Abstract
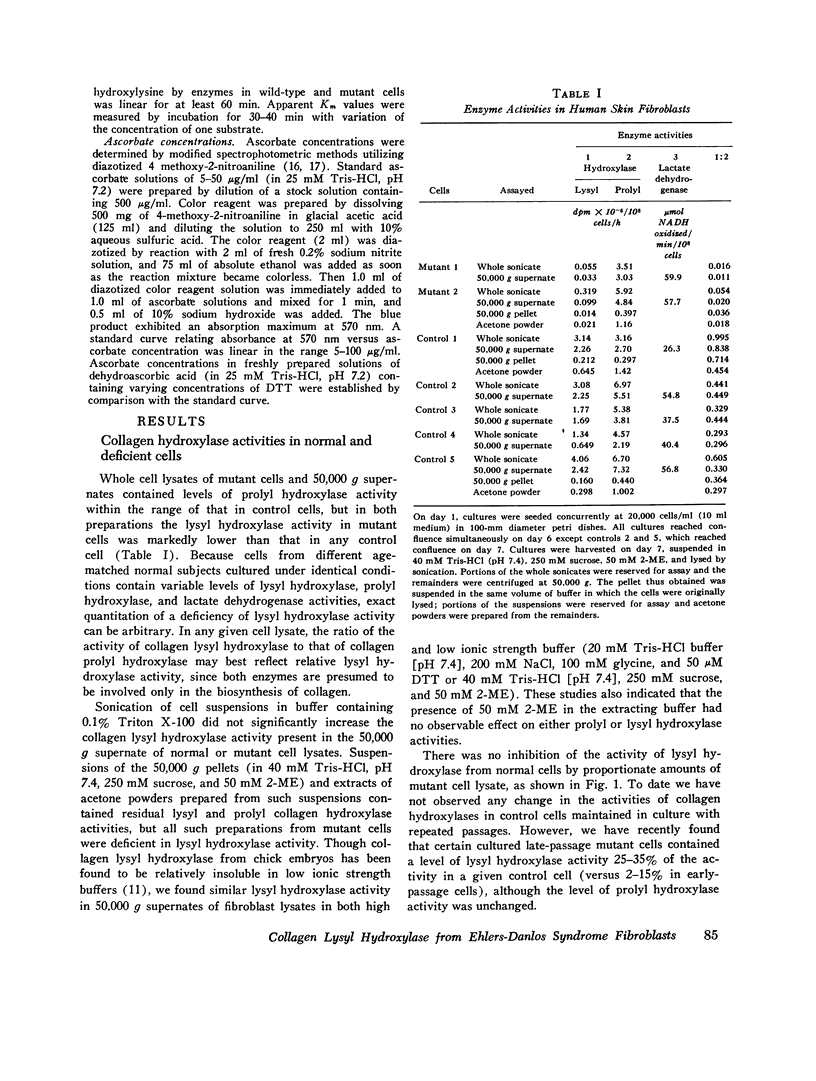
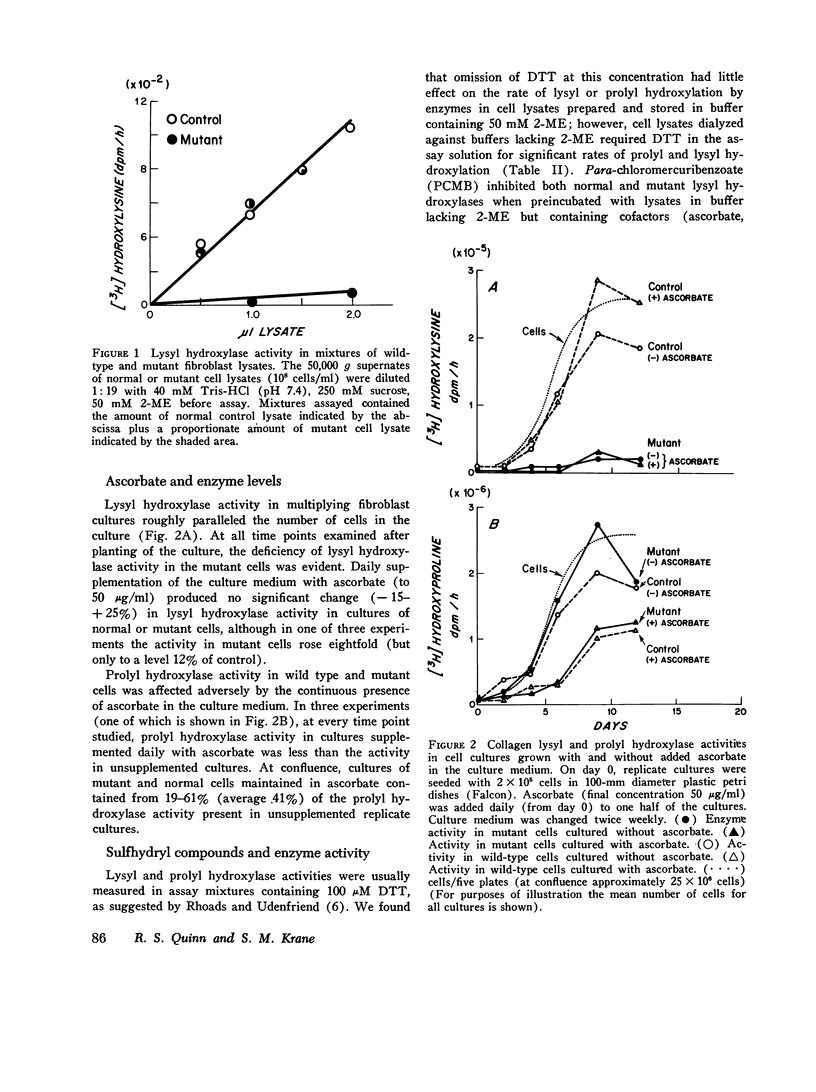
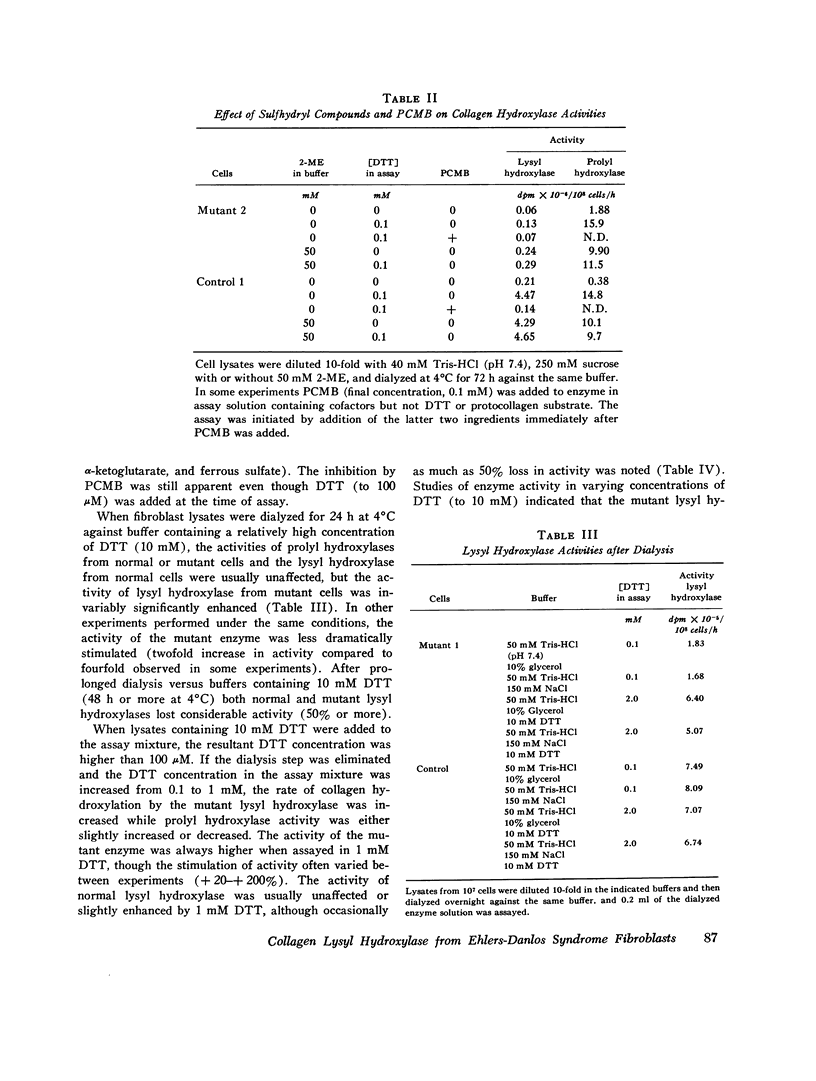
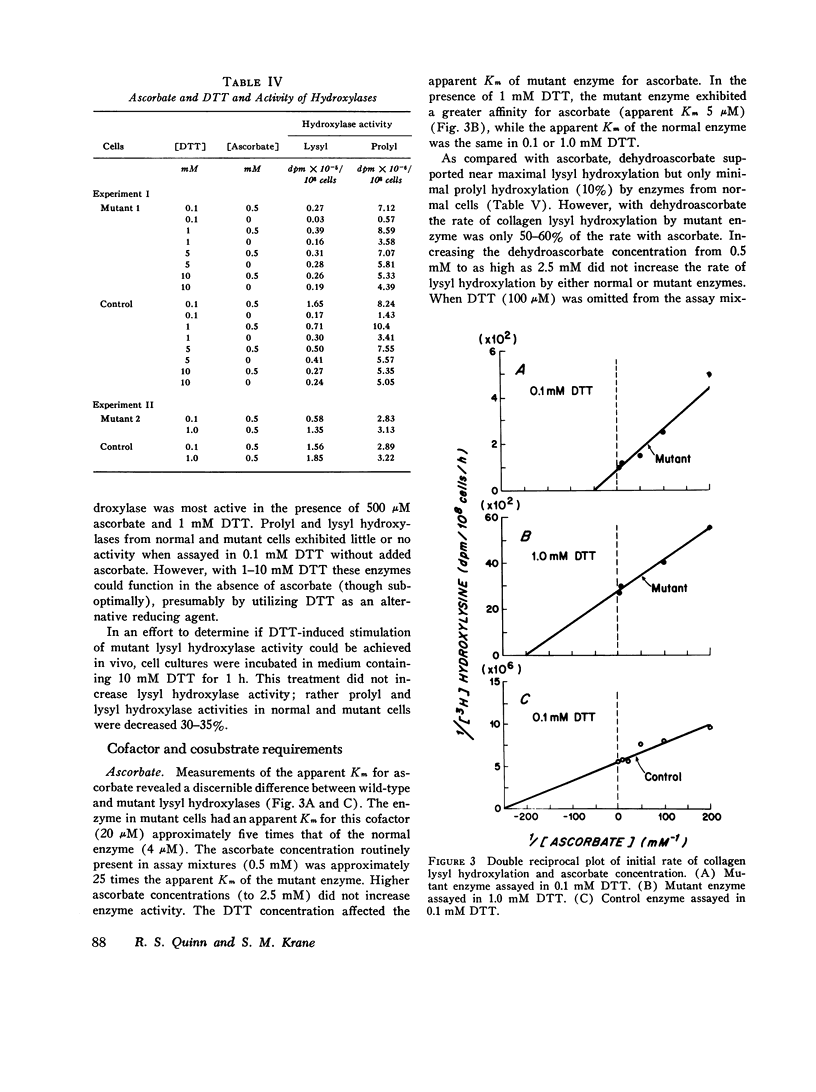
Skin fibroblasts from two siblings with hydroxylysine-deficient collagen collagen (Ehlers-Danlos syndrome, type VI) contained normal levels of collagen prolyl hydroxylase activity but were markedly deficient in collagen lysyl hydroxylase activity. The deficiency was evident in all fractions of cell lysates, in low and high ionic strength buffers, and in detergent. Assays of mixtures of wild-type and mutant cell lysates indicated no activation of mutant enzyme by factors in wild-type cells or inhibition of normal enzyme by material in mutant cells. Wild type or mutant cells cultured with ascorbic acid (50 mug/ml of culture medium, added daily) contained approximately the same level of lysyl hydroxylase activity as cells cultured without ascorbate, but prolyl hydroxylase activity without ascorbate was depressed in both an average of 41%. The mutant lysyl hydroxylase was less stable at 37 degrees C than the wild type and did not form high molecular weight aggregates in low ionic strength buffers, as did the control enzyme. The activity of the mutant enzyme was maximally stimulated after dialysis against buffer solutions containing 10 mM dithiothreitol. When assayed in 100 muM dithiothreitol, the mutant enzyme exhibited a higher apparent Km for ascorbate (20 muM) than the wild type (4 muM). In 1.0 mM dithiothreitol the mutant enzyme's apparent Km for ascorbate was reduced to 5 muM. Wild type and mutant enzymes had the same apparent Km for alpha-keto-glutarate (20 muM). The properties of prolyl hydroxylase in wild type and mutant cells were identical: apparent Km's for ascorbate and alpha-ketoglutarate were 100 muM and 20 muM, respectively. If mutant enzyme protein with altered kinetic properties is the only enzyme functioning to hydroxylate lysyl residues in collagen, the variations in hydroxylysine content observed in collagen from different tissues in the subjects reported here could be in part due to differences in cofactor concentrations and in rate and sequence of events in collagen synthesis in different tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates C. J., Prynne C. J., Levene C. I. Ascorbate-dependent differences in the hydroxylation of proline and lysine in collagen synthesized by 3T6 fibroblasts in culture. Biochim Biophys Acta. 1972 Oct 31;278(3):610–616. doi: 10.1016/0005-2795(72)90025-6. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Blumenkrantz N., Prockop D. J. A rapid assay for 14C-labeled hydroxylysine in collagen and related materials. Anal Biochem. 1969 Sep;30(3):377–385. doi: 10.1016/0003-2697(69)90130-4. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Reducible crosslinks in hydroxylysine-deficient collagens of a heritable disorder of connective tissue. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2594–2598. doi: 10.1073/pnas.69.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman N. A., Cutroneo K. R. Association of prolyl hydroxylase activity with membranes. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1263–1270. doi: 10.1016/0006-291x(73)90637-2. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Ryhänen L., Anttinen H., Bornstein P., Prockop D. J. Further hydroxylation of lysyl residues in collagen by protocollagen lysyl hydroxylase in vitro. Biochemistry. 1973 Nov 20;12(24):4966–4971. doi: 10.1021/bi00748a023. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R., Ruddle F. H. Cyclic changes in enzyme activity in synchronized mammalian cell cultures. Science. 1968 Feb 9;159(3815):634–636. doi: 10.1126/science.159.3815.634. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Pinnell S. R., Erbe R. W. Lysyl-protocollagen hydroxylase deficiency in fibroblasts from siblings with hydroxylysine-deficient collagen. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2899–2903. doi: 10.1073/pnas.69.10.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Langness U., Udenfriend S. Immunological evidence for an inactive precursor of collagen proline hydroxylase in cultured fibroblasts. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1585–1589. doi: 10.1073/pnas.68.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L. Chromatographic separation of the enzymes required for hydroxylation of lysine and proline residues of protocollagen. Arch Biochem Biophys. 1971 Nov;147(1):339–342. doi: 10.1016/0003-9861(71)90342-0. [DOI] [PubMed] [Google Scholar]
- Murphy L., Rosenbloom J. Evidence that chick tendon procollagen must be denatured to serve as substrate for proline hydroxylase. Biochem J. 1973 Sep;135(1):249–251. doi: 10.1042/bj1350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnell S. R., Krane S. M., Kenzora J. E., Glimcher M. J. A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N Engl J Med. 1972 May 11;286(19):1013–1020. doi: 10.1056/NEJM197205112861901. [DOI] [PubMed] [Google Scholar]
- Popenoe E. A., Aronson R. B. Partial purification and properties of collagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):380–386. doi: 10.1016/0005-2744(72)90229-x. [DOI] [PubMed] [Google Scholar]
- Popenoe E. A., Aronson R. B., Van Slyke D. D. The sulfhydryl nature of collagen proline hydroxylase. Arch Biochem Biophys. 1969 Sep;133(2):286–292. doi: 10.1016/0003-9861(69)90456-1. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S., Bornstein P. In vitro enzymatic hydroxylation of prolyl residues in the alpha 1-CB2 fragment of rat collagen. J Biol Chem. 1971 Jul 10;246(13):4138–4142. [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]
- Smith J. A. An improved method of ascorbic acid measurement in the ovarian ascorbic acid depletion assay. J Endocrinol. 1972 Nov;55(2):461–462. doi: 10.1677/joe.0.0550461. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., Udenfriend S. Activation of prolyl hydroxylase in L-929 fibroblasts by ascorbic acid. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1090–1093. doi: 10.1073/pnas.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M., Lichtenstein J. R., Nigra T. P., Martin G. R., McKusick V. A. Hydroxylysine-deficient skin collagen in a patient with a form of the Ehlers-Danlos syndrome. J Bone Joint Surg Am. 1974 Sep;56(6):1228–1234. [PubMed] [Google Scholar]
- Takeuchi T., Prockop D. J. Protocollagen proline hydroxylase in normal liver and in hepatic fibrosis. Gastroenterology. 1969 Apr;56(4):744–750. [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Biosynthesis of cartilage procollagen. Influence of chain association and hydroxylation of prolyl residues on the folding of the polypeptides into the triple-helical conformation. Biochemistry. 1974 Oct 22;13(22):4586–4591. doi: 10.1021/bi00719a018. [DOI] [PubMed] [Google Scholar]


