0.2±0.02 g, p<0.05). Treatment with etanercept after OVX did not alter uterine weight in comparison to OVX mice (Ctrl OVX+Etan: 0.035±0.002 g; SLE OVX+Etan: 0.039±0.01 g).
Figure 3.
Effect of OVX and estradiol (E2) repletion on uterine weight in control mice (A) and SLE mice (B). OVX significantly reduced uterine weight in both control and SLE mice. Repletion of E2 following OVX significantly increased uterine weight in comparison to respective shams. * p<0.05 vs. Sham and OVX+E2. # p<0.05 vs. Sham, OVX, and OVX+Etan.
SLE is characterized by renal injury including albuminuria, and previous work from our laboratory shows that the prevalence of albuminuria in the adult female SLE mice at 34 weeks of age as assessed by dipstick assay is approximately 40% (21, 25). Consistent with this, the prevalence of albuminuria in 34 week old SLE shams in this study reached 37% (Figure 4A). No control mice developed urinary albumin over the course of the study (data not shown). Urinary albumin, as measured by ELISA in samples collected at 34 weeks of age, was increased in SLE mice compared to controls as our laboratory previously showed (19, 21, 25) (Figures 4B and 4C; 3.20±1.5 mg/ml vs. 0.02±0.0 mg/ml, p<0.05 SLE vs. Ctrl, 2 way ANOVA). The percentage of SLE mice with albuminuria was increased to 70% after OVX, and urinary albumin increased compared to SLE shams (10.2±3.9 mg/ml). The OVX SLE mice replete with E2 did not develop albuminuria as measured by dipstick which was confirmed by ELISA (0.01±0.003 mg/ml). The prevalence of albuminuria in OVX SLE mice treated with etanercept was blunted (22%) as was urinary albumin measured by ELISA (1.7±1.5 mg/ml).
Figure 4.
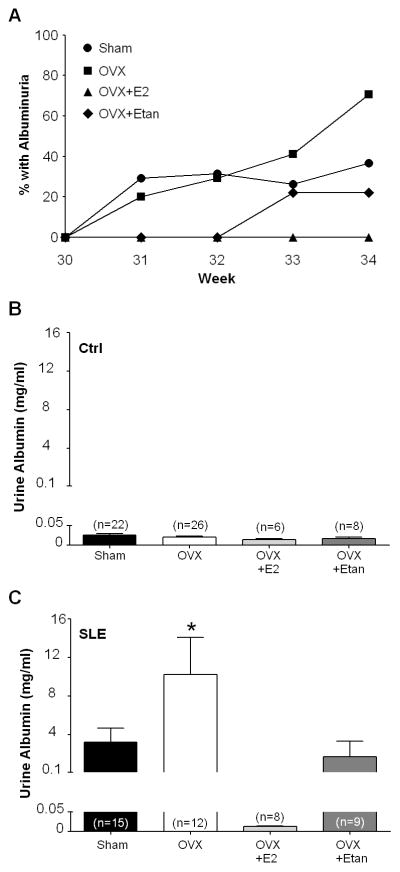
A, Weekly percentage of SLE mice with positive urinary albumin as measured by dipstick assay (n≥8/group). No control mice developed albuminuria. B, Urine albumin in control mice at 34 weeks of age as measured by ELISA (n≥6). Urine albumin was similar between groups. C, Urine albumin in SLE mice at 34 weeks of age as measured by ELISA (n≥8). Urine albumin was increased after ovariectomy (OVX) in comparison to shams. Repletion of estradiol (E2) prevented the OVX induced increase in urine albumin. Treatment with etanercept blunted the increase in urine albumin in OVX SLE mice. * p<0.05 vs. OVX+E2
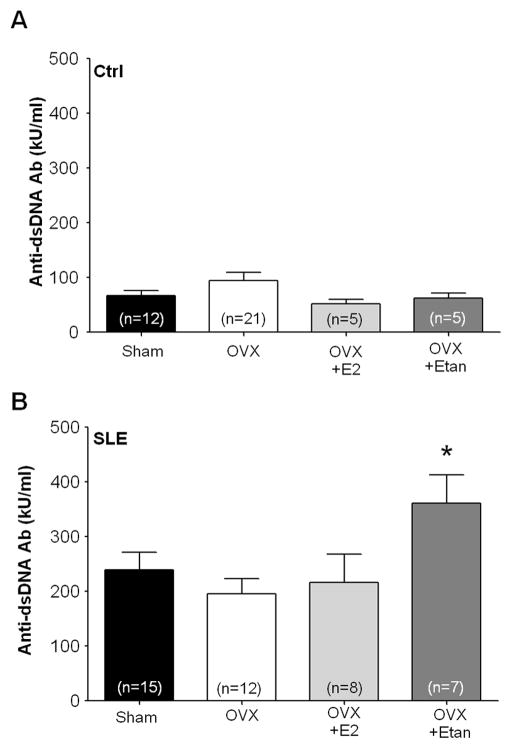
Plasma levels of anti-dsDNA autoantibodies were measured as a marker of SLE disease activity. Plasma anti-dsDNA (IgG) antibodies were higher in SLE mice compared to control Sham (Figure 5A and 5B; 240±32 kU/ml vs. 67±9 kU/ml, p<0.05 SLE vs Ctrl, 2 way ANOVA). OVX in adult female SLE mice did not significantly alter circulating autoantibodies compared with SLE shams (195±28 kU/ml). Plasma anti-dsDNA antibodies in OVX SLE mice replete with E2 were not different from SLE shams (217±51 kU/ml). Circulating antibodies were increased in OVX SLE mice treated with etanercept (Figure 5B; 362±51 kU/ml vs. 195±28 SLE OVX, p<0.05). OVX, repletion of E2, and treatment with etanercept in control mice did not alter plasma anti-dsDNA antibodies compared with control shams (Figure 5A).
Figure 5.
Effect of OVX, estradiol (E2) repletion, and etanercept (Etan) on plasma anti-dsDNA antibodies in control mice (A) and SLE mice (B). A, Plasma anti-dsDNA antibodies were similar between control mice at 34 weeks of age. B, Plasma anti-dsDNA antibodies were increased at 34 weeks of age in SLE mice compared to controls. OVX and repletion of E2 did not significantly alter these levels in comparison to SLE shams. Treatment with etanercept in OVX mice increased antibody levels in comparison to OVX mice. * p<0.05 vs. OVX
Discussion
The prevalence of hypertension is markedly increased in women with SLE (4–8) and represents a major independent risk factor for mortality in this patient population (26); however, the mechanisms contributing to the prevalent hypertension have not been widely studied. Using an established mouse model of SLE, we previously investigated some of the underlying factors that promote hypertension. These include impaired renal hemodynamic function (27), peripheral vascular function (28), inflammatory cytokines (19, 21), and oxidative stress (25). Based on their presumed role to promote SLE disease progression, the present study sought to test whether estrogens could be contributing to development of hypertension. The major new findings of this study are that (1) in adulthood, OVX accelerates rather than protects against the development of hypertension and albuminuria during SLE; (2) the exacerbation of hypertension caused by OVX in adulthood during SLE is mediated by a loss of 17β-estradiol and is associated with increased renal TNF-α expression; (3) the increased renal TNF-α expression mechanistically contributes to the exacerbated hypertension in adult SLE mice because etanercept blunts the effect of OVX. These data provide new insight for understanding the complex role that estrogens have in SLE disease progression and the associated cardiovascular risk factors like hypertension.
Estrogens have been widely implicated in the development of SLE in part based on the strong predilection of the disorder for women of reproductive age. A role for estrogens in autoimmunity has been supported by reports that the production of immunoglobulins (IgG and IgM) is increased in human peripheral blood mononuclear cells incubated with 17β-estradiol, but not other estrogenic compounds (i.e. estrone, estriol) (29). Studies in experimental animal models have also consistently supported an important role for estrogens in SLE disease progression. For example, NZBWF1 estrogen receptor-α knockout mice (ERα KO) exhibited reduced development of anti-dsDNA autoantibodies over time that correlated with increased survival time (30). OVX NZBWF1 mice, treated with a selective ERα agonist, had increased levels of anti-dsDNA IgG specific antibodies (31). These studies support earlier data in the literature, which show that autoantibody production and mortality are increased by estrogen supplementation in female NZBWF1 mice castrated at two weeks of age (11). However, it is worth noting that the dose of E2 (6–7 mg in silastic tubing) used in this previous work is considerably higher than the dose used in the present study and could have caused nonspecific effects including enlarged bladder and associated kidney injury from the chronic muscle relaxation (32, 33). Finally, treatment of NZBWF1 mice with tamoxifen, a selective estrogen receptor modulator, starting at 6–8 weeks of age increased survival but did not impact antibody production (34).
Based on this work, we anticipated that removal of the ovaries would reduce autoantibody production, decrease albuminuria, and protect against the development of hypertension; however, this was not the case. In fact, the data clearly demonstrate a protective role for estrogens against the hypertension and albuminuria, independently of changes in antibody production. The finding that the estradiol has a protective role against the further progression of hypertension is important because blood pressure is a critical factor for increasing cardiovascular risk, the leading cause of death in women with SLE (4, 6–8, 26). Whether this protection translates to delayed mortality could not be addressed with the current experimental design.
A major difference between our study and the work of others that may account for these, initially, unanticipated findings is the timing of estrogen removal. Our study was focused on the role of estrogens in adult female mice (30 weeks of age), whereas previous studies by others were designed to assess the role of estrogens starting in young female mice, either from birth as in the case of Bynote et al. (30), or in mice <8 weeks of age (11, 12, 31, 34). Therefore, the findings of the present study suggest that there may be distinct temporal roles for estrogens in the progression of SLE and its sequelae with estrogens promoting humoral immunity during subclinical disease, but possibly protecting against the tissue inflammation that contributes to disease symptoms in adulthood. There is evidence in the literature supporting distinct temporal effects of estrogen in other diseases. For example, treatment of a mouse model of experimental autoimmune encephalomyelitis with E2 prior to disease onset ameliorated disease severity; however, when treatment was initiated after the development of active disease, the severity of the disease course was not changed (35). Disease severity was also dependent on the timing of treatment with E2 in a study of ovariectomized DBA/1 mice with collagen type II arthritis (36). These studies suggest a high likelihood that estrogen has different temporal effects during inflammatory diseases. One possible explanation for the changing roles of estradiol may be related to alterations in the expression of the ER over time. Roa et al. showed that NZBWF1 mice have fewer ERs per mg cytosol protein than nonautoimmune BALB/c mice (37). ER receptor abundance per mg cytosol protein in the liver was increased in NZBWF1 mice and the parental NZW strain compared to the parental NZB strain while no differences were seen in thymus, spleen, or uterus (38). Another study noted changes in binding affinity and capacity of the ER in MRL mice, a model of lupus, with aging (39). Whether NZBWF1 mice exhibit similar changes with aging is not known.
Although studies in both humans and experimental models of SLE have examined and speculated on the role of estrogens, the impact of estrogens on blood pressure during SLE has not been carefully examined until now. In addition to the fact that hypertension is an independent predictor of mortality, and that blood pressure control is an important clinical consideration for reducing cardiovascular and renal risks in women with SLE, understanding the role of estrogens in blood pressure control in adulthood during SLE is especially important to this patient population because the risk of premature ovarian failure and early menopause is high (13, 40, 41). A number of studies report that hormone therapy in women with SLE is well tolerated (42–45) despite the assumption that estrogens promote SLE disease. In one study assessing the relationship between cardiovascular disease risks and hormone therapy in women with SLE, hormone therapy did not predispose to coronary artery disease (46). In addition, hormone therapy in the SELENA study of 351 women with SLE did not lead to cardiovascular events (42). Similarly, in the LUMINA study hormone therapy was not associated with vascular arterial events (47), and Fernández et al. even speculated that hormone therapy may be a protective factor against the development of arterial vascular events in women with SLE (48). Our data are consistent with these studies suggesting a potential cardioprotective role for estrogens in adulthood during SLE.
In order to gain insight as to the mechanism by which estrogen could protect against the hypertension, we focused on the inflammatory cytokine, TNF-α. Our previously published work showed renal TNF-α to be an important factor in the pathogenesis of hypertension in this model (19). In addition, low levels of estrogen or removal of estrogen promotes a pro-inflammatory response and the production of cytokines (22, 23). For example, TNF-α is increased in postmenopausal women in comparison to premenopausal women (49, 50). In experimental animal studies, estrogen deficient rats have increased serum levels of TNF-α that are reduced following estrogen repletion, and TNF-α inhibition in estrogen deficient rats was associated with improved vascular function (24). Consistent with this data, we show that OVX adult female SLE mice have increased renal cortical protein expression of TNF-α and that the enhanced blood pressure response caused by OVX was largely prevented by treatment with etanercept. While these data suggest that TNF-a is an important mediator of the OVX induced increase in pressure, it is possible that etanercept prevents the increased pressure caused by OVX independently of the blood pressure lowering effects of E2.
Separating changes in albuminuria from changes in blood pressure is always difficult. Female NZBWF1 mice represent a long established model of SLE with immune complex mediated glomerulonephritis; therefore, it is unlikely that the development of albuminuria is strictly related to the hypertension. In support of this, we recently published work showing that bilateral renal denervation in this model ameliorates albuminuria independently of changes in blood pressure (51). In addition, studies in the NZBWF1 model show that lowering blood pressure alone is not sufficient to reduce renal injury (52). Nevertheless, the albuminuria caused by OVX appears to track well with the increase in blood pressure. Interestingly, however, albuminuria was ameliorated in the SLE mice replete with 17β-estradiol, even though the blood pressure is similar to the sham SLE group. The reason for the marked effect of 17β-estradiol on albuminuria is not clear. The dose of estradiol selected for this study was based on previously published work of others (14–18) and the regimen (twice per week) was chosen to better mimic estrous cycling. However, based on the fact that uterine weights were increased over sham controls in animals replete with estradiol, the circulating levels of estradiol achieved were most likely high. We did not measure serum estradiol in this study because levels in mice are very low (<5 pg/ml) which typically falls below the sensitivity of even liquid chromatography-mass spectrometry methods and leads to significant variability in radioimmunoassays, even when comparing OVX to intact animals (53). Irrespective of the absolute circulating levels of estradiol, these data support the idea that the albuminuria in this model is not solely the result of increased arterial pressure. Taken together the results of these studies showing a protective role of estradiol in adulthood during SLE highlight the need to carefully examine the temporal effects of estrogen on disease activity and may help to explain why there is considerable variability in human data with regard to the role of estrogens.
Perspectives
The role of estrogens in adult women with SLE is much less clear than what is commonly presumed. Therefore, it may be particularly informative to consider more carefully this data in the context of what is known about SLE in women. For example, over the course of a normal menstrual cycle, women with SLE report greater disease activity during menses, but not during the normal hormonal surge that is associated with the highest levels of estrogen during the estrus cycle (54) Estrogen based oral contraceptive use is generally safe in women with SLE and does not significantly exacerbate disease activity (55, 56). There are even reports suggesting that estrogens have a protective role against SLE. For example, disease flares were reduced in pregnant women with SLE during the third trimester when estrogen levels are at their highest (57), and in some studies, lower levels of estrogen are associated with increased SLE disease activity (58). In the present study, we show that removal of estrogen during adulthood in female NZBWF1 SLE mice exacerbates hypertension and increases renal injury and renal TNF-α; all of which are prevented by repletion of estrogen. These data suggest that estrogen may play a protective role against a major cardiovascular risk factor in adulthood during SLE. In addition, these data suggest that hormone therapy or repletion of estrogen can be cardioprotective during SLE, while not altering autoantibody levels or encouraging SLE disease activity.
Novelty and Significance.
1. What is new?
In adulthood, loss of estrogen accelerates rather than protects against the development of high blood pressure and kidney injury during lupus disease.
The exacerbation of high blood pressure caused by loss of estrogen in adulthood during SLE is associated with increased inflammation in the kidney.
2. What is relevant?
Cardiovascular disease is the leading cause of death during SLE. Moreover, hypertension, a major cardiovascular risk factor, is highly prevalent in women with SLE for reasons that remain unclear.
The data presented in this study suggest that estrogens protect against the progression of hypertension during adulthood in SLE. These findings contrast with the common assumption that estrogens promote SLE and its sequelae and suggest a more complex role for estrogens in SLE disease progression.
3. Summary
Estrogen plays a protective role during adulthood in SLE at least against progression of hypertension, a major cardiovascular risk factor.
These data provide new insight for understanding the complex role that estrogens have in SLE disease progression and the associated cardiovascular risk factors like hypertension.
Acknowledgments
Sources of Funding
E.L. Gilbert is the recipient of an American Heart Association Greater Southeast Affiliate Predoctoral Fellowship (12PRE12050150). This work was supported by HL085907 and a UMMC Intramural Research Support grant to M.J. Ryan, and HL051971 to UMMC Physiology.
Footnotes
Conflict of Interest/Disclosures
None.
References
- 1.López P, Mozo L, Gutiérrez C, Suárez A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus. 2003;12:860–865. doi: 10.1191/0961203303lu469xx. [DOI] [PubMed] [Google Scholar]
- 2.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995;34:866–872. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 3.Björnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964–95. J Rheumatol. 2004;31:713–719. [PubMed] [Google Scholar]
- 4.Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166–169. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 5.Health, United States. 2012 with chartbook on trends in health of Americans. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 6.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 7.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 8.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, Mediavilla JD, Jiménez-Jáimez J, Díaz-Chamorro A, Jiménez-Alonso J Grupo Lupus Virgen de las Nieves. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026–1032. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 9.Kanda N, Tsuchida T, Tamaki K. Estrogen enhancement of anti-double-stranded DNA antibody and immunoglobulin G production in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:328–337. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Verthelyi D, Ahmed SA. 17β-Estradiol, but not 5α-dihydrotestosterone, augments antibodies to double-stranded deoxyribonucleic acid in nonautoimmune C57BL/6J mice. Endocrinology. 1994;135:2615–2622. doi: 10.1210/endo.135.6.7988450. [DOI] [PubMed] [Google Scholar]
- 11.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect. 2005;113:323–328. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sammaritano LR. Menopause in patients with autoimmune diseases. Autoimmun Rev. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Li J, McMurray RW. Effects of cyclic versus sustained estrogen administration on peripheral immune functions in ovariectomized mice. Am J Reprod Immunol. 2010;63:274–281. doi: 10.1111/j.1600-0897.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 15.Elbourne KB, Keisler D, McMurray RW. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus. 1998;7:420–427. doi: 10.1191/096120398678920352. [DOI] [PubMed] [Google Scholar]
- 16.Carlsten H, Holmdahl R, Tarkowski A, Nilsson LA. Oestradiol- and testosterone-mediated effects on the immune system in normal and autoimmune mice are genetically linked and inherited as dominant traits. Immunology. 1989;68:209–214. [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsten H, Tarkowski A, Holmdahl R, Nilsson LA. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr / lpr mice. Clin Exp Immunol. 1990;80:467–473. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MT. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–10839. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension. 2006;48:988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 21.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–R1289. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers A, Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–34. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- 23.Ralston SH, Russell RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 24.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 25.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginzler EM, Felson DT, Anthony JM, Anderson JJ. Hypertension increases the risk of renal deterioration in systemic lupus erythematosus. J Rheumatol. 1993;20:1694–1700. [PubMed] [Google Scholar]
- 27.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover PH, Ryan MJ. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1286–R1292. doi: 10.1152/ajpregu.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 29.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999;103:282–288. doi: 10.1016/s0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 30.Bynoté KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor-alpha deficiency attenuates autoimmune disease in (NZB x NZW)F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 31.Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on autoimmune disease in lupus-prone NZB/NZW F1 mouse model. Clin Immunol. 2007;123:219–226. doi: 10.1016/j.clim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Walker SE, McMurray RW, Besch-Williford CL, Keisler DH. Premature death with bladder outlet obstruction and hyperprolactinemia in New Zealand black X New Zealand white mice treated with ethinyl estradiol and 17 beta-estradiol. Arthritis Rheum. 1992;35:1387–1392. doi: 10.1002/art.1780351123. [DOI] [PubMed] [Google Scholar]
- 33.Pearse G, Frith J, Randall KJ, Klinowska T. Urinary retention and cystitis associated with subcutaneous estradiol pellets in female nude mice. Toxicol Pathol. 2009;37:227–234. doi: 10.1177/0192623308329281. [DOI] [PubMed] [Google Scholar]
- 34.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52:393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 35.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 36.Jansson L, Mattsson A, Mattsson R, Holmdahl R. Estrogen induced suppression of collagen arthritis. V: Physiological level of estrogen in DBA/1 mice is therapeutic on established arthritis, suppresses anti-type II collagen T-cell dependent immunity and stimulates polyclonal B-cell activity. J Autoimmun. 1990;3:257–270. doi: 10.1016/0896-8411(90)90145-i. [DOI] [PubMed] [Google Scholar]
- 37.Roa R, Greenstein BD. Evidence for pleomorphism of estrogen receptor capacity and affinity in liver and thymus of immature BALB/c and (NZBxNZW) F1 mice, a model of systemic lupus erythematosus. Int J Immunopharmacol. 2000;22:897–903. doi: 10.1016/s0192-0561(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 38.Athreya BH, Moore WC, Wadsworth SA, Gupta C, Goldman AS. Estrogen receptor levels in a murine model of systemic lupus erythematosus. Clin Exp Rheumatol. 1989;7:589–593. [PubMed] [Google Scholar]
- 39.Dhaher YY, Greenstein B, de Fougerolles Nunn E, Khamashta M, Hughes GR. Strain differences in binding properties of estrogen receptors in immature and adult BALB/c and MRL/MP-lpr/lpr mice, a model of systemic lupus erythematosus. Int J Immunopharmacol. 2000;22:247–254. doi: 10.1016/s0192-0561(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 40.Ekblom-Kullberg S, Kautiainen H, Alha P, Helve T, Leirisalo-Repo M, Julkunen H. Reproductive health in women with systemic lupus erythematosus compared to population controls. Scand J Rheumatol. 2009;38:375–380. doi: 10.1080/03009740902763099. [DOI] [PubMed] [Google Scholar]
- 41.Chang YS, Lai CC, Chen WS, Wang SH, Chou CT, Tsai CY. Protein-losing enteropathy and premature ovarian failure in a young woman with systemic lupus erythematosus. Lupus. 2012;21:1237–1239. doi: 10.1177/0961203312449492. [DOI] [PubMed] [Google Scholar]
- 42.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcón GS, Manzi S, Belmont HM, Askanase AD, Sigler L, Dooley MA, Von Feldt J, McCune WJ, Friedman A, Wachs J, Cronin M, Hearth-Holmes M, Tan M, Licciardi F. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 43.Mok CC, Lau CS, Ho CT, Lee KW, Mok MY, Wong RW. Safety of hormonal replacement therapy in postmenopausal patients with systemic lupus erythematosus. Scand J Rheumatol. 1998;27:342–346. doi: 10.1080/03009749850154357. [DOI] [PubMed] [Google Scholar]
- 44.Kreidstein S, Urowitz MB, Gladman DD, Gough J. Hormone replacement therapy in systemic lupus erythematosus. J Rheumatol. 1997;24:2149–2152. [PubMed] [Google Scholar]
- 45.Sánchez-Guerrero J, González-Pérez M, Durand-Carbajal M, Lara-Reyes P, Jiménez-Santana L, Romero-Díaz J, Cravioto MD. Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis Rheum. 2007;56:3070–3079. doi: 10.1002/art.22855. [DOI] [PubMed] [Google Scholar]
- 46.Hochman J, Urowitz MB, Ibañez D, Gladman DD. Hormone replacement therapy in women with systemic lupus erythematosus and risk of cardiovascular disease. Lupus. 2009;18:313–317. doi: 10.1177/0961203308097475. [DOI] [PubMed] [Google Scholar]
- 47.Fernández M, Calvo-Alén J, Bertoli AM, Bastian HM, Fessler BJ, McGwin G, Jr, Reveille JD, Vilá LM, Alarcón GS. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA L II): relationship between vascular events and the use of hormone replacement therapy in postmenopausal women. J Clin Rheumatol. 2007;13:261–265. doi: 10.1097/RHU.0b013e318156bbf5. [DOI] [PubMed] [Google Scholar]
- 48.Fernández M, Calvo-Alén J, Alarcón GS, Roseman JM, Bastian HM, Fessler BJ, McGwin G, Jr, Vilá LM, Sanchez ML, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXI. Disease activity, damage accrual, and vascular events in pre- and postmenopausal women. Arthritis Rheum. 2005;52:1655–1664. doi: 10.1002/art.21048. [DOI] [PubMed] [Google Scholar]
- 49.Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T, Yasui T, Aono T. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol. 2001;184:309–314. doi: 10.1067/mob.2001.109940. [DOI] [PubMed] [Google Scholar]
- 50.Sites CK, Toth MJ, Cushman M, L’Hommedieu GD, Tchernof A, Tracy RP, Poehlman ET. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77:128–135. doi: 10.1016/s0015-0282(01)02934-x. [DOI] [PubMed] [Google Scholar]
- 51.Mathis KW, Venegas-Pont MR, Flynn ER, Williams JM, Maric-Bilkan C, Dwyer TM, Ryan MJ. Hypertension in an experimental model of systemic lupus erythematosus occurs independently of the renal nerves. Am J Physiol Regul Integr Comp Physiol. 2013;305:R711–R719. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl. 1988;6:S684–S686. doi: 10.1097/00004872-198812040-00215. [DOI] [PubMed] [Google Scholar]
- 53.Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Colangelo K, Haig S, Bonner A, Zelenietz C, Pope J. Self-reported flaring varies during the menstrual cycle in systemic lupus erythematosus compared with rheumatoid arthritis and fibromyalgia. Rheumatology (Oxford) 2011;50:703–708. doi: 10.1093/rheumatology/keq360. [DOI] [PubMed] [Google Scholar]
- 55.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, McCune WJ, Hearth-Holmes M, Dooley MA, Von Feldt J, Friedman A, Tan M, Davis J, Cronin M, Diamond B, Mackay M, Sigler L, Fillius M, Rupel A, Licciardi F, Buyon JP. OC-SELENA Trial. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 56.Culwell KR, Curtis KM, del Carmen Cravioto M. Safety of contraceptive method use among women with systemic lupus erythematosus: a systematic review. Obstet Gynecol. 2009;11:341–353. doi: 10.1097/AOG.0b013e3181ae9c64. [DOI] [PubMed] [Google Scholar]
- 57.Stojan G, Baer AN. Flares of systemic lupus erythematosus during pregnancy and the puerperium: prevention, diagnosis and management. Expert Rev Clin Immunol. 2012;8:439–453. doi: 10.1586/eci.12.36. [DOI] [PubMed] [Google Scholar]
- 58.Muñoz JA, Gil A, López-Dupla JM, Vázquez JJ, González-Gancedo P. Sex hormones in chronic systemic lupus erythematosus. Correlation with clinical and biological parameters. Ann Med Interne (Paris) 1994;145:459–463. [PubMed] [Google Scholar]