Abstract
Although estrogen reduces inflammatory-mediated pain responses, the mechanisms behind its effects are unclear. This study investigated if estrogen modulates inflammatory signaling by reducing baseline or inflammation-induced cytokine levels in the injury-site, serum, dorsal root ganglia (DRG) and/or spinal cord. We further tested whether estrogen effects on cytokine levels are in part mediated through hypothalamic– pituitary–adrenal (HPA) axis activation. Lumbar DRG, spinal cord, serum, and hind paw tissue were analyzed for cytokine levels in 17β-estradiol-(20%) or vehicle-(100% cholesterol) treated female rats following ovariectomy/sham adrenalectomy (OVX), adrenalectomy/sham ovariectomy (ADX) or ADX + OVX operation at baseline and post formalin injection. Formalin significantly increased proinflammatory interleukin (IL)-6 levels in the paw, as well as pro- and anti-inflammatory cytokine levels in the DRG, spinal cord and serum in comparison to naïve conditions. Estrogen replacement significantly increased anti-inflammatory IL-10 levels in the DRG. Centrally, estradiol significantly decreased proinflammatory tumor necrosis factor (TNF)-α and IL-1β levels, as well as IL-10 levels, in the spinal cord in comparison to cholesterol treatment. At both sites, most estradiol modulatory effects occurred irrespective of pain or surgical condition. Estradiol alone had no influence on cytokine release in the paw or serum, indicating that estrogen effects were site-specific. Although cytokine levels were altered between surgical conditions at baseline and following formalin administration, ADX operation did not significantly reverse estradiol’s modulation of cytokine levels. These results suggest that estrogen directly regulates cytokines independent of HPA axis activity in vivo, in part by reducing cytokine levels in the spinal cord.
Keywords: Inflammation, Cytokines, Estrogen, Ovariectomy, Adrenalectomy
1. Introduction
Estrogen deficiency renders postmenopausal women vulnerable to degenerative conditions such as osteoarthritis, osteoporosis, atherosclerosis, and Alzheimer’s disease [1–4]. An issue neglected by the current literature is how estrogen alters inflammation-mediated pain responses and reduces neuroinflammatory diseases. This topic is of clinical relevance because elucidating the mechanisms of estrogen’s effects on inflammatory responses will greatly enhance our understanding of painful pathological conditions in women and help to understand why postmenopausal women are more susceptible to these severe chronic conditions.
It has been argued that neuronal inflammation is a pro-inflammatory cytokine-mediated process that results from systemic or direct neuronal tissue injury [5]. During inflammation, pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 are released by a variety of cells and excite nociceptors either by direct action or indirectly by stimulating the release of inflammatory mediators such as bradykinin, prostaglandin (PG) and substance P [6]. While administration of proinflammatory cytokines to rodents produces persistent pain and hyperalgesia, knockout mouse models or antagonists of these cytokines reduce hyperalgesia in animal models of inflammatory and neuropathic pain [7–13]. In the spinal cord, mRNA expression and protein levels of pro-inflammatory cytokines have been identified in response to peripheral or spinal nerve injury [14–17]. On the other hand, the anti-inflammatory cytokine IL-10 produces hypoalgesic responses to inflammatory pain; repeated intrathecal injections of plasmid DNA encoding IL-10 reverses allodynia induced by neuropathic pain [18]. IL-10’s analgesic effect has been postulated to be via inhibition of TNF-α, IL-1β, and PGs in the dorsal root ganglia (DRG) and spinal cord [19,20].
The sex hormone estrogen has been shown to display antiinflammatory and antinociceptive properties during inflammation; for instance, in the formalin assay for inflammatory pain, estrogen administration (via SILASTIC capsule) significantly reduces Phase II nociceptive flinching responses in ovariectomized rats and does so dose-dependently [21–23]. Estradiol administration lowers thermal, mechanical and adjuvant-induced hyperalgesia in rats [24,25]. Furthermore, estradiol reduces carrageenan-induced pleurisy and acute inflammation [26,27]. Parts of the anti-inflammatory effects of estrogen have been linked to its interactions with adrenergic and serotonergic systems [25]. However, the full mechanisms underlying estrogen’s anti-hyperalgesic effects are still under investigation.
Estrogen has been shown to regulate cytokine activity, thereby offering another mechanism for estrogen-mediated analgesia. Estrogen prevents lipopolysaccharide (LPS)-induced microglial toxicity by attenuating the release of TNF-α and IL-1β [28]. Estrogen replacement lowers IL-6 production in ovariectomized mice, while activation of estrogen receptors (ER) ERα and ERβ leads to IL-6 gene suppression [29,30]. Following collagen-induced arthritis, ethinyl estradiol lowers T cell IL-6 and TNF-α secretion and decreases cytokine and chemokine mRNA levels in joint tissue of mice [31]. Pretreatment of 17β-estradiol to rat primary astrocyte cultures attenuates LPS-induced TNF-α and IL-1β release [32]. Moreover, 17β-estradiol stimulates early cytokine release following spinal cord injury in rats [33]. Because of the known involvement of estrogen in cytokine release and production, it is feasible that the attenuation of nociceptive responses after estradiol administration is in part mediated by impeding cytokine activity. Indeed, blocking TNF-α with infliximab reduces ovariectomy-induced mechanical and thermal hyperalgesia in rats, indirectly suggesting that TNF-α plays an important role in estrogen deficient hyperalgesia [34].
Glucocorticoid (GC) hormones released from the hypothalamic– pituitary–adrenal (HPA) axis also contribute to the control of nociception and inflammation by reducing pro-inflammatory cytokine levels [35–37]. Elevated plasma GC levels suppress IL-1 and TNF-α, and increase anti-inflammatory cytokine IL-10 and IL-4 production [35,36]. Chensue et al. [38] found that during LPS administration, decreased TNF-α levels correlate with elevated release of the GC corticosterone (CORT). Kapcala et al. [39] showed that adrenalectomized rats have increased mortality rates following IL-1β administration, but CORT replacement attenuates that lethality. Injection of metyrapone plus aminoglutethimide (GC inhibitors) increases basal hypothalamic IL-1β mRNA expression and alters IL-1β levels following acute stress in rats [40]. Moreover, estrogen has been shown to modulate GC expression; for instance, estradiol increases CORT levels and following adrenalectomy + ovariectomy (ADX + OVX) estradiol leads to increased GC levels in rodent brain and spinal cord tissue [41–44]. Regulation of hormones released from the HPA axis may be another possible mechanism by which estrogen attenuates cytokine activity during inflammation and tissue injury.
We have previously shown that estrogen replacement reduces formalin-induced flinching responses following OVX and/or ADX in female rats [21]. In this paradigm, however, it is not understood if estrogen modulates inflammatory signaling by regulating cytokine activity in combination with or independent from its regulation of the HPA axis. Furthermore, it is not known whether estrogen modulates basal inflammatory mediators before the introduction of inflammation and/or injury. In this study we postulate that estrogen alters inflammatory mediators by reducing the release of pro-inflammatory cytokines and increasing anti-inflammatory IL-10 levels at the site of injury, systemically, as well as in the central nervous system before and following formalin administration. We further investigate whether hormonal influences from the HPA axis mediate estrogen’s effects on regulating cytokine levels.
2. Materials and Methods
2.1. Animals
Eight-week old OVX/sham ADX (denoted as OVX), ADX/sham OVX (denoted as ADX), and ADX + OVX female Sprague-Dawley rats (n = 7–10 per group) were purchased from Taconic (Germantown, NY). Rats were double-housed on a 12-hour light/dark photoperiod (lights on 8 AM EST) with food and water available ad libitum. ADX rats were maintained on water supplemented with 0.9% sodium chloride. Rats weighed about 220 g at the time of sacrifice. Animal treatment was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD) and was approved by the Institutional Animal Care and Use Committee at Hunter College of The City University of New York.
2.2. Estradiol replacement paradigm and formalin administration
Two weeks after OVX and/or ADX, a SILASTIC capsule (1 cm, 0.058 in. ID × 0.077 in. OD, Dow Corning) was inserted into the nape of the rats’ neck. The capsule contained either 20% 17β-Estradiol (1, 3, 5 [10]-Estratriene-3, 17 Beta-diol; Sigma-Aldrich) in cholesterol (5-Cholestin-3Beta-ol; Sigma-Aldrich, St. Louis, MO) for the experimental group or 100% cholesterol for the vehicle group. This dose and manner of estradiol replacement was chosen because (1) serum estradiol levels were maintained at a steady state [23], (2) it produces physiological conditions similar to rats in proestrus [23], and (3) it reduces formalin-induced behavioral responses [21–23,45].
Using a 27½-gauge needle, rats received a 50 µL formalin injection [5% formaldehyde (Sigma); 95% sterile isotonic saline (0.9% Sodium Irrigation USP, Braun Medical, Irving, CA)] subcutaneously into the intraplantar region of the right hind paw while they were manually restrained. Formalin was administered between 9:00 AM and 3:00 PM. This formalin dose and manner of administration has been shown to produce persistent nociception in OVX female rats [21–23,45]. In order to mimic the time that behavior during the formalin assay is collected, OVX, ADX and ADX + OVX rats were sacrificed by rapid decapitation 60 minutes post formalin injection following a brief (20 sec) exposure to CO2. In a separate set of animals, naïve OVX, ADX and ADX + OVX rats were administered either 17β-estradiol or cholesterol and were sacrificed two weeks later to establish baseline hormonal replacement effects. For all procedures, rats were randomly assigned to their respective groups.
2.3. Sample collection and cytokine multiplex assay
Upon decapitation, trunk blood was collected in tubes containing K2-EDTA (BD Vacutainer Systems, Franklin Lakes, NJ), centrifuged at 2600 RPM for 30 min at 4 °C, and serum was stored at −80 °C. Hind paw measurements were taken using a dial caliper (General Tools, New York, NY) measuring the width and depth (the measurement of the plantar to the center dorsal surface) in millimeters (mm). Paws were removed post mortem, weighed and rapidly frozen on dry ice. Spinal cord and DRG tissue were collected from the lumbar region and immediately frozen. All tissue samples were stored at −80 °C until use.
Paw, DRG and spinal cord tissues were manually homogenized in lysing solution that contained cell lysis buffer (from Cell Lysis Kit #171-304012, Bio-Rad, Hercules, CA), a protease inhibitor cocktail provided in the cell lysis kit (Bio-Rad, Hercules, CA), and 500 mM phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, St. Louis, MO) diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). Samples were then stored at −80 °C until use.
Homogenates were thawed and centrifuged at 5000 RPM for 15 min at 4 °C. Supernatants were collected and stored at −80 °C. Total protein concentration was determined using a Bradford kit from Bio-Rad Laboratories (Hercules, CA). In order to control for homogenate variability due to processing of lysing solution, each sample harvested from the same region were homogenized simultaneously.
Tissue homogenates and serum were analyzed for cytokine levels by using the Bio-Plex Cytokine assay along with the Cytokine Reagent Kit (#171-K11070 and #171-304001, respectively, Bio-Rad, Hercules, CA). The cytokine multiplexed immunoassay (IL-1β, IL-2, IL-6, IL-10 and TNF-α) contains premixed beads coated with target capture antibody. Cytokines were chosen according to their known production during inflammatory pain states. Simultaneous cytokine analysis was processed according to the manufacturer’s protocol (for details see [46]). Each sample was run in triplicate. Samples were quantified using the Bio-Plex Suspension Array system and accompanying Bio-Plex Manager software (Bio-Rad, Hercules, CA). The limit of detection was <10 pg/mL and intra-assay coefficients of variance averaged below 20% for each cytokine. For serum, samples were diluted 1:4 with Rat Serum Diluent according to the manufacturer’s protocol (Bio-Rad, Hercules, CA).
2.4. Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed with SPSS 17.0 for Windows and graphed using Graph-Pad Prism 6 Software (San Diego, CA). Paw size is represented as the multiplication of the width and depth of each hind paw expressed in mm2. For naïve rats, right hind paw size measurements were analyzed using One-Way Analysis of Variance (ANOVA). In formalin-treated rats, paw size measurements were analyzed using Repeated Measures ANOVA to compare the ipsilateral to the contralateral hind paw in 17β-estradiol- and cholesterol-treated OVX, ADX and ADX + OVX groups. Cytokine values from tissue homogenates were divided by the protein concentrations obtained from Bradford analysis. Paw, DRG, spinal cord and serum cytokine values were analyzed using three-factor ANOVA to test for differences in cytokine levels following pain condition (naïve vs. formalin; n = 22–24 per group), hormone treatment (estradiol vs. cholesterol-vehicle; n = 22–24 per group) and surgical condition (OVX, ADX or ADX + OVX operation; n = 14–16 per group). Fisher’s Least Significant Difference (LSD) post hoc tests were performed when appropriate. For all analyses, significance was at the p < 0.05 level.
3. Results
3.1. Effects of estradiol and surgery on paw size in naïve rats at baseline and following formalin administration
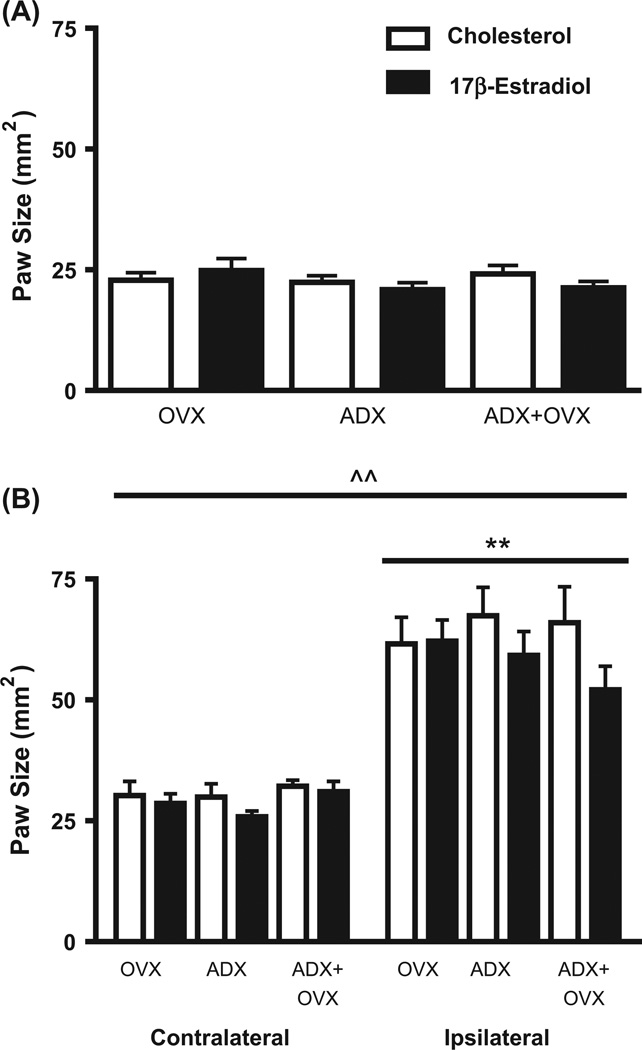
In naïve rats, estradiol administration did not alter paw size at baseline in any of the groups tested (p > 0.05; Fig. 1A). Furthermore, surgery (OVX, ADX or ADX + OVX operation) did not alter paw size at baseline across naive groups (p > 0.05; Fig. 1A). Formalin administration significantly increased ipsilateral paw size as compared to the contralateral paw [F(1,43) = 255.93, p < 0.01; Fig. 1B]. However, no significant main effect on paw size for hormone, surgery or the interaction between factors was observed following formalin administration (all p’s > 0.05).
Fig. 1.
Hind paw size of naïve or formalin-administered ovariectomy/sham adrenalectomy (OVX), adrenalectomy/sham ovariectomy (ADX) and ADX + OVX operated rats following cholesterol or 17β-estradiol treatment. Data shows mean hind paw size values (±SEM) in mm2 of OVX, ADX and ADX + OVX rats subcutaneously implanted with 20% 17β-estradiol (black bars) or 100% cholesterol (vehicle; white bars) and sacrificed one week following hormone replacement. A. Right hind paw measurements obtained from naïve rats at baseline. Estradiol administration did not significantly alter baseline paw size as compared to cholesterol treatment. B. Ipsilateral and contralateral paw size measurements in rats administered formalin to the right hind paw. (^^) Denotes a significant main effect for formalin administration [F(1,43) = 255.93, p < 0.01]. (**) Denotes a significant difference between ipsilateral and contralateral hind paw size following formalin administration (p < 0.01). Each bar represents n = 7–10 per group.
3.2. OVX, ADX and estradiol effects on cytokine values in naïve versus formalin-injected groups
3.2.1. Periphery (paw and dorsal root ganglia)
Formalin administration significantly altered cytokine levels in the paw (Fig. 2A). With the exception of IL-6, paw TNF-α, IL-1β, IL-2 and IL-10 levels were significantly lower post formalin injection when compared to the levels of these cytokines during naïve conditions [TNF-α: F(1,34) = 10.46, p < 0.01; IL-1β: F(1,36) = 20.71, p < 0.001; IL-2: F(1,36) = 5.14, p < 0.05; IL-10: F(1,35) = 9.66, p < 0.01; Fig. 2A]. Alternatively, IL-6 levels in the paw were significantly higher following formalin administration when compared to IL-6 levels in naïve groups [F(1,33) = 16.17, p < 0.001, Fig. 2A]. In the DRG, a main effect of pain condition was observed where TNF-α, IL-1β, IL-6 and IL-10 levels were significantly lower in naïve rats than in formalin-injected rats [TNF-α: F(1,36) = 4.46, p < 0.05; IL-1β: F(1,36) = 11.62, p < 0.01; IL-6: F(1,34) = 144.41, p < 0.001; IL-10: F(1,34) = 38.24, p < 0.001; Fig. 2B].
Fig. 2.
Mean values (±SEM) of TNF-α, IL-1β, IL-6, IL-2 and IL-10 levels (pg/µg) in the paw and dorsal root ganglia (DRG). A and B. Cytokine levels in the paw (A) and DRG (B) of naïve (light bars) and formalin-injected (black bars) rats. (*, **, ***) Denotes a significant main effect of pain condition at p < 0.05, p < 0.01, or p < 0.001, respectively. Each bar represents n = 22–24 per group. C and D. Cytokine levels in the paw (C) and DRG (D) of rats administered 20% 17β-estradiol (gray bars) or 100% cholesterol (vehicle; white bars). In D: (*) Denotes a significant main effect of hormone, p < 0.05. n = 22–24 per group. E and F. Cytokine levels in the paw (E) and DRG (F) following ovariectomy/sham adrenalectomy (OVX; light bars), adrenalectomy/sham ovariectomy (ADX; gray bars) or adrenalectomy + ovariectomy (ADX + OVX; black bars) operation. For both figures, (a) denotes a significant difference to OVX rats; (b) denotes a significant difference to ADX rats; and (c) denotes a significant difference to ADX + OVX rats; p < 0.05 (see text for specific p values). In E: (***) Denotes a significant difference between all surgical groups, p < 0.001. n = 15–16 per group.
Estradiol administration did not significantly alter cytokine levels at the site of injection (Fig. 2C). However, in the DRG, a significant main effect of hormone on anti-inflammatory IL-10 levels was observed; IL-10 DRG levels were significantly higher in estradiol treatment groups when compared to cholesterol treatment [F(1,34) = 4.20, p < 0.05, Fig. 2D]. Moreover, no interactions between hormone × pain and hormone × surgery on IL-10 DRG levels were observed.
A significant main effect of surgical condition was observed in the paw for all cytokines tested (Fig. 2E). TNF-α levels were significantly lower in the paw of ADX or ADX + OVX groups when compared to OVX operation [F(2,34) = 375.58, p < 0.001; Fig. 2E]. Surgery significantly altered paw IL-1β levels across conditions; IL-1β was significantly lowest in the paw tissue of ADX groups but highest in the paw tissue of ADX + OVX groups [F(2,36) = 41.57, p < 0.001; Fig. 2E]. IL-6 levels were significantly lower in the paw of ADX groups when compared to OVX or ADX + OVX operation [F(2,33) = 4.17, p < 0.05; Fig. 2E]. IL-2 levels in the paw were significantly lower in ADX or ADX + OVX rats when compared to OVX rats [F(2,36) = 38.68, p < 0.001; Fig. 2E]. In addition, surgery significantly altered IL-10 levels in the paw [F(2,35) = 63.67, p < 0.001]; paw IL-10 levels were significantly lowest in ADX groups when compared to OVX or ADX + OVX operation (p < 0.001 and p < 0.01, respectively; Fig. 2E). Paw IL-10 levels were significantly lower in ADX + OVX groups when compared to OVX operation (p < 0.001, Fig. 2E).
As shown in Fig. 2F, a significant main effect of surgery was observed for cytokine levels in the DRG. TNF-α levels were significantly lower in the DRG of ADX + OVX groups when compared to OVX or ADX operation [F(2,36) = 6.17, p < 0.01; Fig. 2F]. IL-1β levels were significantly lower in the DRG of ADX + OVX groups than following ADX operation [F(2,36) = 5.06, p < 0.05; Fig. 2F]. IL-6 levels in the DRG of OVX groups were significantly lower when compared to IL-6 DRG levels in ADX or ADX + OVX groups [F(2,34) = 22.86, p < 0.001; Fig. 2F]. IL-2 levels were significantly lower in the DRG of OVX and ADX groups when compared to ADX + OVX operation [F(2,34) = 4.40, p < 0.05; Fig. 2F]. Furthermore, DRG IL-10 levels were significantly lower following OVX and ADX + OVX operation when compared to ADX [F(2,34) = 20.85, p < 0.001; Fig. 2F].
Subsequent comparisons showed significant interactions between pain × surgery conditions at both peripheral sites. At the site of injection, a significant interaction between pain × surgery on TNF-α and IL-1β levels in the paw was observed [TNF-α: F(2,34) = 15.83, p < 0.001; IL-1β: F(2,36) = 4.74, p < 0.05]. During naïve conditions and following formalin administration, TNF-α levels in the paw were significantly lower in ADX and ADX + OVX groups when compared to OVX operation (p < 0.001 for both analyses). In the paw, naïve IL-1β levels were lowest in ADX groups when compared to OVX or ADX + OVX operation (p < 0.01 and p < 0.001, respectively). Following formalin administration, ADX groups had significantly lower IL-1β levels when compared to OVX or ADX + OVX operation (p < 0.001).
In the DRG, a significant pain × surgery interaction was observed for TNF-α, IL-6 and IL-10 levels [TNF-α: F(2,36) = 4.66, p < 0.05; IL-6: F(2,34) = 21.81, p < 0.001; IL-10: F(2,34) = 10.04, p < 0.001]. During naïve conditions, TNF-α levels in the DRG were significantly lower in ADX and ADX + OVX groups when compared to OVX groups (p < 0.001 for both analyses). In formalin-injected rats, TNF-α levels were significantly lower in the DRG of ADX + OVX groups when compared to ADX groups (p < 0.05). Naive OVX and ADX + OVX rats had significantly lower IL-6 levels in the DRG than naïve ADX rats (p < 0.05). In rats injected with formalin, IL-6 levels were significantly lower in the DRG of OVX rats when compared to ADX (p < 0.01) and ADX + OVX (p < 0.001) rats. DRG IL-6 levels in ADX groups were significantly lower post formalin injection than IL-6 levels in ADX + OVX groups (p < 0.05). In naïve rats and following formalin administration, IL-10 levels in the DRG were significantly lower in OVX and ADX + OVX groups when compared to ADX operation (p < 0.01 for all analyses).
3.2.2. Central (spinal cord)
A significant main effect for pain condition was observed for all central cytokines tested [TNF-α: F(1,36) = 20.22, p < 0.001; IL-1β: F(1,34) = 32.41, p < 0.001; IL-6: F(1,36) = 95.12, p < 0.001; IL-2: F(1,36) = 21.30, p < 0.001; IL-10: F(1,33) = 60.70, p < 0.001; Fig. 3A]. TNF-α, IL-1β, IL-6, IL-2 and IL-10 levels in the spinal cord were significantly lower in naïve rats when compared to central cytokine levels in formalin-injected rats (p < 0.001 for all analyses; Fig. 3A).
Fig. 3.
Mean values (±SEM) of TNF-α, IL-1β, IL-6, IL-2 and IL-10 levels (pg/µg) in the spinal cord. A. Cytokine levels in the spinal cord of naïve (light bars) and formalin-injected (black bars) rats. (***) Denotes a significant main effect of pain condition, p < 0.001. Each bar represents n = 22–24 per group. B. Cytokine levels in the spinal cord of rats administered 20% 17β-estradiol (gray bars) or 100% cholesterol (vehicle; white bars). (*) Denotes a significant main effect of hormone, p < 0.05. n = 22–24 per group. C. Cytokine levels in the spinal cord following ovariectomy/sham adrenalectomy (OVX; light bars), adrenalectomy/sham ovariectomy (ADX; gray bars) or adrenalectomy + ovariectomy (ADX + OVX; black bars) operation. (a) Denotes a significant difference to OVX rats; (b) denotes a significant difference to ADX rats; and (c) denotes a significant difference to ADX + OVX rats; p < 0.05 (see text for specific p values). n = 14–16 per group.
A significant main effect of hormone on central cytokine levels was observed (Fig. 3B). Estradiol significantly lowered TNF-α, IL-1β and IL-10 levels in the spinal cord when compared to cholesterol treatment [TNF-α: F(1,36) = 4.94, p < 0.05; IL-1β: F(1,34) = 5.40, p < 0.05; IL-10: F(1,33) = 4.76, p < 0.05; Fig. 3B].
A significant main effect of surgical condition on TNF-α, IL-1β, IL-2 and IL-10 levels in the spinal cord was observed [TNF-α: F(2,36) = 4.94, p < 0.05; IL-1β: F(2,34) = 9.31, p < 0.01; IL-2: F(2,36) = 19.51, p < 0.001; IL-10: F(2,33) = 27.16; p < 0.001; Fig. 3C]. TNF-α levels in the spinal cord were significantly lower in ADX + OVX groups when compared to ADX operation (p < 0.01, Fig. 3C). Central IL-1β levels were significantly lower in OVX or ADX + OVX groups when compared to ADX operation (p < 0.01, Fig. 3C). Central IL-2 levels were significantly lower in ADX and ADX + OVX groups when compared to OVX operation (p < 0.001, Fig. 3C). Furthermore, central IL-10 levels were significantly lower in OVX groups when compared to ADX or ADX + OVX operation (p < 0.001, Fig. 3C).
Subsequent comparisons showed a significant hormone × pain × surgery interaction on IL-1β levels in the spinal cord [F(2,34) = 3.33, p < 0.05]; in estradiol-treated rats, spinal IL-1β levels were higher following formalin injection than during naïve conditions (p < 0.01); however, surgical condition did not alter this affect. In cholesterol-treated rats, central IL-1β levels were higher in formalin-injected groups when compared to naïve conditions (p < 0.001). Furthermore, IL-1β levels in the spinal cord were significantly higher in cholesterol-treated ADX rats when compared to cholesterol-treated OVX or ADX + OVX rats (p < 0.01 for both analyses). A significant hormone × pain interaction on central IL-6 levels was observed [F(1,36) = 5.45, p < 0.05]; following formalin injection, estradiol lowered IL-6 levels in the spinal cord when compared to vehicle but this effect did not reach significance (p = 0.074). Additionally, a significant interaction of surgery × pain [F(2,33) = 6.09, p < 0.01] and surgery × hormone [F(2,33) = 4.61, p < 0.05] on IL-10 levels in the spinal cord was observed. In naïve groups and post formalin injection, IL-10 levels in the spinal cord were significantly lower in OVX rats when compared to ADX or ADX + OVX operation (naïve condition: p < 0.05 and p < 0.01, respectively; formalin condition: p < 0.001 for both analyses). In cholesterol-treated rats, IL-10 spinal cord levels were significantly lower in OVX groups when compared to ADX or ADX + OVX operation (p < 0.01 for both analyses); however no significant effect of surgery was observed in estradiol-treated rats.
3.2.3. Systemic (serum)
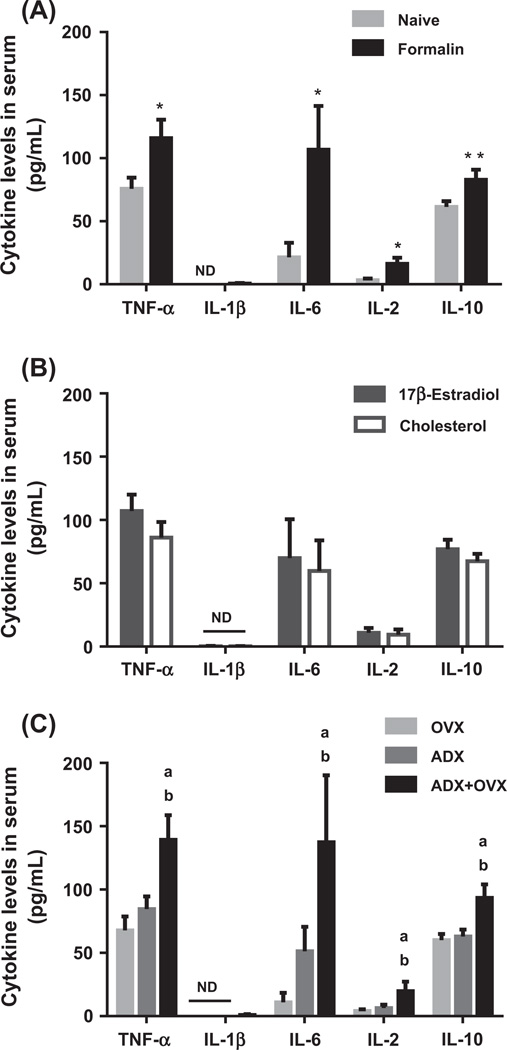
In serum, naïve rats had significantly lower TNF-α, IL-6, IL-2 and IL-10 levels when compared to cytokine serum levels in formalin-injected rats [TNF-α: F(1,35) = 7.20, p < 0.05; IL-6: F(1,34) = 6.17, p < 0.05; IL-2: F(1,33) = 7.18, p < 0.05; IL-10: F(1,36) = 8.75, p < 0.01; Fig. 4A]. Estradiol had no significant effect on systemic cytokine levels (Fig. 4B). A significant main effect of surgical condition was observed for TNF-α, IL-6, IL-2 and IL-10 levels in serum [TNF-α: F(2,35) = 8.49, p < 0.01; p < 0.05; IL-6: F(2,34) = 3.88, p < 0.05; IL-2: F(2,33) = 3.29, p = 0.050; IL-10: F(1,36) = 8.65, p < 0.01; Fig. 4C]. OVX and ADX operation led to significantly lower TNF-α (p < 0.01), IL-6 (p < 0.05), IL-2 (p < 0.05) and IL-10 (p < 0.01) levels in serum when compared to ADX + OVX operation (Fig. 4C).
Fig. 4.
Mean values (±SEM) of TNF-α, IL-1β, IL-6, IL-2 and IL-10 levels (pg/mL) in serum. A. Cytokine levels in serum of naïve (light bars) and formalin-injected (black bars) rats. (*, **) Denotes a significant main effect of pain condition at p < 0.05 or p < 0.01, respectively. Each bar represents n = 21–24 per group. B. Cytokine levels in serum of rats administered 20% 17β-estradiol (gray bars) or 100% cholesterol (vehicle; white bars). No significant effects of hormone were observed. n = 22–24 per group. C. Cytokine levels in serum following ovariectomy/sham adrenalectomy (OVX; light bars), adrenalectomy/sham ovariectomy (ADX; gray bars) or adrenalectomy + ovariectomy (ADX + OVX; black bars) operation. (a) Denotes a significant difference to OVX rats and (b) denotes a significant difference to ADX rats; p < 0.05 (see text for specific p values). n = 14–16 per group. In all figures, (ND) denotes nondetected values.
A pain × surgery interaction was observed for IL-1β, IL-6 and IL-10 levels in serum [IL-1β: F(2,34) = 3.32, p < 0.05; IL-6: F(2,34) = 4.09, p < 0.05; IL-10: F(2,36) = 4.97, p < 0.05]. IL-1β levels were not detected in serum of naïve rats across surgical conditions or in formalin-injected OVX and ADX rats. However, formalin administration led to significantly higher IL-1β levels in ADX + OVX groups when compared to OVX or ADX operation (p < 0.05 for both analyses). Following formalin injection, IL-6 and IL-10 levels were significantly lower in serum of OVX and ADX groups when compared to ADX + OVX operation (IL-6: p < 0.01 and p < 0.05, respectively; IL-10: p < 0.01 for both analyses). A pain × surgery interaction for TNF-α [F(2,35) = 3.04, p = 0.061] and IL-2 [F(2,33) = 3.21, p = 0.053] serum levels approached significance. Furthermore, a hormone × pain interaction for IL-10 levels in serum approached significance [F(1,36) = 3.98, p = 0.054].
4. Discussion
It is well established that estrogen displays analgesic properties in animal models of inflammatory pain and that estrogen antiinflammatory and antinociceptive effects have been postulated to be in part mediated through down regulation of inflammatory modulators [23,47–49]. Here we analyzed the cytokine profile during estradiol administration before and following formalin injection and observed that estrogen directly regulates pro- and antiinflammatory cytokine levels in vivo. We also observed that cytokine activity resulting from estrogen hormone replacement was site specific and can function independently of adrenal activity. Thus, estrogen effects before and during inflammation and/or injury may in part be mediated through its influences on cytokine release and regulation of cytokine-induced pathways.
We hypothesized that exogenous estrogen administration would down-regulate pro-inflammatory cytokines and increase anti-inflammatory cytokine levels at multiple sites and thus block inflammatory signaling. Indeed, anti-inflammatory cytokine IL-10 levels significantly increased in the DRG of estradiol-treated rats when compared to vehicle treatment. During inflammation, antiinflammatory cytokine production is enacted to counteract proinflammatory cytokine expression [60]. The elevation of IL-10 DRG levels in response to estrogen replacement offers a possible mechanism via which pro-inflammatory mediators are hampered in the periphery. Moreover, the present study demonstrated that central TNF-α, IL-1β and IL-10 levels were significantly reduced by estrogen replacement. This inhibition of cytokines by estrogen may explain the decrease of formalin-induced pain responses at the site of central sensitization. Interestingly, central IL-10 levels were also reduced by estradiol, possibly due to estrogen lowering pro-inflammatory cytokine expression and thereby reducing the levels of anti-inflammatory products that need to be released to inhibit inflammatory signaling.
Furthermore, contrary to our hypothesis, the presence of adrenal hormones was not necessary for estrogen effects on cytokines. Although we observed interactions between hormone replacement and surgery on some central cytokine levels examined, these results were mainly due to changes across cytokines in surgically manipulated vehicle groups, but not following estrogen replacement. In this paradigm, the ability for exogenous estradiol to modulate central cytokines in all three surgical conditions (OVX, ADX and ADX + OVX) confirms that estrogen is able to directly alter cytokine levels irrespective of hormonal input from the HPA axis. Therefore, our data found no support of adrenal hormone influences for any estradiol inhibitory effects on centrally-produced cytokines. The HPA axis provides negative feedback to immune challenges via the production of its end products, the GCs. One of the anti-inflammatory effects of GCs is its ability to attenuate inflammatory mediators such as cytokines and prostanoids [61,62]. Estrogen has been shown to regulate HPA activity as well as directly modify cytokine levels; the latter effect was confirmed in our study [43,44,63,64].
Estrogen does not ubiquitously alter all inflammatory mediators. For example, formalin-induced hind paw edema and systemic cytokine levels were not reduced following estrogen replacement. Hunter et al. [21,50] showed that estrogen antinociceptive effects on inflammatory-induced hyperalgesia are minimally altered through activation of prostanoid-mediated mechanisms—COX 1 and 2 antagonists did not block estradiol anti-nociceptive responses. Kuba et al. [45] demonstrated that although PG serum levels are altered after OVX, no differences were seen across COX-1 and COX-2 protein expression in the spinal cord of either naive or formalin-treated rats. While it is not clear how the effects of estrogen on baseline inflammatory mediators alters inflammatory pain states, our results suggest that previously reported estrogen effects on hyperalgesia are in part due to its regulation of basal cytokine levels. Instead of, or in addition to estrogen attenuation of injury-induced behavioral responses, estrogen’s anti-hyperalgesic effects can occur before injury or nociception. That is, in naïve female rats, estrogen alters inflammatory mediators such that before injury responses in the inflammatory pathway are dampened and pain is diminished.
Estradiol regulated some peripheral and central cytokine levels independent of naïve condition or formalin administration, strongly suggesting that pre-nociceptive regulation of cytokine levels by estrogen may contribute in part to its analgesic effects after injury/inflammation. It is possible that estrogen alters the “set-point” for inflammatory pathways such that upon injury the response of these pathways is reduced. We conclude that estrogen’s reduction of inflammatory mediators may in part be nonspecific to nociception and/or injury and in turn due to the general anti-inflammatory effects of this hormone. Thus, the non-specific effects of estrogen dampen or lower the “set-point” for cytokine levels and therefore inhibit the activation of cytokine-mediated pathways during inflammatory pain states. However, our data also suggest that estrogen further attenuates levels and/or activity of these proteins after the inflammatory stimuli. Therefore, modifications to both basal and inflammation-induced factors contribute to the effects of estrogen. Alternatively, if only basal effects were observed, this may indicate that estrogen influences are not nociceptive specific.
We observed that in comparison to naïve groups, formalin administration elevated DRG, central and systemic cytokines in a predicted manner. Cytokines released during inflammation are produced on demand and act locally to induce or facilitate inflammatory pain and hyperalgesia both directly and indirectly [51]. Pro-inflammatory cytokines (i.e. TNF-α, IL-6, IL-1) directly increase nociceptive activity and hyperalgesia at the site of injury [52–54]. Indirectly, pro-inflammatory cytokines act synergistically to induce the expression of inflammatory factors involved in nociception and disease pathology [5,55]. During inflammation, the production of cytokines occurs in a sequence, where TNF-α is produced first, then downstream pro-inflammatory cytokines are released to aid in the propagation of inflammatory signaling [56]. IL-2, also known as T cell growth factor, is an immunoregulatory cytokine that stimulates the production of B, T and Natural Killer cells [57]. Antigens stimulate Th1 cells to produce IL-2 during immune responses; in turn IL-2 induces the proliferation of T cells and the secretion of cytokines such as TNF-α and IL-1 [58,59]. We examined formalin-induced cytokines at the site of injury and found cytokine levels that were lower than basal conditions. This observation should be interpreted with caution since paw tissue was analyzed 60 min post-inflammatory insult and cytokines can show altered patterns of expression over time. After investigating the effect of pain condition across surgical groups, one of the most discernable patterns of cytokine expression was observed systemically. The highest amounts of serum cytokine levels were found in formalin-injected ADX + OVX groups. Although exogenous estradiol did not affect systemic cytokine levels, it is presumed that the presence of endogenous ovarian and adrenal hormones aids in suppressing the release of formalin-induced cytokines in sera.
Our results do not rule out the possibility that multiple variables contribute to the anti-inflammatory effects of estrogen. We must take into account the various systems involved in pain and analgesia that are affected by this hormone. Indeed, estrogen has been shown to increase analgesia via activation of the opioid system as well as reduce nitric oxide levels both in vitro and in vivo [65–69]. This study provides evidence that estrogen modifies nociceptive pathways by targeting upstream mediators (i.e. cytokines) involved in the inflammatory signaling cascade. Delineating the mechanistic functions of estrogen during inflammation and injury is essential for understanding sexually dimorphic responses to inflammatory pain.
5. Conclusion
In summary, this study establishes that in the nervous system estrogen modulates the cytokine pathway at baseline and during inflammatory pain states. Our results further suggest that estrogen effects are not dependent on HPA activity. Taken together, understanding the ways in which ovarian hormones modulate inflammation-induced cytokine levels will offer potential strategies for the treatment of pain perception and responses in women.
Acknowledgments
We thank Oendrila Kamal, Courtney Daly and Sandra Rios for their excellent technical assistance. This work was supported by RR-03037, NF39534 and DA 12136 (to VQJ); UL1-RR024996 and PSC-CUNY (to VQJ, SJ); MBRS-RISE GM60665 and CUNY NSF/AGEP-SBE 0549066 (to KYS).
Footnotes
The authors declare no competing financial interests.
References
- 1.Christenson ES, Jiang X, Kagan R, Schnatz P. Osteoporosis management in postmenopausal women. Minerva Ginecol. 2012;64:181–194. [PubMed] [Google Scholar]
- 2.Chae CU, Derby CA. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am. 2011;38:477–488. doi: 10.1016/j.ogc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Roman-Blas JA, Castaneda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11:241. doi: 10.1186/ar2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–997. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uceyler N, Schafers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 7.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, et al. Interleukin- 1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Zhang H, Dougherty PM. Dynamic effects of TNF-alpha on synaptic transmission in mice over time following sciatic nerve chronic constriction injury. J Neurophysiol. 2013;110:1663–1671. doi: 10.1152/jn.01088.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [Phila Pa 1976]. [DOI] [PubMed] [Google Scholar]
- 16.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 17.Schoeniger-Skinner DK, Ledeboer A, Frank MG, Milligan ED, Poole S, Martin D, et al. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav Immun. 2007;21:660–667. doi: 10.1016/j.bbi.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DA, Barr GA, Amador N, Shivers KY, Kemen L, Kreiter CM, et al. Estradiol-induced antinociceptive responses on formalin-induced nociception are independent of COX and HPA activation. Synapse. 2011;65:643–651. doi: 10.1002/syn.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuba T, Kemen LM, Quinones-Jenab V. Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res. 2005;1047:119–122. doi: 10.1016/j.brainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol. 2010;224:163–169. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda K, Iwasaka H, Hagiwara S, Takeshima N, Takatani J, Uchino T, et al. The antinociceptive effects of estradiol on adjuvant-induced hyperalgesia in rats involve activation of adrenergic and serotonergic systems. J Anesth. 2011;25:392–397. doi: 10.1007/s00540-011-1142-3. [DOI] [PubMed] [Google Scholar]
- 26.Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, et al. 17beta-estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology. 2000;141:1455–1463. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- 27.Esposito E, Iacono A, Raso GM, Pacilio M, Coppola A, Di CR, et al. Raloxifene, a selective estrogen receptor modulator, reduces carrageenan-induced acute inflammation in normal and ovariectomized rats. Endocrinology. 2005;146:3301–3308. doi: 10.1210/en.2005-0375. [DOI] [PubMed] [Google Scholar]
- 28.Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death. Neurochem Res. 2011;36:1587–1593. doi: 10.1007/s11064-010-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor κB transactivation. Cytokine. 2005;31:251–257. doi: 10.1016/j.cyto.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Masiukiewicz US, Mitnick M, Grey AB, Insogna KL. Estrogen modulates parathyroid hormone-induced interleukin-6 production in vivo and in vitro. Endocrinology. 2000;141:2526–2531. doi: 10.1210/endo.141.7.7537. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian S, Tovey M, Afentoulis M, Krogstad A, Vandenbark AA, Offner H. Ethinyl estradiol treats collagen-induced arthritis in DBA/1LacJ mice by inhibiting the production of TNF-alpha and IL-1beta. Clin Immunol. 2005;115:162–172. doi: 10.1016/j.clim.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritz MF, Hausmann ON. Effect of 17b-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 34.Chen BL, Li YQ, Xie DH, He QL, Yang XX. Blocking TNF-alpha with infliximab alleviates ovariectomy induced mechanical and thermal hyperalgesia in rats. Neurol Sci. 2012;33:527–533. doi: 10.1007/s10072-011-0743-9. [DOI] [PubMed] [Google Scholar]
- 35.DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab. 1997;82:2182–2191. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirwan JR, Buttgereit F. Symptom control with low-dose glucocorticoid therapy for rheumatoid arthritis. Rheumatology. 2012;51(Suppl 4):iv14–iv20. doi: 10.1093/rheumatology/kes085. [Oxford]. [DOI] [PubMed] [Google Scholar]
- 38.Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- 39.Kapcala LP, Chautard T, Eskay RL. The protective role of the hypothalamic– pituitary–adrenal axis against lethality produced by immune, infectious, and inflammatory stress. Ann N Y Acad Sci. 1995;771:419–437. doi: 10.1111/j.1749-6632.1995.tb44699.x. [DOI] [PubMed] [Google Scholar]
- 40.Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic–pituitary–adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 42.Niyomchai T, Russo SJ, Festa ED, Akhavan A, Jenab S, Quinones-Jenab V. Progesterone inhibits behavioral responses and estrogen increases corticosterone levels after acute cocaine administration. Pharmacol Biochem Behav. 2005;80:603–610. doi: 10.1016/j.pbb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Ferrini M, Gonzalez S, Nicola AF. Estradiol increases glucocorticoid binding and glucocorticoid induction of ornithine decarboxylase in the rat spinal cord. Life Sci. 1993;52:677–685. doi: 10.1016/0024-3205(93)90460-k. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Bisschop PH, Eggels L, Foppen E, Fliers E, Zhou JN, et al. Intrahypothalamic estradiol modulates hypothalamus-pituitary-adrenal-axis activity in female rats. Endocrinology. 2012;153:3337–3344. doi: 10.1210/en.2011-2176. [DOI] [PubMed] [Google Scholar]
- 45.Kuba T, Hunter D, Zhou L, Jenab S, Quinones-Jenab V. Acute and chronic estradiol replacements differentially alter corticosterone and COX-mediated responses to an inflammatory stimulus in female rats. Ethn Dis. 2010;20:S1–S4. [PMC free article] [PubMed] [Google Scholar]
- 46.Hulse RE, Kunkler PE, Fedynyshyn JP, Kraig RP. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J Neurosci Methods. 2004;136:87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samantaray S, Smith JA, Das A, Matzelle DD, Varma AK, Ray SK, et al. Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem Res. 2011;36:1809–1816. doi: 10.1007/s11064-011-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, et al. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49:441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Torres-Chavez KE, Sanfins JM, Clemente-Napimoga JT, Pelegrini-Da-Silva A, Parada CA, Fischer L, et al. Effect of gonadal steroid hormones on formalin-induced temporomandibular joint inflammation. Eur J Pain. 2012;16:204–216. doi: 10.1016/j.ejpain.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DA, Barr GA, Shivers KY, Amador N, Jenab S, Inturrisi C, et al. Interactions of estradiol and NSAIDS on carrageenan-induced hyperalgesia. Brain Res. 2011;1382:181–188. doi: 10.1016/j.brainres.2011.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 54.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 55.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol. 2005;78:1215–1222. doi: 10.1189/jlb.0405223. [DOI] [PubMed] [Google Scholar]
- 57.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 58.Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res Brain Res Rev. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 59.Sredni-Kenigsbuch D. TH1/TH2 cytokines in the central nervous system. Int J Neurosci. 2002;112:665–703. doi: 10.1080/00207450290025725. [DOI] [PubMed] [Google Scholar]
- 60.Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma. 2013;30:1311–1324. doi: 10.1089/neu.2012.2651. [DOI] [PubMed] [Google Scholar]
- 61.O’Banion MK, Winn VD, Young DA. CDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 63.Martin-Millan M, Castaneda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine. 2013;80:368–373. doi: 10.1016/j.jbspin.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol. 2005;175:6509–6516. doi: 10.4049/jimmunol.175.10.6509. [DOI] [PubMed] [Google Scholar]
- 65.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 66.Lawson KP, Nag S, Thompson AD, Mokha SS. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151:806–815. doi: 10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 68.Nweze IC, Smith JW, Zhang B, Klinge CM, Lakshmanan J, Harbrecht BG. 17beta-Estradiol attenuates cytokine-induced nitric oxide production in rat hepatocyte. J Trauma Acute Care Surg. 2012;73:408–412. doi: 10.1097/TA.0b013e31825a789b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu DZ, et al. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS ONE. 2010;5:e9421. doi: 10.1371/journal.pone.0009421. [DOI] [PMC free article] [PubMed] [Google Scholar]