Abstract
Klebsiella pneumoniae carbapenemases (KPCs) were first identified in 1996 in the USA. Since then, regional outbreaks of KPC-producing K. pneumoniae have occurred in the USA, and have spread internationally. Dissemination of blaKPC involves both horizontal transfer of blaKPC genes and plasmids, and clonal spread. Of epidemiological significance, the international spread of KPC-producing K. pneumoniae is primarily associated with a single multilocus sequence type (ST), ST258, and its related variants. However, the molecular factors contributing to the success of ST258 largely remain unclear. Here, we review the recent progresses in understanding KPC-producing K. pneumoniae that is contributing to our knowledge of plasmid and genome composition and structure among the KPC epidemic clone, and identify possible factors that influence its epidemiological success.
Keywords: Klebsiella pneumoniae carbapenemase, carbapenem-resistant, ST258
Epidemiology and impact of Klebsiella pneumoniae carbapenemases
Carbapenem-resistant Enterobacteriaceae (CRE), have recently emerged as the major class of bacterial pathogens that pose a significant threat to global public health, to high risk patients undergoing life threatening procedures, and to vulnerable patients in long-term care facilities (www.cdc.gov/drugresistance/threat-report-2013/) [1, 2]. It is possible that no infectious agent since the introduction of HIV has threatened our last line therapies more than these pathogens.
Resistance to carbapenems involves multiple mechanisms, including alterations in outer membrane permeability mediated by the loss of porins, upregulation of efflux systems along with hyperproduction of AmpC β-lactamases or extended-spectrum β-lactamases (ESBLs), or more commonly, the production of carbapenemases. Currently, Klebsiella pneumoniae carbapenemase (KPC) is the most clinically significant serine carbapenemase in the United States and its rapid international spread has become a noted public health threat globally [3, 4].
KPC emerged in the late 1990s and was identified in a K. pneumoniae isolate in North Carolina, USA [5]. To date, 22 different KPC enzyme variants have been identified (http://www.lahey.org/Studies/). KPC β-lactamases can hydrolyze all β-lactams, including carbapenems, cephalosporins, cephamycins, monobactams, and clavulanic acid [5, 6]. KPCs have been found in many Gram-negative species, including both Enterobacteriaceae and non-fermenters (e.g. Pseudomonas aeruginosa and Acinetobacter baumannii), with K. pneumoniae the most predominate species. KPCs are frequently found in K. pneumoniae associated with nosocomial infections, such as urinary tract infections, septicemia, pneumonia, and intra-abdominal infections, but are not common in community-acquired infections.
Since its emergence, CREs containing blaKPCs have spread in the Northeastern USA and caused several outbreaks in New York and New Jersey hospitals. In the middle 2000s, these microbes spread from the Northeastern USA to several other countries, including Israel, Greece and Columbia, presumably associated with the travel of patients between advanced care institutions. KPC-producing bacteria are considered to be endemic in certain parts of the world, including the Northeastern USA, Argentina, Brazil, Colombia, Eastern China, Greece, Israel, Italy, Poland and Puerto Rico [4, 7]. The clinical and molecular epidemiology of KPC has been detailed in recent reviews and is not further addressed in this review [3, 4, 7, 8].
Transmission of the KPC gene, blaKPC, can be mediated by different molecular mechanisms, from mobility of small genetic elements (e.g. Tn4401 transposon) to horizontal transfer of plasmids and via clonal spread [9]. Interestingly, similar to the epidemiological success of CTX-M-producing Escherichia coli ST131, the international spread of KPC-producing K. pneumoniae (KPC-Kp) has been linked to a major multilocus sequence type (MLST or ST), namely ST258, and its related variants [10]. ST258 has been reported in more than twenty-five countries from four continents, including the majority of the KPC epidemic countries mentioned previously. To illustrate, ST258 is responsible for >77% of the USA outbreaks and 90% of all KPC-Kp infections in Israel [11, 12]. The factors contributing to the epidemiologic success of ST258 remain unknown; however, chromosomal or plasmid factors, beyond antibiotic resistance, may increase the strain’s fitness and provide an advantage that underlies its prevalence [13, 14]. Identification of these factors is an important step toward understanding the molecular epidemiology of KPC-Kp and will likely contribute to the development of effective measures for infection control and prevention.
Population structure of KPC-Kp strains
Several molecular methods have been used for tracking and characterization of K. pneumoniae isolates; including repetitive sequence-based PCR (rep-PCR), pulsed-field gel electrophoresis (PFGE), and multilocus sequence typing (MLST), with MLST the most common technique. K. pneumoniae MLST is based on genetic variation in seven housekeeping genes (rpoB, gapA, mdh, pgi, phoE, infB, and tonB) that together, provide a relative genetic profile (ST, strain type) among different isolates [15]. In practice, a different allelic number is assigned to each distinct sequence within each locus, and the ST is created by linking the seven different allelic numbers in a standard order. These ST data can be further defined by eBURST (http://eburst.mlst.net/) which groups closely related strains (clonal complexes, CCs), and identifies the founding genotype of each group [16].
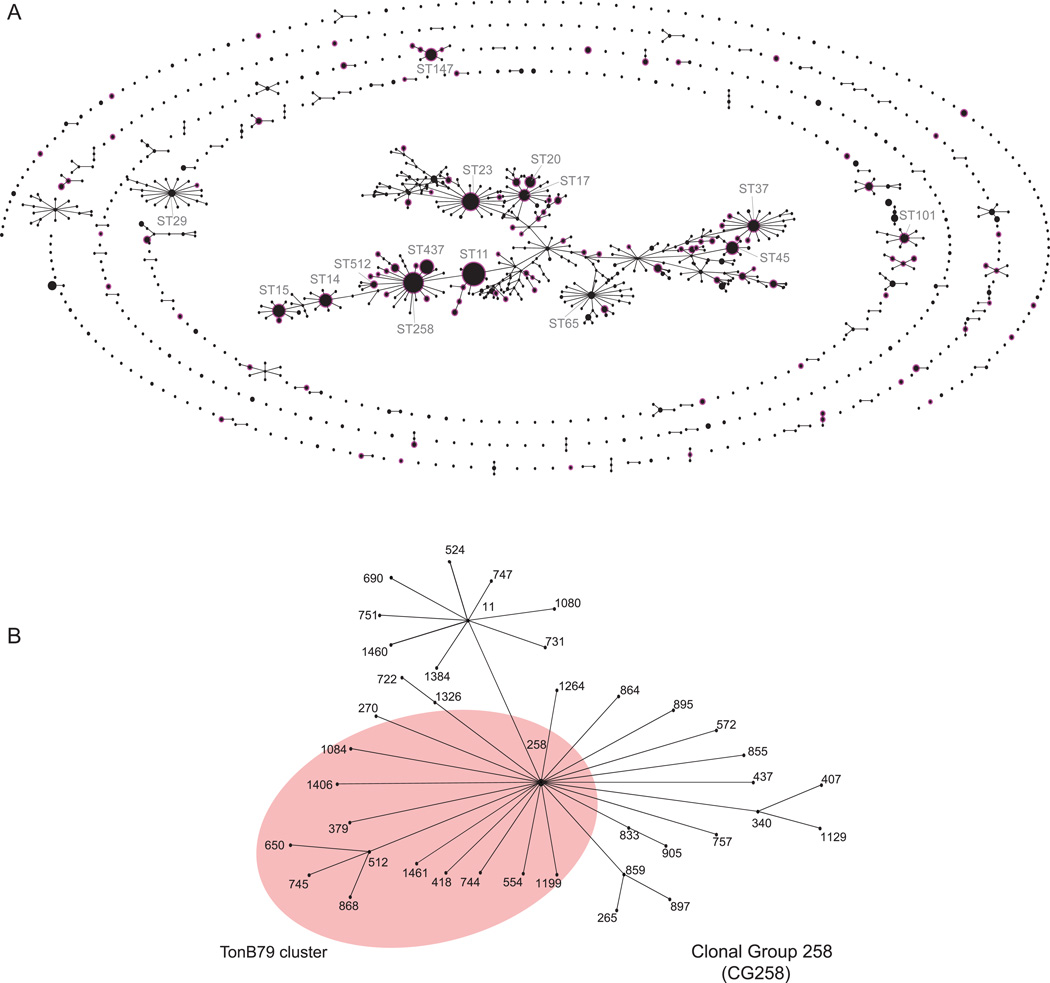
As of April 1, 2014, a total of 1536 STs have been deposited in the K. pneumoniae MLST online database (http://www.pasteur.fr/mlst). The population structure of K. pneumoniae is illustrated in Figure 1A. Using the most stringent criteria, where all members assigned to the same group share identical alleles at 6 of the 7 loci with at least one other member of the group, 136 CCs and 528 singletons (single STs that do not correspond to any CCs) were identified with a central CC comprising of 504 STs (32.8% of all STs). However, it is suggested that the accuracy of the eBURST grouping is questionable if the proportion of STs in a single CC exceeds 25% of all STs for predicting ancestor-descendant links since unrelated groups of STs may join into the same eBURST group [17]. In addition, the presence of a single large heterogeneous and straggly CC also suggests the likelihood of high rate of homologous recombination and DNA transfer between related and unrelated STs, instead of diversification from a single common ancestor [17].
Figure 1.
(A). Population structure of KPC-Kp. The figure represents the population structure of the K. pneumoniae MLST database (http://www.pasteur.fr/mlst) as of April 1, 2014, depicted graphically by eBURST v.3 (http://eburst.mlst.net), and shown in the context of all of the 1,536 STs from 1,924 isolates. KPC-Kp STs are highlighted by a pink halo. (B). Population structure of CG258. The pink shading highlights the STs of CG258-tonB79 cluster.
In an attempt to provide an easily defined epidemiologically meaningful phylogenetic structure in K. pneumoniae, Breurec et al. proposed to subdivide CCs into clonal groups (CGs), where the most prevalent ST would be the central to the CG and include both single-locus variants (SLVs) and their SLVs [18]. The CGs are named according to the central (main) ST. For example, ST258 is the central ST for CG258, ST512 is a SLV when compared to ST258, ST650 is a SLV compared to ST512, and ST650 is still within the CG258 phylogenetic lineage [18] (Figure 1B). Using this approach, Breurec et al. revealed that two major CGs, CG15 (ST15 and ST14) and CG258 (ST340 and ST11), define the predominant international K. pneumoniae clones resistant to third-generation cephalosporins in five African and two Vietnamese cities [18]. A similar population study by Baraniak et al. revealed four major CGs that are responsible for ESBL-producing K. pneumoniae colonizing patients and the genetic diversity in population structures was geographically linked: CG17 in France, CG101 in Italy, CG15 in Spain, and CC147 in Israel [19]. MLST analysis of ESBL isolates have shown that the spread of ESBL-producing K. pneumoniae is largely multi-clonal, in contrast, the international spread of KPC-Kp is limited to specific clones, at least for now.
To date, KPC has been found in more than 115 different STs (~ 7.5 % of all STs), showing a broad heterogeneous distribution (Figure 1A). Nevertheless, the vast majority of KPC-Kp isolates worldwide belong to CG258 and the two predominant sequence types are ST258 (ST allele profile, 3-3-1-1-1-1-79) and ST11 (3-3-1-1-1-1-4). Secondary clones include ST340 (3-3-1-1-1-1-18), ST437 (3-3-1-1-1-1-31), and ST512 (54-3-1-1-1-1-79). ST258 prevails mainly in North America, Latin America, and several countries in Europe, while ST11 is the major KPC-Kp ST in Asia and Latin America [4, 20–22]. ST512 has been discovered in Colombia, Italy and Israel; and ST340 was mainly reported from Brazil and Greece [4]. In contrast, the spread of other non-CG258 KPC-Kp STs are largely limited to certain geographic regions. For example, the recently emerged multi-drug resistant ST442 isolates (e.g strain Kp13) have only been described in Southern Brazil [23].
CG258 is a large group, containing 43 different STs. Among them, sixteen STs (ST258, 379, 418, 512, 554, 650, 744, 745, 868, 1084, 1199, 1406, 1458, 1461, 1481, and 1519) carry a unique MLST tonB allele, tonB79, which is primarily found in ST258 and its SLV and double locus variant (DLV). According to the Breurec et al. nomenclature, we propose to group strains with the tonB79 allele in CG258 and refer to them as the CG258-tonB79 cluster in order to subdivide CG258. Phylogenetically, members of tonB79-CG258 (e.g. ST258, 379, 418 and 512) appear to be more closely related than other members in CG258 (e.g. ST11) [24, 25]. This cluster of STs can be easily identified by a real-time PCR targeting of two single nucleotide polymorphisms (SNPs) [26] or more conventionally, by direct sequencing of the tonB allele.
Comparative K. pneumoniae genomics
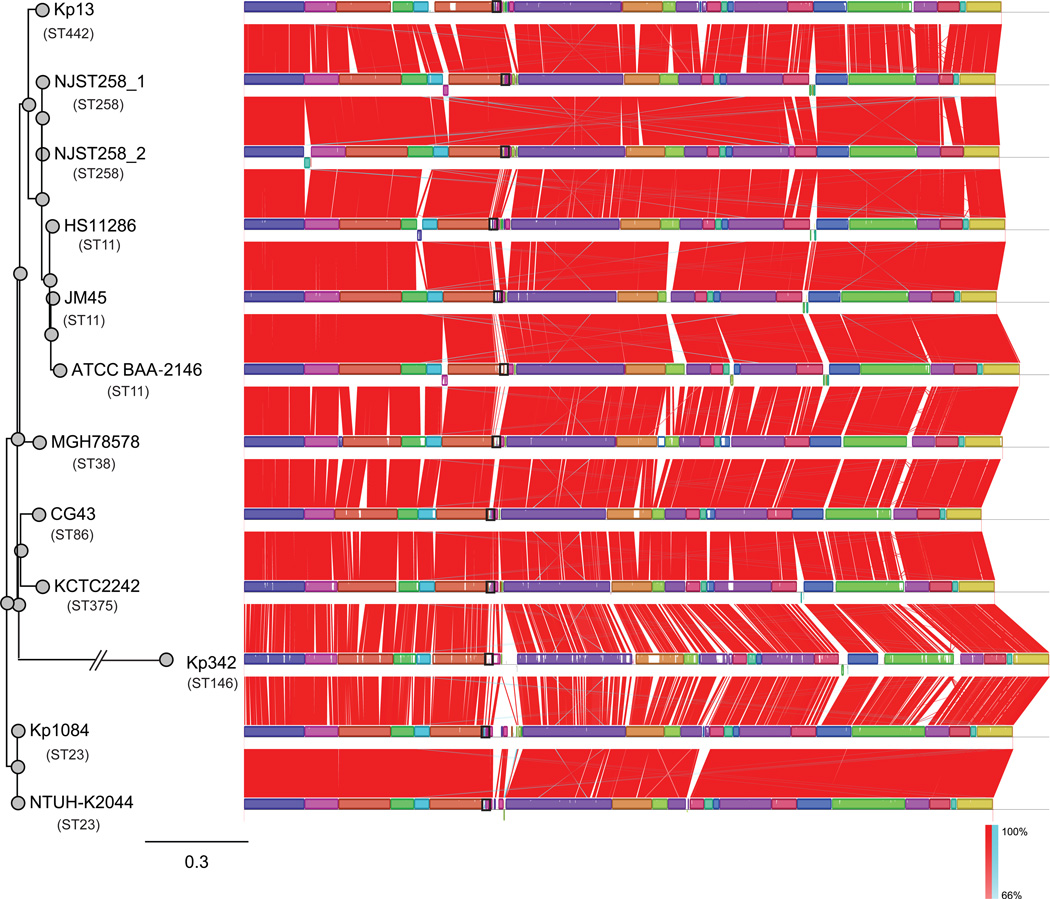
To date (April 1, 2014), thirteen K. pneumoniae genomes have been completely sequenced (ftp://ftp.ncbi.nih.gov/genomes/Bacteria/). A multiple genome alignment obtained using Mauve (http://gel.ahabs.wisc.edu/mauve/) is illustrated in Figure 2. In addition, more than 350 K. pneumoniae draft genomes have been sequenced by next-generation sequencing (http://www.ncbi.nlm.nih.gov/genome/genomes/815).
Figure 2.
Mauve plots of thirteen completely sequenced K. pneumoniae genomes. Boxes with identical colors represent local colinear blocks (LCB), indicating homologous DNA regions shared by two or more chromosomes without sequence rearrangements. LCBs indicated below the horizontal black line represent reverse complements of the reference LCB. Red (forward) and blue (reverse) shading denotes shared regions of homology, and the black box line illustrates the cps region in each genome. ST258 isolates (NJST258_1 and _2; CP006923 and CP006918) [24], ST11 isolate ATCC BAA-2146 (CP006659) [73], ST38 isolate MGH 78578 (CP000647) and one ST146 isolate (Kp 342) (CP000964) [74] were from United States; ST11 isolates HS11286 (CP003200) [29] and JM45 (CP006656) were from mainland China; two ST23 K1 isolates (NTUH-K2044 and Kp 1084; AP006725 and CP003785) and one ST86 K2 isolate (CP006648) were from were Taiwan [75, 76]; and ST375 isolate (KCTC 2242; CP002910) was from Korea [77], and one ST442 isolate (Kp13; CP003999) is from Brazil [23]. The left panel is the maximum likelihood tree generated using the SNPs extracted by Mauve.
Unlike its closely related species, such as Salmonella enterica and Escherichia coli, K. pneumoniae appears to be characterized by a low degree of nucleotide divergence among orthologous genes [23, 27] and as shown in the Mauve plots, the gene synteny among the chromosomes is conserved (Figure 2). A previous comparative genomics analysis of six K. pneumoniae genomes (including both chromosome and plasmids) identified 3,631 proteins in common that accounted for 65 to 75% of the total number of predicted protein-coding genes for any one of the genomes [28]. However, if only the chromosome bearing genes were compared, the K. pneumoniae genomes are more conserved. For example, comparison of K. pneumoniae strain HS11286 chromosome with four other K. pneumoniae chromosomes (MGH 78578, NTUH-K2044, Kp342, and KCTC2242) identified only 422 unique genes, accounting for 8% of a total of 5,316 genes [29]. This finding is consistent with the observation that the diversity in K. pneumoniae genomes is primarily due to the mobile genes that move frequently by horizontal transfer, including plasmids, phages, integrative and conjugative elements (ICEs), and insertion elements (ISs).
DeLeo et al. recently sequenced to closure two ST258 genomes (NJST258_1 and NJST258_2), and compared them with eight other completed genomes in the public databases [24]. The K. pneumoniae genomes have similar chromosomal lengths of ~5.3 Mbp, but vary significantly in the number of mobile genetic elements (MGEs), including plasmids, prophages, ICEs, and IS elements [24]. Notably, the chromosome-borne large MGE structures are similar between ST258 (NJST258_1 and NJST258_2) and ST11 (JM45 and HS11286) genomes. A comparative genomic study further suggests ST258 is a hybrid strain — 80% of the genome originated from ST11-like strains and 20% from ST442-like strains [25], similar to the hybrid pandemic methicillin-resistant S. aureus ST239 strains [30].
Brisse et al. suggested that the evolution of K. pneumoniae is mainly driven by homologous recombination, in contrast to the accumulation of mutations [27]. An example that supports this notion is that the same K type-associated capsular polysaccharide (CPS) synthesis operon is frequently found among unrelated STs, the likely result of horizontal transfer of the cps operon between different STs [27]. Using genomic comparisons based upon high resolution restriction mapping as well as in silico-generated restriction maps of six K. pneumoniae genomes, Ramirez et al. identified a ~160 kb highly heterogeneous region (based on the genome of MGH 78578), designated as a ‘high heterogeneity zone (HHZ)’ in the K. pneumoniae chromosome [31]. The HHZ consists of several ‘hot spot’ recombination regions, including the above mentioned cps operon and the high-pathogenicity genetic island, ICEKp1 (in NTUH-K2044) [31].
In an effort to decipher the molecular evolution of epidemic KPC-Kp ST258 strains, DeLeo et al. sequenced 83 CG258-tonB79 cluster isolates (including ST258, 379, 418 and 512) recovered from patients at diverse geographic locations[24]. These genomes were compared to the two closed ST258 scaffolds [24]. The 83 queried isolates differed from NJST258_1 on average by 350 SNPs (range, 116—784 SNPs) in the core genome, further supporting the idea that CG258-tonB79 strains are closely related. Phylogenetic analysis of the SNPs revealed that ST258 can be segregated into two distinct genetic clades (clade I and II) [24]. Notably, genetic differentiation between the two clades is largely due to a ~215 kb region of divergence that includes genes involved in cps region, and overlaps with the above mentioned HHZ region identified in other K. pneumoniae genomes [31]. Moreover, two distinct cps operons were identified in ST258 clades (ST258 cps1 in clade 1 and cps2 in clade 2). Similar findings were reported in independent and contemporary studies conducted by van Duin et al. [32], using rep-PCR and epidemiological analysis of a KPC surveillance network, and by Wright et al. [33], who examined the population structure of KPC bearing K. pneumoniae strains from the Great Lakes region. Furthermore, ST258 clade I strains may have evolved from a clade II strain as a result of cps region replacement [25]. Therefore, horizontal transfer of the cps region appears to be a key element driving the molecular diversification in K. pneumoniae strains.
blaKPC-bearing genetic elements
The original source of blaKPC remains unknown, but it is likely that this resistance gene was acquired from an ancestral chromosome of an environmental organism. β-lactamases existed long before the antibiotic era [34]. For example, recent metagenomic analyses of rigorously authenticated ancient DNA from 30,000-year-old Beringian permafrost sediments identified the presence of genes encoding resistance to β-lactams [35]. Fevre et al. estimated that blaOXY, the β-lactamase gene in Klebsiella oxytoca, originated as early as 100 million years ago [36]. Therefore, it is plausible that blaKPC may have had an ancient origin associated with an environmental organism, and that its present success is the consequence of its capacity for horizontal transfer, the dramatic and man-made increase in antibiotic selection pressure, and the ability for Enterobacteriaceae to readily accept foreign DNA.
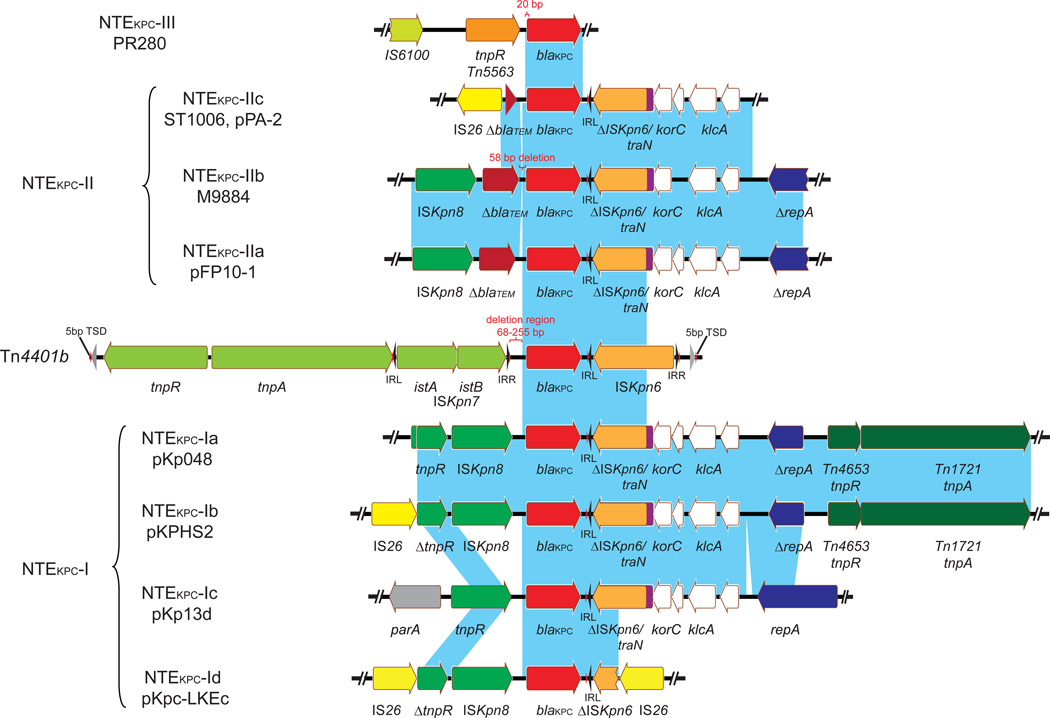
The most common blaKPC-containing mobile element is a Tn3-based transposon, Tn4401 [37]. Tn4401 is 10 kb in length, delimited by two 39-bp imperfect inverted repeat (IR) sequences, and harbors blaKPC, a Tn3 transposase gene (tnpA), a Tn3 resolvase gene (tnpR), and two insertion sequences, ISKpn6 and ISKpn7 [37] (Figure 3). Tn4401 is commonly flanked by a 5-bp target site duplication (TSD), as a result of its integration. Tn4401 is believed to originate from the Tn3-based tnpA and tnpR insertion upstream of blaKPC, followed by the integration of ISKpn6 and ISKpn7 downstream and upstream of blaKPC, respectively [37]. Two sets of IRs and TSDs are adjacent to ISKpn6 and ISKpn7, suggesting the recent insertion of both ISs in the backbone of Tn4401 [37]. Five Tn4401 isoforms (a–e) have been identified, differing by 68- to 255-bp deletions upstream of blaKPC (a, −99 bp; b, no deletion; c, −215 bp; d, −68 bp; e, −255 bp) [38]. Cuzon et al. subsequently showed that Tn4401 is a highly active transposon capable of transposition with a 5-bp TSD and without target site specificity in an in vitro model [39]. However, one common hot-spot for Tn4401 is the transposon Tn1331, creating a hybrid transposon structure that has been observed on plasmids of different backgrounds; notably, IncN, IncI2 and IncFIA plasmids [40–42]. Tn1331 carries Tn3-like transposase and resolvase genes (tnpA and tnpR); aminoglycoside modifying enzyme genes, aac(6’)-Ib and aadA1; and β-lactamase genes, blaOXA-9 and blaTEM-1 [43]. Even without understanding whether Tn4401 has repeatedly inserted at the same location in Tn1331 or whether the hybrid transposon has jumped onto different plasmids, the association of blaKPC with other antibiotic resistance determinants provides a very simple scenario for a carbapenemase to spread as a hitchhiker gene, and most alarmingly, in the absence of carbapenem selection.
Figure 3.
blaKPC-harboring genetic elements (Tn4401 and NTEKPC). Based on the insertion sequence upstream of blaKPC, NTEKPC can be divided into three groups: NTEKPC-I, no insertion [48]; NTEKPC-II, insertion of ΔblaTEM [48]; and NTEKPC-III, insertion of Tn5563/IS6100 [78]. NTEKPC-I can be further classified as -Ia (prototype, pKp048) [48], -Ib (pKPHS2) [29], -Ic (pKp13d) [23] and -Id (pKPC-LKEc) [79] based on the insertion sites of upstream and/or downstream of IS26 and the presence of ISKpn8. NTEKPC-II can be subgrouped as -IIa (pFP10-1, and blaKPC-harboring plasmids from strain M9196 and M11180) [49, 80], -IIb (from strain M9884 and M9988) [49], and -IIc (pPA-2) [50], based on the differences of the length of ΔblaTEM and the deletions. Light-blue shading denotes shared regions of homology.
Moreover, different Tn4401 isoforms appear to be associated with different blaKPC-harboring plasmids. For example, the blaKPC-3-harboring IncFIIK2 plasmid pKpQIL is associated with Tn4401a [44–46], while the blaKPC-3-bearing IncI2 plasmid pBK15692 carries Tn4401b [40]. In addition, the recently reported blaKPC-3-harboring IncFIA plasmids pBK30661 and pBK30683 are associated with Tn4401d [47]. The association of blaKPC variants with specific Tn4401 isoforms can be used as a genetic marker to distinguish different KPC plasmids.
blaKPC has also been found in other non-Tn4401 mobile elements from isolates in China, Argentina and other regions, as well as in other non-K. pneumoniae species [48–50]. To simplify the nomenclature of these novel elements, we propose to name them as blaKPC-bearing non-Tn4401 elements (NTEKPC). As shown in the alignment in Figure 3, seven blaKPC elements that contain genetic remnants of Tn4401 have been characterized and catalogued on the basis of the genes adjacent to blaKPC. Partial ISKpn6 genes, located downstream of blaKPC, are identical in elements subgrouped as types I and II and intact in Tn4401; more importantly, ISKpn6 associated left IR (IRL) is intact among NTEKPC-I, -II and Tn4401, suggesting NTEKPC-I and -II may evolve from Tn4401 by genetic recombination. It is noteworthy that NTEKPCs are primarily found in non-ST258 K. pneumoniae or other non-K. pneumoniae species; whereas, blaKPC in epidemic ST258 K. pneumoniae strains is exclusively carried on Tn4401.
blaKPC-harboring plasmids
blaKPC is typically plasmid-borne, and is carried on plasmids of different incompatibility (Inc) groups, including IncFII, FIA, I2, A/C, N, X, R, P, U, W, L/M and ColE [40, 42, 44, 50–55]. Unlike other carbapenemase genes, blaKPC is present mainly in plasmids in Enterobacteriaceae. However, two separate reports identified blaKPC in the P. aeruginosa chromosome; evidence that the gene can transpose from a plasmid and integrate into the host genome [56, 57].
Currently (April 1, 2014), more than 40 blaKPC-harboring plasmids have been completely sequenced; the majority of these plasmids are from K. pneumoniae (Table 1). These blaKPC-containing plasmids often contain several genes that encode resistance to other antimicrobial agents, such as the aminoglycosides, quinolones, trimethoprim, sulphonamides and tetracyclines. These findings amplify the complexity of controlling the spread of these plasmids, as co-selection leads to the transmission of multidrug resistance among members of the Enterobacteriaceae.
Table 1.
Completely sequenced blaKPC-bearing plasmids in K. pneumoniae
| Plasmid | Accession | ST | Year | Location | Length (bp) | GC (%) | Inc | KPC | KPC element |
|---|---|---|---|---|---|---|---|---|---|
| pKP1433 | JX397875 | 340 | 2009–2010 | Greece | 55,417 | 50.8 | a | KPC-2 | Tn4401b |
| pKpQIL-LS6 | JX442975 | 258 | 2011 | Italy | 78,227 | 54.2 | b | KPC-3 | Tn4401a |
| p15S | FJ223606 | - | 2005 | New York City | 23,753 | 57.0 | ColE1 | KPC-2 | Tn4401a |
| pNJST258C2 | CP006919 | 258 | 2010 | New Jersey | 25,284 | 56.7 | ColE1 | KPC-3 | Tn4401b |
| pBK30661 | KF954759 | 258 | 2010 | New Jersey | 73,635 | 53.9 | FIA | KPC-3 | Tn4401d |
| pNJST258N2 | CP006926 | 258 | 2010 | New Jersey | 73,636 | 53.9 | FIA | KPC-3 | Tn4401d |
| pBK30683 | KF954760 | 963 | 2010 | New Jersey | 139,941 | 54.0 | FIA | KPC-3 | Tn4401d |
| pKPC-LK30 | KC405622 | 11 | 2012 | Taiwan | 86,518 | 56.0 | FIIK | KPC-2 | NTEKPC-Ib |
| pBK32179 | JX430448 | 258 | 2010 | New York City | 165,295 | 52.7 | FIIK1 | KPC-2 | Tn4401a |
| p1-JM45 | CP006657 | 11 | 2010 | China | 317,154 | 53.0 | FIIK1 | KPC-2 | NTEKPC-Ia |
| pKPN101-IT | JX283456 | 101 | 2011 | Italy | 107,748 | 52.7 | FIIK1, R | KPC-2 | Tn4401a |
| pSLMT | HQ589350 | - | - | - | 21,138 | 56.3 | FIIK2 | KPC-2 | Tn4401a |
| pKP1780-kpc | KF874497 | - | - | Greece | 113,622 | 53.9 | FIIK2 | KPC-2 | Tn4401a |
| pKpQIL | GU595196 | 258 | 2006 | Israel | 113,637 | 53.9 | FIIK2 | KPC-3 | Tn4401a |
| pKP1504-kpc | KF874496 | - | - | Greece | 113,640 | 53.9 | FIIK2 | KPC-2 | Tn4401a |
| pKP3913-kpc | KF874499 | - | - | Greece | 113,640 | 53.9 | FIIK2 | KPC-2 | Tn4401a |
| pKpQIL-IT | JN233705 | 258 | 2010 | Italy | 115,300 | 53.9 | FIIK2 | KPC-3 | Tn4401a |
| pKP1870-kpc | KF874498 | - | - | Greece | 116,047 | 54.0 | FIIK2 | KPC-2 | Tn4401a |
| pKPHS2 | CP003224 | - | - | China | 111,195 | 53.3 | FIIK2, R | KPC-2 | NTEKPC-Ib |
| pKP048 | FJ628167 | - | 2006–2007 | China | 151,188 | 51.3 | FIIK5, R | KPC-2 | NTEKPC-Ia |
| pBK15692 | KC845573 | 258 | 2005 | New Jersey | 77,801 | 45.4 | I2 | KPC-3 | Tn4401b |
| pKPC_FCF13/05 | CP004366 | - | 2005 | Brazil | 53,081 | 52.5 | N | KPC-2 | Tn4401bc |
| pKPC_FCF/3SP | CP004367 | - | 2009 | Brazil | 54,605 | 52.9 | N | KPC-2 | Tn4401b |
| pKo6 | KC958437 | - | - | China | 65,549 | 51.0 | N | KPC-2 | NTEKPC-Ic |
| p9 | FJ223607 | - | 2005 | New York City | 70,655 | 54.3 | N | KPC-2 | Tn4401b |
| p12 | FJ223605 | - | 2005 | New York City | 75,617 | 52.8 | N | KPC-3 | Tn4401b |
| pBK31551 | JX193301 | 834 | 2005 | New Jersey | 83,712 | 53.5 | N | KPC-4 | Tn4401b |
| pHS062105-3 | KF623109 | - | 2006 | China | 42,848 | 49.7 | P | KPC-2 | NTEKPC-Ia |
| pKPC-NY79 | JX104759 | 258 | 2011 | New York City | 42,447 | 48.5 | X3 | KPC-2 | Tn4401a |
| pKP13d | CP003997 | 442 | 2009 | Southern Brazil | 45,574 | 46.0 | X3 | KPC-2 | NTEKPC-Ic |
| pKpS90 | JX461340 | 258 | 2009 | France | 53,286 | 49.6 | X3 | KPC-2 | Tn4401a |
| pBK31567 | JX193302 | 429 | 2006 | New Jersey | 47,387 | 49.0 | X5 | KPC-5 | Tn4401b |
, IncN plasmid backbone, but lack the IncN repA replicon.
, IncFIIK2 plasmids are pKpQIL-like, but lack the IncFII repA gene.
, 256 bp insertion in tnpA in Tn4401.
Abbreviation: -, data not available.
blaKPC-harboring plasmids of different Inc groups, e.g. IncFIIK1, FIIK2, FIA, I2, X, A/C, R and ColE1, are also identified in epidemic ST258 isolates. The epidemiology associated with blaKPC plasmids indicates that certain incompatibility groups harboring Tn4401 are more predominant [44–46, 58–60]. The IncFII plasmids are one salient example. They are commonly low copy number, harbor multiple replicons, and are widely distributed in different species of Enterobacteriaceae [61]. This finding is similar to the worldwide dissemination of blaCTX-M-15, which is largely associated with E. coli ST131 and harbored on multidrug-resistant IncFII plasmids [61, 62]. pKpQIL was the first KPC-encoding plasmid described for ST258. It is an IncFIIK2 group plasmid containing Tn4401a; it was initially identified in 2006 in a K. pneumoniae ST258 strain from Israel, and then believed to have spread to Poland, Italy, Colombia, United Kingdom and other countries [44–46, 58–60]. However, pKpQIL-like plasmids spread in the New York and New Jersey area as early as 2003, and a PCR screening of 284 clinical K. pneumoniae isolates identified 35.6% as harboring pKpQIL-like plasmids in nine out of ten surveyed hospitals [45]. This study documented the wide dissemination of pKpQIL in this endemic region [45]. Further support for this observation is the finding that an IncFIIK5 plasmid, pKp048, harboring a blaKPC element variant, is widely disseminated in China and associated with ST11 strains [48, 63].
pBK15692 is the second predominant blaKPC plasmid found among six New York City and New Jersey hospitals. This plasmid is an IncI2 blaKPC-3-harboring plasmid that was identified in 23% of 256 KPC-bearing K. pneumoniae isolates [40]. In addition, novel blaKPC-3-harboring IncFIA plasmids, pBK30661 and pBK30683, were identified in 20% of 491 K. pneumoniae isolates collected between 2002 to 2012 in ten New York City and New Jersey hospitals [47]. Although the spread of pBK15692 and pBK30683 in other geographical region remains unknown, these mobile genetic elements have successfully transferred to different K. pneumoniae genetic backgrounds and into different species, and we assume that their successful transmission is the result of strong antibiotic selection [40, 47].
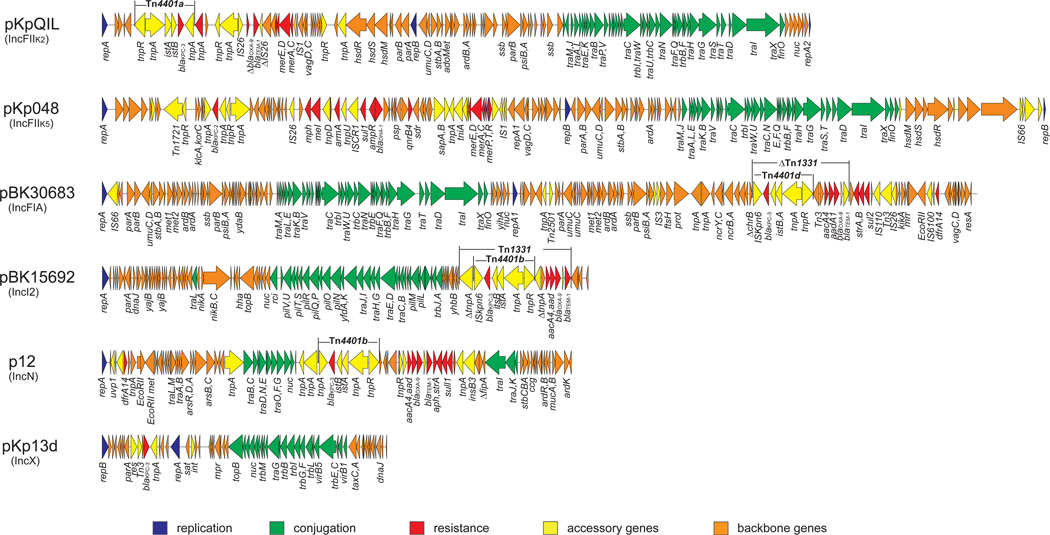
The genetic structures of six blaKPC-harboring plasmids from different incompatibility groups are shown in Figure 4. One common structure shared by these plasmids that is that they all carry a tra operon, which encodes the plasmid conjugation machinery that facilitates the spread of plasmids and resistance to other strains and species. Clearly, the successful epidemiology associated with plasmids that are able to conjugate and harbor selectable resistance genes is evidence that both factors are important for their dissemination.
Figure 4.
Structures of blaKPC-harboring plasmids: p12 (IncN), pKpQIL (IncFIIK2), pKp048 (IncFIIK5), pBK15692 (IncI2), pBK30683 (IncFIA), and pKp13d (IncX3).
Interestingly, there appears to be an association between different plasmid Inc groups and the genome clades in CG258 strains. In a recent genomics study, the pBK15692 (KPC-3)-associated IncI2 plasmids, and pBK30661/30683 (KPC-3)-associated IncFIA plasmids are found exclusively in clade II of CG258 strains [24]. In contrast, the pKpQIL-associated IncFIIK2 plasmids were found in both clade I and clade II [24], whereas clade I strains mainly carry blaKPC-2 and clade II strains primarily harbor blaKPC-3 [24, 64]. These findings clearly suggest that multiple plasmid acquisitions have occurred among strains that are catalogued collectively as the epidemic ST258 clone. These findings also indicate that convergent evolution has occurred within the ST258 lineage, and that the natural selection of different CG258 clade backgrounds with blaKPC-carrying mobile elements or plasmids has given rise to predominant clones. The evolutionary fine-tuning of these associations may help to maintain or increase bacterial fitness of these epidemic clone, as demonstrated previously in CTX-M-producing E. coli ST131 strains [65].
Understanding the success of epidemic ST258
The spread and success of KPC-producing CRE strains is multifactorial, as blaKPC is on a promiscuous transposon, Tn4401, and this transposon has jumped to numerous plasmids that are commonly conjugative. These plasmids have spread to different Enterobacteriaceae species and have found a highly compatible host in the K. pneumoniae ST258 background [10].
The molecular epidemiology of KPC-producing strains indicates that K. pneumoniae is the predominant species, suggesting a unique fitness and selective advantage beyond resistance. The finding that conjugative transfer of blaKPC-carrying plasmids was successful within species of Klebsiella, but not among other Enterobacteriaceae, could explain the observed epidemiology [66]. Given the success of the K. pneumoniae ST258 lineage worldwide (i.e., it is widely disseminated), one could speculate that the fitness of this clone and/or the conjugative efficiency of the blaKPC-harboring plasmids in this genetic background. Identifying the factors contributing to the epidemic success of ST258 remains an important public health question.
One could hypothesize that the success of KPC-Kp ST258 may also be associated with unique virulence traits, or their expression, that facilitates the ability to cause disease and spread. However, this argument is challenged by the recent study that showed ST258 to be virtually avirulent in immunocompetent and neutropenic animal models, highly susceptible to serum killing, and rapidly undergoing phagocytosis in vitro [67]. Genetic analysis by PCR amplification of targeted genes revealed that ST258 strains lack well-characterized K. pneumoniae virulence factors, including K1, K2, and K5 capsular antigen genes, the aerobactin genes, and regulator of mucoid phenotype gene rmpA [67]. The observation that epidemic ST258 strains are not highly virulent in animal models leads to the speculation that its successful spread is due largely to a combination of the genetic background being compatible with plasmids enhanced harboring Tn4401, and that this ‘fitness’ plus its multidrug resistance phenotype provides an advantage.
Undoubtedly, the multi-resistant phenotype of ST258 strains allows them to survive the barrage of antibiotics used in the treatment of hospital infections. Nevertheless, this cannot adequately explain the success of this clone. As described above, blaKPC was identified in more than 100 different STs, including STs that are distinct to ST258, but none of them have spread so widely. Meanwhile, other carbapenemase-producing K. pneumoniae strains, including those that carry blaNDM, blaVIM, blaIMP and blaOXA-48, are frequently identified, but none has disseminated to the extent of K. pneumoniae ST258. This leads us to conclude that, in addition to antibiotic resistance, other ST258 unique genetic factors, either on the chromosome or on specific plasmids, must contribute to the success and rapid spread of this clone.
The two closed ST258 genomes carry seven to eight prophages and two ICEs, and most of these mobile genetic elements are also present in ST11 strains [24]. ICEKp258.1 (harbored by both ST11 and ST258 strains) carries a type IV secretion system, which could potentially promote the transfer of genetic elements such as plasmids [68]. Meanwhile, ICEKp258.2, which is unique to ST258 strains [25], harbors a type IV pilus gene cluster that may facilitate adherence to living and nonliving surfaces, e.g. the gut of humans or the environment, as well as increase the uptake and exchange of DNA (e.g., plasmids) [69]. Moreover, ICEKp258.2 harbors a type III restriction-modification system that could serve as a ‘host specificity’ system that only allows the exchange of certain compatible plasmids [70]. These unique genetic factors may potentially contribute to the dissemination of ST258.
In addition to these attributes, a recent fitness study suggests other host-associated factors may contribute to epidemic success of the ST258 lineage. Benenson et al. compared the fitness of two distinct K. pneumoniae strains (KP314 and KP154) that harbor the same KPC plasmids (pKpQIL) [71]. KP314 is an ST321 isolate, while KP154 is classified as ST512, a member of CG258-tonB79 cluster [71]. In an in vitro model, KP314 (ST321) had a fitness advantage over KP154 (ST512), whereas in the clinical setting KP154 was more successful than KP314. This finding suggests that there are likely host-related factors that explain the discrepancy between the in vitro study and the epidemiological observation for these two related CG258 strains [71].
As described above, the ST258 ‘strain’ is comprised of at least two distinct lineages or clades rather than a single clone. The two clades are differentiated largely by a ~215 Kb region that encodes capsule polysaccharide biosynthesis machinery (cps1 and cps2). Meanwhile, the three closed ST11 genome strains carry three distinct cps regions (Figure 2). The cps locus is one of the primary determinants of antigenicity associated with K. pneumoniae, and capsule switching is a species-specific mechanism used by the microbe to escape the host immune response. DNA exchange in-and-around the cps regions may be an important mechanism used by K. pneumoniae to rapidly diversify and evolve [72]. Thus, chromosomal recombination is likely the major contributing factor to the global success of ST258, ST11 and other strains above and beyond antibiotic resistance.
Concluding remarks
K. pneumoniae was described more than one century ago, and it remains one of the most common pathogens causing healthcare-associated infections. In spite of the long history and considerable worldwide dissemination of K. pneumoniae infections, the population genetics and genomics have not attracted much attention until now. As a consequence of its continual increasing resistance to antibiotics, first by acquisition of ESBLs and now carbapenemases, there is compelling need to understand the plasmid and chromosome architecture of this pathogen. The studies reviewed here highlight recent advances that aim to address these key issues using novel approaches, e.g. comparative genomics, for exploring the determinants that contribute to the success of specific clones and circulating plasmids. Undoubtedly, understanding the molecular evolution of successful KPC-Kp lineages as well as their associated plasmids will lead to improved tracking of resistance, and thus control of the spread of carbapenem resistance (Box 1). Similar to other serious infections that have challenged humanity, KPC and other carbapenemase producing-Gram negative pathogens are changing the global face of resistance.
Box 1. Outstanding questions.
Do blaKPC-harboring plasmids have unique compatibility with K. pneumoniae ST258 or confer a fitness advantage that accounts for the global predominance of this resistant lineage?
Is fitness of ST258 strains – which are hybrid descendants of ST11 and ST442 strains – enhanced compared to either of these two parental strains?
Considering that cps region undergoes rapid change or exchange, is it reasonable to develop a polysaccharide vaccine for prevention and/or treatment of KPC-Kp infections?
What is the mechanism underlying recombination of the cps locus in K. pneumoniae?
Do specific chromosomal and/or plasmid factors explain why some blaKPC-harboring plasmids are more frequently observed in certain K. pneumoniae genetic backgrounds?
Do blaKPC-harboring plasmids contribute to the overall fitness or virulence in K. pneumoniae?
Highlight.
Active spread of Klebsiella pneumoniae carbapenemases (KPCs) occurs through transposons, plasmids, and epidemic clones.
Certain KPC-producing epidemic clones have spread globally.
Numerous factors are responsible for the spread and success of epidemic KPC clones.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grant 1R01AI090155 (to B.N.K.), and by the Intramural Research Program of the NIAID, NIH. This work was also supported by Public Health Service grant R01AI072219 and R01AI063517 (to R.A. Bonomo) from the National Institutes of Health and funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program and the Geriatric Research Education and Clinical Center VISN 10 to R. A. Bonomo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.McKenna M. Antibiotic resistance: the last resort. Nature. 2013;499:394–396. doi: 10.1038/499394a. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn BM. "Nightmare" bacteria on the rise in US hospitals, long-term care facilities. JAMA. 2013;309:1573–1574. doi: 10.1001/jama.2013.2922. [DOI] [PubMed] [Google Scholar]
- 3.Tzouvelekis LS, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz-Price LS, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yigit H, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001;45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp-Wallace KM, et al. Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase. Antimicrob. Agents Chemother. 2010;54:890–897. doi: 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers in Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12719. http://dx.doi.org/10.1111/1469-0691-12719. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann P, et al. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends in molecular medicine. 2012;18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Price LS, Quinn JP. The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids, and transposons. Clin. Infect. Dis. 2009;49:1739–1741. doi: 10.1086/648078. [DOI] [PubMed] [Google Scholar]
- 10.Cuzon G, et al. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 2010;16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwaber MJ, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 2011;52:848–855. doi: 10.1093/cid/cir025. [DOI] [PubMed] [Google Scholar]
- 12.Kitchel B, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 2009;53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmelnitsky I, et al. Unique genes identified in the epidemic extremely drug-resistant KPC-producing Klebsiella pneumoniae sequence type 258. J. Antimicrob. Chemother. 2013;68:74–83. doi: 10.1093/jac/dks370. [DOI] [PubMed] [Google Scholar]
- 14.Cottell JL, et al. Functional genomics to identify the factors contributing to successful persistence and global spread of an antibiotic resistance plasmid. BMC Microbiol. 2014;14:168. doi: 10.1186/1471-2180-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diancourt L, et al. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feil EJ, et al. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner KM, et al. Assessing the reliability of eBURST using simulated populations with known ancestry. BMC Microbiol. 2007;7:30. doi: 10.1186/1471-2180-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breurec S, et al. Klebsiella pneumoniae resistant to third-generation cephalosporins in five African and two Vietnamese major towns: multiclonal population structure with two major international clonal groups, CG15 and CG258. Clin Microbiol Infect. 2013;19:349–355. doi: 10.1111/j.1469-0691.2012.03805.x. [DOI] [PubMed] [Google Scholar]
- 19.Baraniak A, et al. Comparative population analysis of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases colonizing patients in rehabilitation centers in four countries. Antimicrob. Agents Chemother. 2013;57:1992–1997. doi: 10.1128/AAC.02571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade LN, et al. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob. Agents Chemother. 2011;55:3579–3583. doi: 10.1128/AAC.01783-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade LN, et al. Expansion and evolution of a virulent, extensively drug-resistant (polymyxin B-resistant), QnrS1-, CTX-M-2-, and KPC-2-producing Klebsiella pneumoniae ST11 international high-risk clone. J. Clin. Microbiol. 2014;52:2530–2535. doi: 10.1128/JCM.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Y, et al. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 2011;66:307–312. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 23.Ramos PI, et al. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genomics. 2014;15:54. doi: 10.1186/1471-2164-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLeo FR, et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, et al. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. MBio. 2014;5 doi: 10.1128/mBio.01355-14. e01355-e01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, et al. Multiplex real-time PCR for detection of an epidemic KPC-producing Klebsiella pneumoniae ST258 clone. Antimicrob. Agents Chemother. 2012;56:3444–3447. doi: 10.1128/AAC.00316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisse S, et al. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS ONE. 2009;4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, et al. Comparative genomics of Klebsiella pneumoniae strains with different antibiotic resistance profiles. Antimicrob. Agents Chemother. 2011;55:4267–4276. doi: 10.1128/AAC.00052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, et al. Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J. Bacteriol. 2012;194:1841–1842. doi: 10.1128/JB.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson DA, Enright MC. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 2004;186:1060–1064. doi: 10.1128/JB.186.4.1060-1064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez MS, et al. Multidrug-resistant (MDR) Klebsiella pneumoniae clinical isolates: a zone of high heterogeneity (HHZ) as a tool for epidemiological studies. Clin Microbiol Infect. 2012;18:E254–E258. doi: 10.1111/j.1469-0691.2012.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Duin D, et al. Surveillance of Carbapenem-Resistant Klebsiella pneumoniae: Tracking Molecular Epidemiology and Outcomes through a Regional Network. Antimicrob. Agents Chemother. 2014;58:4035–4041. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright MS, et al. Population Structure of KPC-Producing Klebsiella pneumoniae Isolates from Midwestern U.S. Hospitals. Antimicrob. Agents Chemother. 2014;58:4961–4965. doi: 10.1128/AAC.00125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush K. Proliferation and significance of clinically relevant beta-lactamases. Ann. N. Y. Acad. Sci. 2013;1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 35.D'Costa VM, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 36.Fevre C, et al. Six groups of the OXY beta-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob. Agents Chemother. 2005;49:3453–3462. doi: 10.1128/AAC.49.8.3453-3462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naas T, et al. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob. Agents Chemother. 2008;52:1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naas T, et al. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob. Agents Chemother. 2012;56:4753–4759. doi: 10.1128/AAC.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuzon G, et al. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob. Agents Chemother. 2011;55:5370–5373. doi: 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, et al. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 2013;57:5019–5025. doi: 10.1128/AAC.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice LB, et al. The KQ element, a complex genetic region conferring transferable resistance to carbapenems, aminoglycosides, and fluoroquinolones in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2008;52:3427–3429. doi: 10.1128/AAC.00493-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gootz TD, et al. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 2009;53:1998–2004. doi: 10.1128/AAC.01355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarno R, et al. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob. Agents Chemother. 2002;46:3422–3427. doi: 10.1128/AAC.46.11.3422-3427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leavitt A, et al. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 2010;54:4493–4496. doi: 10.1128/AAC.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, et al. Comparative genomic analysis of KPC-encoding pKpQIL-like plasmids and their distribution in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 2014;58:2871–2877. doi: 10.1128/AAC.00120-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Fernandez A, et al. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 2012;56:2143–2145. doi: 10.1128/AAC.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, et al. Molecular survey of the dissemination of two blaKPC-harboring IncFIA plasmids in New Jersey and New York hospitals. Antimicrob. Agents Chemother. 2014;58:2289–2294. doi: 10.1128/AAC.02749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen P, et al. Novel genetic environment of the carbapenem-hydrolyzing beta-lactamase KPC-2 among Enterobacteriaceae in China. Antimicrob. Agents Chemother. 2009;53:4333–4338. doi: 10.1128/AAC.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez SA, et al. Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin Microbiol Infect. 2011;17:1520–1524. doi: 10.1111/j.1469-0691.2011.03600.x. [DOI] [PubMed] [Google Scholar]
- 50.Naas T, et al. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013;68:1757–1762. doi: 10.1093/jac/dkt094. [DOI] [PubMed] [Google Scholar]
- 51.Chen L, et al. Complete nucleotide sequences of blaKPC-4- and blaKPC-5-harboring IncN and IncX plasmids from Klebsiella pneumoniae strains isolated in New Jersey. Antimicrob. Agents Chemother. 2013;57:269–276. doi: 10.1128/AAC.01648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bryant KA, et al. KPC-4 is encoded within a truncated Tn4401 in an IncL/M plasmid, pNE1280, isolated from Enterobacter cloacae and Serratia marcescens. Antimicrob. Agents Chemother. 2013;57:37–41. doi: 10.1128/AAC.01062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida AC, et al. Escherichia coli ST502 and Klebsiella pneumoniae ST11 sharing an IncW plasmid harbouring the bla (KPC-2) gene in an Intensive Care Unit patient. Int. J. Antimicrob. Agents. 2012;40:374–376. doi: 10.1016/j.ijantimicag.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Almeida AC, et al. First description of KPC-2-producing Klebsiella oxytoca in Brazil. Antimicrob. Agents Chemother. 2013;57:4077–4078. doi: 10.1128/AAC.02376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida AC, et al. First description of KPC-2-producing Pseudomonas putida in Brazil. Antimicrob. Agents Chemother. 2012;56:2205–2206. doi: 10.1128/AAC.05268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuzon G, et al. Wide dissemination of Pseudomonas aeruginosa producing β-lactamase blaKPC-2 gene in Colombia. Antimicrob. Agents Chemother. 2011;55:5350–5353. doi: 10.1128/AAC.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villegas MV, et al. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob. Agents Chemother. 2007;51:1553–1555. doi: 10.1128/AAC.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hidalgo-Grass C, et al. KPC-9, a novel carbapenemase from clinical specimens in Israel. Antimicrob. Agents Chemother. 2012;56:6057–6059. doi: 10.1128/AAC.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warburg G, et al. A carbapenem-resistant Klebsiella pneumoniae epidemic clone in Jerusalem: sequence type 512 carrying a plasmid encoding aac(6')-Ib. J. Antimicrob. Chemother. 2012;67:898–901. doi: 10.1093/jac/dkr552. [DOI] [PubMed] [Google Scholar]
- 60.Baraniak A, et al. Molecular characteristics of KPC-producing Enterobacteriaceae at the early stage of their dissemination in Poland, 2008–2009. Antimicrob. Agents Chemother. 2011;55:5493–5499. doi: 10.1128/AAC.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villa L, et al. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 2010;65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 62.Coque TM, et al. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 2008;14:195–200. doi: 10.3201/eid1402.070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Y, et al. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 2010;54:3967–3969. doi: 10.1128/AAC.00137-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, et al. Multiplex PCR for identification of two capsular types in epidemic KPC-producing Klebsiella pneumoniae sequence type 258 strains. Antimicrob. Agents Chemother. 2014;58:4196–4199. doi: 10.1128/AAC.02673-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deschamps C, et al. Multiple acquisitions of CTX-M plasmids in the rare D2 genotype of Escherichia coli provide evidence for convergent evolution. Microbiology. 2009;155:1656–1668. doi: 10.1099/mic.0.023234-0. [DOI] [PubMed] [Google Scholar]
- 66.Siu LK, et al. Virulence and plasmid transferability of KPC Klebsiella pneumoniae at the Veterans Affairs Healthcare System of New Jersey. Microb Drug Resist. 2012;18:380–384. doi: 10.1089/mdr.2011.0241. [DOI] [PubMed] [Google Scholar]
- 67.Tzouvelekis LS, et al. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob. Agents Chemother. 2013;57:5144–5146. doi: 10.1128/AAC.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cascales E, Christie PJ. The versatile bacterial type IV secretion systems. Nat Rev Microbiol. 2003;1:137–149. doi: 10.1038/nrmicro753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giltner CL, et al. Type IV pilin proteins: versatile molecular modules. Microbiol. Mol. Biol. Rev. 2012;76:740–772. doi: 10.1128/MMBR.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao DN, et al. Type III restriction-modification enzymes: a historical perspective. Nucleic Acids Res. 2014;42:45–55. doi: 10.1093/nar/gkt616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benenson S, et al. Comparison of two carbapenem-resistant Klebsiella pneumoniae clones: from a contained outbreak in a paediatric population and from a national epidemic. J. Antimicrob. Chemother. 2012;67:1651–1654. doi: 10.1093/jac/dks115. [DOI] [PubMed] [Google Scholar]
- 72.Croucher NJ, Klugman KP. The emergence of bacterial "hopeful monsters". MBio. 2014;5 doi: 10.1128/mBio.01550-14. e01550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudson CM, et al. Resistance Determinants and Mobile Genetic Elements of an NDM-1-Encoding Klebsiella pneumoniae Strain. PLoS One. 2014;9:e99209. doi: 10.1371/journal.pone.0099209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fouts DE, et al. Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS genetics. 2008;4:e1000141. doi: 10.1371/journal.pgen.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin AC, et al. Complete genome sequence of Klebsiella pneumoniae 1084, a hypermucoviscosity-negative K1 clinical strain. J. Bacteriol. 2012;194:6316. doi: 10.1128/JB.01548-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu KM, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J. Bacteriol. 2009;191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin SH, et al. Complete genome sequence of the 2,3-butanediol-producing Klebsiella pneumoniae strain KCTC 2242. J. Bacteriol. 2012;194:2736–2737. doi: 10.1128/JB.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolter DJ, et al. Phenotypic and enzymatic comparative analysis of the novel KPC variant KPC-5 and its evolutionary variants, KPC-2 and KPC-4. Antimicrob. Agents Chemother. 2009;53:557–562. doi: 10.1128/AAC.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen YT, et al. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J. Antimicrob. Chemother. 2013;69:628–631. doi: 10.1093/jac/dkt409. [DOI] [PubMed] [Google Scholar]
- 80.Li B, et al. First report of Klebsiella oxytoca strain coproducing KPC-2 and IMP-8 carbapenemases. Antimicrob. Agents Chemother. 2011;55:2937–2941. doi: 10.1128/AAC.01670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]