Abstract
Although the use of antihypertensive medications has been associated with reduced risk of Alzheimer’s disease (AD), it remains unclear which class provides the most benefit. The Cache County Study of Memory Health and Aging is a prospective longitudinal cohort study of dementing illnesses among the elderly population of Cache County, Utah. Using waves I to IV data of the Cache County Study, 3417 participants had a mean of 7.1 years of follow-up. Time-varying use of antihypertensive medications including different class of diuretics, angiotensin converting enzyme inhibitors, β-blockers, and calcium channel blockers was used to predict the incidence of AD using Cox proportional hazards analyses. During follow-up, 325 AD cases were ascertained with a total of 23,590 person-years. Use of any anti-hypertensive medication was associated with lower incidence of AD (adjusted hazard ratio [aHR], 0.77; 95% confidence interval [CI], 0.61–0.97). Among different classes of antihypertensive medications, thiazide (aHR, 0.7; 95% CI, 0.53–0.93), and potassium-sparing diuretics (aHR, 0.69; 95% CI, 0.48–0.99) were associated with the greatest reduction of AD risk. Thiazide and potassium-sparing diuretics were associated with decreased risk of AD. The inverse association of potassium-sparing diuretics confirms an earlier finding in this cohort, now with longer follow-up, and merits further investigation.
Keywords: Antihypertensive medications, Diuretics, Alzheimer’s disease
1. Introduction
Over the past 10 years, clinical trials of new treatments for Alzheimer’s disease (AD) have resulted in numerous failures and no approvals of new drugs by the U.S. Food and Drug Administration or the European Union European Medicines Agency (Becker et al., 2008; Greenberg et al., 2013). Although governments increasingly recognize the burgeoning public health burden of AD (Rosow et al., 2011), the promise for new breakthrough treatment or prevention strategies are uncertain at best, given the enormous resources needed for drug discovery and development (e.g., development times for new drug products often exceeding 13 years). New approaches are therefore needed to reduce timelines, decrease costs, and improve the prospects of approval for AD treatments.
In this era of declining resource availability, both public and private, drug repurposing may offer an efficient alternative to new drug development (Collins, 2011). This approach is not without its challenges (National Center for Advancing Translational Sciences, 2011). Yet, the broad class of antihypertensive medications offers multiple agents for investigation in AD, each having extensive safety data as well as pharmacologic, toxicological, and clinical data. Moreover, considerable literature describes a possible role of hypertension and hypertensive vasculopathy as risk factors for both vascular and Alzheimer’s dementia (Kivipelto et al., 2001; Posner et al., 2002; Skoog and Gustafson, 2006). Several studies have therefore examined the potential role of antihypertensive (anti-HTN) medications as modifiers of AD risk.
Prospective observational studies have generally found an inverse association between the use of antihypertensive therapy and risk of dementia (Guo et al., 1999; Haag et al., 2009; in’t Veld et al., 2001; Khachaturian et al., 2006; Ohrui et al., 2004; Sink et al., 2009). Three of these studies found a reduced risk specifically with diuretic use (Guo et al., 1999; Khachaturian et al., 2006; Yasar et al., 2013), whereas others reported similar findings with centrally active angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (Li et al., 2010; Ohrui et al., 2004; Sink et al., 2009). Unfortunately, the effects of antihypertensive therapy on dementia incidence in controlled trials have been conflicting (Anderson et al., 2011; Bosch et al., 2002; Diener et al., 2008; Forette et al., 1998; Lithell et al., 2003; Peters et al., 2008; The SHEP Cooperative Research Group, 1991; Tzourio et al., 2003), likely because of short follow-ups and the fact that incidence of dementia or cognitive decline were frequently secondary outcomes.
The Cache County Study is a large and well-characterized population-based study that has followed 5092 older adults up to 12 years since 1995. Using data collected from the first 2 assessments of the study (waves I and II), we reported that the use of anti-HTN medications was associated with reduced risk of AD over 4 years of follow-up (Khachaturian et al., 2006). However, our previous study only used anti-HTN medication information collected at baseline and had a follow-up period of only 3 years. Two more assessments (waves III and IV) on medication use have subsequently been completed over 7 additional years, allowing the calculation of time-varying exposure of medications. As a result, with more AD cases and longer follow-up, we reevaluate the association between anti-HTN medications and risk of AD.
2. Subjects and methods
2.1. Study overview
The Cache County Study on Memory Health and Aging is a prospective study of the prevalence and incidence of dementia among elderly adults living in Cache County, Utah. The details of study design have been described elsewhere (Breitner et al., 1999). Briefly, all residents of the county aged ≥65 years in 1995 were invited to participate, and 5092 (90%) completed baseline assessments and interviews in 1995–1997 (wave I). Living participants who did not meet criteria for dementia (n = 3024) were then asked to participate in wave II (1998–2000), III (2002–2004), and IV (2005–2007) assessments. We used data collected from all 4 waves for individuals who completed at least 1 follow-up assessment. A buccal sample for genetic material was collected at wave I, and information on various genetic and environmental risk factors including demographics, family history, and medical history was gathered at each wave via detailed interviews. All protocols were approved by the Institutional Review Boards of Utah State University, Duke University, and Johns Hopkins University. Spouses or next of kin gave informed consent when participants were unable to provide it.
2.2. Exposure assessment
Information about medication use was also obtained at each wave using a detailed inventory of all over-the-counter and prescription medications taken during the prior 2 weeks. For community-dwelling participants, interviewers inspected all available medication containers and asked participants questions about the form, dosage, start date, and duration of medication use. If participants were institutionalized, the information was obtained from nursing home medication records. Anti-HTN medications were classified into the following categories: ACE inhibitors, β-blockers, calcium channel blockers, or diuretics. Calcium channel blockers were further classified into dihydropyridines (e.g., nifedipine and amlodipine) or nondihydropyrines (e.g., verapamil, bepridil, diltiazem), whereas diuretics were classified into loop directics (e.g., furosemide, ethacrynic acid, bumetanide), thiazides (e.g., hydrochlorothiazide, chlorthalidone, etc.), and potassium-sparing agents (e.g., spironolactone, triamterene, amiloride). Participants who used more than 1 class of anti-HTN medication or anti-HTN combination pills were categorized as users of each of the individual classes.
2.3. Outcome assessment
At each evaluation, a multistage screening and assessment procedure was used to ascertain the presence and type of dementia. Participants were initially screened with a revised version (Tschanz et al., 2002) of the Modified Mini-Mental State examination (Teng and Chui, 1987) or, for those unable to complete the Modified Mini-Mental State examination, the Informant Questionnaire for Cognitive Decline in the Elderly (Jorm and Jacomb, 1989) was administered to a knowledgeable collateral informant. Participants with screening scores below a predetermined cutoff point were further evaluated (in waves I and II) using the Dementia Questionnaire (Silverman et al., 1986). Participants screening positive for possible cognitive impairment were referred for a detailed clinical evaluation. A 19% designated subsample of participants and all participants older than the age of 90 (waves I and II) or 85 years (waves III and IV) were also sent for a clinical evaluation regardless of their scores on the screening evaluations. The clinical evaluation was conducted by research nurses and psychometricians and included a clinical and medical history, family history of dementia, a brief physical examination, a structured neurologic examination, and a 1-hour battery of neuropsychological tests (Tschanz et al., 2000). Board-certified geriatric psychiatrists and neuropsychologists then reviewed the results of the clinical evaluations and assigned working DSM-III-R (Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition) (APA, 1987) diagnoses of dementia or other cognitive syndromes. Participants with working diagnoses of dementia or other cognitive disorders were asked to undergo a geriatric psychiatric examination and standard laboratory tests for differential diagnosis. Final diagnoses were adjudicated by a consensus panel of expert clinicians using DSM-III-R and National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al., 1984) for AD. Estimated age at onset of dementia was recorded as the year in which participants unambiguously met DSM-III-R criteria. The sensitivity and specificity of the above screening methods for detection of incident dementia have been estimated at 88.9% and 95.6%, respectively (Hayden et al., 2003). In a further validation sub-study, clinical diagnoses were confirmed by autopsy in 84% of the cases that went to autopsy (Plassman et al., 2006).
2.4. Covariates
Information on cardiovascular risk factors and disease was obtained at each wave. Participants were asked about a number of risk factors and history of cardiovascular events including diagnosis of hypertension, hypercholesterolemia, diabetes mellitus, stroke, coronary artery bypass graft, and myocardial infarction. A positive history of each condition was recorded if the participant was ever told by a doctor or nurse that he or she had the condition or received treatment for it. Data on smoking and drinking habits were captured at baseline as never, ever, or current. The response rate for donation of buccal DNA samples at baseline was 97%. APOE genotypes were determined using polymerase chain reaction amplification and a restriction isotyping following the methods described previously (Richards et al., 1993). APOE genotypes were not known to clinicians during the diagnostic process.
2.5. Statistical analysis
Differences in age, sex, education, APOE genotype, baseline smoking and drinking habits, and history of vascular risk factors were compared between anti-HTN medication users and nonusers. Continuous variables were examined using t-tests or analysis of variance, whereas categorical variables were examined via χ2 tests. Kaplan-Meier survival analysis and the log rank test were used to evaluate the differences in AD-free survival between the 2 groups. We then conducted extended Cox models to examine the relationship of time to AD and exposure to anti-HTN medication use while controlling for potential confounders. Anti-HTN medication use was captured as a time-dependent cumulative exposure, such that once a participant first reported using anti-HTN medications, he or she was classified as an anti-HTN medication user from that time forward. If a participant switched from 1 class of anti-HTN to another, he or she was classified as a user of both from that point forward. Age was used as the time-scale, with age at baseline as the origin. Participants who developed AD dementia were captured as having an event at the estimated age of dementia onset. Participants who survived without dementia or were lost to follow-up were censored at the age of their last wave of assessment, whereas participants who developed other forms of dementia were censored at their estimated age of dementia onset. We adjusted for potential confounders including age at baseline, gender, years of education, number of APOE ε4 alleles, baseline smoking and drinking habits, and history of stroke, hypercholesterolemia, diabetes, coronary artery bypass graft, and myocardial infarction. Results were presented as hazard ratios (HRs) with 95% confidence intervals (CIs) to provide relative risk of use of anti-HTN medications compared with nonusers although in the analyses for each class of anti-HTN medications, the reference group comprised participants who used other classes of anti-HTN medications or nonusers. To address the possibility of confounding by indication, we repeated the analyses but this time restricted the sample to only anti-HTN medication users. All analyses were performed using STATA version 11 software (Stata-Corp, College Station, TX, USA). Two-sided p-values <0.05 were considered statistically significant.
3. Results
Of the 5092 elderly individuals who completed a baseline assessment, 359 were found to have prevalent dementia and 1309 were lost to follow-up after wave I. These latter individuals tended to be male (χ2 = 6.05; p = 0.014), older (t = 19.7; p < 0.0001), and less well educated (t = −7.04; p < 0.0001). Thus, 3424 individuals completed a baseline evaluation and had at least 1 follow-up assessment, permitting their inclusion in the current analysis. Of these, 327 cases of incident AD were identified over a mean follow-up period of 5.39 years (range 0.02–12.08 years; standard deviation 3.17 years).
Only 7 participants (<1%, 2 with AD, 1 with another type of dementia, and 4 without a diagnosis of dementia) included in the current analysis had missing data on anti-HTN medication use. Among the remaining 3417 individuals with complete medication information, 1992 (58.3%) had used anti-HTN medications at some point. Of these, 801 (40.2%) had used an ACE inhibitor, 717 (36%) a β-blocker, 670 (33.6%) a calcium channel blocker (47.3% dihydropyridine type), and 1253 (62.9%) a diuretic (of these, 75% thiazides, 38% potassium-sparing, and 30.8% loop). About 66.8% of individuals had used more than 1 class of anti-HTN medications during the entire follow-up period.
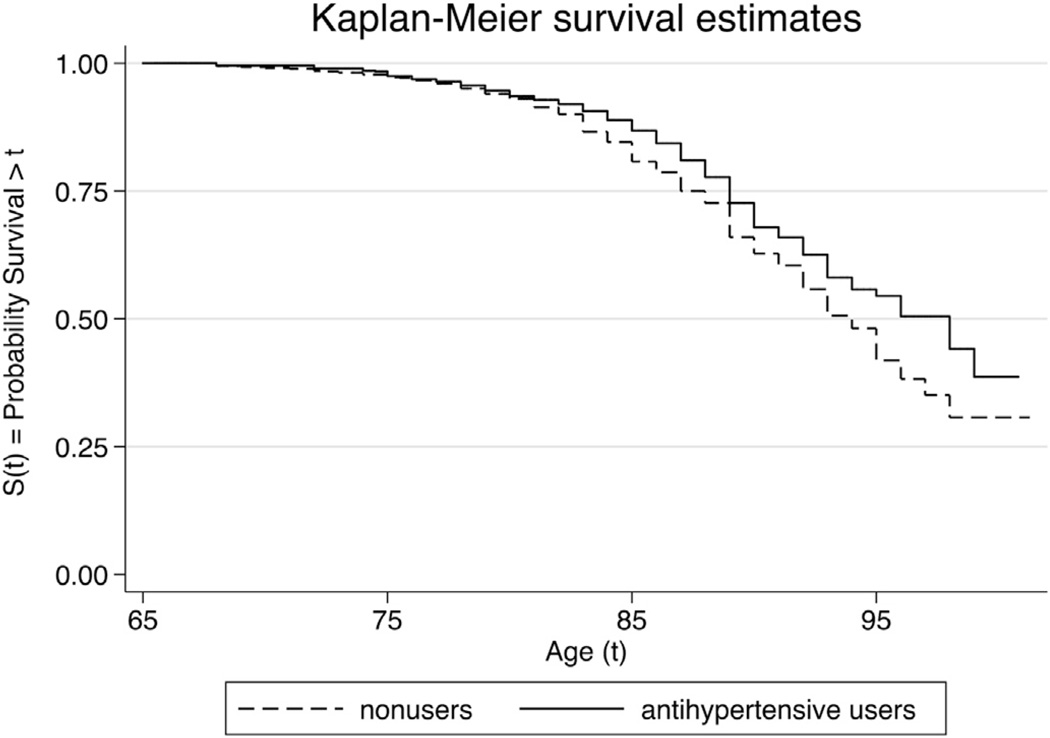
We compared the characteristics between anti-HTN medication users and nonusers to examine differences that might be related to risk of AD (Table 1). Anti-HTN medication users were more likely to be women and, as expected, to have a history of stroke, higher blood cholesterol, diabetes, myocardial infarction, or coronary artery bypass graft. AD-free survival Kaplan-Meier curves for participants with anti-HTN medication users versus nonusers are displayed in Fig. 1. Anti-HTN medication users had a significantly higher AD-free survival rate than nonusers (log rank test; p = 0.03).
Table 1.
Characteristics of study participants (n = 3417) by anti-HTN medication use
| Characteristics | Nonusers (n = 1425) | Anti-HTN medication users (n = 1992) | p |
|---|---|---|---|
| Male, n (%) | 662 (46.5) | 768 (38.6) | <0.001 |
| Age, (y) mean ± SD | 74.2 ± 6.5 | 74.9 ± 6.4 | 0.002 |
| Education, (y) mean ± SD | 13.6 ± 2.9 | 13.2 ± 2.9 | 0.002 |
| Smoking habits, n (%)a | |||
| Nonsmoker | 1138 (80.0) | 1619 (81.4) | 0.006 |
| Ever smoker | 237 (16.7) | 336 (16.9) | |
| Current smoker | 48 (3.4) | 33 (1.7) | |
| Drinking habits, n (%)a | |||
| Nondrinker | 1193 (83.9) | 1674 (84.2) | 0.515 |
| Ever drinker | 167 (11.7) | 243 (12.2) | |
| Current drinker | 62 (4.4) | 72 (3.6) | |
| Number of ε4 alleles, n (%)a | |||
| 0 | 938 (66.7) | 1392 (70.3) | 0.065 |
| 1 | 434 (30.9) | 538 (27.2) | |
| 2 | 35 (2.5) | 50 (2.5) | |
| Prevalence of vascular risk factors, n (%)a | |||
| Hypertension | 341 (33.2) | 1582 (85.1) | <0.001 |
| High cholesterol | 476 (46.3) | 985 (61.2) | <0.001 |
| Diabetes mellitus | 151 (15.6) | 486 (32.0) | <0.001 |
| Stroke | 82 (8.9) | 260 (17.9) | <0.001 |
| CABG | 63 (6.9) | 273 (18.6) | <0.001 |
| MI | 108 (11.4) | 446 (29.4) | <0.001 |
| Alzheimer’s disease, n (%) | 159 (11.2) | 166 (8.3) | 0.003 |
Key: CABG, coronary artery bypass graft; MI, myocardial infarction; SD, standard deviation.
This is the percentage of the total number with non-missing data.
Fig. 1.
Kaplan-Meier curves for Alzheimer’s disease-free survival for individuals with and without antihypertensive medication use.
The unadjusted and adjusted HRs from Cox proportional hazards model are shown in Table 2. In the unadjusted and adjusted (first for baseline characteristics and then baseline characteristics plus history of vascular factors) models, there was a significant lower risk of AD among anti-HTN medication users compared with nonusers (adjusted hazard ratio [aHR], 0.77; 95% confidence interval [CI], 0.61–0.97). The observed relationship between anti-HTN medication usage and AD did not vary significantly with reported duration of use (data not shown). The results of a sensitivity analysis, in which exposure to anti-HTN medications was modeled as a time-dependent but noncumulative exposure, were quantitatively similar to those of the primary analyses in which anti-HTN medication use was modeled as a time-dependent cumulative exposure as described previously in Section 2 (data not shown).
Table 2.
Association between anti-HTN medication use and incident AD from Cox proportional hazards model in the whole sample and in anti-HTN medication users
| Number of users | Number of users | AD/person-years | Whole samplea | Anti-HTN medicationb users | ||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR1 (95% CI)c | Adjusted HR2 (95% CI)d | Adjusted HR2 (95% CI) | |||
| Antihypertensive | 1992 | 166/12,212 | 0.78 (0.62–0.97)e | 0.75 (0.60–0.94)e | 0.76 (0.60–0.96)e | NA |
| ACE inhibitors | 801 | 59/4430 | 0.90 (0.68–1.19) | 0.91 (0.68–1.22) | 0.95 (0.71–1.29) | 1.06 (0.76–1.50) |
| β-Blockers | 717 | 56/4125 | 0.90 (0.67–1.20) | 0.86 (0.64–1.16) | 0.90 (0.67–1.21) | 1.02 (0.73–1.43) |
| Calcium channel blockers | 670 | 51/4115 | 0.77 (0.57–1.03) | 0.73 (0.54–0.98)e | 0.75 (0.55–1.04) | 0.85 (0.60–1.20) |
| Dihydropyridine | 317 | 22/1823 | 0.75 (0.49–1.16) | 0.69 (0.45–1.07) | 0.75 (0.48–1.17) | 0.84 (0.53–1.34) |
| Non-dihydropyridine | 373 | 32/2370 | 0.88 (0.61–1.27) | 0.87 (0.60–1.26) | 0.88 (0.60–1.30) | 0.99 (0.66–1.49) |
| Diuretics | 1253 | 95/7289 | 0.75 (0.59–0.96)e | 0.74 (0.58–0.95)e | 0.72 (0.56–0.93)e | 0.77 (0.56–1.06) |
| Loop | 386 | 36/1848 | 1.02 (0.72–1.45) | 1.00 (0.70–1.43) | 0.98 (0.67–1.43) | 1.09 (0.73–1.64) |
| Thiazide | 940 | 67/5697 | 0.74 (0.57–0.97)e | 0.71 (0.54–0.94)e | 0.70 (0.53–0.93)e | 0.75 (0.54–1.04) |
| Potassium sparing | 476 | 34/2869 | 0.69 (0.49–0.99)e | 0.67 (0.46–0.96)e | 0.69 (0.48–0.99)e | 0.74 (0.50–1.10) |
Key: ACE, angiotensin-converting enzyme; AD, Alzheimer’s disease; CABG, coronary artery bypass graft; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; NA, not applicable.
The reference group of each class of anti-HTNs comprises use of other anti-HTNs and nonusers.
The reference group of each class of anti-HTNs comprises use of other anti-HTNs.
Adjusted relative hazard estimated from models that control for age, sex, education, number of APOE ε4 alleles, and smoking and drinking habits at baseline.
Adjusted relative hazard estimated from models that control for variables above plus history of high cholesterol, diabetes, stroke, CABG, and MI.
p ≤ 0.05.
We further examined the associations between different classes of anti-HTN medications and risk of AD. There was a trend toward reduced risk of AD for calcium channel blockers (aHR, 0.75; 95% CI, 0.55–1.04) and a statistically significant reduction in the risk of AD for diuretics (aHR, 0.72; 95% CI, 0.56–0.93). Among subclasses of diuretics, the association was mainly driven by the use of thiazide diuretics (aHR, 0.71; 95% CI, 0.53–0.94) and potassium sparing diuretics (aHR, 0.70; 95% CI, 0.48–1.00), rather than loop diuretics (aHR, 0.98; 95% CI, 0.67–1.43).
We further investigated the associations of thiazide and potassium-sparing diuretics used alone and in combination with incident AD (Table 3). The use of thiazide and potassium-sparing diuretics in combination was associated with a significant decrease in AD risk (aHR, 0.63; 95% CI, 0.42–0.94). The use of either thiazide diuretics (aHR, 0.69; 95% CI, 0.47–1.02) or potassium-sparing diuretics (aHR, 0.56; 95% CI, 0.18–1.76) alone was associated with similar reductions in AD risk, but these associations were not significant, presumably because of smaller numbers particularly for the use of potassium-sparing diuretics alone. Moreover, the aHRs in these 3 groups were very similar suggesting that thiazide diuretics and potassium-sparing diuretics were equally associated with reduced risk of AD.
Table 3.
Association between thiazide and potassium-sparing diuretics and incident AD from Cox proportional hazards model in both the whole sample and anti-HTN users
| Drug category | Number of users | Whole samplea | Anti-HTN drug users | ||
|---|---|---|---|---|---|
| aHR (95% CI)b | p | aHR (95% CI)b | p | ||
| Thiazide and potassium-sparing diuretics in combination | 433 | 0.63 (0.42–0.94) | 0.023 | 0.69 (0.45–1.06) | 0.094 |
| Thiazide without potassium-sparing diuretics | 507 | 0.69 (0.47–1.02) | 0.061 | 0.78 (0.51–1.17) | 0.225 |
| Potassium-sparing without thiazide diuretics | 43 | 0.56 (0.18–1.76) | 0.322 | 0.64 (0.20–2.02) | 0.443 |
| Other anti-HTN drugs | 1054 | 0.88 (0.67–1.16) | 0.364 | REF | REF |
Key: AD, Alzheimer’s disease; aHR, adjusted hazard ratio; CABG, coronary artery bypass graft; CI, confidence interval; MI, myocardial infarction; NA, not applicable; REF, reference group.
The reference group is nonusers of anti-HTN drugs.
Adjusted relative hazard estimated from models that control for age, sex, education, number of APOE ε4 alleles, smoking and drinking habits at baseline, history of high cholesterol, diabetes, stroke, CABG, and MI.
Finally, we repeated the previously mentioned analyses restricted to anti-HTN medication users only (Tables 2 and 3). The results were qualitatively similar to those using the whole sample. The use of diuretics was still associated with a reduced risk of AD with marginal statistical significance, and this association was primarily driven by thiazide and potassium-sparing diuretics. A sensitivity analysis using data from only waves II–IV, which have not been described previously (Khachaturian et al., 2006) gave similar results (data not shown), demonstrating that the apparent protective effect of thiazide and potassium-sparing diuretics persisted throughout the entire follow-up of the study.
4. Discussion
In this large, prospective cohort study of older adults who were followed up to a maximum of 12 years, anti-HTN medication use was associated with a reduced risk of AD. The greatest effect was seen specifically with thiazide and potassium-sparing diuretics, which were associated with a 30% reduction in risk of AD. The observed associations were unchanged after adjustment for demographic differences and cardiovascular risk factors, suggesting they were not mediated by these other factors.
With longer follow-up and AD cases, the present study strengthens several previous findings, including our own (Khachaturian et al., 2006). For example, The Kungsholmen Project (Guo et al., 1999), a Swedish cohort of 1810 persons aged ≥75 years found that individuals taking diuretics had an adjusted relative risk of 0.7 for all-cause dementia. In the Ginkgo Evaluation of Memory study of a group of nondemented, community-dwelling older adults, use of diuretics was found to be associated with lower risk of AD dementia in participants with normal cognitive function or mild cognitive impairment (Yasar et al., 2013). So far, the HYVET-COG trial (Peters et al., 2008) is the only randomized-controlled trial testing the effect of diuretics on dementia. It was performed in a population of hypertensive patients older than 80 years and they were treated with a thiazide diuretic (indapamide) with or without an additional ACE inhibitor. Rates of dementia were 38 per 1000 patient-years in the placebo group versus 33 per 1000 patient-years in those taking antihypertensive therapy (hazard ratio: 0.86 [0.67–1.09]). Unfortunately, the effect did not reach statistical significance because the trial was terminated early (2.2 years) when the investigators found a substantial reduction in total mortality and stroke among the treatment group.
Several lines of evidence suggest that potassium levels might also have important effects on cognitive decline. Hypokalemia has been shown to be associated with decreased cerebrospinal fluid amyloid-beta (Abeta42), which is a biological marker of AD (Mielke et al., 2006). Potassium supplementation improves learning and memory deficits in chronic cerebral hypoperfused rats (Zhao et al., 2013). And, in the Hisayama Study, higher self-reported dietary intake of potassium was linked to lower risk of dementia (Ozawa et al., 2012). These results together with those from the Cache County Study suggest that further investigation into possible protective effects of potassium-sparing diuretics is also warranted.
Distinct from previous studies, our study has several notable strengths. First, because of the large sample size and long follow-up, we were able to examine the effects of different classes of anti-HTN medications over an extended period of time although controlling for important potential confounders such as age, gender, APOE genotype, and cardiovascular comorbidities. In addition, we are the first to apply time-varying exposure of each class of antihypertensive medications to predict AD. Second, misclassification of the outcome was minimized because of the standardized, multi-stage diagnostic procedure, supported by laboratory and neuroimaging studies and adjudicated by a panel of experts. This process has demonstrated good sensitivity and specificity for diagnosis of AD. Finally, selection bias is reduced in our study because we studied an entire population rather than a volunteer or convenience sample.
Our study also has several potential limitations. First, because this is an observational study, there is the possibility that its findings reflected measured or unmeasured confounding. We therefore attempted to control for important measured confounders in our analytic models, and this did not change the findings. To address further possibility of confounding by indication (i.e., the observed inverse association with potassium-sparing diuretics and thiazide diuretics was because of the indications for which these medications are taken), we have restricted the analysis only to participants who were taking anti-HTN. Second, it is possible that differential mortality among the users of different classes of anti-HTN may have explained the observed inverse associations. Indeed, anti-HTN users were more likely to die over the course of follow-up than nonusers (HR, 1.40; 95% CI, 1.13–1.73). This is expected because of the cardiovascular indications for taking anti-HTN in the first place. However, among all anti-HTN users, those taking potassium-sparing diuretics (HR, 0.99; 95% CI, 0.75–1.31) or thiazide (HR, 1.16; 95% CI, 0.92–1.48) were no more likely to die than users of other classes of anti-HTN medications. Third, we did not have blood pressure measurements for all participants, which prevented us from systematically examining whether blood pressure control mediated the apparent protective effects of the potassium-sparing diuretics and thiazide diuretics on AD risk. In our previous report, we were able to look at the blood pressure measurements in a small subgroup of participants and found that controlling for blood pressure did not change the findings, suggesting that this was not the case. Moreover, a recent study has showed the protective effect of antihypertensive drug on risk of dementia persisted even in individuals with high blood pressure (Yasar et al., 2013). Finally, this population is predominantly white and highly educated, which may limit the generalizability of results to the general population.
In summary, use of potassium-sparing diuretics and thiazide diuretics may decrease the risk of AD dementia. This finding supports the practice of using thiazide diuretics, especially when combined with potassium-sparing agents, as the first-line treatment for hypertension in the elderly individuals, yielding improved cognitive as well as cardiovascular outcome (ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group and The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, 2002). Most importantly, the current finding should motivate further research to determine whether these diuretics can be used in future for prevention of dementia.
Acknowledgements
The authors thank all the study participants for their contribution to the study. They are grateful to the neurogenetics laboratory of the Bryan Alzheimer’s Disease Research Center at Duke University for APOE genotyping. They thank Nancy Sassano, PhD (Utah State University); Linda Sanders, MPH and Michelle McCart, BA (Duke University); and Cara Brewer, BA, Tony Calvert, BSC, Tiffany Newman, BA, Roxane Pfister, MS, Russell Ray, MS, Sarah Schwartz, MS, Leslie Toone, MS, and Joslin Werstak, BA (Utah State University) for their expert technical assistance in study coordination and data collection, entry, and analysis. John C.S. Breitner, Kathleen Hayden, Chris Corcoran, JoAnn Tschanz, Maria Norton, Ron Munger, Kathleen Welsh-Bohmer, and Peter P. Zandi are funded by grants from the National Institutes of Health (R0I AG 11380). The work on this study was supported by a grant from the National Institute on Aging (R01 AG11380).
Disclosure statement
Yi-Fang Chuang, Yen-Ling Chiu, and Ara Khachaturian report no disclosures.
Appendix A
Coinvestigator appendix
Other Cache County Study investigators include: James Anthony, PhD (Michigan State University); Erin Bigler, PhD (Brigham Young University); Ron Brookmeyer, PhD (UCLA); James Burke, MD, PhD (Duke University); Eric Christopher, MD (Duke University); Jane Gagliardi, MD (Duke University); Robert Green, MD (Harvard University), Michael Helms, MS (Duke University); Christine Hulette, MD (Duke University); Ara Khachaturian (Alzheimer’s & Dementia), PhD, Liz Klein, MPH (Utah State University); Carol Leslie, MS (Utah State University); Constantine Lyketsos, MD, MHS (Johns Hopkins University); John Morris, MD (Washington University in Saint Louis); Chiadi Onyike, MD, MHS (Johns Hopkins University); Truls Ostbye, MD, PHD, MPH (Duke University); Ron Petersen, MD (Mayo Clinic); Kathy Piercy, PhD (Utah State University); Carl Pieper, Dr PH (Duke University); Brenda Plassman, PhD (Duke University); Peter Rabins, MD (Johns Hopkins University); Pritham Raj, MD (Duke University); Ingmar Skoog, MD, PhD (University of Gothenburg); David Steffens, MD, MHS (Duke University); Martin Steinberg, MD (Johns Hopkins University); Marty Toohill, PhD (Utah State University); Leslie Toone, MS (Utah State University); Jeannette Townsend, MD (University of Utah); Michael Williams, MD (Duke University); and Bonita Wyse, PhD (Utah State University).
References
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, Commerford P, Dyal L, Schumacher H, Pogue J, Paolasso E, Holwerda N, Chazova I, Binbrek A, Young J, Yusuf S. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-iii-r. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Becker RE, Greig NH, Giacobini E. Why do so many drugs for Alzheimer’s disease fail in development? Time for new methods and new practices? J. Alzheimers Dis. 2008;15:303–325. doi: 10.3233/jad-2008-15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, Davies R, Ostergren J, Probstfield J. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324:699–702. doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Collins FS. Mining for therapeutic gold. Nat. Rev. Drug Discov. 2011;10:397. doi: 10.1038/nrd3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW Prevention Regimen for Effectively Avoiding Second Strokes (PRo-FESS) study group. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Carrillo MC, Ryan JM, Gold M, Gallagher K, Grundman M, Berman RM, Ashwood T, Siemers ER. Improving Alzheimer’s disease phase II clinical trials. Alzheimers Dement. 2013;9:39–49. doi: 10.1016/j.jalz.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Guo Z, Fratiglioni L, Zhu L, Fastbom J, Winblad B, Viitanen M. Occurrence and progression of dementia in a community population aged 75 years and older: relationship of antihypertensive medication use. Arch. Neurol. 1999;56:991–996. doi: 10.1001/archneur.56.8.991. [DOI] [PubMed] [Google Scholar]
- Haag MD, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Duration of antihypertensive drug use and risk of dementia: a prospective cohort study. Neurology. 2009;72:1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC. Characteristics of a two-stage screen for incident dementia. J. Clin. Epidemiol. 2003;56:1038–1045. doi: 10.1016/s0895-4356(03)00247-6. [DOI] [PubMed] [Google Scholar]
- in’t Veld BA, Ruitenberg A, Hofman A, Stricker BH, Breteler MM. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol. Aging. 2001;22:407–412. doi: 10.1016/s0197-4580(00)00241-4. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch. Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J. Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Zandi PP, Blennow K, Gustafson D, Sjogren M, Rosengren L, Skoog I. Low serum potassium in mid life associated with decreased cerebrospinal fluid Abeta42 in late life. Alzheimer Dis. Assoc. Disord. 2006;20:30–36. doi: 10.1097/01.wad.0000201848.67954.7d. [DOI] [PubMed] [Google Scholar]
- National Center for Advancing Translational Sciences. NIH-Industry Round-table: Exploring New Uses for Abandoned and Approved Therapeutics. 2011 http://www.ncats.nih.gov/files/exploring_new_uses_for_abandoned_and_approved_therapeutics.pdf.
- Ohrui T, Matsui T, Yamaya M, Arai H, Ebihara S, Maruyama M, Sasaki H. Angiotensin-converting enzyme inhibitors and incidence of Alzheimer’s disease in Japan. J. Am. Geriatr. Soc. 2004;52:649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Ninomiya T, Ohara T, Hirakawa Y, Doi Y, Hata J, Uchida K, Shirota T, Kitazono T, Kiyohara Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama Study. J. Am. Geriatr. Soc. 2012;60:1515–1520. doi: 10.1111/j.1532-5415.2012.04061.x. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Khachaturian AS, Townsend JJ, Ball MJ, Steffens DC, Leslie CE, Tschanz JT, Norton MC, Burke JR, Welsh-Bohmer KA, Hulette CM, Nixon RR, Tyrey M, Breitner JC. Comparison of clinical and neuropathologic diagnoses of Alzheimer’s disease in 3 epidemiologic samples. Alzheimers Dement. 2006;2:2–11. doi: 10.1016/j.jalz.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad RB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum. Mol. Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Rosow K, Holzapfel A, Karlawish JH, Baumgart M, Bain LJ, Khachaturian AS. Countrywide strategic plans on Alzheimer’s disease: developing the framework for the international battle against Alzheimer’s disease. Alzheimers Dement. 2011;7:615–621. doi: 10.1016/j.jalz.2011.09.226. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am. J. Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC., Jr Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch. Intern. Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol. Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- The SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol. Behav. Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Skoog I, West N, Norton MC, Wyse BW, Nickles R, Breitner JC. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County study. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, Dekosky ST, Carlson MC Ginkgo Evaluation of Memory (GEM) Study Investigators. Antihypertensive drugs decrease risk of Alzheimer disease: ginkgo evaluation of memory study. Neurology. 2013;81:896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Xu S, Peng Y, Ji X, Cao D, Li J, Liu B, Shi Q, Wang L, Wang X. Potassium 2-(1-hydroxypentyl)-benzoate improves learning and memory deficits in chronic cerebral hypoperfused rats. Neurosci. Lett. 2013;541:155–160. doi: 10.1016/j.neulet.2013.01.053. [DOI] [PubMed] [Google Scholar]