Abstract
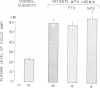
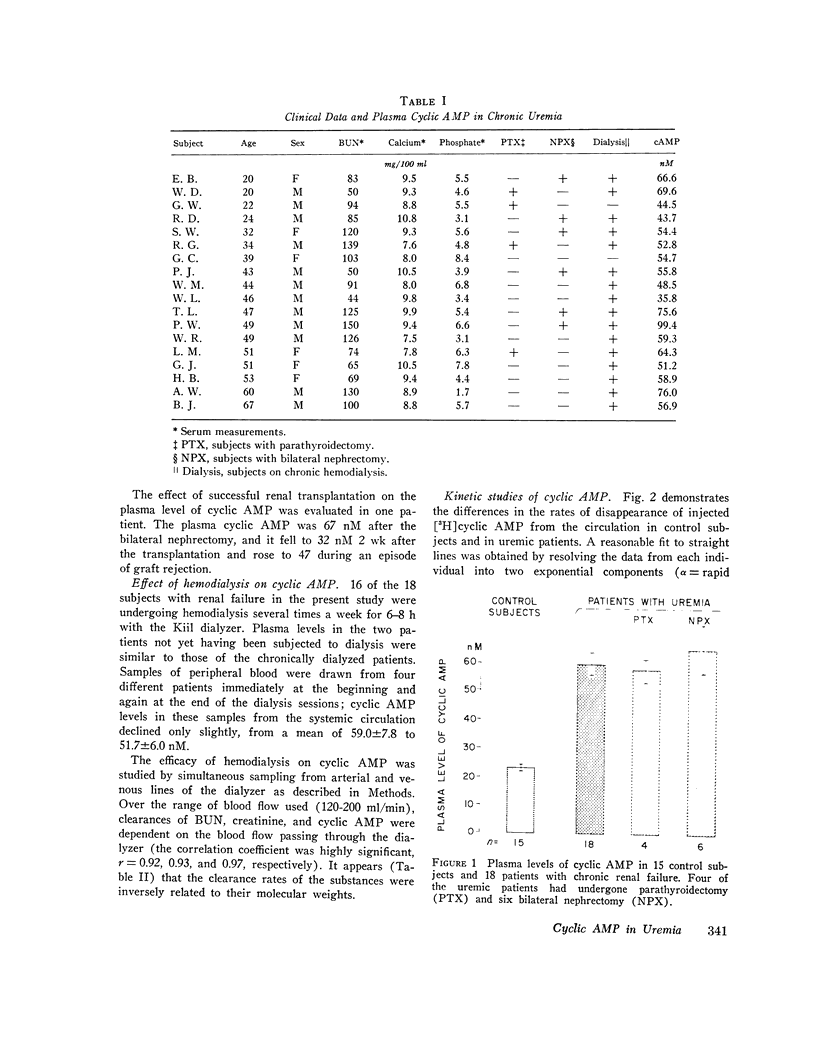
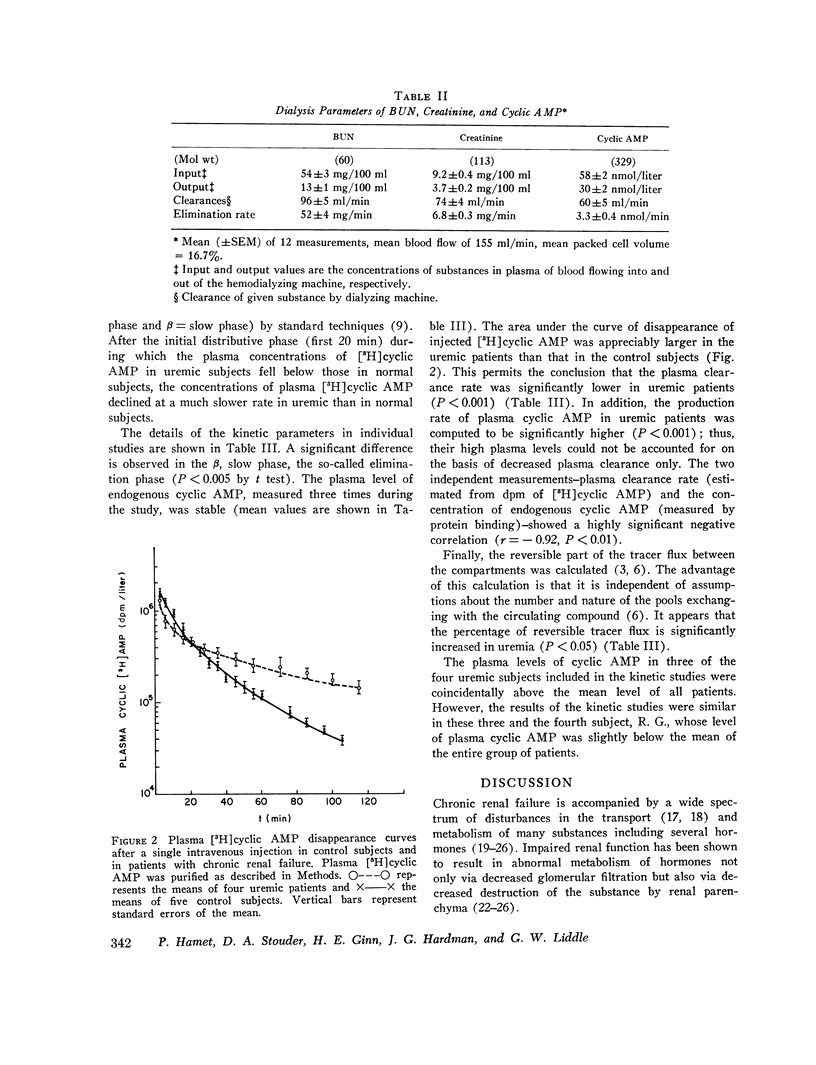
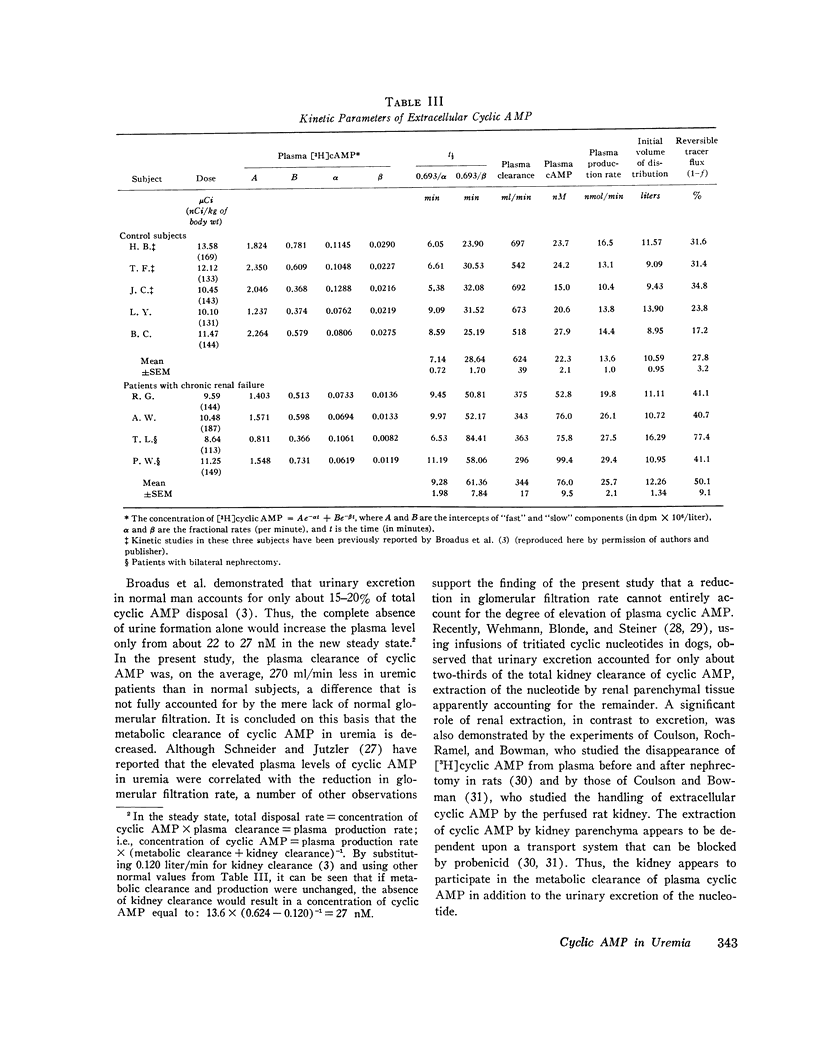
This study was designed to elucidate the mechanism of elevation of plasma cyclic AMP in uremic man. Plasma cyclic AMP was measured in 15 normal subjects and in 18 patients with severe renal failure. In some members from both groups the kinetic parameters of the metabolism of extracellular cyclic AMP were measured. Plasma cyclic AMP was elevated from 23 nM in control subjects to 59 nM in uremic patients, regardless of the presence or absence of the kidneys or parathyroid glands. A single pass of uremic blood through a Kiil hemodialyzer decreased plasma cyclic AMP from 58 to 30 nM. The clearance of cyclic AMP by the dialyzer correlated directly with the blood flow passing through the machine. Hemodialysis for 6 h decreased plasma cyclic AMP levels in the systemic circulation by only 12%. Studies with tritiated cyclic AMP revealed a plasma clearance rate of 624 ml/min in normal subjects and of 344 ml/min in patients with uremia. Such a large decrease in plasma clearance rate cannot be explained by a failure of urinary excretion of cyclic AMP and suggests impairment of "metabolic clearance." In addition, the "plasms production rate" of cyclic AMP was 65% higher in patients with renal failure than in normal subjects. It is concluded that the elevation of plasma cyclic AMP in uremic man is due to a combination of: (a) lack of urinary excretion, (b) decreases metabolic clearance, and (c) increased production of plasma cyclic AMP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beck L. V., Fedynskyj N. Evidence from combined immunoassay and radioautography procedures that intact insulin-125-I molecules are concentrated by mouse kidney proximal tubule cells. Endocrinology. 1967 Sep;81(3):475–485. doi: 10.1210/endo-81-3-475. [DOI] [PubMed] [Google Scholar]
- Blonde L., Wehmann R. E., Steiner A. L. Plasma clearance rates and renal clearance of 3H-labeled cyclic AMP and 3H-labeled cyclic GMP in the dog. J Clin Invest. 1974 Jan;53(1):163–172. doi: 10.1172/JCI107534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker N. S., Bourgoignie J. J., Klahr S. A humoral inhibitor of sodium transport in uremic serum. A potential toxin? Arch Intern Med. 1970 Nov;126(5):860–864. [PubMed] [Google Scholar]
- Broadus A. E., Kaminsky N. I., Hardman J. G., Sutherland E. W., Liddle G. W. Kinetic parameters and renal clearances of plasma adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2222–2236. doi: 10.1172/JCI106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain M. J., Stimmler L. The renal handling of insulin. J Clin Invest. 1967 Jun;46(6):911–919. doi: 10.1172/JCI105597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B., Robison G. A., Hartmann R. C. The role of cyclic AMP in platelet function. Ann N Y Acad Sci. 1971 Dec 30;185:477–487. doi: 10.1111/j.1749-6632.1971.tb45274.x. [DOI] [PubMed] [Google Scholar]
- Cooke C. R., Ruiz-Maza F., Kowarski A., Migeon C. J., Walker W. G. Regulation of plasma aldosterone concentration in anephric man and renal transplant recipients. Kidney Int. 1973 Mar;3(3):160–166. doi: 10.1038/ki.1973.24. [DOI] [PubMed] [Google Scholar]
- Coulson R., Bowman R. H. Excretion and degradation of exogenous adenosine 3',5'-monophosphate by isolated perfused rat kidney. Life Sci. 1974 Feb 1;14(3):545–566. doi: 10.1016/0024-3205(74)90369-5. [DOI] [PubMed] [Google Scholar]
- Danovitch G. M. Uric acid transport in renal failure. A review. Nephron. 1972;9(5):291–299. doi: 10.1159/000180160. [DOI] [PubMed] [Google Scholar]
- Eknoyan G., Wacksman S. J., Glueck H. I., Will J. J. Platelet function in renal failure. N Engl J Med. 1969 Mar 27;280(13):677–681. doi: 10.1056/NEJM196903272801301. [DOI] [PubMed] [Google Scholar]
- Gill J. R., Jr, Casper A. G. Renal effects of adenosine 3',5'-cyclic monophosphate and dibutyryl adenosine 3',5'-cyclic monophosphate. Evidence for a role for adenosine 3',5'-cyclic monophosphate in the regulation of proximal tubular sodium reabsorption. J Clin Invest. 1971 Jun;50(6):1231–1240. doi: 10.1172/JCI106600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamet P., Kuchel O., Fraysse J., Genest J. Plasma adenosine 3',5'-cyclic monophosphate in human hypertension. Can Med Assoc J. 1974 Aug 17;111(4):323–328. [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Orloff J. Factors involved in the action of cyclic AMP on the permeability of mammalian kidney and toad urinary bladder. Ann N Y Acad Sci. 1971 Dec 30;185:345–350. doi: 10.1111/j.1749-6632.1971.tb45260.x. [DOI] [PubMed] [Google Scholar]
- Horowitz H. I. Uremic toxins and platelet function. Arch Intern Med. 1970 Nov;126(5):823–826. [PubMed] [Google Scholar]
- KESSLER G., WOLFMAN M. AN AUTOMATED PROCEDURE FOR THE SIMULTANEOUS DETERMINATION OF CALCIUM AND PHOSPHORUS. Clin Chem. 1964 Aug;10:686–703. [PubMed] [Google Scholar]
- Kaminsky N. I., Broadus A. E., Hardman J. G., Jones D. J., Jr, Ball J. H., Sutherland E. W., Liddle G. W. Effects of parathyroid hormone on plasma and urinary adenosine 3',5'-monophosphate in man. J Clin Invest. 1970 Dec;49(12):2387–2395. doi: 10.1172/JCI106458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Gurpide E., Sciarra J. J., Grumbach M. M. Metabolic clearance rate and production rate of chorionic growth hormone-prolactin in late pregnancy. J Clin Endocrinol Metab. 1968 Oct;28(10):1450–1460. doi: 10.1210/jcem-28-10-1450. [DOI] [PubMed] [Google Scholar]
- Kerr D. N., Hoenich N. A., Frost T. H., Clayton C. B., Jolly D. Which dialyser? Nephron. 1974;12(5):368–392. doi: 10.1159/000180351. [DOI] [PubMed] [Google Scholar]
- Koutras D. A., Marketos S. G., Rigopoulos G. A., Malamos B. Iodine metabolism in chronic renal insufficiency. Nephron. 1972;9(1):55–65. doi: 10.1159/000180133. [DOI] [PubMed] [Google Scholar]
- Kövér G., Tost H., Stimácz E. Effect of cyclic 3'-5'-adenosine monophosphate and theophylline on renal function. Acta Physiol Acad Sci Hung. 1972;42(2):111–117. [PubMed] [Google Scholar]
- MARSH W. H., FINGERHUT B., MILLER H. AUTOMATED AND MANUAL DIRECT METHODS FOR THE DETERMINATION OF BLOOD UREA. Clin Chem. 1965 Jun;11:624–627. [PubMed] [Google Scholar]
- Martin T. J., Melick R. A., de Luise M. The effect of nephrectomy on the metabolism of labelled parathyroid hormone. Clin Sci. 1969 Aug;37(1):137–142. [PubMed] [Google Scholar]
- O'Brien J. P., Sharpe A. R., Jr The influence of renal disease on the insulin I-131 disappearance curve in man. Metabolism. 1967 Jan;16(1):76–83. doi: 10.1016/0026-0495(67)90161-8. [DOI] [PubMed] [Google Scholar]
- Parsons V. Divalent ion metabolism and the kidney. Nephron. 1973;10(2):157–173. doi: 10.1159/000180184. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Reita R. E., Deftos L. J., Kaye M. B., Richardson J. A., Buckle R. M., Aurbach G. D. Secondary hyperparathyroidism in chronic renal disease. Arch Intern Med. 1969 Oct;124(4):408–412. [PubMed] [Google Scholar]
- Rescigno A., Gurpide E. Estimation of average times of residence, recycle and interconversion of blood-borne compounds using tracer methods. J Clin Endocrinol Metab. 1973 Feb;36(2):263–276. doi: 10.1210/jcem-36-2-263. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Spitz I. Role of the kidney in insulin metabolism and excretion. Diabetes. 1968 Mar;17(3):161–169. doi: 10.2337/diab.17.3.161. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Levine L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. II. Effect of N6-2'-o-dibutyryl cyclic 3',5'-adenosine monophosphate on platelet function. J Clin Invest. 1971 Jan;50(1):131–141. doi: 10.1172/JCI106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Jutzler G. A. Letter: Implications of cyclic adenosine 3',5'-monophosphate in chronic renal failure. N Engl J Med. 1974 Jul 18;291(3):155–155. doi: 10.1056/NEJM197407182910315. [DOI] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Davis B. B., Pawlson L. G., Josimovich J. B., Mintz D. H. Factors influencing the urinary excretion of 3',5'-adenosine monophosphate in humans. J Clin Endocrinol Metab. 1970 Mar;30(3):316–324. doi: 10.1210/jcem-30-3-316. [DOI] [PubMed] [Google Scholar]
- Wehmann R. E., Blonde L., Steiner A. L. Sources of cyclic nucleotides in plasma. J Clin Invest. 1974 Jan;53(1):173–179. doi: 10.1172/JCI107535. [DOI] [PMC free article] [PubMed] [Google Scholar]