Abstract
Objective
To determine neonatal immunologic factors that correlate with mother-to-child-transmission of HIV-1.
Design
This case–control study compared cord blood natural killer (NK) and T-cell populations of HIV-1 exposed infants who subsequently acquired infection by 1 month (cases) to those who remained uninfected by 1 year of life (controls). Control specimens were selected by proportional match on maternal viral load.
Methods
Cryopreserved cord blood mononuclear cells (CBMCs) were thawed and stained for multiparameter flow cytometry to detect NK and T-cell subsets and activation status. CBMCs were also used in a viral suppression assay to evaluate NK cell inhibition of HIV-1 replication in autologous CD4+ T cells.
Results
Cord blood from cases contained a skewed NK cell repertoire characterized by an increased proportion of CD16−CD56+ NK cells. In addition, cases displayed less-activated CD16−CD56+ NK cells and CD8+ T cells, based on HLA-DR+CD38+ costaining. NK cell suppression of HIV-1 replication ex vivo correlated with the proportion of acutely activated CD68+CD16−CD56+ NK cells. Finally, we detected a higher proportion of CD27−CD45RA− effector memory CD4+ and CD8+ T cells in cord blood from cases compared with controls.
Conclusion
When controlled for maternal viral load, cord blood from infants who acquired HIV-1 had a higher proportion of CD16−CD56+ NK cells, lower NK cell activation and higher levels of mature T cells (potential HIV-1 targets) than control infants who remained uninfected. Our data provide evidence that infant HIV-1 acquisition may be influenced by both innate and adaptive immune cell phenotypes and activation status.
Keywords: CD38, HLA-DR, Kenya, mother-to-child HIV-1 transmission, natural killer cell, Tem, viral suppression assays
Introduction
Although HIV-1 transmission predominantly occurs at mucosal sites, repeated exposures are typically required for the establishment of an infection, making mucosal HIV-1 transmission a relatively rare event (reviewed in [1]). Transmitter infectiousness and host protective mechanisms lead to differential susceptibility of the host and ultimately influence the likelihood of a productive HIV-1 infection. Therefore, a better understanding of host immune factors associated with HIV-1 transmission or protection from transmission is needed to inform vaccine design. The mother-to-child HIV-1 transmission (MTCT) model provides a unique opportunity to explore cellular immune cofactors of HIV-1 transmission due to systematic access to transmitter viral load data and relative precision regarding timing of infection.
There is evidence that infant acquisition of HIV-1 infection is influenced by target cell activation status and location (reviewed in [2]). On one hand, clinical conditions associated with enhanced inflammation lead to increased HIV-1 susceptibility [3–5]. Further, cord blood lymphocyte activation in response to maternal helminth coinfection has been correlated with increased MTCT [6]. Alternatively, activation of immune cells and the presence of immune modulators that can inhibit HIV-1 replication, such as interferons or defensins, may protect infants from HIV-1 infection [7,8].
Natural killer (NK) cells are a component of the innate immune response and capable of identifying and killing virally infected cells. Three functionally distinct populations of NK cells can be defined on the basis of differential cell surface expression of CD16 and CD16+CD56+ cells make up to 90% of adult NK cells and contain high concentrations of perforin, reflecting their cytotoxic nature. NK cells lacking CD16, including CD56bright and CD56dim NK cells, make up approximately 10% of adult NK cells. This subset is poorly cytotoxic and is capable of producing large amounts of cytokines (reviewed in [9,10]). Finally, CD16+CD56−NK cells are thought to reflect an anergic or progenitor state of NK cells that are poorly cytotoxic and are expanded in HIV-1 or hepatitis C-infected adults [11–13]. To date, few studies have focused on the role of NK cells in HIV-1 transmission. Studies of highly HIV-1 exposed seronegative adults (HESNs) have shown that NK cells from HESNs have increased cytolytic activity and increased interferon-gamma (IFNγ), tumour necrosis factor-alpha (TNFα) and β-chemokine secretion compared with both unexposed controls and seroconverters [14]. In addition, maternal and infant HIV-1 peptide-specific NK cell responses have been associated with a reduced risk of MTCT [15]. Together, these findings suggest a role for NK cell functionality in protecting against HIV-1 infection.
In contrast to the beneficial antiviral effects associated with NK cell activation, an activated or mature T-cell repertoire likely enhances susceptibility to HIV-1, potentially through the generation of HIV-1 target cells, while a dormant or quiescent T-cell phenotype is critical in resisting HIV-1 infection [6,16–19]. Thus, immune activation may serve as a ‘double-edged sword’ in the context of HIV-1 infection, where maintaining an optimal balance between activated cells with antiviral capacity and activated HIV-1 target cells may be critical for preventing HIV-1 infection.
Here, we conducted a case–control study to compare NK and T cells in cord blood of infants who were HIV-1-uninfected at birth but acquired HIV-1 infection by 1 month to those who remained uninfected in order to determine associations of preexisting infant immune cellular parameters that associate with MTCT. Because maternal viral load is highly correlated with transmission [20], we controlled for maternal viral load to evaluate the contribution of cord blood NK and T-cell phenotypic subsets and activation status to infant HIV-1 transmission independent of maternal viral load.
Materials and methods
Cohort and specimen selection
This study used specimens from an MTCT cohort collected between 1999 and 2005 in Nairobi, Kenya, as described previously [21,22]. Women were recruited during pregnancy, provided written informed consent for participation and storage of specimens, and received zidovudine (ZDV) prophylaxis for preventing MTCT [23]. Maternal peripheral blood was collected at 32 weeks gestation, prior to initiation of ZDV, for baseline viral load. Infants were examined at birth, cord blood was collected at delivery and peripheral blood was collected within 48 h of life and at 1 month, 3 months and quarterly thereafter to test for HIV-1 infection status.
We used a case–control design based on proportional representation across quartiles of maternal viral load, as it is the most significant risk factor associated with vertical transmission [24]. Selected cases were HIV-1 uninfected at birth but HIV-1 infected by 1 month of age, had multiple cryopreserved vials of cord blood mononuclear cells (CBMCs) and CBMC viability more than 40% upon thaw (n = 7). One sample was lost during staining for the NK cell panel, and therefore, only six infant samples could be assessed for NK cell phenotype and activation status. Approximately four controls were selected to match maternal viral load quartile per case, while also meeting sample quality criteria above (n = 24). Selection criteria and viral load quartile cutoffs are detailed in the participant flow chart (supplemental digital content 1, http://links.lww.com/QAD/A505).
All components of this study were approved by the Kenyatta National Hospital Ethics and Research Committee and the University of Washington Institutional Review Board.
Cord blood collection and preservation
Approximately 40 ml of umbilical cord blood was collected by venipuncture after clamping the cord in two places. Cord blood mononuclear cells (CBMCs) were isolated by density gradient purification and washed in RPMI-1640 medium; lymphocytes were enumerated by morphology and were cryopreserved in 10% dimethyl sulfoxide-90% foetal calf serum (FCS; all Sigma-Aldrich, St. Louis, Missouri, USA).
Infant HIV-1 diagnosis
Infants were diagnosed with HIV-1 infection as previously described [21]. Briefly, an infant was considered HIV-1 infected if either HIV-1gag DNA was detected from blood spotted onto filter papers by PCR [25] or HIV-1 RNA was detected in plasma with the Gen-Probe HIV-1 Viral Load Assay (Gen-Probe Inc, San Diego, California, USA) [26]. Infection was considered peripartum if the birth specimen collected within 48 h of life had undetectable HIV-1 DNA or RNA and the 1-month specimen was HIV-1 DNA or RNA positive. All peripartum infections were later confirmed by retesting the birth plasma specimens using a real-time transcription-mediated amplification HIV-1 RNA viral load assay under development by Gen-Probe.
Cord blood mononuclear cell sample preparation and multiparameter flow cytometric phenotypic analysis
CBMCs were thawed according to the HIV-1 Vaccine Trials Network standard operating procedure [27]. Cell number and viability was determined using trypan blue (CellgroMediatech, Fisher, Pittsburgh, Pennsylvania, USA) exclusion, and samples with more than 40% viability were used for further analysis. Dead cells were identified and excluded using LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen, Eugene, Oregon, USA). All antibodies were from BD Bioscience, (San Jose, California, USA) unless otherwise noted. The gating strategy for both NK and T-cell subsets first selected singlets and viable cells. NK cells were then identified using anti-CD16 AlexaFluor647 (clone 3G8) and anti-CD56 PE-Cy5 (clone B159) while not expressing CD20 (anti-CD20 PerCPCy5.5, clone 2H7) or CD3 (anti-CD3 ECD, clone UCHT1; Beckman Coulter, Indianapolis, Indiana, USA), and appearing in a low-side scatter (SSC) lymphocyte gate. T cells were identified via anti-CD3 ECD (clone UCHT1; Beckman Coulter), anti-CD4 PE-Cy5 (clone RPA-T4) and anti-CD8 APC (clone RPA-T8). Anti-CD27 APC-Cy7 (clone O323; Biolegend, San Diego, California, USA) and anti-CD45RA PE (clone 5H9) were used to distinguish effector and memory populations. Anti-CD38 FITC (clone AT-1; StemCell Technologies, Vancouver, Canada), anti-CD69 PE (clone L78) and anti-HLA-DR PE-Cy7 (clone L243) were used to identify activated NK cells and T cells. Positivity gates were established using fluorescence-minus-one (FMO) staining for each panel. To evaluate cell populations in greater detail, a secondary gating strategy was undertaken to differentiate changes based on the fluorescence intensity. The CD56bright NK cells and CD56dim NK cells that have previously been demonstrated to be phenotypically and functionally distinct [28] were evaluated, as were the CD16brightCD56neg and CD16dimCD56neg populations (supplemental digital content 2, http://links.lww.com/QAD/A505). For all flow cytometric analyses, the conjugated antibodies were incubated with the cells for 30 min on ice prior to being fixed in 2% paraformaldehyde and acquired on a BD-LSRII.
Viral suppression assay
NK cell suppression of viral replication in autologous, activated CD4+ T cells was performed as previously described for CD8+ T cells [29]. Briefly, CBMCs were thawed and specimens with viability more than 66% were enriched by negative selection using Human CD4+T-cell Enrichment Kit (EasySep; StemCell Technologies). Selected cells (2 × 106 cells/ml) were stimulated with phytohemagglutinin (PHA; 2 μg/ml2; Remel, Thermo Scientific, Lenexa, Kansas, USA) and cultured in R10 supplemented with recombinant IL-2 (50 U/ml, R&D, Minneapolis, Minnesota, USA). After 3 days, cells were washed in R10 and infected with HIV-1CSFJR at an MOI of 0.01 using Viromag-magnetofection (OZ Biosciences, San Diego, California, USA). On day 3, autologous CBMCs were thawed for NK effector cell enrichment using the Human NK cell enrichment Kit (EasySep; StemCell Technologies). A minimum of triplicate cultures were plated in the presence of autologous effector NK cells at an effector:target ratio of 5 : 1 in 96-well plates. Uninfected CD4+ T cells alone served as a negative control. The level of HIV-1 p24 in the day 7 supernatants was quantified using an HIV-1 p24 Antigen ELISA Kit (Perkin Elmer, Waltham, Massachusetts, USA) and the ability of NK cells to suppress viral replication was calculated as follows:
Data analysis
Flow cytometry data were analysed using Flowjo (Treestar v. 8.8.6; Ashland, Oregon, USA) and statistical analyses were performed using GraphPad Prism (San Diego, California, USA). The Mann–Whitney U test was used to compare proportions of NK cell and T-cell population and NK cell subset populations were modelled as continuous variables (P-values are two-tailed, α = 0.05).
Results
Cohort description
Our case–control cohort (n = 31), selected on the basis of proportional matching of maternal viral load as described above (see supplemental digital content 1, http://links.lww.com/QAD/A505), included seven infants who acquired HIV-1 by 1 month of life (cases) and 24 infants who remained uninfected during the first year of life (controls). The mothers of infants selected into the cohort were young, moderately immunosuppressed, and their infants were of normal birth weight and maturity (Table 1). As a result of proportional maternal viral load selection, baseline maternal viral load measured at 32 weeks gestation did not differ significantly between cases and controls (5.0 vs. 5.1 log10 copies HIV-1 RNA/ml, P = 0.5). There were no other significant differences in maternal or infant characteristics (Table 1) [30].
Table 1.
Characteristics of the cohort.
| Cases median (IQR) or N (%) | Controls median (IQR) or N (%) | P* | |
|---|---|---|---|
| Maternal characteristics | N = 7 | N =24 | |
| Age (years) | 21 (19–28) | 27 (24–29) | 0.1 |
| Parity | 1 (1–2) | 1 (1–2) | 0.7 |
| Pregnancy CD4+ cell count | 365 (236–732) | 345 (242–495) | 0.8 |
| Pregnancy CD4+ cell percentage | 24 (10–33) | 22 (16–28) | 0.9 |
| Pregnancy HIV viral load, log10 copies/ml plasma | 5.0 (4.7 –5.7) | 5.1 (4.8–5.4) | 0.5 |
| Vaginal delivery | 6 (86%) | 19 (79%) | 1.0 |
| Infant characteristics | |||
| Birthweight (kg) | 3.5 (2.9–3.8) | 3.2 (2.9–3.4) | 0.3 |
| Dubowitz/Ballard score | 55 (48–57) | 56 (52–60) | 0.3 |
| Estimated maturity (weeks) | 39 (38–40) | 39 (39–40) | 0.3 |
| Bw04 HLA type | 4 (57%) | 16 (66%) | 0.6 |
| Month 1 plasma viral load, log10 copies/ml plasma | 6.5 (5.7 –7.2) | ND | |
IQR, interquartile range; ND, none detected.
P values determined using Kruskal–Wallis for 2 independent comparisons or Fisher’s exact test for mode of delivery.
Cord blood natural killer subsets and activation marker expression in HIV-1 exposed infants
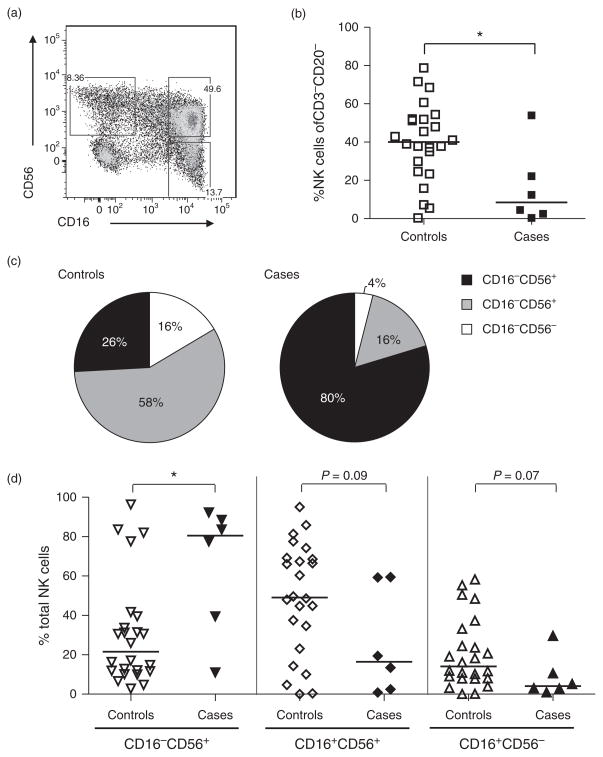
Cord blood from cases had a lower proportion of total NK cells, defined by the percentage of CD3−CD20− cells expressing CD16 and/or CD56 (median 8.5 compared with 40% for controls; P = 0.03; Fig. 1b). The NK cell subset distribution also differed between cases and controls (Fig. 1c), with infants who went on to acquire HIV-1 showing a significant overrepresentation of CD16−CD56+ NK cells (80.5 vs. 21.5%; P = 0.04, Fig. 1d), a phenotype associated with cytokine secretion [10]. Upon further assessment, this increase in the cases was determined to be primarily within the CD16−CD56dim NK cells in cases, whereas the CD56bright NK cells were similar between the two groups (supplemental digital content 2, http://links.lww.com/QAD/A505). Cases also exhibited a trend towards under-representation of CD16+CD56+ NK cells (16.5 vs. 49.1%; P = 0.09), a phenotype associated with cytotoxicity. Interestingly, cases trended towards underrepresentation of CD16+CD56− NK cells (4.1 and 14.1%; P = 0.07) compared with controls, and these findings were also found in the refined gating strategy analysis (supplemental digital content 2, http://links.lww.com/QAD/A505). This population has been previously described as immature [12,13,31,32], suggesting a potential protective effect of having a larger pool of NK cells able to undergo differentiation into mature NK cells associated with effector functions.
Fig. 1. Natural killer cell frequency and subset distribution in cord blood of HIV-1 uninfected infants grouped by subsequent HIV-1 acquisition.
Cord blood mononuclear cells were gated on singlets and live cells prior to exclusion of CD3+ and CD20+ lymphocytes. NK cells subsets were defined by the expression of surface markers CD56 and/or CD16 (a). The proportion of CD3−CD20− cells defined as NK cells found in cases and controls (b). The median frequencies of the phenotypic distribution of NK cell subsets shown for controls and cases (c). Detailed distribution of individual data points for comparison of NK cell subsets between controls and cases (d). Lines represent the medians for a given subset. Statistics were generated via a Mann–Whitney test (*P <0.05).
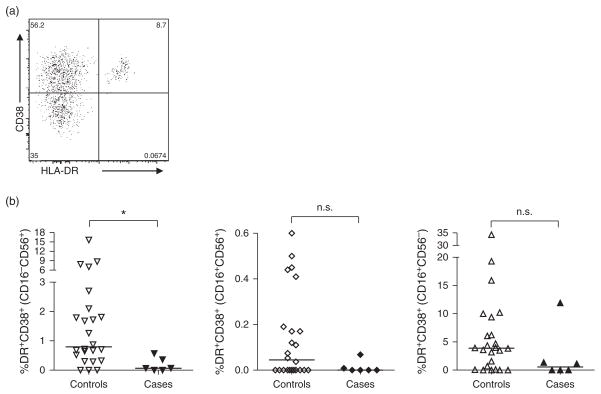
We also evaluated cord blood NK cell subsets for expression of surface markers associated with chronic activation, HLA-DR and CD38 (Fig. 2a). Although we observed a consistent trend towards decreased activation for all NK cell subsets in cases compared with controls, this reached statistical significance only in the CD16−CD56+ (activated/cytokine-secreting) NK subset (0.07% cases vs. 0.8% controls; P = 0.01; Fig. 2b). NK cell expression of the acute activation marker, CD69, was not different between cases and controls (data not shown).
Fig. 2. Natural killer cell activation status by subset.
NK cell subsets were assessed for coexpression HLA-DR and CD38 (a) as indicators of chronic activation in cases and controls in CD16−CD56+, CD16+CD56+ and CD16+CD56− NK cells (b). Lines represent medians for a given subset. Statistics were generated via a Mann–Whitney test (*P <0.05; n.s. P >0.1).
Cord blood natural killer cell suppression of HIV-1 replication in autologous CD4+ T cells
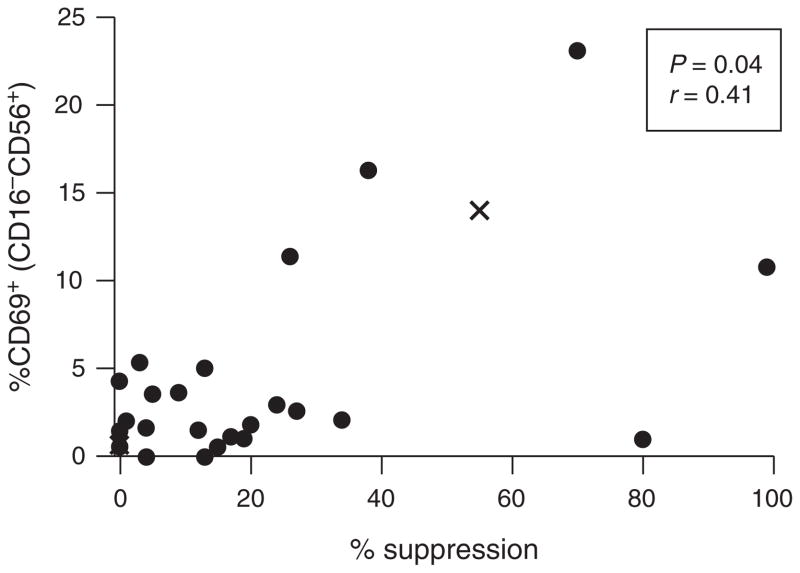
We performed a functional analysis to measure the ability of infant cord blood NK cells to suppress HIV-1 replication in autologous CD4+ T cells infected with HIV-1 in vitro. Bulk NK suppression of HIV-1 replication ranged from 0 to 99% in our cohort (Fig. 3). A requirement for a large number of cells and high cell viability limited our power to detect differences between cases and controls. We were, however, able to detect a correlation between the suppressive capacity of bulk NK cells and the percentage of acutely activated (CD69+) CD16−CD56+ NK cells (r = 0.41; P = 0.04; Fig. 3), consistent with the cytokine secretory capacity of this population. We did not detect any correlation between the capacity of bulk NK cells to suppress HIV-1 replication in autologous CD4+ T cells and the proportion or activation status of other NK subsets (supplemental digital content 3, http://links.lww.com/QAD/A505).
Fig. 3. Viral suppressive capacity of cord blood natural killer cells.
NK cells were assessed for their ability to suppress HIV-1 replication in autologous CD4+ T cells (n = 26). Suppressive capacity was correlated with NK activation (CD69+ NK cells). Each point represents the average suppressive capacity of NK cells from each cord blood sample’s replicates. Solid dots represent infants who remained HIV-1 uninfected (controls) while ‘x’s’ represent infants who subsequently became HIV-1 infected (cases). Statistics were generated using a Spearman correlation.
Cord blood T-cell activation and differentiation in HIV-1 exposed infants
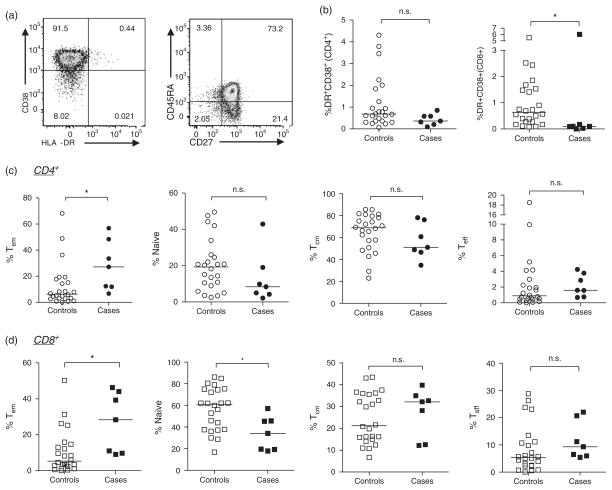
We were also interested in determining the relationship between T-cell maturation status, activation and HIV-1 acquisition (Fig. 4). Infants who acquired HIV-1 infection displayed similar proportions of bulk CD3+CD4+CD8− T cells (median 69.7 vs. 68.4%, respectively) and CD3+CD4−CD8+ T cells (24.8 vs. 27.2%, respectively) compared with those who remain uninfected (data not shown). Although the proportion of CD4+ T cells expressing both chronic activation markers HLA-DR and CD38 was similar between the groups, cases exhibited a significantly lower proportion of activated (HLA-DR+CD38+) CD8 T cells than controls (0.095 vs. 0.63, respectively; P = 0.02; Fig. 4b, right).
Fig. 4. CD4+ and CD8+ T-cell activation and maturation status in cryopreserved cord blood mononuclear cell by subsequent HIV-1 acquisition.
CD4+ or CD8+ lymphocytes were assessed for activation and maturation status by expression of HLA-DR and CD38 (a, left) as well as CD27 and/or CD45RA (a, right). Activation of CD4+ (b, circles, left) and CD8+ (b, squares, right) T cells between cases and controls as well as the proportion of cells in maturation states (from left to right): effector memory (CD27−CD45RA−), naive (CD27+CD45RA+), central memory (CD27+CD45RA−) or effector (CD27−CD45RA+) CD4+ T cells (c) or CD8+ T cells (d). CD4+ T cells are represented by circles and CD8+ T cells are represented by squares; controls are open symbols, cases, closed symbols. Lines represent medians for each group, and statistics were generated using a Mann–Whitney test (*P <0.05, n.s. P >0.1).
Our assessment of T-cell activation identified a very high percentage of cord blood T cells singly expressing CD38 (HLA-DR−) in all cord blood samples examined (supplemental digital content 4a, b, http://links.lww.com/QAD/A505). As CD38 can also serve as a marker of immaturity, this likely reflects the overall immature status of T cells in cord blood [33,34]. There was a trend towards an increased percentage of CD38+HLA-DR−CD4+ T cells in controls compared with cases (medians 95 vs. 91%, respectively; P = 0.08; supplemental digital content 4a, http://links.lww.com/QAD/A505) and a significantly increased proportion of CD8+CD38+ T cells in controls compared with cases (median 87 vs. 74%, P = 0.02), suggesting that cases have fewer immature CD4+ and CD8+ T cells than controls. The proportion of T cells expressing only HLA-DR was small and not significantly different between the groups (supplemental digital content 4c, d, http://links.lww.com/QAD/A505).
CD4+ and CD8+ T-cell populations were also assessed for perturbation in naive and memory phenotypes. Consistent with the finding that cases had a lower proportion of CD38+ (immature) T cells, these individuals had an increased frequency of effector memory (Tem) (CD27−CD45RA−) CD4+ T cells (27.2 vs. 6.3%; P = 0.02; Fig. 4c, left) and CD8+ T cells (28.4 vs. 5.3%; P = 0.01; Fig. 4d, left), and a concomitant lower frequency of naive (CD27+CD45RA+) CD8+ T cells (34.1 vs. 61%; Fig. 4d, second panel from left) than controls. Although cases also displayed a trend towards decreased CD4+ naive T cells, this did not reach statistical significance (Fig. 4c). The median frequencies of central memory (Tcm) (CD27+CD45RA−) and effector (Teff) (CD27−CD45RA+) phenotypes were similar between groups for bothCD4+ and CD8+ T cells (Fig. 4c, d).
Discussion
MTCT provides a unique setting for identifying factors critical for HIV-1 transmission, as it permits sampling of HIV-1 exposed individuals before transmission occurs, often within a month of the transmission event. This precision of preinfection sampling is less possible using adult HIV-1 transmission models. Further, this setting provides systematic access to the transmitter (mother) viral load. Using a historical MTCT cohort, we identified several cellular immune correlates of HIV-1 transmission. We found that cord blood from cases contained an elevated proportion of CD16−CD56+ NK cells, fewer activated HLA-DR+CD38+ CD16−CD56+ NK cells and CD8+ T cells and, finally, a higher proportion of Tem CD27−CD45RA− CD4+ and CD8+ T cells than the cord blood from controls. These data provide new insights into innate immune correlates of HIV-1 transmission and indirectly support the model of immune quiescence in resistance to HIV-1 infection, as cases had an increased proportion of Tem CD4+ T cells compared with controls.
Here, we found an expansion in CD16+CD56+ NK cells, which play a key role in the production of NK-derived cytokines (reviewed in [9,10]). When examined for CD56 expression levels, a difference in the proportion of CD16−CD56dim NK cells was observed with the cases containing higher levels; however, no change in CD56bright cells was observed between the groups (Supplemental Digital Content 2, http://links.lww.com/QAD/A505). Although CD56bright NK cells are most often associated with cytokine secretion, CD56dim NK cells are also important cytokine producers following target cell recognition [10]. Thus, the expansion of the CD16−CD56+ NK cell subset (to nearly 80% of NK cells, Fig. 1) in infants who go on to become HIV-1 infected may reflect the propensity of NK cell derived proinflammatory cytokines, such as interferon-γ and TNF-α, to activate HIV-1 target cells, ultimately facilitating HIV-1 transmission. In addition, this expansion was also associated with a contraction in the overall proportion of cytotoxic (CD16+CD56+) NK cells in cord blood of the case infants who went on to acquire HIV-1 infection, consistent with the hypothesis that NK cell killing functionality may be critical in defending against HIV-1 acquisition in the context of repeated exposures, such as MTCT. Together, our data argue that the relative frequency of NK cell subsets may be important in defending against HIV-1, with an advantageous outcome among infants with an expanded population of mature, cytotoxic, NK cells able to directly lyse virally infected target cells to limit cellular infection and viral dissemination.
Because infants in our cohort acquired HIV-1 by 1 month of life, acquisition likely occurred during delivery or through breastfeeding. HIV-specific antibodies commonly found in breast milk have been shown to mediate antibody-dependent cellular cytotoxicity (ADCC) and are associated with a reduced risk of MTCT [35]. Interestingly, in our study, both subsets of CD16+ NK cells expressing the Fc-γ receptor CD16 and therefore capable of mediating ADCC (CD56+ or CD56−) displayed trends towards underrepresentation in infants who went on to become infected compared with control infants. As NK cells are found at mucosal sites, including the gut mucosa [36], the capacity for NK cells to mediate ADCC in the context of exposure to HIV through breast milk may represent an important component of protection from infection.
The identification of a lower proportion of activated NK cells and CD8+ T cells in cord blood from infants who went on to become HIV-1 infected suggests that maintaining an activated repertoire of these cells, both non-HIV-1 targets, may be protective in the context of MTCT. Although we found a consistent trend towards lower activation of all NK cell subsets in infants who later acquired HIV-1, this only reached statistical significance in the cytokine-secreting (CD16−CD56+) NK population. Consistent with this observation, enhanced cytokine secretion and cytotoxicity by NK cells has previously been shown to correlate with protective immunity against HIV-1 transmission in adults [37]. Therefore, although cord blood from infants in our cohort had an expanded population of CD16−CD56+(cytokine-secreting) NK cells, they were less activated than NK cells in cord blood from controls, suggesting that activation of these cells, rather than solely their presence, may be important for protection from HIV-1 transmission. Together, these results suggest a beneficial effect of activated NK and CD8+ T cells in the context of repeated HIV-1 exposure.
Through the viral suppression assays, we found that cord blood NK cells from different individuals display a marked variation in the ability to suppress HIV-1 replication in autologous CD4+ T cells (Fig. 4; min 0% suppression to max 99% suppression), similar to a previous report of neonate and adult NK cells [38]. We also observed a modest correlation between the suppressive capacity of bulk NK cells and the percentage of acutely activated CD69+CD16−CD56+ NK cells from all infants in our cohort. This finding again suggests that activation of these cytokine-producing NK cells may be an important component for suppressing viral replication in this assay, in agreement with previous studies [12,38]. Further studies are warranted to examine the direct role of NK cell derived cytokines in HIV-1 transmission.
Finally, consistent with previous work [17], our assessment of T-cell subsets identified a significant increase in the proportion of CD4+ Tem cells in CBMC from infants who went on to become HIV-1 infected. As the HIV-1+ coreceptor, CCR5, is primarily expressed on Tem CD4 cells, this suggests a protective effect in having a lower proportion of mature, HIV-1 vulnerable T cells that can contribute to initial cellular infection as well as the spread of infection to draining lymph nodes in the periphery [39]. Although the underlying mechanism for alterations in these different immune cell parameters is not known, the maternal cytokine milieu may drive or support HIV-1 replication [40], or the activation or development of neonatal NK and T cells. Although the initial site of HIV-1 transmission in MTCT is likely mucosal, our findings derived from peripheral blood offer insight into potential protective mechanisms. Improved antenatal care to address underlying maternal health issues that induce an activated adaptive cell phenotype may prove a beneficial role in reducing foetal T-cell maturation and, thus, alter the risk of MTCT.
Perinatal transmission of HIV-1 is influenced by virologic and immunologic factors in both infant and the mother. To our knowledge, our study is the first to characterize the role of preexisting NK cell and T-cell immune status in influencing MTCT. We have identified three immune phenotypes in CBMC associated with an increased risk of MTCT during the first month of life, including an increased frequency of CD16−CD56+ NK cells, decreased activation of non-HIV-1 target NK and CD8+ T cells, and an increased frequency of Tem CD4+ cells, which may serve as HIV-1 target cells. Together, these findings provide evidence that the HIV-1 acquisition in infants is influenced by both innate and adaptive immune cell phenotypes and activation status.
Supplementary Material
Acknowledgments
This work was supported by the Elizabeth Glaser Pediatric AIDS Foundation grant MV-00-9-900-01872-0-00 (B.L.-P.), National Institutes of Health grants HD-23412 (G.J.-S.), TW-006080 (B.L.-P.), the University of Washington Center For AIDS Research grant AI 027757 and the Viral Pathogenesis Training Grant T32-AI083203-01 (M.A.G.). We thank the clinical, data and retention teams who operated the CTL study in Nairobi and Seattle from 1999 to 2006. We thank the women and infants of the CTL study for their participation. We thank Sandy Emery for the infant HIV-1 diagnosis, Steve DeRosa for reviewing the flow cytometry results, Sarah Rowland-Jones and Adriana Weinberg for helpful discussions, and Vasudha Sundaravaradan, Heather Jaspan and Lianna Wood for critical reading of the manuscript.
Footnotes
Conflicts of interest
The authors declare there are no conflicts of interest.
References
- 1.Dosekun O, Fox J. An overview of the relative risks of different sexual behaviours on HIV transmission. Curr Opin HIV AIDS. 2010;5:291–297. doi: 10.1097/COH.0b013e32833a88a3. [DOI] [PubMed] [Google Scholar]
- 2.Wood LF, Chahroudi A, Chen HL, Jaspan HB, Sodora DL. The oral mucosa immune environment and oral transmission of HIV/SIV. Immunol Rev. 2013;254:34–53. doi: 10.1111/imr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KE, Sherman ME, Ssempiija V, Tobian AA, Zenilman JM, Duggan MA, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS. 2009;23:1807–1815. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 5.Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 7.Lohman-Payne B, Slyker JA, Moore S, Maleche-Obimbo E, Wamalwa DC, Richardson BA, et al. Breast milk cellular HIV-specific interferon gamma responses are associated with protection from peripartum HIV transmission. AIDS. 2012;26:2007–2016. doi: 10.1097/QAD.0b013e328359b7e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 10.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson A, Bell F, Lejarcegui N, Mitchell C, Frenkel L, Horton H. Healthy neonates possess a CD56-negative NK cell population with reduced anti-viral activity. PLoS One. 2013;8:e67700. doi: 10.1371/journal.pone.0067700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verneris MR, Miller JS. The phenotypic and functional characteristics of umbilical cord blood and peripheral blood natural killer cells. Br J Haematol. 2009;147:185–191. doi: 10.1111/j.1365-2141.2009.07768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott-Algara D, Troung LX, Versmisse P, David A, Luong TT, Nguyen N, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 15.Tiemessen CT, Shalekoff S, Meddows-Taylor S, Schramm DB, Papathanasopoulos MA, Gray GE, et al. Cutting edge: unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–5918. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaren PJ, Ball TB, Wachihi C, Jaoko W, Kelvin DJ, Danesh A, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+T-cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;202 (Suppl 3):S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 17.Koning FA, Otto SA, Hazenberg MD, Dekker L, Prins M, Miedema F, et al. Low-level CD4+T-cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005;175:6117–6122. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 18.Begaud E, Chartier L, Marechal V, Ipero J, Leal J, Versmisse P, et al. Reduced CD4T-cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner K, Myrie L, Malhotra I, Mungai P, Muchiri E, Dent A, et al. Fetal immune activation to malaria antigens enhances susceptibility to in vitro HIV infection in cord blood mononuclear cells. J Infect Dis. 2010;202:899–907. doi: 10.1086/655783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obimbo EM, Wamalwa D, Richardson B, Mbori-Ngacha D, Overbaugh J, Emery S, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51:209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John-Stewart GC, Mbori-Ngacha D, Lohman-Payne B, Farquhar C, Richardson BA, Emery S, et al. HIV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J Infect Dis. 2009;199:889–898. doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otieno PA, Brown ER, Mbori-Ngacha DA, Nduati RW, Farquhar C, Obimbo EM, et al. HIV-1 disease progression in breast-feeding and formula-feeding mothers: a prospective 2-year comparison ofT-cell subsets, HIV-1 RNA levels, and mortality. J Infect Dis. 2007;195:220–229. doi: 10.1086/510245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaffer N, Chuahchoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomized clinical trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 24.Obimbo E, Wamalwa D, Richardson BA, Mbori-Ngacha D, Overbaugh J, Emery S, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. J Acquir Immune Defic Syndr. 2009;51:209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVange Panteleeff D, John GC, Nduati R, Mbori-Ngacha D, Richardson BA, Kreiss JK, et al. Rapid method for screening dried blood samples on filter paper for human immuno-deficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bull M, Lee D, Stucky J, Chiu Y-L, Rubin A, Horton H, et al. Defining blood procession parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Meth. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang OO. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 32.Gaddy J, Broxmeyer HE. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180:132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 33.D’Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197–203. [PubMed] [Google Scholar]
- 34.Rabian-Herzog C, Lesage S, Gluckman E. Characterization of lymphocyte subpopulations in cord blood. Bone Marrow Transplant. 1992;9 (Suppl 1):64–67. [PubMed] [Google Scholar]
- 35.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breast-milk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein HB, Kinter AL, Jackson R, Fauci AS. Neonatal natural killer cells produce chemokines and suppress HIV replicatioin in vitro. AIDS Res Hum Retrovirus. 2004;20:1189–1195. doi: 10.1089/aid.2004.20.1189. [DOI] [PubMed] [Google Scholar]
- 39.Groot F, van Capel TM, Schuitemaker J, Berkhout B, de Jong EC. Differential susceptibility of naive, central memory and effector memory T-cells to dendritic cell-mediated HIV-1 transmission. Retrovirology. 2006;3:52. doi: 10.1186/1742-4690-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King CC, Ellington SR, Kourtis AP. The role of co-infections in mother-to-child transmission of HIV. Curr HIV Res. 2013;11:10–23. doi: 10.2174/1570162x11311010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.